Abstract
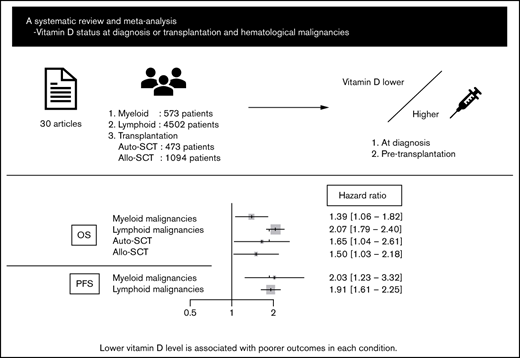
Vitamin D deficiency impairs prognosis in many types of cancer; however, its significance in each subtype of hematological malignancies is unclear. In addition, data on the association between pretransplant vitamin D levels and outcomes of hematopoietic stem cell transplantation (HSCT) are inconsistent. This systematic review and meta-analysis aimed to elucidate the impact of vitamin D levels at diagnosis or pre-HSCT on the prognosis of hematological malignancies. Thirty articles and abstracts were extracted from PubMed, Embase, and Cochrane Library databases and conference proceedings. Fixed and random effect models were used to analyze primary outcomes: overall survival (OS) and progression-free survival (PFS). Lower vitamin D level was significantly associated with poorer OS and PFS in myeloid malignancies (hazard ratio [HR], 1.39; 95% confidence interval [CI], 1.06-1.82 and HR, 2.03; 95% CI, 1.23-3.32, respectively) and lymphoid malignancies (HR, 2.07; 95% CI, 1.79-2.40 and HR, 1.91; 95% CI, 1.61-2.25, respectively), as well as outcomes for several lymphoma subtypes individually. Furthermore, a lower pretransplant vitamin D level was associated with poorer OS in autologous and allogeneic HSCT (HR, 1.65; 95% CI, 1.04-2.61 and HR, 1.50; 95% CI, 1.03-2.18, respectively). Despite the relatively small number of studies evaluated, these data suggest the importance of vitamin D status in outcomes of hematological malignancies (PROSPERO registration number: CRD42020205821).
Introduction
Vitamin D is produced in sun-exposed skin or taken in from the diet, hydroxylated in the liver and the proximal renal tubule to 1,25(OH)2D3, and acts as a steroid hormone by binding to the vitamin D receptor.1 It plays an important role in skeletal health, as well as in tumorigenesis by controlling cell proliferation, apoptosis, differentiation, angiogenesis, invasive and metastatic potential, and tumor immunity.2-4 The association between circulating vitamin D levels and cancer outcomes has been investigated in many types of cancer, and some meta-analyses revealed that higher vitamin D levels result in better outcomes in several cancers, including colorectal,5 breast,5 and prostate cancer6 and melanoma.7
The role of vitamin D in hematological malignancies has also been studied in clinical settings, because in vitro analysis showed the ability of vitamin D to induce differentiation of human acute myeloid leukemia (AML) cells into mature myeloid cells.8 Although clinically meaningful data using vitamin D and its analogs as differentiation therapy for AML are limited,9 some meta-analyses have revealed that vitamin D deficiency in hematological malignancies was associated with poorer prognosis.5,10 Hematological malignancies include many subtypes of myeloid and lymphoid malignancies; thus, the influence of vitamin D on each subtype should be examined separately, but a detailed analysis to address this issue has not been performed.
In addition, the effects of vitamin D levels on autologous and allogeneic hematopoietic stem cell transplantation (HSCT) have been assessed in several studies, and the significance remains controversial11 ; thus, a comprehensive analysis is warranted. We performed a systematic review and meta-analysis to determine the impact of vitamin D level at diagnosis or pre-HSCT on the prognosis of each subtype of hematological malignancies. This is the first meta-analysis focusing on each subtype of lymphoid malignancies, as well as examining transplant outcomes.
Methods
Search strategy
This study was registered with PROSPERO (CRD42020205821) and conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis guidelines.12 PubMed, EMBASE, and the COCHRANE registry of clinical trials databases were searched through 17 February 2021 without language restriction, using the following terms: “vitamin D” AND (“lymphoma” OR “leukemia” OR “myeloma” OR “myelodysplastic syndrome” OR “hematological malignancy” OR “hematopoietic stem cell transplantation” OR “bone marrow transplantation”) AND (“progression-free survival” OR “overall survival” OR “PFS” OR “OS” OR “survival” OR “prognosis”). We also searched conference proceedings of the American Society of Hematology (2004-2020), the American Society of Clinical Oncology (2011-2020), and the European Hematology Association (2009-2020) and scanned references of identified articles and reviews for further studies.10,11
Study selection and quality assessment
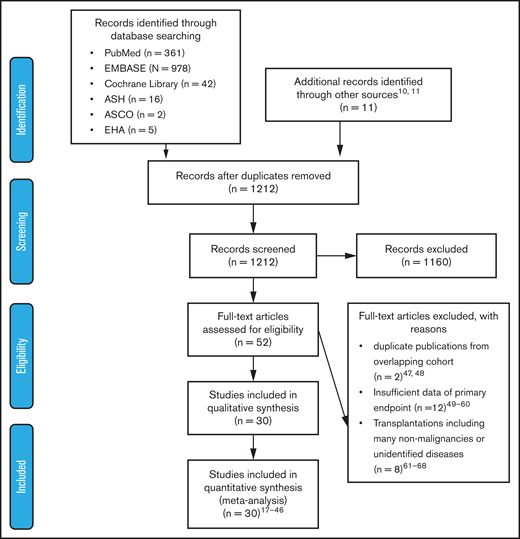
Two authors (Y.I. and A.H.) independently assessed the titles and abstracts of all of the identified studies by searching electronic databases. Subsequently, we screened the full texts of the potentially eligible articles. We excluded studies that lacked information needed to evaluate hazard ratios (HRs) of outcomes, were duplicate publications using overlapping patient cohorts, or included many nonmalignant patients undergoing allogeneic HSCT (allo-HSCT). Any discrepancies between the authors were resolved through a discussion including a third author (M.K.) until consensus was reached. The outline of the data extraction is described in Figure 1. The Newcastle-Ottawa scale was used to assess the quality of the nonrandomized trials.13
Preferred Reporting Items For Systematic Reviews And Meta-Analysis (PRISMA) flow diagram of study selection. After screening the titles and abstracts of 1212 articles, 52 articles were considered relevant. Among them, 22 articles were excluded for various reasons; 30 articles were included in the analysis. ASCO, American Society of Clinical Oncology; ASH, American Society of Hematology, EHA, European Hematology Society.
