Abstract
Chimeric antigen receptor T-cell (CAR-T) therapy has transformed treatment paradigms for relapsed/refractory (r/r) B-cell acute lymphoblastic leukemia (B-ALL) in children and younger adults. We performed a systematic review to investigate the published literature on efficacy and toxicity of CAR-T therapy in adults with r/r B-ALL. We searched MEDLINE, Embase, and the Cochrane Library for prospective interventional studies and included published studies of ≥5 patients with median age at enrollment of ≥18 years. Risk of bias was assessed with a modified Institute of Health Economics tool. A total of 2566 records were assessed; 16 studies involving 489 patients were included in the final analysis. The mean complete remission (CR) rate was 81% and the measurable residual disease (MRD)–negative remission rate was 81% at 4 weeks after CAR-T infusion. With median follow-up across studies of 24 months, the cumulative 12-month probabilities of progression-free survival (PFS) and overall survival (OS) were 37% (95% CI, 26-48) and 57% (95% CI, 49-65), respectively. Relapse occurred in 40.3% of cases; target antigen was retained in 73.2% of relapses. Across studies, any grade of cytokine release syndrome (CRS) occurred in 82% of patients (95% CI, 61-95) and grade 3 or higher CRS in 27% (95% CI, 18-36). Neurotoxicity of any grade occurred in 34% of patients (95% CI, 24-47) and grade 3 or higher in 14% (95% CI, 1-25). In summary, CAR-T therapy achieves high early remission rates in adults with r/r B-ALL and represents a significant improvement over traditional salvage chemotherapy. Relapses are common and durable response remains a challenge.
Introduction
Although cytotoxic multiagent chemotherapy cures >90% of B-cell acute lymphoblastic leukemia (B-ALL) in children, B-ALL in older patients remains a challenge, with a large proportion of adults developing leukemia that is either refractory to or recurs after front-line therapy.1,2 The development and ultimate approval of chimeric antigen receptor T-cell therapy (CAR-T) represents a striking breakthrough in ALL therapeutics, where the availability of commercial CD19-targeting CAR-T therapy has transformed treatment paradigms for r/r B-ALL in children and younger adults. In the pivotal single-arm phase 2 multicenter ELIANA trial, 75 children and adolescents up to age 21 received tisagenlecleucel (Kymriah; Novartis). Remission was achieved in 82%; the 12-month event free survival (EFS) and overall survival (OS) were 50% and 76%, respectively; and the median duration of remission was not reached.3 Recently presented “real world” outcomes with tisagenlecleucel confirm similar response and survival outcomes in pediatric r/r B-ALL.4 Currently, only a fraction of patients with r/r B-ALL receive commercial CAR-T therapy in the United States, as the indication is restricted to patients under the age of 26 years. Thus, extrapolation of the results of the pediatric CAR-T experience to adults with r/r B-ALL is limited.
Despite the lack of a commercially available CAR-T for adults with r/r ALL in the United States, a variety of groups around the world have reported results of prospective, early-phase studies of various CAR-T constructs in r/r B-ALL in adults. Recognizing that CAR-T outcomes in adult ALL are likely to differ from pediatric reports, we perceived a need for a comprehensive evaluation of CAR-T in r/r adult B-ALL. To overcome the challenges of interpreting data across heterogenous studies, we conducted a systematic review focused on the efficacy and toxicity of CAR-T therapy in adults with r/r B-ALL. Other systematic reviews of CAR-T therapy in pediatric and general r/r ALL have been published; however, the focus of our analysis is on studies with primarily adult patient populations.5,6 The results of our study will aid clinicians in caring for adults with r/r B-ALL by providing a review of overall response and survival estimates of published CAR-T trials and will provide a general benchmark for investigators studying CAR-T therapies in adult ALL.
