Key Points
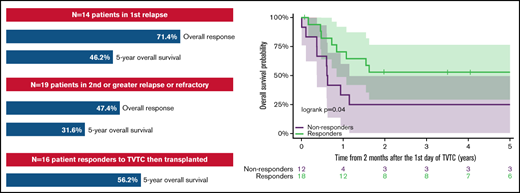
The overall response rate for children and young adults with AML receiving TVTC in first relapse was 71.4%.
Eighty-four percent of responders were bridged to hematopoietic stem cell transplant without anthracycline and 56.2% had long-term survival.
Abstract
Effective reinduction regimens are needed for children with relapsed and refractory acute myeloid leukemia (AML), as outcomes remain poor. Therapeutic options are limited in this heavily pretreated patient population, many of whom have reached lifetime recommended doses of anthracycline chemotherapy. The development of effective non-anthracycline–based salvage regimens is crucial to these patients who are at significant risk of life-threatening cardiotoxicity. We previously reported results of a phase 2 trial of a clofarabine-based regimen with topotecan, vinorelbine, and thiotepa (TVTC) in patients with relapsed acute leukemias. Here we report on an expanded bicenter cohort of 33 patients, <25 years of age, with relapsed/refractory AML treated with up to 2 cycles of the TVTC reinduction regimen from 2007 to 2018. The overall response rate, defined as complete remission or complete remission with partial recovery of platelet count, was 71.4% (95% confidence interval [CI], 41.9-91.6) for those patients in first relapse (n = 14) and 47.4% (95% CI, 24.4-71.1) for patients in second or greater relapse or with refractory disease. Responses were seen across multiple high-risk cytogenetic and molecular subtypes, with 84% of responders successfully bridged to allogeneic stem cell transplantation. The 5-year overall survival for patients in first relapse was 46.2% (95% CI, 19.1-73.3) and 50.0% (95% CI, 26.9-73.1) for patients who responded to TVTC. For pediatric and young adult patients with relapsed/refractory AML, TVTC reinduction compares favorably with currently used salvage regimens and warrants further exploration.
Introduction
Newly diagnosed children with acute myeloid leukemia (AML) have long-term, event-free survival rates ranging from 50% to 63% when treated with contemporary frontline regimens.1-4 Children with relapsed disease, albeit a heterogenous patient population, carry a poor prognosis despite intensive salvage regimens.5,6 Among survivors of relapsed AML, more than one-third will develop late cardiotoxicity from cumulative anthracycline-containing frontline and salvage therapy regimens.7,8 There is an unmet need for effective and minimally cardiotoxic salvage regimens for children with relapsed AML.
Clofarabine is a deoxyadenosine analogue, similar in structure to fludarabine and cladribine but modified to enhance cytotoxic activity as well as stability.9 Clofarabine was granted US Food and Drug Administration approval for the treatment of relapsed acute lymphoblastic leukemia in 2004 following the results of a phase 2 single-agent study.10
We previously reported the results of a phase 1 and phase 2 trial of a clofarabine-based regimen with topotecan, vinorelbine, and thiotepa (TVTC) in a cohort of patients with relapsed acute leukemias.11,12 In the phase 2 study, 8 (67%) of 12 patients with relapsed or refractory (R/R) AML achieved a complete response (CR) or complete response with incomplete platelet recovery (CRp). The current article describes the outcomes of an expanded retrospective cohort of 33 patients with R/R AML treated with the TVTC regimen.
Methods
Patients
Children and young adults aged <25 years with R/R AML were included in this bicenter retrospective analysis from 2007 to 2018. This included 11 patients with AML treated on a previously published phase 2 clinical trial.12 Patients were included from both the Memorial Sloan Kettering Cancer Center (MSKCC) and the University of California San Francisco Benioff Children’s Hospital of Oakland. The institutions’ review boards approved the study and granted waivers of consent.