Preferred Reporting Items For Systematic Reviews And Meta-Analysis (PRISMA) flow diagram of study selection. After screening the titles and abstracts of 1212 articles, 52 articles were considered relevant. Among them, 22 articles were excluded for various reasons; 30 articles were included in the analysis. ASCO, American Society of Clinical Oncology; ASH, American Society of Hematology, EHA, European Hematology Society.
End points
The primary outcomes in this review were HRs of overall survival (OS) and progression-free survival (PFS), alternatively termed event-free survival, relapse-free survival, or leukemia-free survival in some articles. Secondary outcomes were time-to-treatment (TTT) for patients with chronic lymphocytic leukemia (CLL), relapse rate, and nonrelapse mortality (NRM) for allo-HSCT. When an article reported univariate and multivariate analyses, multivariate data were preferred. When HR was not available, it was estimated using the methods described by Tierney et al.14 When the cohort was divided into >2 groups according to vitamin D level, the data comparing groups with the highest and lowest levels were used. With regard to the measurement of vitamin D level, serum 25-hydroxyvitamin D [25(OH)D] is the major circulating vitamin D metabolite and is used to assess vitamin D status in this meta-analysis; 1 ng/mL of vitamin D corresponds to 2.5 nmol/L.
Statistical analysis
Statistical analyses were performed using EZR software.15 For each trial, the impact of vitamin D deficiency was calculated using HRs with 95% confidence intervals (CIs); mean HRs and the upper 95% CI from each study were input into EZR software for statistical analysis. An HR > 1 favored the higher vitamin D arm. We used the random effect model according to the method of DerSimonian and Laird.16 When the P value for heterogeneity exceeded .10, we preferred the Mantel-Haenszel (fixed effect) method. We assessed the trial results using the χ2 test of heterogeneity and the I2 measure of inconsistency. Heterogeneity was considered statistically significant at P < .10 or I2 > 50%. Potential sources of heterogeneity were investigated using subgroup analyses. Publication bias was examined using funnel plots, coupled with Egger’s test.
Results
Study selection
A literature search of PubMed, EMBASE, and Cochrane Library databases and 3 conference proceedings (American Society of Hematology, American Society of Clinical Oncology, and European Hematology Association) identified 1212 articles after duplicates were eliminated, of which 52 were considered relevant through the evaluation of titles and abstracts. Among them, 30 articles fulfilled the criteria for this meta-analysis: 5 articles on myeloid malignancies,17-21 20 articles on lymphoid malignancies,18,22-40 3 articles on autologous HSCT (ASCT),41-43 and 3 articles on allo-HSCT.44-46 A flow diagram of the article selection process is shown in Figure 1. Twenty-two articles were excluded for the following reasons: duplicate publications from overlapping cohorts,47,48 insufficient data about primary end points,49-60 and transplantations including many nonmalignancies or unidentified diseases.61-68 The characteristics of each study are summarized in Table 1.
Characteristics of the studies included in the meta-analysis
| Study . | Study period . | Country . | Disease . | Age, median (range), y . | Total N . | Low N . | High N . | Mid N . | Vitamin D threshold . | Measuring method . | Median f/u . | Outcome . | NOS . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hu et al, 202017 | 2014-2016 | China | AML in the elderly (w/o APL, secondary AML) | 70 (60-89) | 68 | 25 | 43 | 0 | 8.84 ng/mL | ECL | 2 y | OS | 9 |
| Jackmann et al, 202018 | 1990-2016 | Sweden | ALL in children | 7 (0.4-17.8) | 232 | 71 | 161 | 0 | 50 nmol/L | CLIA | n.a. | OS | 8 |
| AML in children | 52 | 22 | 30 | 0 | |||||||||
| CML/JMML in children | 11 | 5 | 6 | 0 | |||||||||
| Radujkovic et al, 201719 | 2006-2014 | Germany | MDS, oligoblastic AML | 69 (31-83) | 58 | 29 | 29 | 0 | 32.6 nmol/L | CLIA | 29 mo | OS | 7 |
| Lee et al, 201420 | n.a. | US | AML (w/o APL) | 60 (19-91) | 97 | 29 | 34 | 34 | <20 ng/mL, ≥32 ng/mL | RIA | 15.6 mo | OS, RFS | 8 |
| Pardanani et al, 201121 | n.a. | US | PMF | 63 (14-83) | 247 | 118 | 129 | 0 | 25 ng/mL | LC-MS/MS | 34 mo | OS, LFS | 8 |
| MDS | 72 (44-89) | 74 | 21 | 53 | 0 | 25 mo | |||||||
| Mao et al, 202122 | 2014-2019 | China | ENKTL | 55 (21-92) | 93 | 55 | 38 | 0 | 50 nmol/L | ECL | 23 mo | OS, PFS | 8 |
| Wang et al, 202023 | 2016-2018 | China | DLBCL | 58 (19-85) | 208 | 142 | 66 | 0 | 52.5 nmol/L | ECL | 29 mo | OS, PFS | 9 |
| Chen et al, 202133 | 2011-2018 | China | DLBCL | n.a. | 332 | 111 | 111 | 110 | <11.5 ng/mL, ≥18.7 ng/mL | LC-MS/MS | 34.2 mo | OS, PFS | 9 |
