Key Points
Historical monotherapy trials with azacitidine or decitabine confirm generally low CR rates (14% of patients).
The DNMTI used may influence responses; more patients treated with azacitidine achieved HI, whereas the marrow ORR was higher with decitabine.
Abstract
DNA methyltransferase inhibitors (DNMTIs) for patients with higher risk myelodysplastic syndromes (HR-MDS) have low complete remission rates and are not curative. Early DNMTI combination clinical trials in HR-MDS are often termed “promising,” but many randomized trials subsequently failed to show benefit. Clearer understanding of when a combination is likely to improve upon DNMTI monotherapy would inform randomized studies. We reviewed MDS azacitidine or decitabine monotherapy studies. We collected baseline demographics including International Prognostic Scoring System (IPSS) risk, DNMTI, disease characteristics; and response variables including survival and marrow and hematologic responses. Aggregate estimates across studies were calculated using meta-analyses techniques. Using a binomial design, we estimated the necessary operating characteristics to design a phase 2 study showing improved efficacy of a combination over monotherapy. Among 1908 patients, the overall response rate (ORR) was 24% (n = 464; 95% confidence interval [CI], 0.22-0.26): 267 complete response (CR, 14%), 68 partial response (4%), and 129 marrow complete remission (7%). Among 1604 patients for whom a hematologic response was reported, 476 (30%; 95% CI, 0.27-0.32) reported hematologic improvement (HI). More patients treated with azacitidine achieved HI (38%; 95% CI, 0.35-0.41) compared with decitabine (15%; 95% CI, 0.13-0.19), whereas the marrow ORR rate was higher with decitabine (29%; 95% CI, 0.26-0.33) compared with azacitidine (21%; 95% CI, 0.19-0.23). CR rates were similar between DNMTIs: 13% with azacitidine and 16% with decitabine. Variables that influence MDS response include the specific DNMTI backbone and the distribution of IPSS risk of patients enrolled on a trial. Considering these factors can help identify which early combination approaches are worth assessing in larger randomized trials.
Introduction
The myelodysplastic syndromes (MDS) include a group of hematopoietic neoplasms for which therapeutic options are limited and inadequate; no therapy other than allogeneic hematopoietic cell transplantation offers the possibility of cure.1,2 Physicians consider both patient and disease characteristics when selecting treatment, including an estimation of the likelihood of a morbid or mortal complication, using risk stratification tools such as the International Prognostic Scoring System (IPSS) and its revisions.3,4 Patients with lower risk MDS are usually treated with supportive measures such as transfusions and low-intensity therapies such as hematopoietic growth factors to ameliorate sequelae of MDS such as complications of anemia, neutropenia, or thrombocytopenia.5-9 Although symptomatic burden and complications of cytopenias are also a concern for higher risk MDS (HR-MDS), because life-expectancy is shorter, more intensive therapies are often considered to modify disease and prolong life.
Currently, the standard initial therapy for patients with HR-MDS is one of the DNA methyltransferase inhibitors (DNMTIs), also termed hypomethylating agents.10-13 The most commonly used DNMTIs are azacitidine and decitabine. Azacitidine was associated with an improvement in survival compared with supportive care, low-dose cytarabine or intensive chemotherapy in 2 randomized study of HR-MDS and acute myeloid leukemia (AML) (AZA-001, NCT #00071799), and either agent can delay AML progression or induce durable periods of disease remission in a subset of patients, including reduction of marrow myeloblast burden and recovery of blood counts. Nonetheless, remission duration is usually short, and few patients (<5%) are alive and in remission 5 years after starting DNMTI therapy.14
There are many ongoing efforts continue to improve upon current DNMTI therapy for HR-MDS, most commonly by adding a second agent to a DNMTI “backbone.” The second drug may be added to try to prevent emergence of a resistant clone, may target a specific disease-associated mutation, or may show synergistic cell killing in preclinical assays. Despite many trials to date, no such combinations have yet proven superior to DNMTI alone.15,16
One challenge in developing new drugs for HR-MDS is that combination studies are often conducted without a monotherapy comparator at first. Given the heterogeneity of prior DNMTI monotherapy studies in MDS, it can be difficult to know if a combination will indeed be superior until tested in a randomized fashion.17 Combination therapies may suggest early evidence of increased response rates or more durable responses compared with historical controls, but enrolled patient selection bias or other factors may contribute to these higher response rates.17 Response rates and survival after DNMTI therapy are influenced by characteristics of patients who were enrolled to a given study, such as differences in disease risk, age, comorbidities, genetic mutation profiles, and prior therapies.18-21
Azacitidine and decitabine were approved by the US Food and Drug Administration for MDS therapy more than 15 years ago, but even today it remains challenging to know whether a response rate or survival signal seen during an early-phase trial is truly promising and likely to be better than would be expected with azacitidine or decitabine alone. The small numbers of patients on such early-phase studies, along with disease and participant heterogeneity, limit ability to draw conclusions. Despite high response rates in nonrandomized studies, combinations of azacitidine with various histone deacetylase inhibitors (entinostat, vorinostat, pracinostat, panobinostat, valproic acid) and with lenalidomide all failed to improve outcomes compared with azacitidine alone,17,22-25 and the combination approaches were associated with more toxicity. More recently, pevonedistat26 and eprenetapopt (APR-246),27 which also showed promise in early-phase studies in MDS in combination with azacitidine, failed to meet primary outcomes in a randomized setting.
