Key Points
Dasatinib-based 2-step induction resulted in a 100% CR rate with minimal toxicities and 53% MRD negativity.
This protocol treatment increased the number of HSCTs in CR1, thereby improving 3-year EFS.
Abstract
The standard treatment for adults with Philadelphia chromosome–positive (Ph+) acute lymphoblastic leukemia (ALL) in Japan is imatinib-based chemotherapy followed by allogeneic hematopoietic stem cell transplantation (HSCT). However, ∼40% of patients cannot undergo HSCT in their first complete remission (CR1) because of chemotherapy-related toxicities or relapse before HSCT or older age. In this study, we evaluated dasatinib-based 2-step induction with the primary end point of 3-year event-free survival (EFS). The first induction (IND1) was dasatinib plus prednisolone to achieve CR, and IND2 was dasatinib plus intensive chemotherapy to achieve minimal residual disease (MRD) negativity. For patients who achieved CR and had an appropriate donor, HSCT during a consolidation phase later than the first consolidation, which included high-dose methotrexate, was recommended. Patients with pretransplantation MRD positivity were assigned to receive prophylactic dasatinib after HSCT. All 78 eligible patients achieved CR or incomplete CR after IND1, and 52.6% achieved MRD negativity after IND2. Nonrelapse mortality (NRM) was not reported. T315I mutation was detected in all 4 hematological relapses before HSCT. Fifty-eight patients (74.4%) underwent HSCT in CR1, and 44 (75.9%) had negative pretransplantation MRD. At a median follow-up of 4.0 years, 3-year EFS and overall survival were 66.2% (95% confidence interval [CI], 54.4-75.5) and 80.5% (95% CI, 69.7-87.7), respectively. The cumulative incidence of relapse and NRM at 3 years from enrollment were 26.1% and 7.8%, respectively. Dasatinib-based 2-step induction was demonstrated to improve 3-year EFS in Ph+ ALL. This study was registered in the UMIN Clinical Trial Registry as #UMIN000012173.
Introduction
The standard treatment for adults with Philadelphia chromosome–positive (Ph+) acute lymphoblastic leukemia (ALL) is tyrosine kinase inhibitor (TKI)–based chemotherapy.1,2 Although promising results were reported using a chemotherapy-only strategy with a TKI plus hyperCVAD (cyclophosphamide, vincristine, doxorubicin, and dexamethasone administered as hyperfractionated therapy) at MD Anderson Cancer Center,3-5 a US intergroup study using dasatinib plus hyperCVAD followed by allogeneic hematopoietic stem cell transplantation (HSCT) demonstrated significantly superior survival advantages for patients undergoing transplantation.6 Therefore, for adults who achieve complete remission (CR), HSCT in the first CR (CR1) is recommended if they have an appropriate donor.
The introduction of TKIs in the treatment of Ph+ ALL led to a high rate of stable CR, which enabled more patients to undergo HSCT in CR1. However, severe therapy-related toxicities and relapse before HSCT and older age remain obstacles to undergoing HSCT in CR1.1,2,7 The Japan Adult Leukemia Study Group previously introduced imatinib into an HSCT-based strategy, which is currently the standard strategy for adults with Ph+ ALL in Japan.8-10 Dasatinib is a more potent inhibitor against BCR-ABL1 kinase than imatinib11,12 and is active against imatinib-resistant mutations, except for T315I.13-16 Therefore, dasatinib was expected to improve the efficacy of the current HSCT-based strategy using imatinib. However, a study comparing hyperCVAD plus dasatinib with hyperCVAD plus imatinib found no significant difference in long-term survival outcomes.17 Chemotherapy combined with a TKI in induction therapy should be selected in consideration of early therapy-related toxicities. A TKI plus intensive chemotherapy can lead to CR in >95% of patients and minimal residual disease (MRD) negativity in up to 70%, but rapid eradication of leukemic cells causes severe treatment-related toxicities, and the rate of nonrelapse mortality (NRM) has been reported to be 2% to 6%.8,9,18,19 A series of trials by the Gruppo Italiano Malattie Ematologiche dell’Adulto revealed that almost all patients, even elderly patients, can achieve CR using a TKI plus steroids without severe toxicities.20,21 Although achieving MRD negativity predicts long-term survival,17,22 the rate of MRD negativity with TKI plus steroid induction is low, resulting in relapse without effective consolidation.20 The Group for Research on Adult Acute Lymphoblastic Leukemia reported a randomized phase 3 study comparing imatinib plus reduced-intensity chemotherapy with imatinib plus hyperCVAD as induction therapy. Both arms were followed by imatinib plus high-dose methotrexate (MTX)/cytarabine therapy, and patients who had a donor were eligible for HSCT. Patients allocated to the imatinib plus reduced-intensity chemotherapy arm had a significantly higher CR rate and lower early death rate than those in the imatinib plus hyperCVAD arm.23 The Group for Research on Adult Acute Lymphoblastic Leukemia study demonstrated that intensive chemotherapy is not essential for induction with a TKI if followed by consolidations with a TKI plus intensive therapy. Thus, TKI plus steroid induction followed by a TKI plus intensive chemotherapy may reduce toxicities without attenuating the molecular response before HSCT and improve survival outcomes. Therefore, we designed the phase 2 Ph+ALL213 study to assess whether dasatinib could improve efficacy compared with imatinib and whether 2-step induction therapy could reduce toxicities, thereby improving 3-year event-free survival (EFS).
Patients and methods
Patients
Patients age between 15 and 64 years with newly diagnosed BCR-ABL1+ ALL were included in this study. Eligibility criteria were the same as in the previous Ph+ALL208 study. Patients were excluded if they had previous chronic-phase chronic myelogenous leukemia, other active malignancies, viral infections (HIV or hepatitis B surface antigen positivity), or concurrent diseases that may affect dasatinib toxicities. All patients provided written informed consent before enrollment.
Study design
This was a single-arm, multi-institutional phase 2 study. The protocol was reviewed and approved by the institutional review board of each participating institution and conducted according to the Declaration of Helsinki.
Study treatments
Treatment schedules are listed in Table 1 and were the same as in our previous Ph+ALL208 study,9 but dasatinib was used instead of imatinib, and the induction phase was separated into 2 steps. The first induction therapy (IND1) was started with prednisolone after the diagnosis of precursor B-cell ALL. In the case of BCR-ABL1 positivity, dasatinib was started on day 8 to achieve hematological CR. IND1 was followed by IND2 to achieve MRD negativity, at which time dasatinib was administered in combination with the same 4-drug intensive chemotherapy24 as in our previous studies.8,9 After IND2, 2 types of consolidation therapy, dasatinib in combination with high-dose MTX/cytarabine in C1 or a CHOP-like (cyclophosphamide, doxorubicin, vincristine, and prednisone) regimen in C2, were administered alternatingly for 4 cycles. Maintenance therapy was 12 cycles of dasatinib plus prednisolone with vincristine. Intrathecal injection of MTX and dexamethasone was administered on day 22 of IND1, on the first day of IND2, and at each consolidation. Dose modification for patients age 60 to 64 years is described in Table 1.