| Xu et al, 202034 | 2014-2018 | China | MCL | 61 (39-77) | 70 | 40 | 30 | 0 | 50 nmol/L | ECL | 25.5 mo | OS, PFS | 8 |
| Yellapragada et al, 202035 | n.a. | US | MM | 68.9 | 1889 | 582 | 1307 | 0 | 20 ng/mL | n.a. | n.a. | OS | 8 |
| Borchmann et al, 201936 | 1993-1998 | Germany | HL | 32 (16-75) | 351 | 175 | 176 | 0 | 30 nmol/L | ELISA | 13 y | OS, PFS | 8 |
| Kim et al, 201837 | 2008-2016 | Korea | PTCL, ENKTL | 17-85 | 251 | 105 | 146 | 0 | 10 ng/mL | LC-MS/MS | 35.8 mo | PFS | 8 |
| Djurasinovic et al, 201838 | 2014- 2016 | Serbia | Lymphoid malignancy (DLBCL, FL, CLL/SLL, HL) | 58 (18-84) | 133 | n.a. | n.a. | 0 | 19.6 nmol/L | CLIA | 20 mo | PFS | 8 |
| Hohaus et al, 201839 | 2013-2016 | Italy | Aggressive BCL | 65 | 154 | 104 | 50 | 0 | 20 ng/mL | CLIA | n.a. | EFS | 7 |
| Ferrari et al, 201740 | 2013-2016 | Italy | DLBCL | 70 (24-93) | 50 | n.a. | n.a. | 0 | 15 ng/mL | n.a. | 20 mo | OS, PFS | 6 |
| Tracy et al, 201724 | 2002-2012 | US | FL | 60 (23-93) | 642 | 120 | 522 | 0 | 20 ng/mL | LC-MS/MS | 59 mo | OS | 8 |
| Cuccaro et al, 201725 | 2014-2016 | Italy | HL | 33 | 76 | 9 | 67 | 0 | 10 ng/mL | CLIA | 12 mo | PFS | 7 |
| Kelly et al, 201526 | 1998-2008 | US | FL (SWOG cohort) | n.a. | 183 | 28 | 155 | 0 | 20 ng/mL | LC-MS/MS | 5.4 y | PFS, OS | 9 |
| 2004-2007 | — | FL (LYSA cohort) | n.a. | 240 | 60 | 180 | 0 | 10 ng/mL | 6.6 y | ||||
| Bittenbring et al, 201427 | 2000-2005 | Germany | DLBCL with R #1 in the elderly | 61-80 | 184 | 81 | 103 | 0 | 8 ng/mL | CLIA | 34.5 mo | PFS, OS | 9 |
| DLBCL without R in the elderly | 61-80 | 175 | 70 | 105 | 0 | ||||||||
| 2005-2007 | DLBCL with R #2 in the elderly | 61-80 | 63 | 9 | 54 | 0 | 39 mo | ||||||
| Aref et al, 201328 | n.a. | Egypt | B-CLL | 57 (50-60) | 75 | 54 | 21 | 0 | 20 ng/mL | ELISA | n.a. | OS | 8 |
| NHL (DLBCL, MCL, FL, LPL, BL) | 61 (52-67) | 120 | 64 | 56 | 0 | 5 y | |||||||
| Molica et al, 201229 | 1998-2008 | Italy | CLL | 68 (43-87) | 130 | n.a. | n.a. | 0 | 13.5 ng/mL | CLIA | 39 m | TTT | 7 |
| Tretli et al, 201230 | 1984-2008 | Norway | Lymphoma | 56.3 (37-79) | 145 | 40 | 28 | 77 | <46 nmol/L, >81 nmol/L | RIA | n.a. | OS | 7 |
| Shanafelt et al, 201131 | 1994-2008 | US | CLL | n.a. | 543 | 180 | 363 | 0 | 25 ng/mL | LC-MS/MS | n.a. | OS, TTT | 9 |
| Drake et al, 201032 | 2002-2008 | US | NHL (DLBCL, TCL, MCL, FL, other) | 62 (19-94) | 983 | 436 | 547 | 0 | 25 ng/mL | LC-MS/MS | 34.8 mo | EFS, OS | 9 |
| Eicher et al, 202041 | 2012-2018 | Switzerland | ASCT (lymphoma, myeloma) | 60 (24-77) | 183 | 102 | 81 | 0 | 52 nmol/L | CLIA | n.a. | OS | 8 |
| Rakhee et al, 201642 | 2010-2015 | US | ASCT (myeloma) | n.a. | 158 | 94 | 64 | 0 | 23 ng/mL | n.a. | n.a. | OS | 6 |
| Clairmont et al, 201443 | 2009- 2010 | US | ASCT (lymphoma, myeloma) | n.a. | 132 | n.a. | n.a. | 0 | n.a. | n.a. | n.a. | OS | 5 |
| Bajwa et al, 201944 | 2012-2017 | US | Allo-HSCT in children | n.a. | n.a. | 48 | 78 | n.a. | ≤20 ng/mL, >30 ng/mL | n.a. | n.a. | OS | 7 |
| Radujkovic et al, 201745 | 2002-2013 | Germany | Allo-HSCT (myeloid #1) | 17-75 | 242 | 188 | 54 | 0 | 20 ng/mL | CLIA | 51.2 mo | OS, RR, NRM | 9 |
| Allo-HSCT (lymphoid) | 17-75 | 250 | 208 | 42 | 0 | ||||||||
| 2009-2013 | Allo-HSCT (myeloid #2) | 17-73 | 398 | 348 | 50 | 0 | 51.3 mo | ||||||
| von Bahr et al, 201546 | 2005-2011 | Sweden | Allo-HSCT | 12-68 | 166 | 19 | 59 | 88 | <25 nmol/L, ≥50 nmol/L | CLIA | 71 mo | OS | 8 |
| Study . | Study period . | Country . | Disease . | Age, median (range), y . | Total N . | Low N . | High N . | Mid N . | Vitamin D threshold . | Measuring method . | Median f/u . | Outcome . | NOS . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hu et al, 202017 | 2014-2016 | China | AML in the elderly (w/o APL, secondary AML) | 70 (60-89) | 68 | 25 | 43 | 0 | 8.84 ng/mL | ECL | 2 y | OS | 9 |
| Jackmann et al, 202018 | 1990-2016 | Sweden | ALL in children | 7 (0.4-17.8) | 232 | 71 | 161 | 0 | 50 nmol/L | CLIA | n.a. | OS | 8 |
| AML in children | 52 | 22 | 30 | 0 | |||||||||
| CML/JMML in children | 11 | 5 | 6 | 0 | |||||||||
| Radujkovic et al, 201719 | 2006-2014 | Germany | MDS, oligoblastic AML | 69 (31-83) | 58 | 29 | 29 | 0 | 32.6 nmol/L | CLIA | 29 mo | OS | 7 |
| Lee et al, 201420 | n.a. | US | AML (w/o APL) | 60 (19-91) | 97 | 29 | 34 | 34 | <20 ng/mL, ≥32 ng/mL | RIA | 15.6 mo | OS, RFS | 8 |
| Pardanani et al, 201121 | n.a. | US | PMF | 63 (14-83) | 247 | 118 | 129 | 0 | 25 ng/mL | LC-MS/MS | 34 mo | OS, LFS | 8 |
| MDS | 72 (44-89) | 74 | 21 | 53 | 0 | 25 mo | |||||||
| Mao et al, 202122 | 2014-2019 | China | ENKTL | 55 (21-92) | 93 | 55 | 38 | 0 | 50 nmol/L | ECL | 23 mo | OS, PFS | 8 |
| Wang et al, 202023 | 2016-2018 | China | DLBCL | 58 (19-85) | 208 | 142 | 66 | 0 | 52.5 nmol/L | ECL | 29 mo | OS, PFS | 9 |