Patients with R/R AML were defined as those having at least 5% leukemic burden in the bone marrow (BM) according to morphologic assessment, and those who were treated with at least one cycle of TVTC were included in the analysis. Patients with combined medullary relapse with central nervous system involvement were included as well as those patients who received a prior hematopoietic stem cell transplant (HSCT). A total of 33 patients were identified who met these criteria. The population was further stratified into 2 groups: Group A (n = 14), defined as those in first relapse (after having previously achieved a remission) with no additional reinduction attempt; and Group B (n = 19) as all other patients, including patients in second or greater relapse, in first relapse and refractory to at least one reinduction attempt, or with primary refractory disease. Patients with therapy-related AML, mixed phenotype acute leukemia, and myeloid leukemias of Down syndrome were excluded from this analysis. Patients with previous clofarabine exposure were not included.
Total anthracycline exposure was calculated by using a conversion multiplier of 4 daunorubicin equivalents for mitoxantrone per the Children’s Oncology Group (COG) guidelines.13 Genetic prognostic markers were identified based on the risk stratification for cytogenetic abnormalities in the ongoing COG AAML1831 trial for de novo AML.14,15
Treatment and response assessment
Patients received the phase 2 recommended schedule of the TVTC regimen as follows: topotecan 1 mg/m2 per day (120-hour continuous infusion, days 0-4), vinorelbine 20 mg/m2 per dose (days 0, 7, and 14), thiotepa 15 mg/m2 per dose (day 2), and clofarabine 40 mg/m2 per day (days 3-7) (supplemental Figure 1). Patients >21 years of age or pediatric patients receiving a second cycle of TVTC were treated with reduced clofarabine dosing of 30 mg/m2 per day. Dexamethasone was given on day 3 before starting clofarabine to reduce the risk of capillary leak syndrome. Granulocyte colony-stimulating factor was administered until signs of neutrophil recovery. Response was assessed through BM evaluation and was performed at the point of hematologic recovery defined as absolute neutrophil count >0.5 K/mcL and platelet count >75 K/mcL. Outcomes of interest were defined as the following: overall response rate (ORR) as defined by complete remission (CR) of BM blasts to <5% by morphology and normal count recovery or CR without platelet recovery (CRp) after up to 2 cycles of TVTC. Minimal residual disease (MRD) was assessed by multiparameter flow cytometry using the “different from normal” method as previously described with a threshold for positivity of 0.05%.16,17 In the absence of multiparameter flow, alternate methods of MRD assessment, including quantitative reverse transcription polymerase chain reaction (PCR) for inv(16) and t(8;21), and PCR for FMS-like tyrosine kinase 3–internal tandem duplication (FLT3-ITD) detection, were used.
Statistical analysis
Overall survival (OS) was defined as time from the initiation of TVTC to death from any cause. Patients alive were censored at their date of last follow-up. The 95% confidence intervals (95% CIs) for the response rates were calculated by using the exact method (Pearson-Klopper method). To assess the OS according to response, a landmark analysis was conducted, with a landmark time at 2 months, assessing the impact of a response in the first 2 cycles.