Methods
Information sources and search strategy
We performed a systematic review according to the Cochrane Collaboration Guidelines, and the findings are reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.7,8
MEDLINE, Embase, and Cochrane Library were searched for records discussing CAR-T therapy and B-ALL. After 4 gold-standard articles were analyzed with Yale Mesh Analyzer (https://mesh.med.yale.edu/), search strategies were created and run by a librarian who used a combination of keywords and controlled vocabulary to search the databases MEDLINE (EBSCOhost), Embase (Elsevier), and Cochrane. No filters or limits were applied to the search. All search strategies were completed and run on 16 November 2020. Records were uploaded to and deduplicated through EndNote. Once the initial deduplication was completed, 2574 records were then uploaded to Covidence for a secondary deduplication and analysis. Eight additional duplicates were found in Covidence, meaning 2566 unique results were assessed with the inclusion and exclusion criteria. Full search strategies can be found in the supplemental Materials. One study, published after the search inclusion date, was subsequently included in the analyses because of its potential to make a significant impact in the field.9 The protocol was registered with the international prospective register of systematic reviews (PROSPERO), in accordance with PRISMA-P guidelines (PROSPERO CRD42021226121).10,11
Eligibility criteria and outcome measures
We included prospective interventional studies that reported data on the outcomes of adults with B-ALL treated with CAR-T therapy, if they met the following inclusion criteria: (1) studies enrolling adults only (≥18 years) or both pediatric and adult patients if the median age of the study population was ≥18 years and (2) studies enrolling ≥5 patients. Both single and multi-institutional studies were included. If more than 1 publication reported on the same clinical trial, the published manuscript with the most recently updated results was included. Two studies that met the criteria were excluded: the first one because of insufficient reporting on survival outcomes and the second one because of detected data inconsistencies. Unpublished gray literature, as well as published abstracts, commentaries, reviews, editorials, and articles in languages other than English were also excluded. The efficacy outcomes were rate of complete remission (CR), measurable residual disease (MRD)–negative remission, progression free survival (PFS), and overall survival (OS). The toxicity outcomes were cytokine release syndrome (CRS) and neurotoxicity at any time after CAR-T therapy.
Data collection process
Two review authors (P.G. and O.V.) independently screened the retrieved titles and abstracts from the search results according to the inclusion and exclusion criteria. Full texts were reviewed to confirm that the inclusion criteria were met. A standardized data extraction template was used, and data were extracted independently by 2 investigators (P.G. and O.V.). Any discrepancies during the screening or data extraction process were resolved by discussion with other investigators (L.M. and E.C.). Extracted study characteristics included journal title, the first author, trial design, study sample size and patient characteristics (age, prior therapies, disease characteristics before CAR-T therapy, presence of extramedullary or central nervous system disease, and proportion of Philadelphia chromosome–positive [Ph+] patients). CAR-T characteristics including T-cell origin, antigen target, CD19 scFV clone, costimulatory domain, dose, and lymphodepletion regimen were also extracted. The data extracted for efficacy outcomes included the number of patients who achieved complete remission and MRD-negative remission within 30 days of CAR-T infusion and the number of patients with no evidence of disease and those alive at the 12-month follow-up. The number of patients who relapsed, antigen expression at relapse, and the number of patients who underwent allogeneic stem cell transplant after CAR-T therapy were also extracted. Data extracted for toxicity included proportion of patients who experienced CRS or neurotoxicity at any time after infusion and the study-specific toxicity grading system used.
Study qualitative assessment
A modified Institute of Health Economics (IHE) risk of bias tool was used to evaluate all included studies.12 This modified tool contains 19 items for assessment of study objectives, study design, intervention, outcomes measures, statistical analysis, results, and conflicts of interest. The risk-of-bias assessment was performed independently by 2 review authors (P.G. and O.V.), and disagreements were resolved through discussion with other study authors (E.C. and L.M.).
Statistical Analysis
For time-to-event end points (OS/PFS), we performed parametric meta-analyses assuming exponential survival distribution for event time in individual study. The log-transformed hazard rate from individual study follows a common normal distribution, center of which is the parameter of interest. Hazard rate estimates from individual studies were extracted from the reported 12-month survival probability, the 24-month survival probability, median survival time, reported individual patient data, or reconstructed individual patient data from the published Kaplan-Meier curves. For binary end points (CR, MRD, CRS, and neurotoxicity), we fit the β-binomial random effects model, which enables between-study heterogeneity to construct the exact 95% confidence interval (CI) for the mean incidence rate across studies.13 Additionally, we repeated the meta analyses in subgroups of studies with adult patients only, and subgroups of studies limited to receipt of CD19-targeting CAR-T constructs, and the exact CI was constructed under the normal-normal random effects model considering the small number of studies.14 The median of maximum follow-up time from individual studies was calculated. The study-specific maximum follow-up time was obtained, either by reported individual patient level data or the Kaplan-Meier curves. Last, we performed meta regression analysis to study the association of hazard rate for time-to-event end points or incidence rate for binary end points with median age, median pre-CAR-T blast percentage, prior allogeneic hematopoietic cell transplant (HCT) rate, T-cell construct type, and T-cell origin.