| Chen et al, 202133 | 2011-2018 | China | DLBCL | n.a. | 332 | 111 | 111 | 110 | <11.5 ng/mL, ≥18.7 ng/mL | LC-MS/MS | 34.2 mo | OS, PFS | 9 |
| Xu et al, 202034 | 2014-2018 | China | MCL | 61 (39-77) | 70 | 40 | 30 | 0 | 50 nmol/L | ECL | 25.5 mo | OS, PFS | 8 |
| Yellapragada et al, 202035 | n.a. | US | MM | 68.9 | 1889 | 582 | 1307 | 0 | 20 ng/mL | n.a. | n.a. | OS | 8 |
| Borchmann et al, 201936 | 1993-1998 | Germany | HL | 32 (16-75) | 351 | 175 | 176 | 0 | 30 nmol/L | ELISA | 13 y | OS, PFS | 8 |
| Kim et al, 201837 | 2008-2016 | Korea | PTCL, ENKTL | 17-85 | 251 | 105 | 146 | 0 | 10 ng/mL | LC-MS/MS | 35.8 mo | PFS | 8 |
| Djurasinovic et al, 201838 | 2014- 2016 | Serbia | Lymphoid malignancy (DLBCL, FL, CLL/SLL, HL) | 58 (18-84) | 133 | n.a. | n.a. | 0 | 19.6 nmol/L | CLIA | 20 mo | PFS | 8 |
| Hohaus et al, 201839 | 2013-2016 | Italy | Aggressive BCL | 65 | 154 | 104 | 50 | 0 | 20 ng/mL | CLIA | n.a. | EFS | 7 |
| Ferrari et al, 201740 | 2013-2016 | Italy | DLBCL | 70 (24-93) | 50 | n.a. | n.a. | 0 | 15 ng/mL | n.a. | 20 mo | OS, PFS | 6 |
| Tracy et al, 201724 | 2002-2012 | US | FL | 60 (23-93) | 642 | 120 | 522 | 0 | 20 ng/mL | LC-MS/MS | 59 mo | OS | 8 |
| Cuccaro et al, 201725 | 2014-2016 | Italy | HL | 33 | 76 | 9 | 67 | 0 | 10 ng/mL | CLIA | 12 mo | PFS | 7 |
| Kelly et al, 201526 | 1998-2008 | US | FL (SWOG cohort) | n.a. | 183 | 28 | 155 | 0 | 20 ng/mL | LC-MS/MS | 5.4 y | PFS, OS | 9 |
| 2004-2007 | — | FL (LYSA cohort) | n.a. | 240 | 60 | 180 | 0 | 10 ng/mL | 6.6 y | ||||
| Bittenbring et al, 201427 | 2000-2005 | Germany | DLBCL with R #1 in the elderly | 61-80 | 184 | 81 | 103 | 0 | 8 ng/mL | CLIA | 34.5 mo | PFS, OS | 9 |
| DLBCL without R in the elderly | 61-80 | 175 | 70 | 105 | 0 | ||||||||
| 2005-2007 | DLBCL with R #2 in the elderly | 61-80 | 63 | 9 | 54 | 0 | 39 mo | ||||||
| Aref et al, 201328 | n.a. | Egypt | B-CLL | 57 (50-60) | 75 | 54 | 21 | 0 | 20 ng/mL | ELISA | n.a. | OS | 8 |
| NHL (DLBCL, MCL, FL, LPL, BL) | 61 (52-67) | 120 | 64 | 56 | 0 | 5 y | |||||||
| Molica et al, 201229 | 1998-2008 | Italy | CLL | 68 (43-87) | 130 | n.a. | n.a. | 0 | 13.5 ng/mL | CLIA | 39 m | TTT | 7 |
| Tretli et al, 201230 | 1984-2008 | Norway | Lymphoma | 56.3 (37-79) | 145 | 40 | 28 | 77 | <46 nmol/L, >81 nmol/L | RIA | n.a. | OS | 7 |
| Shanafelt et al, 201131 | 1994-2008 | US | CLL | n.a. | 543 | 180 | 363 | 0 | 25 ng/mL | LC-MS/MS | n.a. | OS, TTT | 9 |
| Drake et al, 201032 | 2002-2008 | US | NHL (DLBCL, TCL, MCL, FL, other) | 62 (19-94) | 983 | 436 | 547 | 0 | 25 ng/mL | LC-MS/MS | 34.8 mo | EFS, OS | 9 |
| Eicher et al, 202041 | 2012-2018 | Switzerland | ASCT (lymphoma, myeloma) | 60 (24-77) | 183 | 102 | 81 | 0 | 52 nmol/L | CLIA | n.a. | OS | 8 |
| Rakhee et al, 201642 | 2010-2015 | US | ASCT (myeloma) | n.a. | 158 | 94 | 64 | 0 | 23 ng/mL | n.a. | n.a. | OS | 6 |
| Clairmont et al, 201443 | 2009- 2010 | US | ASCT (lymphoma, myeloma) | n.a. | 132 | n.a. | n.a. | 0 | n.a. | n.a. | n.a. | OS | 5 |
| Bajwa et al, 201944 | 2012-2017 | US | Allo-HSCT in children | n.a. | n.a. | 48 | 78 | n.a. | ≤20 ng/mL, >30 ng/mL | n.a. | n.a. | OS | 7 |
| Radujkovic et al, 201745 | 2002-2013 | Germany | Allo-HSCT (myeloid #1) | 17-75 | 242 | 188 | 54 | 0 | 20 ng/mL | CLIA | 51.2 mo | OS, RR, NRM | 9 |
| Allo-HSCT (lymphoid) | 17-75 | 250 | 208 | 42 | 0 | ||||||||
| 2009-2013 | Allo-HSCT (myeloid #2) | 17-73 | 398 | 348 | 50 | 0 | 51.3 mo | ||||||
| von Bahr et al, 201546 | 2005-2011 | Sweden | Allo-HSCT | 12-68 | 166 | 19 | 59 | 88 | <25 nmol/L, ≥50 nmol/L | CLIA | 71 mo | OS | 8 |
ALL, acute lymphoblastic leukemia; APL, acute promyelocytic leukemia; BCL, B-cell lymphoma; BL, Burkitt lymphoma; CLIA, chemiluminescence immunoassay; CLL, chronic lymphocytic leukemia; CML, chronic myeloid leukemia; DLBCL, diffuse large B-cell lymphoma; ECL, electrochemiluminescence; EFS, event-free survival; ELISA, enzyme-linked immunosorbent assay; ENKTL, extranodal natural killer/T-cell lymphoma; FL, follicular lymphoma; f/u, follow-up; High N, patient number with high vitamin D level; HL, Hodgkin lymphoma; JMML, juvenile myelomonocytic leukemia; LC-MS/MS, liquid chromatography–tandem mass spectrometry; LFS, leukemia-free survival; Low N, patient number with low vitamin D level; LPL, lymphoplasmacytic lymphoma; MCL, mantle cell lymphoma; MDS, myelodysplastic syndrome; Mid N, patient number with middle vitamin D level; MM, multiple myeloma; n.a., not available; NHL, non-Hodgkin lymphoma; NOS, Newcastle-Ottawa scale; PMF, primary myelofibrosis; PTCL, peripheral T-cell lymphoma; R, rituximab; RFS, relapse-free survival; RIA, radioimmunoassay; RR, relapse rate; SLL, small lymphocytic lymphoma; TCL, T-cell lymphoma; Total N, total patient number; US, United States; w/o, without.