Treatment schedule in the JALSG Ph+ALL213 study
| Drug . | Dose . | Schedule . |
|---|---|---|
| Prephase | ||
| PSL | 60 mg/m2 per d orally | D −7 to −1 |
| IND1 | ||
| Dasatinib | 140 mg once per d orally | D 1-28 |
| PSL | 60 mg/m2 per d orally | D 1-14 and taper |
| CNS prophylaxis | 15 mg of MTX + 4 mg of dexamethasone IT | D 22 |
| IND2 | ||
| CPM | 1200 (900*) mg/m2, dip, 3 h | D 1 |
| DNR | 45 (30*) mg/m2, dip, 1 h | D 1-3 |
| VCR | 1.3 mg/m2 (max 2 mg/body) IV | D 1, 8, 15, 22 |
| PSL | 60 (45*) mg/m2 per d orally | D 1-21 and taper |
| Dasatinib | 100 mg once per d orally | D 4-31 |
| CNS prophylaxis | 15 mg of MTX + 4 mg of dexamethasone IT | D 1 |
| C1 | ||
| MTX | 1000 mg/m2, dip, 24 h | D 1 |
| Cytarabine | 2000 (1000*) g/m2, dip, 3 h, every 12 h | D 2-3 |
| mPSL | 50 mg/body, every 12 h IV | D 1-3 |
| Dasatinib | 100 mg once per d orally | D 4-24 |
| CNS prophylaxis | 15 mg of MTX + 4 mg of dexamethasone IT | D 1 |
| C2 | ||
| CPM | 1200 mg/m2, dip, 3 h | D 1 |
| DNR | 45 mg/m2, dip, 1 h | D 1 |
| VCR | 1.3 mg/m2 (max 2 mg/body) IV | D 1 |
| PSL | 60 mg/m2 per d orally | D 1-7 and taper |
| Dasatinib | 100 mg once per d orally | D 2-22 |
| CNS prophylaxis | 15 mg of MTX + 4 mg of dexamethasone IT | D 1 |
| Maintenance33 | ||
| VCR | 1.3 mg/m2 (max 2 mg/body) IV | D 1 |
| PSL | 60 mg/m2 per d orally | D 1-7 and taper |
| dasatinib | 100 mg once per d orally | D 1-28 |
| Post-HSCT dasatinib | ||
| Dasatinib | 50, 70, or 100 mg per d orally | D 1-28 every 35 d × 10 cycles |
| Posttherapy for molecular relapse | ||
| Dasatinib | 100 max 180 mg once per d orally | Until physician decision |
| Drug . | Dose . | Schedule . |
|---|---|---|
| Prephase | ||
| PSL | 60 mg/m2 per d orally | D −7 to −1 |
| IND1 | ||
| Dasatinib | 140 mg once per d orally | D 1-28 |
| PSL | 60 mg/m2 per d orally | D 1-14 and taper |
| CNS prophylaxis | 15 mg of MTX + 4 mg of dexamethasone IT | D 22 |
| IND2 | ||
| CPM | 1200 (900*) mg/m2, dip, 3 h | D 1 |
| DNR | 45 (30*) mg/m2, dip, 1 h | D 1-3 |
| VCR | 1.3 mg/m2 (max 2 mg/body) IV | D 1, 8, 15, 22 |
| PSL | 60 (45*) mg/m2 per d orally | D 1-21 and taper |
| Dasatinib | 100 mg once per d orally | D 4-31 |
| CNS prophylaxis | 15 mg of MTX + 4 mg of dexamethasone IT | D 1 |
| C1 | ||
| MTX | 1000 mg/m2, dip, 24 h | D 1 |
| Cytarabine | 2000 (1000*) g/m2, dip, 3 h, every 12 h | D 2-3 |
| mPSL | 50 mg/body, every 12 h IV | D 1-3 |
| Dasatinib | 100 mg once per d orally | D 4-24 |
| CNS prophylaxis | 15 mg of MTX + 4 mg of dexamethasone IT | D 1 |
| C2 | ||
| CPM | 1200 mg/m2, dip, 3 h | D 1 |
| DNR | 45 mg/m2, dip, 1 h | D 1 |
| VCR | 1.3 mg/m2 (max 2 mg/body) IV | D 1 |
| PSL | 60 mg/m2 per d orally | D 1-7 and taper |
| Dasatinib | 100 mg once per d orally | D 2-22 |
| CNS prophylaxis | 15 mg of MTX + 4 mg of dexamethasone IT | D 1 |
| Maintenance33 | ||
| VCR | 1.3 mg/m2 (max 2 mg/body) IV | D 1 |
| PSL | 60 mg/m2 per d orally | D 1-7 and taper |
| dasatinib | 100 mg once per d orally | D 1-28 |
| Post-HSCT dasatinib | ||
| Dasatinib | 50, 70, or 100 mg per d orally | D 1-28 every 35 d × 10 cycles |
| Posttherapy for molecular relapse | ||
| Dasatinib | 100 max 180 mg once per d orally | Until physician decision |
Cyclophosphamide (CPM) was diluted in 500 mL of normal saline. Daunorubicin (DNR) was diluted in 100 mL of normal saline. High-dose MTX and cytarabine were diluted in 500 mL of 5% glucose solution. High-dose MTX was followed by a rescue with 15 mg of folinic acid (IV) every 6 h 8 times, starting 36 h after starting MTX perfusion. C1 and C2 were alternatively repeated for 4 cycles (C1-1, C2-1, C1-2, C2-2, C1-3, C2-3, C1-4, C2-4).
CNS, central nervous system; IT, intrathecally; JALSG, Japan Adult Leukemia Study Group; mPSL, methyl-prednisolone; PSL, prednisolone; VCR, vincristine.
Dose modification for patients age >59 years.
Patients who achieved CR and had an appropriate donor underwent HSCT. The timing of HSCT was recommended to be during the consolidation phase, after the end of cycle 1 of the first consolidation (C1-1). The procedure for HSCT, including the conditioning regimen and prophylaxis for graft-versus-host disease (GVHD), was determined by each institution. We defined a reduced-intensity conditioning (RIC) regimen as having the following dosage levels: <9 mg/kg of busulfan, <140 mg/m2 of melphalan, and total-body irradiation (TBI) at <500 (single) or 500 to 800 cGy (fractionated).25 An RIC regimen was allowed when the patient was age ≥55 years or when myeloablative conditioning (MAC) was impossible because of poor physical condition. Patients who did not want to undergo HSCT or who had no satisfactory donor proceeded to the remaining rounds of consolidation and maintenance therapy. Patients with pretransplantation MRD positivity were assigned to receive prophylactic dasatinib after HSCT. For patients with pretransplantation MRD negativity, dasatinib was administered preemptively upon molecular relapse. Dasatinib was started at a dose of 50 mg per day and increased up to 100 mg if tolerable and continued for 4 weeks, which was repeated every 5 weeks for 10 cycles.
Detection of BCR-ABL1 transcripts and MRD monitoring
BCR-ABL1 positivity at presentation was confirmed by multiplex real-time quantitative polymerase chain reaction (RQ-PCR) at the central laboratory (SRL, Inc., Tokyo, Japan). Copy numbers of major and minor BCR-ABL1 messenger RNA RQ-PCR were normalized by glyceraldehyde 3-phosphate dehydrogenase expression and converted into copies per μg of RNA. MRD was categorized into the following three ranges: quantitative, nonquantitative, and negative with no significant signal. The threshold of quantitation was 50 copies per μg of RNA, corresponding to a sensitivity of 10−5.9
MRD was monitored centrally using bone marrow samples after IND1, IND2, C1-1, C2-4, and the last course of maintenance therapy. For patients who underwent HSCT, MRD was monitored centrally within 2 weeks before HSCT (pretransplantation MRD) and 30 days after HSCT (posttransplantation MRD). Reverse transcriptase nested PCR was assessed when pretransplantation MRD was negative to determine the sensitivity of RQ-PCR negativity. Mutation of the ABL1 gene was analyzed using direct sequencing when pretransplantation MRD remained within the quantitative range or at the first hematological relapse.