Results
Baseline patient and CAR-T characteristics
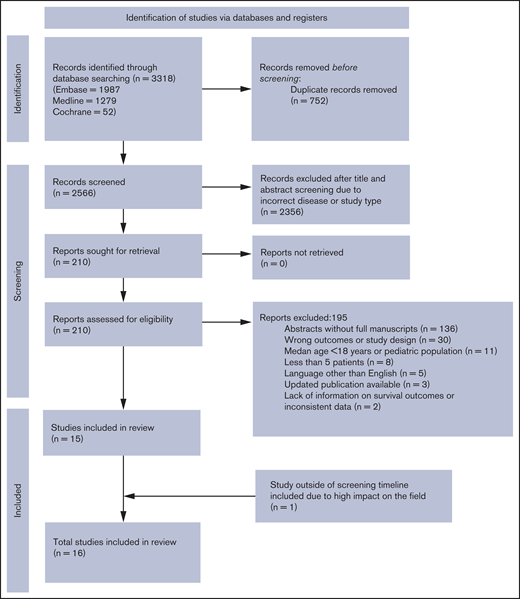
Our search identified 2566 unique records. Of those, 16 studies involving 489 patients met the inclusion criteria and were included in the final analysis.15-29 The flowchart illustrating the literature search process is presented in Figure 1.8 Baseline characteristics of patients and CAR-T studies are presented in Table 1 and supplemental Tables 1 and 2. All studies were single-arm trials of patients with r/r B-ALL who had received at least 1 prior line of therapy. The majority of studies (11 of 16) included both pediatric and adult patients (n = 283), but 5 of the studies enrolled only adult patients (n = 206). The median age of patients in the 16 studies ranged from 18-44 years, with most studies (15 of 16) having a median patient age of >23 years. Median prior lines of therapy ranged from 2 to 4 across studies (range, 1-11). Rates of prior allotransplant and blinatumomab among all participants across studies were 40% and 19%, respectively. Most of the patients were in morphological relapse or had >5% blasts in the bone marrow before CAR-T therapy (70.5%); however, 22% were in CR, with or without MRD. Extramedullary disease was reported in 13.5% patients across 9 studies; 13 patients (8.6%) across 6 studies were reported to have CNS disease at time of CAR-T therapy. CAR-T characteristics are summarized in supplemental Table 2. The majority of the studies used autologous CAR-T cells with 41BB costimulatory domain targeted against CD19. Fludarabine/cyclophosphamide alone or in combination with other chemotherapy was the most frequently used lymphodepletion regimen.
Baseline patient and CAR-T characteristics
| Study . | Patients, n . | Age . | Prior HSCT n (%) . | Ph+ n, (%) . | Median pre-CAR-T blast, % . | CAR-T construct type . | T-cell origin . | Antigen target . | Median dose . | |
|---|---|---|---|---|---|---|---|---|---|---|
| Median age . | Combined/adult-only . | |||||||||
| Dai et al, 202015 | 6 | 23.5 (17-44) | Combined | 0 | 1 (16.67) | 46.97 | 41BB | Autologous | Bispecific C19/22 | 2 × 106 per kg |
| Dai et al, 201516 | 9 | 35 (15-65) | Combined | 3 (33.33) | 5 (55.56) | 73.44 | 41BB | Autologous/ allogeneic* | CD19 | 4.5 × 106 per kg |
| Frey et al, 202017 | 35 | 34 (21-70) | Adult | 13 (37.14) | 3 (8.57) | NA | 41BB | Autologous | CD19 | Total dose: 5 × 108 |
| Gu et al, 202018 | 20 | 18 (3-52) | Combined | NA | 2 (10) | 35.50 | 41BB | Autologous | CD19 | 5 × 106 per kg |
| Hay et al, 201919 | 53 | 39 (20-76) | Adult | 23 (43.40) | 11 (20.75) | 28 | 41BB | Autologous | CD19 | 2 × 105per kg |
| Hua et al, 202020 | 11 | 28 (12-39) | Combined | 11 (100) | 5 (45.45) | NA | 41BB | Allogeneic | CD19 | 5 × 106per kg |