We set out to systematically evaluate reported clinical trials evaluating either azacitidine or decitabine as monotherapy to determine hematologic and marrow response rates, and then to model the likelihood of an early-phase response rate of combination therapy being superior to historical controls. This primary aim of this study is to create a reference for baseline outcomes with either azacitidine or decitabine monotherapy to estimate what may be clinically meaningful responses in DNMTI combination arms according to the trial participant composition and study enrollment.
Methods
We identified published studies treating patients with HR-MDS administering azacitidine and decitabine as monotherapy. We accessed clinicaltrials.gov (initial access date 9 December 2019) and identified trials using the search terms “MDS” for the “Condition or disease” field (which includes synonyms “myelodysplastic syndromes,” “preleukemia,” “myelodysplasia,” “dysmyelopoietic syndrome,” “myelodysplastic neoplasm,” and “myeloid dysplasia”) and either “azacitidine” or “decitabine” in the “other terms” field (which also includes synonyms for each agent). We included trials regardless of dose schedule (eg, decitabine daily days 1-5 or every 8 hours on days 1-3, or different azacitidine schedules).
We limited inclusion to adult patients with MDS and manually reviewed trials to select those that primarily enrolled patients with HR-MDS, defined as either IPSS intermediate-2 and high risk, and Revised IPSS intermediate, high, and very high risk.3,4 We did not exclude studies that, in addition to HR-MDS, also enrolled other subsets of patients including AML or lower risk MDS, given the evolution of the World Health Organization (WHO) classification of MDS during the period studied.28 We categorized clinical trials as either “completed” or “ongoing.” Completed trials included those that had been “completed,” “suspended,” “terminated,” or “withdrawn;” these were reviewed individually to identify studies with published manuscripts including a monotherapy treatment arm with at least 5 patients. Ongoing studies were categorized as those with a status of “recruiting,” “enrolling by invitation,” and “active, not recruiting.” Studies with unknown status were reviewed for best assignment.
Historical outcomes were extracted from the text of each published manuscript. These included the year of study initiation, the number of patients enrolled on monotherapy, drug and treatment schedule, IPSS risk category, cytogenetic risk category, median absolute neutrophil count (ANC), platelet, and hemoglobin levels, and French-American-British (FAB) or WHO category. We recategorized patients with refractory anemia with excess blasts in transformation (20%-30% blasts) as AML for this analysis. Because some studies did not delineate between MDS with excess blasts (MDS-EB) 1 and 2, or combined refractory anemia/refractory anemia with ring sideroblasts/refractory cytopenia with multilineage dysplasia categories, and given variations in classification over the study periods, we also created combined groups of low blast count MDS and high blast count MDS. Papers that did not report FAB or WHO categories or blast counts were listed as MDS unspecified (distinct from the WHO category of MDS, unclassifiable). Reported responses were assessed as complete remission (CR), partial remission (PR), marrow complete remission (mCR), hematopoietic improvement (HI), transfusion independence, time to response, duration of response, progression-free survival, and overall survival (OS). We analyzed rates of CR both as an independent response and in combination with other responses used in clinical trials, though with less clear clinical benefit, mCR, and PR, to provide a broader comparison with past trials and because these criteria have been used in trial reporting.