Vitamin D level in myeloid malignancies at diagnosis
The OS data for myeloid malignancies were available in 5 articles17-21 that included 573 patients with AML, chronic myeloid leukemia, juvenile myelomonocytic leukemia, myelodysplastic syndrome, and primary myelofibrosis. Patients with lower vitamin D levels had significantly poorer OS (HR, 1.39; 95% CI, 1.06-1.82) with substantial heterogeneity (I2 = 57%; P = .03; Figure 2A-B). PFS was analyzed in 384 patients from 3 cohorts;20,21 it was significantly poorer in the group with lower vitamin D status (HR, 2.03; 95% CI, 1.23-3.32) without heterogeneity (I2 = 0%, P = .57; Figure 2C).
Outcomes in myeloid malignancies. HR of OS survival in myeloid malignancies (A) and funnel plot (B). (C) HR of progression-free survival in myeloid malignancies. CML, chronic myeloid leukemia; JMML, juvenile myelomonocytic leukemia; MDS, myelodysplastic syndrome; PMF, primary myelofibrosis; seTE, standard error of treatment estimate; TE, estimated treatment effect.
Outcomes in myeloid malignancies. HR of OS survival in myeloid malignancies (A) and funnel plot (B). (C) HR of progression-free survival in myeloid malignancies. CML, chronic myeloid leukemia; JMML, juvenile myelomonocytic leukemia; MDS, myelodysplastic syndrome; PMF, primary myelofibrosis; seTE, standard error of treatment estimate; TE, estimated treatment effect.
Vitamin D level in lymphoid malignancies at diagnosis
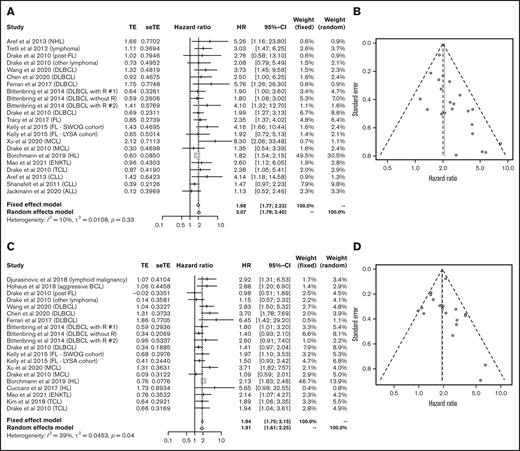
Data for 4502 patients from 14 articles were eligible for the analysis of OS in lymphoid malignancies.18,22-24,26-28,30-34,36,40 OS was significantly poorer in the group with lower vitamin D status (HR, 2.07; 95% CI, 1.79-2.40) without heterogeneity (I2 = 10%; P = .33; Figure 3A). The funnel plot suggested a publication bias (P = .002; Figure 3B). PFS analyzed in 3436 patients from 13 articles22,23,25-27,32-34,36-40 was also significantly poorer in those with lower vitamin D levels (HR, 1.91; 95% CI, 1.61-2.25) with heterogeneity (I2 = 39%; P = .04; Figure 3C). The funnel plot did not show a publication bias (P = .69; Figure 3D).
Outcomes in lymphoid malignancies. HR of OS in lymphoid malignancies (A) and funnel plot (B). HR of progression-free survival in lymphoid malignancies (C) and funnel plot (D). ALL, acute lymphoblastic leukemia; BCL, B-cell lymphoma; DLBCL, diffuse large B-cell lymphoma; ENKTL, extranodal natural killer/T-cell lymphoma; FL, follicular lymphoma; HL, Hodgkin lymphoma; MCL, mantle cell lymphoma; NHL, non-Hodgkin lymphoma; R, rituximab; seTE, standard error of treatment estimate; TCL, T-cell lymphoma; TE, estimated treatment effect.
Outcomes in lymphoid malignancies. HR of OS in lymphoid malignancies (A) and funnel plot (B). HR of progression-free survival in lymphoid malignancies (C) and funnel plot (D). ALL, acute lymphoblastic leukemia; BCL, B-cell lymphoma; DLBCL, diffuse large B-cell lymphoma; ENKTL, extranodal natural killer/T-cell lymphoma; FL, follicular lymphoma; HL, Hodgkin lymphoma; MCL, mantle cell lymphoma; NHL, non-Hodgkin lymphoma; R, rituximab; seTE, standard error of treatment estimate; TCL, T-cell lymphoma; TE, estimated treatment effect.
Vitamin D level in DLBCL
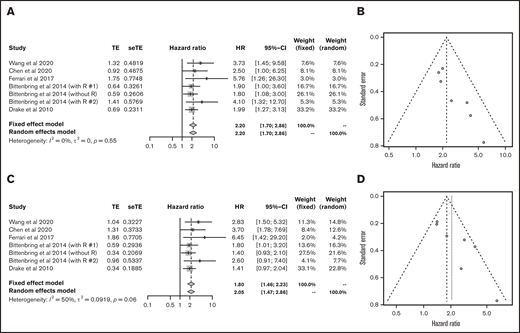
Next, we analyzed each subtype of lymphoid malignancy. Data on 1272 patients with diffuse large B-cell lymphoma (DLBCL) from 5 articles were eligible for analysis of OS and PFS.23,27,32,33,40 Lower vitamin D status was associated with poorer OS (HR, 2.20; 95% CI, 1.70-2.86) and PFS (HR, 2.05; 95% CI, 1.47-2.86) (Figure 4).