| Hu et al, 201721 | 15 | 32 (7-57) | Combined | 7 (46.67) | 4 (26.67) | 63.50 | 41BB | Autologous | CD19 | 3.7 × 106per kg |
| Jiang et al, 201922 | 58 | 28 (10-65) | Combined | 3 (5.17) | 7 (12.07) | 12.10 | 41BB | Autologous | CD19 | 1.66 × 106 per kg |
| Li et al, 201823 | 10 | 33 (18-59) | Adult | 1 (10) | 2 (20) | 12.75 | 41BB, CD28† | Autologous | CD19 | 0.62 × 106 per kg |
| Ma et al, 202024 | 9 | 34.1 (16-57) | Combined | 0 | 2 (22.22) | 5.20 | 41BB | Autologous | CD19 | 1 × 106 per kg |
| Ortíz-Maldonado et al, 202025 | 38§ | 24.5 (3-67) | Combined | 33 (86.84) | NA | NA | 41BB | Autologous | CD19 | NA |
| Park et al, 201826 | 53 | 44 (23-64) | Adult | 19 (35.85) | 16 (30.19) | 63 | CD28 | Autologous | CD19 | 3 × 106 per kg |
| Shah et al, 20219 | 55 | 40 (IQR 28-52) | Adult | 23 (42) | 15 (27) | 60 | CD28 | Autologous | CD19 | 1 × 106 per kg |
| Wang et al, 202027 | 23 | 42 (10-67) | Combined | 0 | 7 (30.43) | 40.40 | 41BB | Autologous | CD19 | 1 × 106 per kg |
| Wang et al, 202028 | 51 | 27 (9 to 62) | Combined | 9 (17.65) | 13 (25.49) | 59 | Third generation | Autologous | Sequential CD19 followed by CD22 | CAR19: 2.6 × 106 per kg CAR22: 2.7 × 106 per kg |
| Zhang et al, 202029 | 43 | 24 (4 to 60) | Combined | 43 (100) | 5 (11.63) | NA | 41BB, CD28‡ | Allogeneic | CD19 | 1.76 106 per kg |
| Study . | Patients, n . | Age . | Prior HSCT n (%) . | Ph+ n, (%) . | Median pre-CAR-T blast, % . | CAR-T construct type . | T-cell origin . | Antigen target . | Median dose . | |
|---|---|---|---|---|---|---|---|---|---|---|
| Median age . | Combined/adult-only . | |||||||||
| Dai et al, 202015 | 6 | 23.5 (17-44) | Combined | 0 | 1 (16.67) | 46.97 | 41BB | Autologous | Bispecific C19/22 | 2 × 106 per kg |
| Dai et al, 201516 | 9 | 35 (15-65) | Combined | 3 (33.33) | 5 (55.56) | 73.44 | 41BB | Autologous/ allogeneic* | CD19 | 4.5 × 106 per kg |
| Frey et al, 202017 | 35 | 34 (21-70) | Adult | 13 (37.14) | 3 (8.57) | NA | 41BB | Autologous | CD19 | Total dose: 5 × 108 |
| Gu et al, 202018 | 20 | 18 (3-52) | Combined | NA | 2 (10) | 35.50 | 41BB | Autologous | CD19 | 5 × 106 per kg |
| Hay et al, 201919 | 53 | 39 (20-76) | Adult | 23 (43.40) | 11 (20.75) | 28 | 41BB | Autologous | CD19 | 2 × 105per kg |
| Hua et al, 202020 | 11 | 28 (12-39) | Combined | 11 (100) | 5 (45.45) | NA | 41BB | Allogeneic | CD19 | 5 × 106per kg |
| Hu et al, 201721 | 15 | 32 (7-57) | Combined | 7 (46.67) | 4 (26.67) | 63.50 | 41BB | Autologous | CD19 | 3.7 × 106per kg |
| Jiang et al, 201922 | 58 | 28 (10-65) | Combined | 3 (5.17) | 7 (12.07) | 12.10 | 41BB | Autologous | CD19 | 1.66 × 106 per kg |
| Li et al, 201823 | 10 | 33 (18-59) | Adult | 1 (10) | 2 (20) | 12.75 | 41BB, CD28† | Autologous | CD19 | 0.62 × 106 per kg |