Results
A total of 33 patients with R/R AML treated with the TVTC regimen were identified between the 2 centers (MSKCC, n = 31; University of California San Francisco Benioff Children’s Hospital of Oakland, n = 2). Fourteen patients were treated in first relapse (Group A). Nineteen patients were treated in second or greater relapse or with R/R or primary refractory disease (Group B). Patient characteristics of the entire cohort are shown in Table 1. The median age at the start of TVTC was 11 years (range, 1-24 years). All patients had an isolated BM relapse except for 2 patients who had a combined BM and central nervous system relapse. Both patients received additional weekly intrathecal treatment during therapy. Four patients in Group A (28.6%) had received prior allogeneic HSCT and 5 (26.3%) in Group B. In those patients who received TVTC in first relapse, 7 had an early relapse (within 1 year from diagnosis), and 7 patients relapsed >1 year from diagnosis.18
Study population characteristics
| Characteristic . | N = 33* . |
|---|---|
| Age at start of TVTC, y | 11.0 (1-24) |
| Sex | |
| Female | 17 (51.5) |
| Male | 16 (48.5) |
| BM blasts, % | 32 (10-99) |
| Prior HSCT | |
| No | 24 (72.7) |
| Yes | 9 (27.3) |
| Prior anthracycline (cumulative dose, mg/m2) | 342 (60-492) |
| Group A | n = 14 |
| Length of CR1, d | |
| <180 | 1 (7.1) |
| 180-365 | 6 (50) |
| >365 | 7 (42.9) |
| Prior HSCT | 4 (28.6) |
| Group B | n = 19 |
| Prior HSCT | 5 (26.3) |
| Characteristic . | N = 33* . |
|---|---|
| Age at start of TVTC, y | 11.0 (1-24) |
| Sex | |
| Female | 17 (51.5) |
| Male | 16 (48.5) |
| BM blasts, % | 32 (10-99) |
| Prior HSCT | |
| No | 24 (72.7) |
| Yes | 9 (27.3) |
| Prior anthracycline (cumulative dose, mg/m2) | 342 (60-492) |
| Group A | n = 14 |
| Length of CR1, d | |
| <180 | 1 (7.1) |
| 180-365 | 6 (50) |
| >365 | 7 (42.9) |
| Prior HSCT | 4 (28.6) |
| Group B | n = 19 |
| Prior HSCT | 5 (26.3) |
Group A = first relapse; Group B = second or greater R/R/primary refractory.
Statistics presented as median (minimum-maximum) or n (%).
The ORR for patients in Group A and Group B was 71.4% (10 of 14; 95% CI, 41.9-91.6) and 47.4% (9 of 19; 95% CI, 24.4-71.1), respectively. All responses were seen after 1 cycle of TVTC except in a single patient who achieved CR after a second cycle of TVTC. The distribution of prognostic features by relapse subgroups, including cytogenetic and molecular findings, is presented in Tables 2 and 3. Patients in both Groups A and B presented with high-risk cytogenetic or molecular features. Responses were seen across multiple high-risk cytogenetic subtypes, but only 1 response was seen among patients with monosomy 7 or ETV6 fusions (1 of 5). The cohort included 6 (19%) patients with favorable cytogenetics (4 in Group A and 2 in Group B), for whom the ORR was 100%. Supplemental Tables 1 and 2 provide detailed individual patient characteristics of the 2 relapse subgroups.