Response definition
CR and relapse were defined by standard criteria, as described in our previous study.9 CR with incomplete blood count recovery, defined as meeting all criteria for CR except platelet count and/or neutrophils, was used only for response evaluation after IND1. Molecular relapse was defined as the reappearance of a BCR-ABL1 signal, which was confirmed consecutively twice with an interval longer than 1 week. Hematological response to IND1 was evaluated on day 42, and molecular response to IND2 was evaluated on day 38.
End points and statistical analysis
The primary end point was 3-year EFS. Secondary end points were 3-year overall survival (OS); hematological response after IND1; MRD negativity rates after IND1, IND2, and C1-1 and MRD negativity rate pre- and posttransplantation; 3-year EFS and OS after HSCT; toxicities in IND1, IND2, C1-1, and C2-1; NRM; hematological and CNS relapse rates; frequencies and types of ABL1 mutations in patients with refractory or relapsed disease; and impact of risk factors at presentation and at HSCT. Toxicities were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0; version translated by Japan Clinical Oncology Group).
Statistical analysis was performed in all evaluable cases. EFS was defined as the time from the date of enrollment to an event, which was relapse or death resulting from any cause. Patients with no events were censored at the time of the last follow-up. OS was defined as the time from the date of enrollment to the date of death resulting from any cause. Patients who survived or were lost to follow-up were censored at the time of the last follow-up. Survival curves were plotted using the Kaplan-Meier method and compared by log-rank test. Fisher’s exact test was used for categorical variables. Cumulative incidence probabilities of relapse and NRM were calculated using a competing-risk setting treating events as follows: for relapse, death without relapse was the competing-risk event, and for NRM, relapse was the competing-risk event.26
Sixty-nine evaluable patients with Ph+ ALL were required to test the null hypothesis of 45% as the threshold and an alternative of 60%, with a 1-sided type 1 error of 5% and 80% statistical power. The threshold value of 45% was decided based on our previous Ph+ALL202 study. Considering a dropoff rate of 10%, 77 patients were targeted to be enrolled in this study.
Analyses were performed according to the intention-to-treat principle for all eligible patients using GraphPad Prism 6 (GraphPad Software, Inc., La Jolla, CA). The data cutoff date was 1 July 2019.
Results
Patient characteristics
Eighty-one patients were enrolled consecutively from 46 institutions between November 2013 and April 2016. Three patients were not included because of ineligibility (Ph− ALL), lack of prerequired central diagnostic test, or inappropriate informed consent. The baseline characteristics of 78 eligible patients are listed in Table 2. Their median age was 44.5 years (range, 16-64), with 25 patients (32.1%) age ≥55 years. Major-type BCR-ABL1 transcripts were observed in 22 patients (28.2%). Cytogenetic abnormalities (CAs) were noted in 69 patients (88.5%), and 50 (72.5%) had additional CAs.
Patient characteristics
| . | All patients . |
|---|---|
| Eligible patients | 78 |
| Median age (range), y | 44.5 (16-64) |
| ≥55 | 25 (32.1) |
| Sex | |
| Male | 37 (47.4) |
| Female | 41 (52.6) |
| ECOG PS | |
| 0 | 44 (56.4) |
| 1 | 29 (37.2) |
| 2 | 4 (5.1) |
| 3 | 1 (1.3) |
| Median WBC count (range), ×109/L | 32.5 (0.9-443.2) |
| >30 | 40 (51.3) |
| Median PB blast (range), % | 66.0 (0.0-98.0) |
| Median BM blast (range), % | 92.7 (0.0-100) |
| Surface marker | |
| CD10+ | 75 (96.2) of 78 |
| CD13 | 34 (43.5) of 78 |
| CD19 | 79 (100) of 79 |
| CD20 | 17 (21.5) of 79 |
| CD33 | 32 (41.0) of 78 |
| CD56 | 3 (3.8) of 78 |
| HLADR | 75 (98.7) of 76 |
| Cytoplasmic IgM* | |
| Positive | 49 (94.2) |
| Negative | 3 (5.8) |
| Not tested | 25 |
| Cytogenetics | |
| Isolated Ph positivity | 19 (24.4) of 78 |
| Ph+ and others (additional CAs) | 50 (64.1) of 78 |
| +Der(22)t(9;22) | 21 (26.6) of 78 |
| Deletion 7 | 10 (12.6) of 78 |
| Normal | 7 (9.0) of 78 |
| No mitosis | 2 (2.6) of 78 |
| BCR-ABL1 | |
| Breakpoints | |
| Minor | 56 (71.8) |
| Major | 17 (21.8) |
| Major + minor | 5 (6.4) |
| Copy number, copy/μg RNA | |
| Average minor (SD), × 105 | 5.5 (5.4) |
| Average major (SD), × 105† | 2.8 (1.3) |
| . | All patients . |
|---|---|
| Eligible patients | 78 |
| Median age (range), y | 44.5 (16-64) |
| ≥55 | 25 (32.1) |
| Sex | |
| Male | 37 (47.4) |
| Female | 41 (52.6) |
| ECOG PS | |
| 0 | 44 (56.4) |
| 1 | 29 (37.2) |
| 2 | 4 (5.1) |
| 3 | 1 (1.3) |
| Median WBC count (range), ×109/L | 32.5 (0.9-443.2) |
| >30 | 40 (51.3) |
| Median PB blast (range), % | 66.0 (0.0-98.0) |
| Median BM blast (range), % | 92.7 (0.0-100) |
| Surface marker | |
| CD10+ | 75 (96.2) of 78 |
| CD13 | 34 (43.5) of 78 |
| CD19 | 79 (100) of 79 |
| CD20 | 17 (21.5) of 79 |
| CD33 | 32 (41.0) of 78 |
| CD56 | 3 (3.8) of 78 |
| HLADR | 75 (98.7) of 76 |
| Cytoplasmic IgM* | |
| Positive | 49 (94.2) |
| Negative | 3 (5.8) |
| Not tested | 25 |
| Cytogenetics | |
| Isolated Ph positivity | 19 (24.4) of 78 |
| Ph+ and others (additional CAs) | 50 (64.1) of 78 |
| +Der(22)t(9;22) | 21 (26.6) of 78 |
| Deletion 7 | 10 (12.6) of 78 |
| Normal | 7 (9.0) of 78 |
| No mitosis | 2 (2.6) of 78 |
| BCR-ABL1 | |
| Breakpoints | |
| Minor | 56 (71.8) |
| Major | 17 (21.8) |
| Major + minor | 5 (6.4) |
| Copy number, copy/μg RNA | |
| Average minor (SD), × 105 | 5.5 (5.4) |
| Average major (SD), × 105† | 2.8 (1.3) |
Data are presented as n (%) unless otherwise indicated.
BM, bone marrow; ECOG PS, Eastern Cooperative Oncology Group performance status; PB, peripheral blood; SD, standard deviation; WBC, white blood cell.
Percentage of those tested.
Patients with major + minor types were included.