Outcomes in DLBCL. HR of OS in DLBCL (A) and funnel plot (B). HR of progression-free survival in DLBCL (C) and funnel plot (D). R, rituximab; seTE, standard error of treatment estimate; TE, estimated treatment effect.
Outcomes in DLBCL. HR of OS in DLBCL (A) and funnel plot (B). HR of progression-free survival in DLBCL (C) and funnel plot (D). R, rituximab; seTE, standard error of treatment estimate; TE, estimated treatment effect.
Vitamin D level in FL at diagnosis
For follicular lymphoma (FL), data on 1065 patients from 3 cohorts were eligible for the analysis of OS.24,26 Tracy et al performed an expanded analysis of their prior cohort; thus, we used their expanded data.24,32 Lower vitamin D status was also associated with significantly poorer OS (HR, 2.55; 95% CI, 1.68-3.88) without heterogeneity (Figure 5A). PFS data were available for 423 patients from 2 cohorts;26 lower vitamin D status was associated with significantly poorer PFS (HR, 1.67; 95% CI, 1.16-2.42) without heterogeneity (Figure 5B).
Outcomes in each subtype of lymphoid malignancies. HRs of OS (A) and PFS (B) in FL. HRs of OS (C) and PFS (D) in MCL. (E) HR of PFS in HL. HRs of OS (F) and PFS (G) in TCL. HRs of OS (H) and TTT (I) in CLL. seTE, standard error of treatment estimate; TE, estimated treatment effect.
Outcomes in each subtype of lymphoid malignancies. HRs of OS (A) and PFS (B) in FL. HRs of OS (C) and PFS (D) in MCL. (E) HR of PFS in HL. HRs of OS (F) and PFS (G) in TCL. HRs of OS (H) and TTT (I) in CLL. seTE, standard error of treatment estimate; TE, estimated treatment effect.
Vitamin D level in MCL at diagnosis
For mantle cell lymphoma (MCL), the data on 141 patients from 2 articles were available32,34 ; they did not show any significant association between vitamin D level and OS (HR, 3.10; 95% CI, 0.53-8.24) or PFS (HR, 1.98; 95% CI, 0.60-6.59) with substantial heterogeneity (Figure 5C-D).
Vitamin D level in HL at diagnosis
For Hodgkin lymphoma (HL), OS data were available for 351 patients from only 1 article,36 which reported that lower vitamin D status was related to impaired OS (HR, 1.82; 95% CI, 1.53-2.15). The data on 427 patients from 2 articles were eligible for analysis of PFS,25,36 which revealed poor PFS in patients with lower vitamin D level (HR, 2.31; 95% CI, 1.36-3.93; Figure 5E).
Vitamin D level in TCL at diagnosis
For T-cell lymphoma (TCL), data for 163 patients from 2 articles22,32 and 414 patients from 3 articles22,32,37 were eligible for the analysis of OS and PFS, respectively. Lower vitamin D status was associated with poor OS and PFS (HR, 2.49; 95% CI, 1.38-4.48 and HR, 1.97; 95% CI, 1.38-2.82, respectively; Figure 5F-G). Kim et al further divided TCL into peripheral TCL and extranodal natural killer/T-cell lymphoma37 and showed that vitamin D deficiency impaired prognosis only in the latter group of patients; thus, further subdivision of TCL may be required for more accurate examination.
Vitamin D level in CLL at diagnosis
For CLL, the data for 618 patients from 2 articles were eligible for the analysis of OS,28,31 which showed that lower vitamin D status was not significantly associated with OS (HR, 2.07; 95% CI, 0.80-5.36; Figure 5H). There were no articles reporting on PFS, and TTT data were available from 2 articles with 673 patients.29,31 TTT was significantly shorter in the group of patients with lower vitamin D status (HR, 1.55; 95% CI, 1.19-2.00; Figure 5I).
Vitamin D level in MM at diagnosis
For multiple myeloma (MM), only 1 article was included in our search;35 it reported that lower vitamin D status was significantly associated with poor OS (HR, 1.34; P = .008). The investigators showed that its impact on OS was observed in white patients (HR, 1.45; P = .005), but not in African Americans, suggesting a difference between ethnic groups.
Threshold of vitamin D level in the studies of lymphoid malignancies
There is no agreed upon cutoff value for the definition of vitamin D deficiency,11 and the studies included in this meta-analysis used various thresholds based on different guidelines (Table 1). To examine this discrepancy, we performed subgroup analyses based on the cutoff values in lymphoid malignancies. Eight studies defined 50 nmol/L (20 ng/mL) as a threshold18,22,24,26,28,34,35,39 ; patients with vitamin D levels less than this value had poorer OS (HR, 2.83; 95% CI, 1.81-4.42) and PFS significantly (HR, 2.48; 95% CI, 1.76-3.51; supplemental Figure 1A-B) without heterogeneity. Three studies defined 25 nmol/L (10 ng/mL) as a threshold;25,26,37 a vitamin D level less than this value was associated with poorer PFS (HR, 1.75; 95% CI, 1.19-2.59; supplemental Figure 1C). Two studies used 62.5 nmol/L (25 ng/mL) as a threshold;31,32 a vitamin D level less than this value was also associated with poorer OS (HR, 1.77; 95% CI, 1.36-2.29) and PFS (HR, 1.32; 95% CI, 1.04-1.68; supplemental Figure 1D-E). All subgroup analyses demonstrated that lower vitamin D levels were associated with poorer outcomes.
The impact of geographical distribution on vitamin D level in lymphoid malignancies
Geographical factors, such as latitude and sunlight exposure, affect the levels of vitamin D in the normal population11 ; thus, we performed subgroup analysis in lymphoid malignancies for the United States,24,26,31,32,35 China,22,23,33,34 Italy,25,29,39,40 and Germany.27,36 A lower vitamin D level was associated with significantly poorer OS (HR, 1.96; 95% CI, 1.56-2.46) and PFS (HR, 1.40; 95% CI, 1.12-1.75) in the United States, significantly poorer OS (HR, 3.26; 95% CI, 2.00-5.30) and PFS (HR, 2.99; 95% CI, 2.12-4.21) in China, significantly poorer PFS (HR, 3.80; 95% CI, 1.90-7.60) in Italy, and significantly poorer OS (HR, 1.85; 95% CI, 1.59-2.15) and PFS (HR, 1.92; 95% CI, 1.54-2.39) in Germany (supplemental Figure 2).