Group A characteristics
| Characteristic . | Responders (n = 10) . | Nonresponders (n = 4) . |
|---|---|---|
| Cytogenetic/molecular features | ||
| Normal | 1 | 0 |
| Favorable inv(16), t(8;21) | 4 | 0 |
| 11q23 | 0 | 1 |
| Del5q/monosomy 5 | 1 | 0 |
| Monosomy 7 | 0 | 1 |
| FLT3-ITD | 1 | 2 |
| Other | 3 | 0 |
| Prior HSCT | ||
| No | 9 (90%) | 1 (25%) |
| Yes | 1 (10%) | 3 (75%) |
| Characteristic . | Responders (n = 10) . | Nonresponders (n = 4) . |
|---|---|---|
| Cytogenetic/molecular features | ||
| Normal | 1 | 0 |
| Favorable inv(16), t(8;21) | 4 | 0 |
| 11q23 | 0 | 1 |
| Del5q/monosomy 5 | 1 | 0 |
| Monosomy 7 | 0 | 1 |
| FLT3-ITD | 1 | 2 |
| Other | 3 | 0 |
| Prior HSCT | ||
| No | 9 (90%) | 1 (25%) |
| Yes | 1 (10%) | 3 (75%) |
Group B characteristics
| Characteristic . | Responders (n = 9) . | Nonresponders (n = 10) . |
|---|---|---|
| Cytogenetic/molecular features | ||
| Normal | 1 | 0 |
| Favorable inv(16), t(8;21) | 2 | 0 |
| 11q23 | 2 | 0 |
| Del5q/monosomy 5 | 1 | 1 |
| Monosomy 7 | 1 | 2 |
| FLT3-ITD | 0 | 2 |
| NUP98-NSD1 | 1 | 0 |
| DEK-NUP214 | 1 | 0 |
| ETV6 rearrangement | 0 | 2 |
| Other | 0 | 3 |
| Prior HSCT | ||
| No | 5 (55.5%) | 9 (90%) |
| Yes | 4 (44.4%) | 1 (10%) |
| Characteristic . | Responders (n = 9) . | Nonresponders (n = 10) . |
|---|---|---|
| Cytogenetic/molecular features | ||
| Normal | 1 | 0 |
| Favorable inv(16), t(8;21) | 2 | 0 |
| 11q23 | 2 | 0 |
| Del5q/monosomy 5 | 1 | 1 |
| Monosomy 7 | 1 | 2 |
| FLT3-ITD | 0 | 2 |
| NUP98-NSD1 | 1 | 0 |
| DEK-NUP214 | 1 | 0 |
| ETV6 rearrangement | 0 | 2 |
| Other | 0 | 3 |
| Prior HSCT | ||
| No | 5 (55.5%) | 9 (90%) |
| Yes | 4 (44.4%) | 1 (10%) |
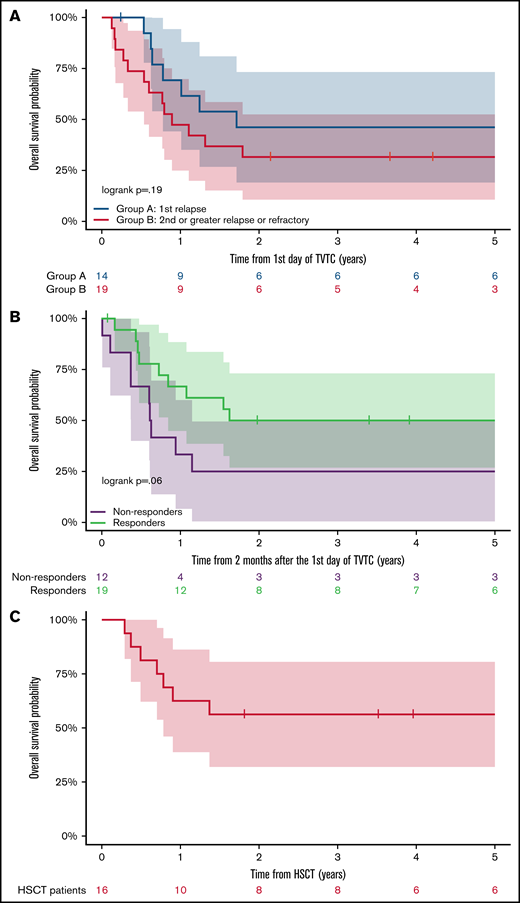
The 5-year OS of patients in Group A and Group B was 46.2% (95% CI, 19.1-73.3) and 31.6% (95% CI, 10.7-52.5), respectively (Figure 1A). Patients who responded to TVTC had a 5-year OS of 50.0% (95% CI, 26.9-73.1) vs 25% for nonresponders (95% CI, 0.5-49.5) (Figure 1B). Of the 19 responders to TVTC in the entire cohort, 16 (84.0%) proceeded to receive allogeneic HSCT. The other 3 patients had disease recurrence before HSCT. The 5-year OS of responders who proceeded to allogeneic HSCT (n = 16) was 56.2% (95% CI, 31.9-80.6) (Figure 1C), which included patients from Groups A and B (n = 9 and 7). Of the 7 patients who died after HSCT, 4 were due to relapsed disease and 3 from transplant-related toxicities.
Outcomes of relapsed/refractory patients with AML treated with TVTC. (A) Five-year OS of patients in Group A vs Group B. (B) Five-year OS according to response to TVTC. (C) Five-year OS of responders who proceeded to allogeneic HSCT (n = 16).
Outcomes of relapsed/refractory patients with AML treated with TVTC. (A) Five-year OS of patients in Group A vs Group B. (B) Five-year OS according to response to TVTC. (C) Five-year OS of responders who proceeded to allogeneic HSCT (n = 16).