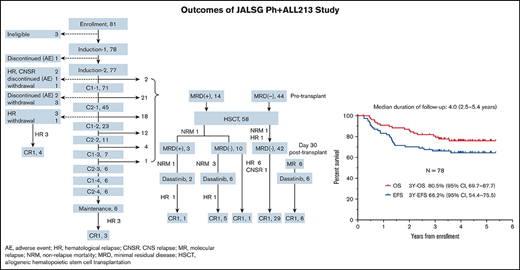
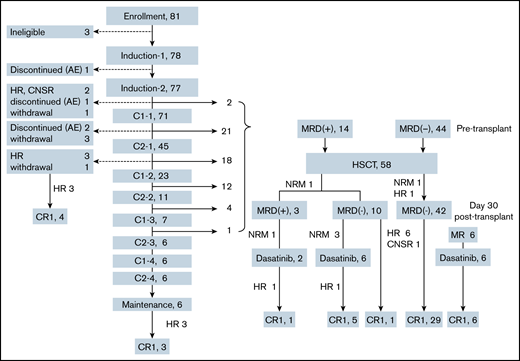
Patient flow and conditions
Patient flow and conditions are shown in Figure 1. Six patients (7.7%) completed the protocol chemotherapies without undergoing HSCT. Fifty-eight patients (73.4%) underwent HSCT in CR1. Five patients (6.4%) discontinued treatment before HSCT because of hematological relapse (n = 4) or CNS relapse (n = 1). Four patients (5.1%) withdrew from the protocol treatments because of severe toxicities (n = 3) or intolerable toxicities (n = 1). Five patients (6.4%) withdrew because of patient decision (n = 1), physician decision (n = 2), or transfer to nonparticipating institution (n = 2).
Patient flow and conditions. AE, adverse event; CNSR, central nervous system relapse; HR, hematological relapse; MR, molecular relapse.
Patient flow and conditions. AE, adverse event; CNSR, central nervous system relapse; HR, hematological relapse; MR, molecular relapse.
Hematological and molecular responses
Results of the treatments from IND1 to C2-1 are summarized in Table 3. At day 42 of IND1, all patients achieved either CR (n = 74) or CR with incomplete blood count recovery (n = 4). The MRD negativity rates after IND1, IND2, and C1-1 were 21.8%, 52.6%, and 57.7%, respectively. Among 58 patients who underwent HSCT in CR1, MRD negativity rates pre- and posttransplantation were 75.9% (44 of 58) and 94.5% (52 of 55), respectively. Among 14 patients with pretransplantation MRD positive, 7 had quantitative MRD and 7 had nonquantitative MRD. Among 44 patients with pretransplantation MRD negativity, 42 had posttransplantation MRD negativity.
Results of pretransplantation treatments (from IND1 to C2-1)
| Treatment . | n (%) . | Result . |
|---|---|---|
| IND1 | ||
| n of patients | 78 | |
| Rasburicase | 25 (32.1) | |
| Thrombomodulin-α | 23 (29.5) | |
| Dasatinib, dose down | 6 (7.7) | Transaminase elevation (n = 4), skin rash (n = 2) |
| Average total dose (SD), % of planned 3920 mg | 94 (13.6) | |
| D 8 PB blast <5% | 34 (43.6) | |
| D 22 PB blast <5% | 73 (93.6) | |
| D 42 response (CR + CRi) | 78 (100) | |
| CR | 74 (94.9) | |
| CRi | 4 (5.1) | |
| MRD negative* | 17 (21.8) of 78 | |
| IND2 | ||
| n of patients | 77 | |
| Delay of start | 13 (16.8) | Myelosuppression (n = 5), infection (n = 5), transaminase elevation (n = 1), social (n = 2) |
| Dasatinib, dose down | 3 (3.9) | FN (n = 1), unknown (n = 1), dose down as IND1 (n = 1) |
| Average total dose (SD), % of planned 2713 mg | 96.9 (14.6) | |
| MR* | 41 (56.2) of 73 | |
| Proceeded to HSCT | 2 (2.6) | |
| C1-1 | ||
| n of patients | 71 | |
| Delay of start | 20 (28.2) | Neutropenia (n = 8), infection (n = 4), liver damage (n = 2), social (n = 2), patient decision (n = 4) |
| Dasatinib, dose down | 8 (11.3) | Transaminase elevation (n = 3), other toxicities (n = 3), bridged to HSCT (n = 2) |
| Average total dose (SD), % of planned 2100 mg | 94.9 (16.1) | |
| MR* | 45 (63.4) of 71 | |
| Proceeded to HSCT | 21 (26.9) | |
| C2-1 | ||
| n of patients | 45 | |
| Delay of start | 6 (13.3) | Thrombocytopenia (n = 2), bridging to HSCT (n = 2), herpes infection (n = 1), patient decision (n = 1) |
| Dasatinib, dose down | 5 (11.1) | Transaminase elevation (n = 1), as previous course (n = 2), bridging to HSCT (n = 1), relapse (n = 1) |
| Average total dose (SD), % of planned 2100 mg | 91 (4.6) | |
| MRD negative* | 24 (64.9) of 37 | |
| Proceeded to HSCT | 18 (23.1) |
| Treatment . | n (%) . | Result . |
|---|---|---|
| IND1 | ||
| n of patients | 78 | |
| Rasburicase | 25 (32.1) | |
| Thrombomodulin-α | 23 (29.5) | |
| Dasatinib, dose down | 6 (7.7) | Transaminase elevation (n = 4), skin rash (n = 2) |
| Average total dose (SD), % of planned 3920 mg | 94 (13.6) | |
| D 8 PB blast <5% | 34 (43.6) | |
| D 22 PB blast <5% | 73 (93.6) | |
| D 42 response (CR + CRi) | 78 (100) | |
| CR | 74 (94.9) | |
| CRi | 4 (5.1) | |
| MRD negative* | 17 (21.8) of 78 | |
| IND2 | ||
| n of patients | 77 | |
| Delay of start | 13 (16.8) | Myelosuppression (n = 5), infection (n = 5), transaminase elevation (n = 1), social (n = 2) |
| Dasatinib, dose down | 3 (3.9) | FN (n = 1), unknown (n = 1), dose down as IND1 (n = 1) |
| Average total dose (SD), % of planned 2713 mg | 96.9 (14.6) | |
| MR* | 41 (56.2) of 73 | |
| Proceeded to HSCT | 2 (2.6) | |
| C1-1 | ||
| n of patients | 71 | |
| Delay of start | 20 (28.2) | Neutropenia (n = 8), infection (n = 4), liver damage (n = 2), social (n = 2), patient decision (n = 4) |
| Dasatinib, dose down | 8 (11.3) | Transaminase elevation (n = 3), other toxicities (n = 3), bridged to HSCT (n = 2) |
| Average total dose (SD), % of planned 2100 mg | 94.9 (16.1) | |
| MR* | 45 (63.4) of 71 | |
| Proceeded to HSCT | 21 (26.9) | |
| C2-1 | ||
| n of patients | 45 | |
| Delay of start | 6 (13.3) | Thrombocytopenia (n = 2), bridging to HSCT (n = 2), herpes infection (n = 1), patient decision (n = 1) |
| Dasatinib, dose down | 5 (11.1) | Transaminase elevation (n = 1), as previous course (n = 2), bridging to HSCT (n = 1), relapse (n = 1) |
| Average total dose (SD), % of planned 2100 mg | 91 (4.6) | |
| MRD negative* | 24 (64.9) of 37 | |
| Proceeded to HSCT | 18 (23.1) |
CRi, CR with incomplete blood count recovery; FN, febrile neutropenia; PB, peripheral blood; SD, standard deviation.