Vitamin D level at ASCT
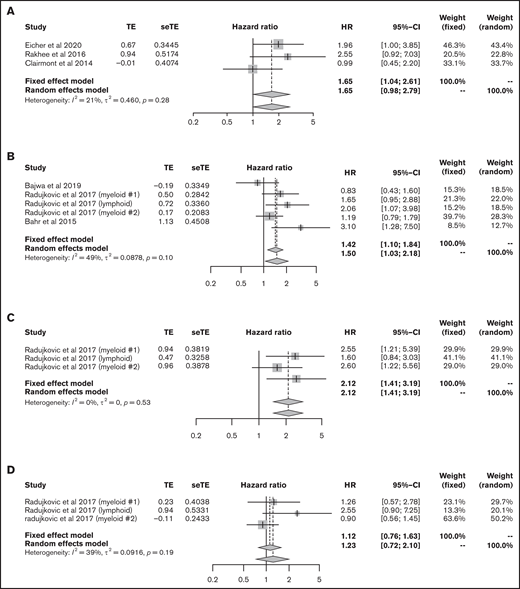
We then focused on the significance of vitamin D levels during HSCT. First, we analyzed the data from patients with lymphoma and myeloma who underwent ASCT. The data for 141 lymphoma patients and 332 myeloma patients from 3 articles were eligible for analysis of OS;41-43 lower vitamin D status was associated with significantly poorer OS (HR, 1.65; 95% CI, 1.04-2.61, using the fixed effect model), with low heterogeneity (I2 = 21%, P = .28) (Figure 6A).
Outcomes in HSCT. (A) HR of OS in ASCT. HRs of OS (B), relapse rate (C), and NRM (D) in allo-HSCT. seTE, standard error of treatment estimate; TE, estimated treatment effect.
Outcomes in HSCT. (A) HR of OS in ASCT. HRs of OS (B), relapse rate (C), and NRM (D) in allo-HSCT. seTE, standard error of treatment estimate; TE, estimated treatment effect.
Vitamin D level at allo-HSCT
Next, we extracted allo-HSCT data for hematological malignancies. The data for 1094 patients from 5 cohorts were eligible for the analysis of OS.44 -46 Bajwa et al analyzed OS in patients with malignancy and nonmalignancy separately; thus, we only used data for patients with malignancy.44 The meta-analysis showed that lower vitamin D status was associated with significantly poorer OS (HR, 1.50; 95% CI, 1.03-2.18; Figure 6B). Data for relapse rate and NRM were available from 3 cohorts in only 1 article45 that included 890 patients; lower vitamin D status was related to a high relapse rate (HR, 2.12; 95% CI, 1.41-3.19) but not to NRM (HR, 1.23; 95% CI, 0.72-2.10; Figure 6C-D).
Discussion
This meta-analysis comprehensively investigated the impact of circulating vitamin D levels at diagnosis on the prognosis of hematological malignancies and each subset of lymphoid malignancy. We showed that lower vitamin D level at diagnosis was related to a significantly impaired prognosis for myeloid and lymphoid malignancies, as previously reported.5,10 Moreover, further subgroup analysis revealed that a lower vitamin D level was associated with a poorer prognosis for several lymphoma subtypes: DLBCL, FL, HL, and TCL. Although the survival data for patients with MCL or CLL did not reach statistical significance, lower vitamin D status shortened TTT in CLL.
Vitamin D is associated with bone homeostasis, as well as with tumorigenesis via many mechanisms.2,3,11 Several reports have confirmed the direct antitumor effect of vitamin D against leukemia and lymphoma cells in vitro: an antiproliferation effect in non-HL69 and myeloma70 and induction of apoptosis in B-cell CLL71 . Vitamin D also exerts synergistic effects with other anticancer agents (eg, azacytidine) against myeloid cell lines19 and etoposide and doxorubicin against HL cell lines.36 Moreover, vitamin D potentiates antitumor immunity by activating natural killer cells27 and macrophages72 ; thus, vitamin D has a variety of protective mechanisms against hematological malignancies, and further investigation is warranted to understand the entire spectrum.73
Several meta-analyses have been performed to elucidate the role of vitamin D in hematological malignancies (Table 2). Vitamin D deficiency was consistently associated with poorer OS and PFS in leukemia and lymphoma patients,5,10,74 Also, some meta-analyses focused on the relationship between vitamin D status and the risk of lymphoma but did not find any significant correlation.75-77
Characteristics of previous meta-analyses of the role of vitamin D status in hematological malignancies
| Study . | Disease . | No. of articles included . | HR (95% CI) . |
|---|---|---|---|
| This study | Myeloid malignancy | 5 | OS, 1.39 (1.06-1.82); PFS, 2.03 (1.23-3.32) |
| Lymphoid malignancy | 20 | OS, 2.07 (1.79-2.40); PFS, 1.91 (1.61-2.25) | |
| ASCT | 3 | OS, 1.65 (1.04-2.61) | |
| Allo-HSCT | 3 | OS, 1.50 (1.03-2.18) | |
| Tao et al, 202174 | Lymphoma | 12 | OS, 1.94 (1.71-2.19); PFS, 2.06 (1.82-2.32) |
| Chiengthong et al, 202079 | HSCT | 8 | aGVHD, 1.07 (0.74-1.53); cGVHD, 1.75 (0.72-4.26) |
| Wang et al, 201510 | Hematological cancers | 7 | OS, 1.85 (1.54-2.23); RFS, 1.45 (1.25-1.70) |
| Leukemia | 3 | OS, 2.17 (1.54-3.05); RFS, 1.74 (1.34-2.27) | |
| Lymphoma | 4 | OS, 1.95 (1.47-2.59); RFS, 1.25 (1.02-1.54) | |
| Li et al, 20145 | Lymphoma | 2 | OS, 2.08 (1.56-2.78) |
| Study . | Disease . | No. of articles included . | HR (95% CI) . |
|---|---|---|---|
| This study | Myeloid malignancy | 5 | OS, 1.39 (1.06-1.82); PFS, 2.03 (1.23-3.32) |
| Lymphoid malignancy | 20 | OS, 2.07 (1.79-2.40); PFS, 1.91 (1.61-2.25) | |
| ASCT | 3 | OS, 1.65 (1.04-2.61) | |
| Allo-HSCT | 3 | OS, 1.50 (1.03-2.18) | |
| Tao et al, 202174 | Lymphoma | 12 | OS, 1.94 (1.71-2.19); PFS, 2.06 (1.82-2.32) |
| Chiengthong et al, 202079 | HSCT | 8 | aGVHD, 1.07 (0.74-1.53); cGVHD, 1.75 (0.72-4.26) |
| Wang et al, 201510 | Hematological cancers | 7 | OS, 1.85 (1.54-2.23); RFS, 1.45 (1.25-1.70) |
| Leukemia | 3 | OS, 2.17 (1.54-3.05); RFS, 1.74 (1.34-2.27) | |
| Lymphoma | 4 | OS, 1.95 (1.47-2.59); RFS, 1.25 (1.02-1.54) | |
| Li et al, 20145 | Lymphoma | 2 | OS, 2.08 (1.56-2.78) |
aGVHD, acute graft-versus-host disease; cGVHD, chronic graft-versus-host disease; RFS, relapse-free survival.