MRD data after treatment with TVTC were available in 12 of the 19 responders. Eight of 12 (67%) patients were MRD negative after 1 cycle of TVTC according to either flow cytometry or quantitative reverse transcription PCR. All 8 patients who achieved MRD negativity proceeded to transplant and are alive without relapse at their last follow-up. Of the 4 patients with MRD-positive disease, all proceeded to HSCT but eventually died of progressive disease within 6 months.
Discussion
The thiotepa, vinorelbine, and topotecan (TVT) backbone was originally developed to provide an anthracycline-free chemotherapeutic regimen of novel active agents to minimize risk of drug resistance. These agents have been shown in earlier studies to have single-agent tumor activity in patients with refractory leukemias and activity when combined.19-23 An initial study of TVT combined with dexamethasone alone or with dexamethasone and gemcitabine in children and young adults with relapsed acute leukemia reported a CR rate of 25% and 36%, respectively.23 After a phase 1 study showing the safety of adding clofarabine to the TVT backbone, a phase 2 study of TVTC reported an ORR as defined by CR or CRp in 8 (67%) of 12 children and young adults with R/R AML.12 In this retrospective analysis, we sought to further explore the efficacy of TVTC in an expanded cohort of patients with R/R AML.
Anthracycline-related cardiotoxicity is well established as a frequent consequence in survivors of childhood AML.24 The cumulative incidence of late-onset cardiotoxicity has been estimated to be >25% in long-term survivors of AML.7 The incidence is significantly higher in survivors of relapsed AML.8 Recent studies have also identified mitoxantrone, an agent frequently used in frontline or relapsed AML regimens, as being associated with significantly higher long-term cardiotoxicity risk than previously recognized.25
Several strategies have been used to reduce anthracycline-related cardiotoxicity. The use of the antioxidant scavenger dexrazoxane in patients receiving frontline anthracycline-containing therapy on the COG AAML1031 study led to smaller reductions in ejection and shortening fractions in treated patients.26 Based on these results, dexrazoxane is administered for all patients receiving standard anthracyclines on the ongoing COG AAML1831 trial for children with newly diagnosed AML. Liposomal preparations of daunorubicin have also been studied in multiple relapsed AML trials for children in an attempt to reduce anthracycline-associated cardiotoxicity.6,27 Cooper et al in AAML1421 reported a 76% CR/CRp/CR with incomplete blood count recovery after 1 cycle of CPX-351 (now approved as Vyxeos [Jazz Pharmaceuticals]), a liposomal preparation of daunorubicin and cytarabine, in children with AML in first relapse, with a CR rate similar to that in patients treated with TVTC in this study (71.4%). In AAML1421, all evaluable patients with grade 2 or higher echocardiographic evidence of left ventricular systolic dysfunction had recovery of ejection fractions to >50%. CPX-351 is currently being evaluated in the frontline setting on COG AAML1831 to establish the short- and long-term cardioprotective benefits compared with standard daunorubicin. Finally, Rubnitz et al28 recently published the results of the St. Jude Children’s Research Hospital AML08 study, which randomized children with newly diagnosed AML to receive a first round of induction with clofarabine and cytarabine instead of standard induction with cytarabine, daunorubicin, and etoposide. There was no statistically significant difference in 3-year event-free survival or OS between the 2 arms, suggesting that anthracycline use may be safely reduced in children with newly diagnosed AML in an attempt to decrease short- and long-term cardiotoxicity.