Percentage of those tested.
Toxicity
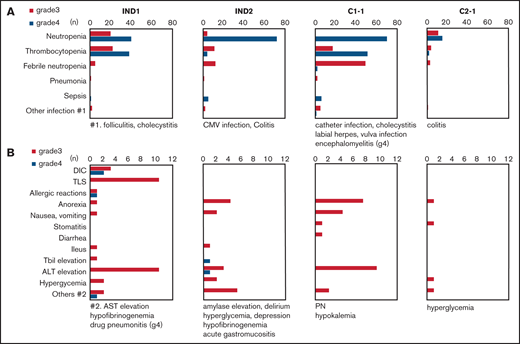
Grade 3/4 hematological and nonhematological toxicities from IND1 to C2-1 are shown in Figure 2. Major toxicities were myelosuppression, infections, and increased transaminase. Grade 3/4 pleural effusion and QTc elongation were not reported. NRM during chemotherapy was not reported. Toxicities in IND1 were relatively mild, but laboratory tumor lysis syndrome developed in 13%, even though rasburicase was used prophylactically in 32.1% of patients, and grade 3/4 disseminated intravascular coagulation developed in 8%. Grade 4 thrombocytopenia was reported in 48.7% of patients. One case of grade 4 allergic pneumonitis with high fever and eruption was noted. Toxicities in IND2 and C1-1 were more severe than those in IND1; grade 4 neutropenia and sepsis were reported in 94% and 5% of patients in IND2 and 99% and 9% of those in C1-1, respectively. However, grade 4 thrombocytopenia was noted in only 5.2% of patients in IND2, but in 71.8% of those in C1-1. Toxicities in C2-1 were mild.
Frequency of grade 3/4 toxicities. (A) Hematological toxicities and infections. (B) Nonhematological toxicities other than infections. No grade 3/4 pleural effusion or QTc elongation were reported. CMV, XXX; DIC, disseminated intravascular coagulopathy; PN, peripheral neuropathy; Tbil, total bilirubin; TLS, tumor lysis syndrome.
Frequency of grade 3/4 toxicities. (A) Hematological toxicities and infections. (B) Nonhematological toxicities other than infections. No grade 3/4 pleural effusion or QTc elongation were reported. CMV, XXX; DIC, disseminated intravascular coagulopathy; PN, peripheral neuropathy; Tbil, total bilirubin; TLS, tumor lysis syndrome.
Survival
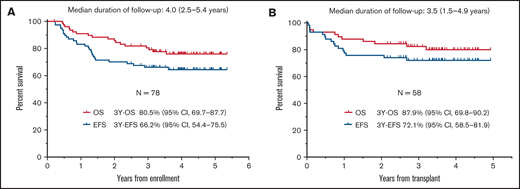
The median follow-up duration for survivors was 4.0 years (range, 2.5-5.4). The 3-year EFS and OS rates were 66.2% (95% confidence interval [CI], 54.4-75.5) and 80.5% (95% CI, 69.7-87.7), respectively (Figure 3A). The lower limit of the 90% CI of 3-year EFS was 56.4% and exceeded the threshold 3-year EFS. At a median follow-up of 3.5 years (range, 1.5-4.9), the 3-year EFS and OS rates in patients who underwent HSCT in CR1 were 72.1% (95% CI, 58.5-81.9) and 87.9% (95% CI, 69.8-90.2), respectively (Figure 3B).
Survival curves. EFS and OS of the 78 eligible patients (A) and of the 58 patients who underwent HSCT in CR1 (B).
Survival curves. EFS and OS of the 78 eligible patients (A) and of the 58 patients who underwent HSCT in CR1 (B).
Relapse and NRM
Eighteen patients relapsed during protocol treatment (23.1%), 5 during consolidation, 3 after maintenance therapy, and 10 after HSCT in CR1. Six patients died as a result of relapse (33.3%), 2 after maintenance therapy and 4 after HSCT in CR1. Six patients died as a result of NRM after HSCT in CR1 (7.7%), 1 from sepsis, 1 from sinusoidal obstruction syndrome, and 3 from infections after gut acute GVHD. Of 9 patients who withdrew from the protocol treatments, 3 died as a result of relapse and 3 died as a result of NRM after HSCT in CR1 or CR2. As a result, 21 (26.9%) of the eligible patients relapsed, 9 (11.5%) died as a result of relapse, and 9 (11.5%) died as a result of NRM after HSCT. The cumulative incidences of relapse and NRM at 3 years from enrollment were 26.1% (95% CI, 16.8-36.6) and 7.8% (95% CI, 3.2% to 15.2%), respectively.
HSCT
The median age of the 58 patients who underwent HSCT in CR1 was 43 years (range, 16-63), including 13 (22.4%) age ≥55 years. HSCT from related and unrelated donors accounted for 29.3% and 70.7%, respectively. Cord blood transplantation accounted for 20.7%. RIC regimens were used in 17 patients (29.3%). The most common regimen for MAC was cyclophosphamide/TBI (31 [75.6%] of 41), and that for RIC was fludarabine/melphalan/TBI (9 [52.9%] of 17). The median duration from enrollment to stem cell transplantation was 173.5 days (range, 84-381). Acute and chronic GVHD developed in 31 (53.4%) and 17 patients (29.3%), respectively.
Among 14 patients who were assigned to prophylactic dasatinib (Figure 1), 2 of 3 with posttransplantation MRD positivity received dasatinib at day 28 for 17 days and day 39 for 39 days. They did not respond to dasatinib, but 1 responded to donor lymphocyte infusion. Grade 3 and 4 thrombocytopenia and grade 3 gastrointestinal bleeding were reported. Six of 10 patients with posttransplantation MRD negativity received prophylactic dasatinib at a median of 50 days (range, 40-69) from HSCT, and all except 1 ended as MRD−. Grade 3 thrombocytopenia (n = 2), gastrointestinal bleeding (n = 1), and increased transaminase (n = 1) were reported. Among 44 patients who were assigned to preemptive dasatinib, 42 (87.5%) were posttransplantation MRD−; 29 (65.9%) did not require dasatinib, 7 (15.9%) relapsed before receiving dasatinib and did not respond to dasatinib, and 6 (13.6%) received dasatinib preemptively at a median of 303 days (range, 110-661) from HSCT and ended as MRD− after a median of 657 days (range, 32-1032). Grade 3 anemia (n = 1) and gastroenteritis (n = 1) were reported.
T315I mutation
T315I mutation was detected in all 4 patients who relapsed hematologically before HSCT. They underwent HSCT after withdrawing from the protocol treatments, and 3 achieved CR2. T315I mutation was not detected in 11 patients with pretransplantation MRD positivity. Four BCR-ABL1 mutations, 3 T315I mutations, and 1 F317I mutation were detected in 10 patients who relapsed after HSCT in CR1. Two achieved CR2 by the second HSCT. Overall, 5 (62.5%) of the 8 patients who relapsed with a BCR-ABL1 mutation underwent successful HSCT rescue.