We also performed the first meta-analysis of the association between pretransplant vitamin D levels and prognosis, which showed a significant negative impact of lower vitamin D status on OS in ASCT and allo-HSCT patients, despite the limited number of studies. Lower vitamin D level at allo-HSCT was not associated with NRM, but it was associated with a higher relapse rate, which might have resulted in a worse prognosis. Studies on pediatric transplantation that included a substantial proportion of nonmalignant diseases were excluded from this meta-analysis. Some of them concluded that vitamin D deficiency was associated with worse outcome62,66 ; thus, subgroup analyses that differentiate between malignant and nonmalignant diseases are needed in the future. Pretransplant vitamin D deficiency has also been implicated in the pathogenesis of graft-versus-host disease via immunomodulatory effects,46,78 but a recent meta-analysis did not show a statistically significant association79 (Table 2).
Evidence for an impact of vitamin D levels on cancer prognosis has been accumulating gradually, but it remains uncertain whether vitamin D supplementation can improve cancer prognosis. A recent meta-analysis demonstrated a clinically meaningful benefit for vitamin D supplementation on colorectal cancer survival outcomes.80 With regard to hematological malignancies, some studies suggested that vitamin D supplementation was associated with improved event-free survival in DLBCL patients39 and relapse-free survival after ASCT.81 Larger randomized clinical trials are needed to establish additional evidence.
This meta-analysis has several limitations. First, the threshold for vitamin D level differed between articles. The threshold is determined based on clinical effects in bone health, such as osteoporosis, bone mineral density, and hip fractures, or the inverse relationship between serum parathyroid hormone and vitamin D level.82,83 Several studies calculated different thresholds using their own clinical data respectively; thus, a consensus has not been achieved.82 For example, Endocrine Society clinical practice guidelines define deficiency as <20 ng/mL, insufficiency as 21 to 29 ng/mL, and sufficiency as >30 ng/mL.84 Another report defines severe deficiency as <5 ng/mL, moderate deficiency as 5 to 10 ng/mL, mild deficiency as 10 to 20 ng/mL, and replete as >20 ng/mL.83 Other reviews suggest 10 ng/mL, 12 ng/mL, 20 ng/mL,11 or 30 ng/mL85 as a cutoff. The definition is different among guidelines and reviews, and the studies in our meta-analyses referred to them individually, resulting in the discrepancies shown in Table 1. Vitamin D levels are also influenced by geographic region, diet, environmental factors, and lifestyle,86 and the optimal target remains unclear.11,26 In MM patients, vitamin D deficiency was associated with poor OS in white, but not in African American,35 patients, suggesting the importance of racial differences. To clarify the significance of these discrepancies, we performed subgroup analysis classified by each cutoff value (25, 50, and 62.5 nmol/L) and each country (United States, China, Italy, and Germany) in lymphoid malignancies, which confirmed the significant relationship between lower vitamin D levels and poorer outcomes in all subgroups. Second, the measurement method was also different between articles. Liquid chromatography-tandem mass spectrometry is recommended as the most accurate method, but immunoassays were used in some articles;87 thus, the measurement method should be agreed upon. Third, the number of studies specific to each subtype of leukemia and lymphoma remains insufficient. With regard to myeloid malignancies, we could not analyze patients with AML, MDS, chronic myeloid leukemia/juvenile myelomonocytic leukemia, or primary myelofibrosis individually. These subtypes are different with regard to disease biology; thus, they should be analyzed separately. Considering lymphoid malignancies, the data on DLBCL patients were relatively abundant: 5 articles, including 7 cohorts, but other lymphoma subgroups were studied in only 1 to 3 articles. In addition, the condition of transplantation is quite different according to the type of disease, disease status, age, donor source, preconditioning regimen, and so on; thus, it should be adjusted in future investigations. Fourth, 25(OH)D is commonly used as a reliable indicator of vitamin D status; however, some articles recommend bioavailable 25(OH)D level as a precise biomarker.33 Moreover, Peter et al showed that peritransplant 1,25(OH)2D3 levels, but not 25(OH)D levels, predicted survival after stem cell transplantation.49 Therefore, the most precise biomarker of vitamin D levels in predicting the outcome of malignancies should be investigated further. Fifth, although our meta-analysis clarified the association between low vitamin D level and poorer PFS and OS, it does not establish causality and cannot exclude the possibility of residual confounding or reverse causality. Sixth, some patients with vitamin D deficiency were prescribed vitamin D supplementation,39 but most articles did not mention whether patients received supplementation. Thus, we could not include this factor in this meta-analysis. However, even if patients with vitamin D deficiency received supplementation, our results still suggest that lower vitamin D status at diagnosis has a worse prognosis. The significance of vitamin D supplementation needs to be addressed in future prospective trials.
In conclusion, this meta-analysis demonstrated that lower vitamin D status at diagnosis was associated with a significantly worse prognosis for myeloid and lymphoid malignancies, as well as several lymphoma subtypes, including DLBCL, FL, HL, and TCL. We also showed that pretransplant vitamin D level was an important factor for prognosis in ASCT and allo-HSCT. Further studies focusing on each subtype of hematological malignancy are warranted.
Authorship
Contribution: Y.I. conceptualized and designed the study, performed literature research, analyzed data, performed statistical analyses, and wrote the manuscript; A.H. performed literature review and critically revised the manuscript; and M.K. supervised the study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mineo Kurokawa, Department of Hematology and Oncology, Graduate School of Medicine, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8655, Japan; e-mail: kurokawa-tky@umin.ac.jp.
References
Author notes
The full-text version of this article contains a data supplement.