| Ma et al, 202024 | 9 | 34.1 (16-57) | Combined | 0 | 2 (22.22) | 5.20 | 41BB | Autologous | CD19 | 1 × 106 per kg |
| Ortíz-Maldonado et al, 202025 | 38§ | 24.5 (3-67) | Combined | 33 (86.84) | NA | NA | 41BB | Autologous | CD19 | NA |
| Park et al, 201826 | 53 | 44 (23-64) | Adult | 19 (35.85) | 16 (30.19) | 63 | CD28 | Autologous | CD19 | 3 × 106 per kg |
| Shah et al, 20219 | 55 | 40 (IQR 28-52) | Adult | 23 (42) | 15 (27) | 60 | CD28 | Autologous | CD19 | 1 × 106 per kg |
| Wang et al, 202027 | 23 | 42 (10-67) | Combined | 0 | 7 (30.43) | 40.40 | 41BB | Autologous | CD19 | 1 × 106 per kg |
| Wang et al, 202028 | 51 | 27 (9 to 62) | Combined | 9 (17.65) | 13 (25.49) | 59 | Third generation | Autologous | Sequential CD19 followed by CD22 | CAR19: 2.6 × 106 per kg CAR22: 2.7 × 106 per kg |
| Zhang et al, 202029 | 43 | 24 (4 to 60) | Combined | 43 (100) | 5 (11.63) | NA | 41BB, CD28‡ | Allogeneic | CD19 | 1.76 106 per kg |
Seven (77.77%) patients received autologous CAR-T, and 2 (22.22%) received allogeneic CAR-T.
Five (50%) patients received 41BB CAR-T cells and 5 (50%) received CD28 CAR-T cells.
§ Efficacy outcome analysis done for 27 patients who were >18 years, toxicity analysis done for all 38 patients (adult and pediatric) since toxicity data for only adult patients was not available
Twenty-five (58.13%) patients received 41BB CAR T cells, and 18 (41.87%) received CD28 CAR T cells.
Study quality
Risk of bias is summarized in supplemental Figure 1. Most studies were single center. Characteristics of patients included in the study, interventions, and relevant outcomes were well defined in most studies. Detailed eligibility criteria, additional interventions, and adverse events were less uniformly reported.
Efficacy
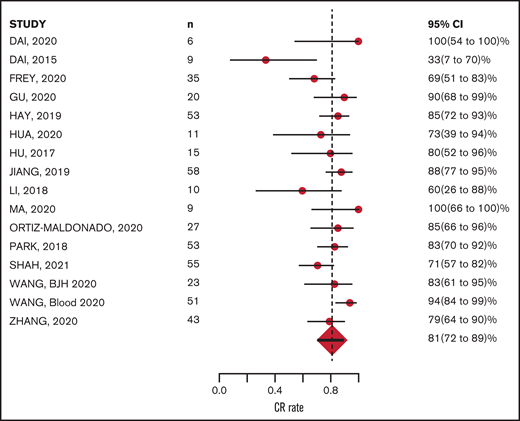
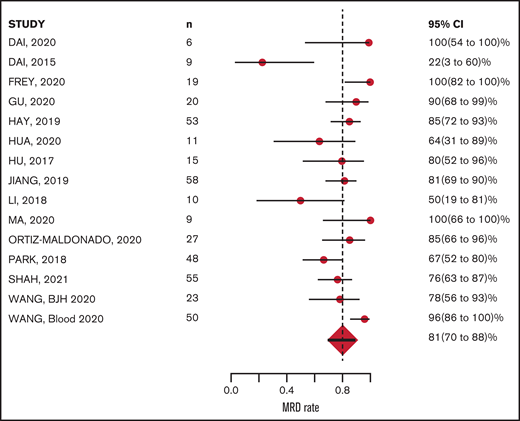
The CR rate was available for all 16 studies and MRD-negative remission rate was available for 15 of the 16 studies. Response was measured 28 to 30 days after CAR-T infusion in 12 studies; however, in 4 studies, response was measured at earlier time points: Wang et al27 measured response 14 days after CAR-T infusion, Zhang et al29 at 21 days, Park et al26 and Li et al23 between 20 and 30 days. Of 489 evaluable patients, 400 achieved CR, with a mean CR rate of 81% (95% CI, 72-89; Figure 2). Of 428 evaluable patients, 341 achieved MRD-negative remission; the mean MRD negative remission rate was 81% (95% CI, 70-89; Figure 3). Relapse was reported in 183 patients (40%) during study follow-up. Target antigen status at the time of relapse was reported in 131 patients across 11 studies: antigen loss occurred in 26.7%, whereas 73.2% were reported to have a target antigen-positive relapse (supplemental Table 3).