Risk factors for survival outcomes
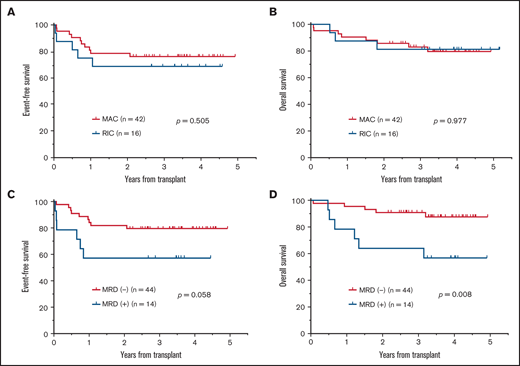
Patients with additional CAs at presentation did not have significantly different EFS (hazard ratio [HR], 2.04; 95% CI, 0.85-4.89; P = .179; Figure 4A), but they had significantly unfavorable OS (HR, 6.59; 95% CI, 2.27-19.09; P = .035; Figure 4B) compared with those with only isolated Ph positivity. Those patients with major BCR-ABL1 transcripts did not have significantly different EFS (HR, 0.65; 95% CI, 0.29-1.47; P = .349; Figure 4C) or OS (HR, 0.49; 95%CI, 0.18-1.36; P = .251; Figure 4D) from those with minor BCR-ABL1 transcripts. Among patients undergoing HSCT in CR1, the intensity of conditioning, MAC or RIC, did not result in significantly different EFS (P = .505; Figure 5A) or OS (P = .977; Figure 5B). Patients with pretransplantation MRD positivity did not have significantly different EFS (HR, 2.61; 95% CI, 0.74-9.19; P = .057; Figure 5C), but they had significantly unfavorable OS (HR, 4.39; 95% CI, 1.04-18.50; P = .008; Figure 5D) compared with those with pretransplantation MRD negativity. Those with pretransplantation MRD positivity had a similar relapse rate (18.2% vs 14.3%; P = 1.000) but had a significantly higher NRM rate (35.7% vs 2.3%; P = .002) than those with pretransplantation MRD negativity.
Impact of additional CAs and type of BCR-ABL1 transcript on survival outcomes. (A) EFS curves of patients with additional CAs and isolated Ph positivity; 3-year EFS rates from enrollment were 61.8% (95% confidence interval [CI], 46.8-73.7) and 77.8% (95% CI, 51.1-91.0), respectively. (B) OS curves of patients with additional CAs and isolated Ph positivity; 3-year OS rates from enrollment were 73.9% (95% CI, 59.3-83.9) and 100%, respectively. (C) EFS curves of patients with major BCR-ABL1 and minor BCR-ABL1 transcripts; 3-year EFS rates from enrollment were 75.9% (95% CI, 51.4-89.2) and 62.4% (95% CI, 48.4-73.7), respectively. (D) OS curves of patients with major BCR-ABL1 and minor BCR-ABL1 transcripts; 3-year OS rates from enrollment were 90.5% (95% CI, 67.0-97.5) and 76.7% (95% CI, 63.3-85.8), respectively.
Impact of additional CAs and type of BCR-ABL1 transcript on survival outcomes. (A) EFS curves of patients with additional CAs and isolated Ph positivity; 3-year EFS rates from enrollment were 61.8% (95% confidence interval [CI], 46.8-73.7) and 77.8% (95% CI, 51.1-91.0), respectively. (B) OS curves of patients with additional CAs and isolated Ph positivity; 3-year OS rates from enrollment were 73.9% (95% CI, 59.3-83.9) and 100%, respectively. (C) EFS curves of patients with major BCR-ABL1 and minor BCR-ABL1 transcripts; 3-year EFS rates from enrollment were 75.9% (95% CI, 51.4-89.2) and 62.4% (95% CI, 48.4-73.7), respectively. (D) OS curves of patients with major BCR-ABL1 and minor BCR-ABL1 transcripts; 3-year OS rates from enrollment were 90.5% (95% CI, 67.0-97.5) and 76.7% (95% CI, 63.3-85.8), respectively.
Impact of conditioning intensity for HSCT and MRD status at HSCT in CR1 on survival outcomes. (A) EFS for patients who received MAC or RIC. (B) OS for patients who received MAC or RIC. (C) EFS for patients who were MRD− or MRD+. (D) OS for patients who were MRD− or MRD+.
Impact of conditioning intensity for HSCT and MRD status at HSCT in CR1 on survival outcomes. (A) EFS for patients who received MAC or RIC. (B) OS for patients who received MAC or RIC. (C) EFS for patients who were MRD− or MRD+. (D) OS for patients who were MRD− or MRD+.
Discussion
This study demonstrated that dasatinib-based 2-step induction followed by HSCT is effective in improving 3-year EFS. By introducing dasatinib, all eligible patients achieved CR and 52.6% achieved MRD negativity after 2-step induction. Moreover, postremission relapse during chemotherapy developed in only 5 patients (6.4%), which was less than half of the 14.7% reported in the Ph+ALL208 study9 and 16.2% in the Ph+ALL202 study.8,10,27 All 4 hematological relapses occurred with T315I mutations. The rate of pretransplantation MRD negativity was 75.9% in this study and 65.0% in the Ph+ALL202 study. Two-step induction successfully reduced toxicities during induction. Grade 4 nonhematological toxicities accounted for <2%, and NRM, which was observed in 4.4% in the Ph+ALL208 study and 2.0% in the Ph+ALL202 study, was not reported. Thus, stable molecular remission with minimal toxicities achieved by dasatinib-based 2-step induction increased the number of patients undergoing HSCT in CR1 to 77.4% of eligible patients, which was higher than the 63.2% reported in the Ph+ALL208 study and 59.6% in the Ph+ALL202 study.27
Administration of TKIs before HSCT significantly improved the long-term survival outcomes for adults with Ph+ ALL.28-30 The 3-year EFS rates from HSCT were similar among the 4 studies: 72.1% in this study and 76% in the US intergroup study,6 which used dasatinib, and 71.4% in the Ph+ALL208 study and 59.9% in the Ph+ALL202 study, which used imatinib. No difference in EFS between imatinib and dasatinib was observed after HSCT when performed. HSCT has become more accessible because of the use of unrelated donors31 and RIC regimens.32-34 Compared with the Ph+ALL202 study, the significantly higher rate of RIC regimens (32.8% vs 10.2%; P = .011) increased the chance of HSCT for older patients. In this study, in addition to the 58 patients who underwent HSCT in CR1, all 5 patients who relapsed before HSCT and 6 of 9 who discontinued or withdrew from the protocol treatments underwent HSCT. As a result, 69 (88.5%) of the eligible patients underwent HSCT in CR1 (n = 64) and CR2 (n = 5). The number of patients who died as a result of relapse after HSCT was 5 (7.2%) of 69, whereas 9 (13.0%) of 69 died as a result of NRM after HSCT. HSCT was used maximally in this study, but the indication for HSCT has always been a subject of discussion. HSCT in CR1 has not been the standard choice of therapy for pediatric patients with Ph+ ALL.35,36 The Children’s Oncology Group AALL062 trial, which used dasatinib and intensive chemotherapy for 60 pediatric patients with Ph+ ALL (median age, 10.2 years; range, 1.5-27.6), reported 5-year ESF and OS rates of 60% and 86%, respectively. HSCT was recommended for patients with a matched sibling donor or with high-risk features based on MRD, and 19 patients underwent HSCT. Patients receiving chemotherapy and dasatinib only and those undergoing HSCT had similar 5-year EFS (60% vs 61%) and OS rates (88% vs 83%).36 Pediatric studies raised the clinical question of whether younger adults need to undergo HSCT in CR1.