The activity of clofarabine in relapsed childhood AML has been studied as both a single agent and in combination with multiple chemotherapeutic agents. Clofarabine showed modest single-agent activity in a phase 2 study, wherein 1 (2%) of 42 patients achieved CR and 10 (24%) of 42 had a partial response. Patients were heavily pretreated in this study, with 36 of 42 children having received ≥2 prior induction regimens.29 A phase 1 study30 and 2 relatively small studies of clofarabine combined with cyclophosphamide and etoposide in children with multiply relapsed AML reported CR/CRp rates close to 30%.31,32 The phase 2 portion of COG AAML0523 evaluated the efficacy of clofarabine with cytarabine for children in first relapse or refractory to induction. The combination resulted in a CR/CRp rate of 48% and a 3-year OS of 46% for responders.33 In a phase 1/2 study investigating GCSF priming, high-dose cytarabine, and clofarabine (GCLAC), CRs were seen in 21 (46%) of the 46 evaluable patients.34 The Innovative Therapies for Children with Cancer (ITCC) consortium conducted a phase 1B study of clofarabine combined with liposomal daunorubicin for children with R/R AML. Of the 31 patients evaluable for efficacy, 68% responded with CR/CRi after 1 cycle of therapy. When evaluated according to disease phase, 87% of patients with early first relapse responded compared with 50% in second or greater relapse. The 2 year OS was 48%.35
The toxicities of TVTC have been described in the phase 1 and 2 publications of the regimen and included a substantial increase in high-grade infections.11,12 In 2012, after these results, there was universal implementation of broad-spectrum antimicrobial prophylaxis for patients receiving this regimen at MSKCC. In this expanded retrospective cohort, there was a reduction in serious adverse events seen from the published phase 2 study of TVTC, with 22 patients developing grade 3 or greater febrile neutropenias (66.7%) and 5 documented cases of bacteremia (15.1%). These findings are comparable to other studies of intensive salvage chemotherapy regimens for R/R AML.5,36,37 One patient developed an abdominal mucormycosis infection. One patient developed BM aplasia and died of sepsis 45 days after receiving TVTC.
The outcomes reported herein of 33 children and young adults with R/R AML represent a substantial expansion of the cohort previously described in the phase 2 study of TVTC. The results compare favorably to other published salvage regimens, both non-anthracycline regimens and those using anthracyclines (with or without liposomal preparations). The ORR for patients in first relapse was 71.4%, with significant response rates reported in patients with high-risk features such as those with unfavorable cytogenetics as well with relapses <1 year after achieving remission. Furthermore, almost one-half of all patients with second or greater relapse or primary refractory disease responded to TVTC. The regimen was successful as a bridge to transplant for the vast majority of responders, with a long-term OS nearing 50% for patients in first relapse.
Of note, 5 patients with FLT3-ITD were treated with TVTC, 4 of whom did not respond and 1 had a brief response but ultimately died of progressive disease before being able to receive an HSCT. These patients were treated at a time when targeted FLT3 kinase inhibitors were not as readily available for children with relapsed AML. The poor outcomes of these 5 patients is not unexpected given the known poor response rates of children with FLT3-ITD–relapsed AML treated with reinduction chemotherapy regimens.38 Contemporary strategies for these patients usually incorporate FLT3-directed targeted therapies, and these patients are largely ineligible for trials using chemotherapy alone. Limitations of this analysis are related to it being a retrospective study with the majority of patients treated at a single institution. Furthermore, MRD evaluation was unavailable for a significant proportion of the patient population. However, these encouraging results in an expanded cohort of 33 patients warrant further evaluation of TVTC as a backbone regimen for relapsed AML, with particular potential utility in children who cannot tolerate additional anthracycline exposure.
Acknowledgments
The authors thank Joseph Olechnowicz, editor, Department of Pediatrics, MSKCC, for editorial assistance. The authors acknowledge support from the National Institutes of Health, National Cancer Institute Cancer Center Support Grant (P30 CA008748).
Authorship
Contribution: All authors substantially contributed to conception and design of the work; contributed to the acquisition of data, and analysis and interpretation of the data; assisted with drafting and provided critical review and revision of the manuscript; and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Neerav Shukla, 1275 York Ave, New York, NY 10065; e-mail: shuklan@mskcc.org.
References
Author notes
Presented as an abstract (“Clofarabine with topotecan, vinorelbine, and thiotepa [TVTC] in children and young adults with relapsed or refractory acute myeloid leukemia”) in Blood. 2018;132(suppl 1):79. https://doi.org/10.1182/blood-2018-99-115185.
Requests for data sharing may be submitted to the corresponding author (ramaswak@mskcc.org).
The online version of this article contains a data supplement.