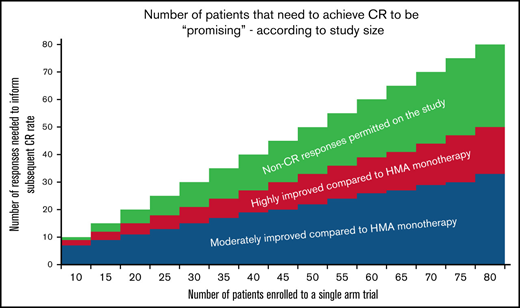
Statistical analysis was performed in R (4.0.0) using the meta package. All response rate estimates were performed in a generalized linear mixed model framework, using a random effect for each study to adjust for between study differences. Any additional exploration of covariate space was done using a 5% type I error rate to determine significance within the generalized linear mixed model framework. Simulations of trial designs were explored using effect sizes learned from the meta-analyses. For estimations of improvement in response compared with baseline, we prespecified moderately improved (CR 30%, CR+PR+mCR 50%, HI 60%) and highly improved response rates (CR 50%, CR+PR+mCR 70%, HI 75%) and estimated the number of responses needed across various sample sizes (n = 10, 15, 20, 25, 30, 35, 40, 45, 50, 55, 60, 65, 70, 75, or 80 in a single-arm study) to achieve statistical significance in MDS trials using a binomial design assuming either a 5% type I error or 10% type I error, and at minimum 90% power.
Results
We identified a total of 30 completed “azacitidine” and “MDS” clinical trials, and 26 completed “decitabine” and “MDS” clinical trials registered on clinicaltrials.gov. After excluding studies for other indications (lower risk MDS, transplant, AML; n = 7), without a monotherapy arm (n = 21), or lacking CR reporting (n = 4), we evaluated a total of 14 studies with azacitidine monotherapy arms and 10 studies with decitabine monotherapy, for a total of 1908 DNMTI-treated patients (Table 1).10-13,17,22,24,25,29-43 These studies included 1137 patients treated with azacitidine monotherapy and 771 patients treated with decitabine monotherapy. IPSS risk was reported for 19 of 22 studies and was available for a total of 1324 patients (69% of all patients, 59% of patients on azacitidine studies, and 85% of patients given decitabine). Similarly, a total of 16 studies reported MDS subtype by FAB or WHO classification. Ten studies had baseline median hematologic parameters available in the manuscript. The median ANC (weighted by study enrollment) was 1.5, median hemoglobin was 8.3 g/dL, and median platelet count was 65 000. A total of 11 studies reported outcomes of more than 75 patients and accounted for 74% of all treated patients in this analysis; these studies are shown in Table 2.
Monotherapy DNMTI studies/arms in higher risk MDS
| Trial No. . | Drug . | Description . | Regimen . | Start year . | N (monotherapy) . |
|---|---|---|---|---|---|
| CALGB 9221 | Azacitidine | CALGB AZA | d1-7 | 1994 | 99 |
| NCT01522976 | Azacitidine | SWOG S1117 | d1-7 | 2012 | 92 |
| NCT01305460 | Azacitidine | Intensified Aza | d1-14 | 2011 | 27 |
| NCT01599325 | Azacitidine | China HR-MDS | d1-7 | 2012 | 72 |
| NCT00384956 | Azacitidine | 5-d Aza | d1-5 | 2006 | 22 |
| NCT01201811 | Azacitidine | Taiwan HR-MDS | d1-7 | 2010 | 44 |
| NCT00071799 | Azacitidine | Aza-001 | d1-7 | 2004 | 179 |
| NCT00102687 | Azacitidine | Alternate dosing | 5-2-2, 5-2-5, 1-5 | 2005 | 151 |
| NCT00313586 | Azacitidine | Aza ± entinostat | d1-10 | 2006 | 74 |
| NCT00321711 | Azacitidine | Aza ± Nplate | d1-7 | 2006 | 40 |
| NCT02158936 | Azacitidine | Aza ± eltrombopag | d1-7 | 2014 | 177 |
| NCT00946647 | Azacitidine | Aza ± panobinostat | d1-7 | 2009 | 42 |
| NCT00744757 | Decitabine | Taiwan DAC | d1-5 | 2008 | 37 |
| NCT01751867 | Decitabine | China DAC | d1-3 or d1-5 | 2009 | 132 |