Regarding risk factors for survival outcomes at presentation, additional CAs were reported as a risk factor for relapse.37-39 In this study, patients with additional CAs did not have significantly different EFS, but they had significantly unfavorable OS compared with those with isolated Ph positivity (Figure 4A-B). The impact of BCR-ABL1 type on survival outcome is controversial.27,40 In this study, patients with the major BCR-ABL1 type did not have significantly different EFS or OS from those with the minor BCR-ABL1 type (Figure 4C-D). Nishiwaki et al41 recently reported that patients with Ph+ ALL with multilineage BCR-ABL1, which was present in 81% of cases of the major type and 19% of cases of the minor type, had significantly better EFS and OS than those with Ph+ ALL with unilineage BCR-ABL1. Studies focusing on the prevalence and prognosis of patients with multilinage BCR-ABL1 are needed. Regarding risk factors at HSCT, the presence of pretransplantation MRD was associated with significantly unfavorable OS (Figure 5C-D). Two retrospective studies using registry data30,42 and 1 retrospective analysis on the Ph+ALL202 study27 reported an unfavorable impact of pretransplantation MRD positivity on relapse rate. However, the patients with pretransplantation MRD positivity in this study had a similar relapse rate to those with pretransplantation MRD negativity. This may be related to patients with pretransplantation MRD positivity having more frequent NRM than those with pretransplantation MRD negativity (35.7% vs 2.3%; P = .002), which may attenuate the relapse rate. The cause of frequent NRM in 5 patients with pretransplantation MRD positivity was not identified.
Posttransplantation TKIs are expected to reduce relapse after HSCT.43-46 The Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation proposed that patients with posttransplantation MRD positivity can be treated using imatinib either prophylactically or preemptively upon molecular relapse.45 In this study, among 14 patients who were assigned to prophylactic dasatinib, 2 with posttransplantation MRD positivity received dasatinib but did not respond to dasatinib with grade 3/4 toxicities, and 6 with posttransplantation MRD negativity received prophylactic dasatinib, 5 of whom ended as MRD−, 4 (66.7%) with grade 3 toxicities. Among 44 patients who were assigned to preemptive dasatinib, 6 were treated using preemptive dasatinib, resulting in 2 patients (16.7%) with grade 3 toxicities, but 7 relapsed before receiving dasatinib with no response, although 4 survived in CR2 (3 after second HSCT and 1 after whole-brain irradiation). Considering the 29 of 44 patients with pretransplantation MRD negativity who did not require dasatinib, 4 survivors in CR2 among the 7 relapsed patients, and grade 3/4 toxicities observed after prophylactic dasatinib, we cannot recommend prophylactic dasatinib for patients with pretransplantation MRD negativity.
We introduced dasatinib instead of imatinib to improve the efficacy of treatment before HSCT. Ponatinib, the most potent BCR-ABL1 inhibitor against both wild-type and mutated BCR-ABL1, including T315I, is now available for relapsed and refractory Ph+ ALL in Japan. Ponatinib provided deeper molecular remission than dasatinib and prevented relapse of the T315I clone during chemotherapy in this study.47,48 Two-step induction successfully reduced toxicities, but grade 3/4 toxicities remained frequent. Recently, the Gruppo Italiano Malattie Ematologiche dell’Adulto reported that consolidation by blinatumomab after induction by dasatinib plus prednisone resulted in excellent molecular response with minimal severe toxicities.49 Of the 63 enrolled patients with a median age of 54 years (range, 24-82), the CR rate was 98%. The molecular response rate after 2 cycles of blinatumomab was 60%. This study suggested that chemotherapy-free treatment can further reduce the toxicities of treatment with a high molecular response before HSCT.
In summary, dasatinib and 2-step induction increased the efficacy and reduced toxicities of treatment before HSCT, thereby increasing the number of patients undergoing HSCT in CR1. As a result, 3-year EFS significantly improved. However, further reduction of toxicities using monoclonal antibodies is expected. HSCT was used maximally, but NRM after HSCT remains an issue. Therefore, the indication for HSCT in CR1, especially in younger adult patients, should be clarified.
Acknowledgments
The authors thank the patients for participating in the study, all participating physicians of the Japan Adult Leukemia Study Group (JALSG) for collaborating on the study, and all staff of JALSG, as well as Satoshi Nishiwaki and Ryuzo Ohno for critical reading and editing of the manuscript.
This study was supported by the following grants: a research program of the Project for Development of Innovative Research on Cancer Therapeutics, Ministry of Education, Culture, Sports, Science and Technology of Japan under grant JP15cm0106055 (2013-2015); the National Cancer Center Research and Development Fund (23-A-23 and 26-A-24); and the Japan Agency for Medical Research and Development under grants JP16ck0106129 and JP19ck0106331.
Authorship
Contribution: The Ph+ALL213 Study Committee of the Japan Adult Leukemia Study Group (JALSG) designed the study; I.S. was the primary investigator of the study; all data were collected and monitored by the JALSG Data Center. I.S. analyzed data fixed on 1 July 2019 under the statistical advice of Y.A. and wrote the manuscript with the committee members of the Ph+ALL213 study; and all authors participated in analyzing and interpreting the data and checked the final version of the manuscript and are fully responsible for the content and editorial decisions pertaining to this manuscript.
Conflict-of-interest disclosure: I.S. has received honoraria from Takeda Pharmaceutical, Novartis Pharma, Bristol-Myers Squibb, and Pfizer Japan. Y.S. has received research funding from Chugai Pharmaceutical, Novartis Pharma, Kyowa Kirin, Bayer, Eisai, Ono Pharma, Otsuka Pharmaceutical, Pfizer Japan, Amgen BioPharma, Celgene, and Takeda Pharmaceutical. S.K. has received honoraria from Bristol-Myers Squibb. H.Y. has received research funding from Astellas Pharma and honoraria from Astellas Pharma, AbbVie, Takeda Pharmaceutical, Janssen Pharma, Celgene, Bristol-Myers Squibb, and Daiichi Sankyo. Y.I. has received honoraria from AbbVie, Astellas Pharma, Celgene, Chugai Pharmaceutical, FUJIFILM, Kyowa Kirin, and Novartis Pharma. Y.T. has received research funding from Eisai. Y.O. has received honoraria from Pfizer Japan, Astellas Pharma, Novartis Pharma, and Kyowa Kirin. Y.U. has acted as a consultant for Otsuka Pharmaceutical and Sanofi. S.F. has received research funding from Astellas Pharma and Pfizer Japan and honoraria from Bristol-Myers Squibb, Novartis Pharma, and Pfizer Japan. Y.H. has received honoraria from Bristol-Myers Squibb and Novartis Pharma. N. Dobashi has received research funding from AbbVie, Astellas Pharma, Chugai Pharmaceutical, Daiichi Sankyo, Eisai, Kyowa Kirin, Otsuka Pharmaceutical, Pfizer Japan, and Zenyaku Kogyo. Y.A. has received honoraria from Astellas Pharma, Mochida Pharmaceutical, and Meiji Seika Pharma. Y.K. has received research funding from Pfizer Japan, Nippon-Shinyaku, SymBio, and Amgen. F.H. has received research funding from Chugai Pharmaceutical and Daiichi Sankyo Foundation of Life Science. T.N. has received research funding from Astellas Pharma, Daiichi Sankyo, and FUJIFILM and honoraria from Astellas Pharma, Nippon Shinyaku, Bristol-Myers Squibb, Sysmex, Pfizer Japan, Otsuka Pharmaceutical, and FUJIFILM. Y.M. has received research funding from Sumitomo-Dainippon and honoraria from Novartis Pharma, Celgene, Sumitomo-Dainippon, Chugai Pharmaceutical, Otsuka Pharmaceutical, Astellas Pharma, Kyowa Kirin, Amgen, Pfizer Japan, Nippon Shinyaku, Janssen Pharma, Bristol-Myers Squibb, Takeda Pharmaceutical, and Daiichi Sankyo. The remaining authors declare no competing financial interests.