MRD-negative remission at ∼4 weeks after CAR-T infusion: 81% (range, 70% to 88%).
MRD-negative remission at ∼4 weeks after CAR-T infusion: 81% (range, 70% to 88%).
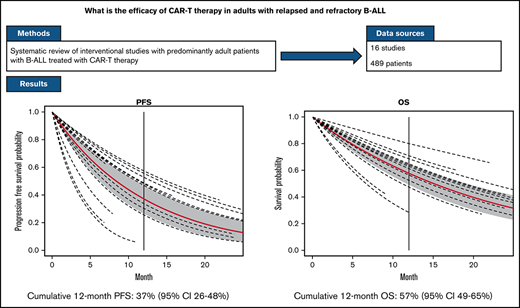
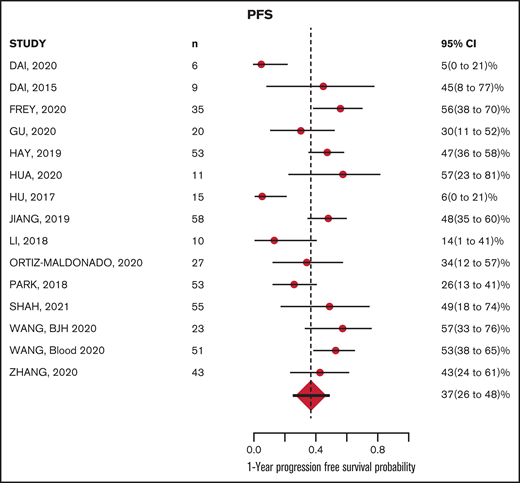
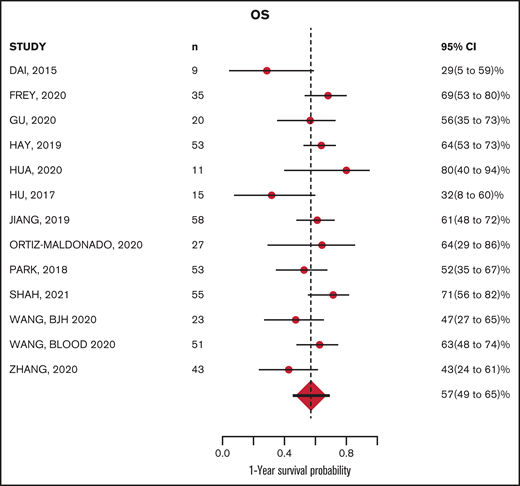
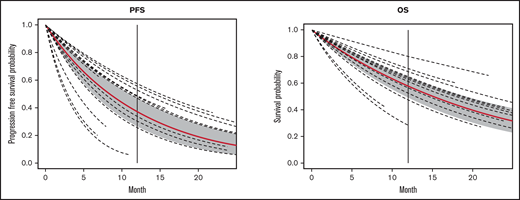
The median follow-up from time of infusion across studies was 24 months (with range of median follow-up from 7 to 65 months). The estimated median PFS and OS were 8.4 (6.2-11.3) months and 15 (11.6-19.4) months, respectively. The estimated PFS and OS at 12 months after CAR-T infusion were available in 15 and 13 studies, respectively (Figures 4-5; supplemental Tables 5 and 6). The cumulative 12-month PFS probability was 37% (95% CI, 26-48), and the 12-month OS probability was 57% (95% CI, 49-65; Figure 6). A sensitivity analysis was performed to evaluate outcomes in the studies limited to CD19-directed CAR-T constructs (n = 14), with similar findings observed: CR rate was 80% (95% CI, 70-88), MRD-negative remission rate was 78% (95% CI, 69-87), 12-month PFS was 37% (95% CI, 27-47), and 12-month OS was 56% (95% CI, 47-64). An additional analysis limited to the 5 studies that excluded patients <18 years of age demonstrated a 12-month PFS probability of 39% (95% CI, 18-60) and a 12-month OS probability of 64% (95% CI, 52-74) (supplemental Figures 6 and 7). We were unable to determine any significant associations between patient and CAR-T characteristics and outcomes in meta regression analyses (supplemental Table 4). Although 26% of patients were reported to have undergone allogeneic HCT after CAR-T, censoring at HCT was not performed uniformly across studies, which limited our ability to capture the impact of consolidative HCT on survival outcomes.