Correspondence: Isamu Sugiura, Division of Hematology and Oncology, Toyohashi Municipal Hospital, 50 Hachiken-nishi, Aotake-cho, Toyohashi, Aichi-prefecture, Japan 441-8570; e-mail: isugiura@med.e-mail.ne.jp.
Appendix
Japan Adult Leukemia Study Group (JALSG) Ph+ALL213 Study Committee members: Isamu Sugiura, Shin Fujisawa, Nobuaki Dobashi, Yoshihiro Hatta, Noboru Asada, Kazuteru Ohashi, Yasushi Onishi, Koh Shiro, Satoshi Nishiwaki, Yoshiko Atsuta, Fumihiko Hayakawa, Kiyotoshi Imai, Shyuichi Mizuta, Chiaki Nakaseko, and Yasutaka Aoyama. Biostatistician: Yoshiko Atsuta, Japanese Data Center for Hematopoietic Cell Transplantation, Nagoya, Japan, and Department of Registry Science for Transplant and Cellular Therapy, Aichi Medical University School of Medicine, Nagakute, Japan. Office of JALSG: H. Kiyoi and S. Amano. Data Center, S. Sato and R. Fujiyoshi, Nagasaki University, Nagasaki, Japan; S. Ohtake, Kanazawa University, Kanazawa, Japan. Collaborating hospitals: Tokyo Metropolitan Cancer and Infectious Diseases Center, Komagome Hospital, Bunkyoku, Japan; Nagoya University Hospital, Nagoya, Japan; Komaki City Hospital, Komaki, Japan; JA Aichi Konan Kosei Hospital, Konan, Japan; Toyohashi Municipal Hospital, Toyohashi, Japan; Japanese Red Cross Nagoya First Hospital, Nagoya, Japan; Kindai University Hospital, Osakasayama, Japan; Osaka International Cancer Institute, Osaka, Japan; Nagasaki University Hospital, Nagasaki, Japan; Sasebo City General Hospital, Sasebo, Japan; University of Fukui Hospital, Yoshidagun, Japan; Kurashiki Central Hospital, Kurashiki, Japan; National Cancer Center Hospital, Chuoku, Japan; International Medical Center, Saitama Medical University, Hidaka, Japan; Chiba University Hospital, Chiba, Japan; Chiba Aoba Municipal Hospital, Chiba, Japan; Chibaken Saiseikai Narashino Hospital, Narashino, Japan; Jikei University Hospital, Minatoku, Japan; National Hospital Organization Nagoya Medical Center, Nagoya, Japan; Kochi Medical School Hospital, Nangoku, Japan; Shiga University of Medical Science Hospital, Otsu, Japan; Shinshu University School of Medicine, Matsumoto, Japan; Hamamatsu University School of Medicine, Hamamatsu, Japan; Kanazawa University Hospital, Kanazawa, Japan; Tokyo Medical University Hospital, Shizyuku, Japan; Sapporo Hokuyu Hospital, Sapporo, Japan; Gunma Saiseikai Maebashi Hospital, Maebashi, Japan; Fuchu Hospital, Izumi, Japan; National Hospital Organization Kyushu Cancer Center, Fukuoka, Japan; Keio University Hospital, Shinjuku, Japan; Osaka City General Hospital, Osaka, Japan; NTT Medical Center Tokyo, Shinagawaku, Japan; Yokohama City University Hospital, Yokohama, Japan; Kanagawa Cancer Center, Yokohama, Japan; Nagasaki Medical Center, Ohmura, Japan; Miyagi Cancer Center, Natori, Japan; National Hospital Organization Matsumoto Medical Center, Matsumoto, Japan; Otsu Red Cross Hospital, Otsu, Japan; Jichi Medical University Saitama Medical Center, Saitama, Japan; Yokohama City Minato Red Cross Hospital, Yokohama, Japan; PL General Hospital, Tondabayashi, Japan; Matsusaka Chuo General Hospital, Matsusaka, Japan; Otemae Hospital, Osaka, Japan; Toyota Kosei Hospital, Toyota, Japan; Saga University Hospital, Saga, Japan; Yamanashi Prefectural Central Hospital, Kofu, Japan; National Hospital Organization Sendai Medical Center, Sendai, Japan; Saiseikai Yokohama Nanbu Hospital, Yokohama, Japan; Shonan Kamakura General Hospital, Kamakura, Japan; and Kobe City Medical Center General Hospital, Kobe, Japan.
References
Author notes
Data sharing requests should be sent to corresponding author Isamu Sugiura (email: isugiura@med.e-mail.ne.jp).
A list of the members of the Japan Adult Leukemia Study Group PH+ALL 213 Study Committee appears in “Appendix.”

![Impact of additional CAs and type of BCR-ABL1 transcript on survival outcomes. (A) EFS curves of patients with additional CAs and isolated Ph positivity; 3-year EFS rates from enrollment were 61.8% (95% confidence interval [CI], 46.8-73.7) and 77.8% (95% CI, 51.1-91.0), respectively. (B) OS curves of patients with additional CAs and isolated Ph positivity; 3-year OS rates from enrollment were 73.9% (95% CI, 59.3-83.9) and 100%, respectively. (C) EFS curves of patients with major BCR-ABL1 and minor BCR-ABL1 transcripts; 3-year EFS rates from enrollment were 75.9% (95% CI, 51.4-89.2) and 62.4% (95% CI, 48.4-73.7), respectively. (D) OS curves of patients with major BCR-ABL1 and minor BCR-ABL1 transcripts; 3-year OS rates from enrollment were 90.5% (95% CI, 67.0-97.5) and 76.7% (95% CI, 63.3-85.8), respectively.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/6/2/10.1182_bloodadvances.2021004607/4/m_advancesadv2021004607f4.png?Expires=1769093963&Signature=W6E158nYKSmFy7vVxzZvo2K5Eq8u-hO8UpVkuKTnT2SZyQfV~fs8VJZCKXqDvs9Yd~GzzG3pzdQpJYuGieseB5vTVRJ5r4FrT8TFWowKrfo1eV1vxqXPIqyxGrFGEUFj88AUZwIrGJiIgmBixLOVNLR9iM1ndecNEMBQ4vs9h2LliKxLruS5iGIN0cs-b5ud4xODbc6qelwwSUxr2ht56QYojRP~gHirn~Dif2KYCE3ZkHJUxWEVxzhmEYWXj1iDFqeC9CGdyjjX-jyaZvQdYwzU5LxGZaf4kIm7oRGvBquUCJ9qOa0Zwq-OyTfxw3j739sxSs6tWtcbHMxrXYMEsw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)