Survival curves for PFS and OS. Vertical line, 12-months; red line, the population survival curve from the meta-analysis; dotted lines, the survival curves from individual studies with different follow-up times.
Survival curves for PFS and OS. Vertical line, 12-months; red line, the population survival curve from the meta-analysis; dotted lines, the survival curves from individual studies with different follow-up times.
CRS and neurotoxicity
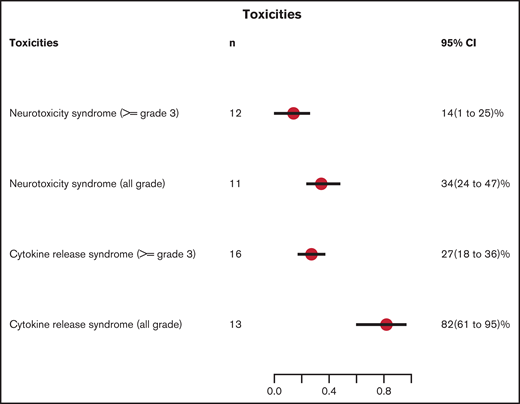
The incidence of any grade of CRS and grade 3 or higher CRS were reported in 13 and 16 studies, respectively, using a variety of CRS grading scales (supplemental Table 3).26,30-33 The cumulative incidence of any grade CRS was 82% (95% CI, 61-95) and grade 3 or higher CRS was 27% (95% CI, 18-36; supplemental Figures 2 and 3). The incidence of any grade of neurotoxicity and grade 3 or higher neurotoxicity was reported in 11 and 12 studies, respectively. Neurotoxicity grading systems differed across studies, with the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) used most commonly (supplemental Table 3). The cumulative incidence of any grade neurotoxicity was 34% (95% CI, 24-47) and grade 3 or higher was 14% (95% CI, 1-25; supplemental Figures 4 and 5). The toxicity data are summarized in Figure 7.
Cumulative incidence of toxicities. n, number of studies with available data.
Discussion
Although CAR-T therapies have been pursued in adult r/r B-ALL for several years, the published reports in this population have consisted of largely single-institution series of patients treated with a variety of CAR-T constructs and approaches. To summarize the literature and facilitate a comprehensive assessment of outcomes, we conducted a systematic review focused on published CAR-T trials in predominantly adult r/r B-ALL patients. Our analysis confirms very high responses across CAR-T therapies in r/r adult B-ALL and provides a benchmark for PFS and OS at 12 months of ∼37% and ∼57%, respectively. Despite the heterogeneity across published trials, the data provide evidence-based estimates of CAR-T outcomes for patients and clinicians treating adult patients with ALL and for investigators seeking to further advance knowledge in the field.
The treatment paradigm for r/r B-ALL in adults has changed dramatically over the past decade. Whereas historical salvage chemotherapy resulted in remission rates of ∼30%,34,35 pivotal phase 3 clinical trials of the newer targeted immunotherapies blinatumomab and inotuzumab ozogamicin demonstrate CR rates of 45% to 80%, respectively, predominantly MRD-negative.36,37 These responses translate into median and 1-year survival of ∼7.7 months and 35%, respectively. The data presented in this systematic review of CAR-T outcomes are not directly comparable to the randomized phase 3 trial data of targeted immunotherapeutic agents, but provide general estimates for consideration. Approximately 25% of the patients included in these CAR-T studies underwent allogeneic HCT after CAR. Although the current commercially available salvage agents for r/r B-ALL generally serve as a bridge to HCT, CAR-T therapies aspire to be stand-alone immunotherapies in the relapsed setting. We were unable to assess the impact of consolidative HCT on CAR-T outcomes in the current analysis, but prior studies have described mixed findings. The optimal sequencing of targeted therapies, HCT, and CAR-T, when they become commercially available in adult B-ALL, remains unknown and requires careful study.
Although most of the larger clinical trial and “real-world” population reports of CAR-T therapies in B-ALL are predominantly focused on children and adolescent/young adults (AYAs), our study provides insights that may differ from pediatric CAR-T results. First, although we did not conduct a statistical comparative analysis, the estimated 12-month PFS and OS in our report of ∼37% and ∼57% appear generally lower than has been reported in pediatric series, with PFS exceeding 50% and OS of ∼75%.3,4,38 A meta-analysis of CAR-T therapies in both pediatric and adult ALL demonstrated similar trends6 ; however, it is important to note that much of the pediatric CAR-T data in ALL are generated from a single product studied at a recommended phase 2 dose.3 Second, we found that ∼25% of the patients in our cohort relapsing after CAR had lost CD19 expression at relapse. The rates of CD19-negative relapse in pediatric reports have been closer to 50%.38 It is possible that CAR-T cell persistence is reduced in heavily pretreated adults with ALL. This hypothesis requires additional study, as CAR-T cell persistence was not well described across studies. Finally, the safety profile in terms of CRS and neurotoxicity reported in our study demonstrated grade 3 or higher CRS in ∼25% of patients and grade 3 or higher in 12%. Although comparison with pediatric CAR-T trials is limited by different toxicity grading systems and evolution of toxicity management over time, these findings at least support that CAR-T–associated toxicity is reasonable in adult B-ALL. Recent data have demonstrated that both the efficacy and safety profile of CAR-T in r/r B-ALL in children/AYA was improved when patients entered therapy with <5% marrow blasts.38 Evaluation of CAR-T as a potentially definitive therapy in MRD-positive B-ALL in adults warrants exploration.
Our systematic review has several limitations and leaves several important questions in the field unanswered. As mentioned, the current literature reporting CAR-T results in adults with r/r B-ALL is limited to smaller, often single-institution studies, with significant heterogeneity across CAR-T constructs. Further, we chose to include several smaller studies that did include children, so long as the median study population age was >18 years. Although the small number of patients under 18 years may have influenced outcomes, a sensitivity analysis that included 206 patients from 5 studies of exclusively adult patients showed no substantial differences in PFS or OS relative to the entire cohort. All of the clinical trials included were relatively small, single-arm clinical trials, often from single centers. Importantly, the lack of individual patient data limited robust subgroup analysis, which would have been informative. In our limited regression analyses, we lacked power to identify any patient or CAR-T factors that could have been significantly associated with outcome. Identifying patient- and disease-based predictors of response to CAR-T in adult ALL is important and will require larger multicenter trials and/or real-world registry/consortiums. The lack of consistent patient censoring or reporting of HCT makes it difficult to assess the influence of HCT on post-CAR outcomes. Finally, long-term outcome reports in this population will be useful in assessing overall durability associated with this therapy.
In summary, CAR-T therapy is associated with high rates of MRD-negative response across the reported clinical trials in r/r B-ALL in adults. Strategies aimed at improving durability and long-term outcomes through improved CAR T-cell persistence, multiantigen targeting, and optimization of patient selection will propel the next phase of CAR-T trials for adults with B-ALL.
Authorship
Contributions: P.G., O.V., E.C., and L.M. designed the study; M.P., P.G., and O.V. collected the data; L.T. and R.S. performed the statistical analyses; all authors contributed to writing the manuscript; and all authors critically reviewed and approved the final version of the manuscript.
Conflict-of-interest disclosure: L.M. has received research funding from Jasper, Astellas, and Adaptive; has been a consultant to Amgen and Pfizer; and has received honoraria from UptoDate.
Correspondence: Lori Muffly, Division of Blood and Marrow Transplantation and Cellular Therapy, Stanford University, 300 Pasteur Dr, H0101, Stanford, CA 94305-5623; e-mail: lmuffly@stanford.edu.
References
Author notes
Presented in abstract form at the ninth annual meeting of the Society of Hematologic Oncology, Houston, TX, 8-10 September 2021.
For original data, please contact the corresponding author (lmuffly@stanford.edu).
The full-text version of this article contains a data supplement.