| NCT00796003 | Decitabine | Japan DAC | d1-5 | 2008 | 37 |
| NCT00260065 | Decitabine | ADOPT | d1-5 | 2005 | 99 |
| NCT00043381 | Decitabine | BSC vs DAC | d1-3 | 2001 | 89 |
| NCT00067808 | Decitabine | 3 DAC schedules | d1-10, d1-5 | 2003 | 95 |
| NCT01687400 | Decitabine | 10-d DAC | d1-10 | 2013 | 26 |
| NCT00414310 | Decitabine | DAC vs VPA | d1-5 | 2006 | 31 |
| NCT00321711 | Decitabine | DAC ± Nplate | d1-5 or d1-3 | 2008 | 29 |
| NCT00043134 | Decitabine | Europe DAC | d1-3 | 2002 | 119 |
| NCT01041846 | Decitabine | Korea DAC | d1-5 | 2008 | 103 |
| Trial No. . | Drug . | Description . | Regimen . | Start year . | N (monotherapy) . |
|---|---|---|---|---|---|
| CALGB 9221 | Azacitidine | CALGB AZA | d1-7 | 1994 | 99 |
| NCT01522976 | Azacitidine | SWOG S1117 | d1-7 | 2012 | 92 |
| NCT01305460 | Azacitidine | Intensified Aza | d1-14 | 2011 | 27 |
| NCT01599325 | Azacitidine | China HR-MDS | d1-7 | 2012 | 72 |
| NCT00384956 | Azacitidine | 5-d Aza | d1-5 | 2006 | 22 |
| NCT01201811 | Azacitidine | Taiwan HR-MDS | d1-7 | 2010 | 44 |
| NCT00071799 | Azacitidine | Aza-001 | d1-7 | 2004 | 179 |
| NCT00102687 | Azacitidine | Alternate dosing | 5-2-2, 5-2-5, 1-5 | 2005 | 151 |
| NCT00313586 | Azacitidine | Aza ± entinostat | d1-10 | 2006 | 74 |
| NCT00321711 | Azacitidine | Aza ± Nplate | d1-7 | 2006 | 40 |
| NCT02158936 | Azacitidine | Aza ± eltrombopag | d1-7 | 2014 | 177 |
| NCT00946647 | Azacitidine | Aza ± panobinostat | d1-7 | 2009 | 42 |
| NCT00744757 | Decitabine | Taiwan DAC | d1-5 | 2008 | 37 |
| NCT01751867 | Decitabine | China DAC | d1-3 or d1-5 | 2009 | 132 |
| NCT00796003 | Decitabine | Japan DAC | d1-5 | 2008 | 37 |
| NCT00260065 | Decitabine | ADOPT | d1-5 | 2005 | 99 |
| NCT00043381 | Decitabine | BSC vs DAC | d1-3 | 2001 | 89 |
| NCT00067808 | Decitabine | 3 DAC schedules | d1-10, d1-5 | 2003 | 95 |
| NCT01687400 | Decitabine | 10-d DAC | d1-10 | 2013 | 26 |
| NCT00414310 | Decitabine | DAC vs VPA | d1-5 | 2006 | 31 |
| NCT00321711 | Decitabine | DAC ± Nplate | d1-5 or d1-3 | 2008 | 29 |
| NCT00043134 | Decitabine | Europe DAC | d1-3 | 2002 | 119 |
| NCT01041846 | Decitabine | Korea DAC | d1-5 | 2008 | 103 |
ADOPT, Alternative Dosing for Outpatient Treatment; Aza, azacitidine; BSC, best supportive care; CALGB, Cancer and Leukemia Group B; d, day; DAC, decitabine.
Selected outcomes of monotherapy azacitidine or decitabine (n > 75 patients)
| Trial . | CALGB 9221 . | NCT01522976 . | NCT00071799 . | NCT00102687 . | NCT02158936 . | NCT01751867 . | NCT00260065 . | NCT00043381 . | NCT00067808 . | NCT00043134 . | NCT01041846 . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Drug | Azacitidine | Azacitidine | Azacitidine | Azacitidine | Azacitidine | Decitabine | Decitabine | Decitabine | Decitabine | Decitabine | Decitabine |
| N | 99 | 92 | 179 | 151 | 177 | 132 | 99 | 89 | 95 | 119 | 103 |
| IPSS | |||||||||||
| Low | 2 | 3 | 0 | 1 | |||||||
| INT-1 | 21 | 25 | 5 | 61 | 57 | 52 | 28 | 19 | 8 | 52 | |
| INT-2 | 9 | 40 | 76 | 83 | 58 | 23 | 38 | 26 | 64 | 35 | |
| High | 7 | 20 | 82 | 33 | 20 | 23 | 23 | 11 | 46 | 13 | |
| Cytogenetic risk | |||||||||||
| Good | 29 | 83 | 81 | 49 | 38 | 65 | |||||
| Intermediate | 16 | 37 | 22 | 15 | 9 | 15 | |||||
| Poor | 33 | 50 | 32 | 29 | 57 | 19 | |||||
| MDS subtype | |||||||||||
| MDS SLD | 17 | 0 | 65 | 27 | 20 | 12 | 5 | 4 | |||
| MDS RS | 5 | 0 | 21 | 4 | 17 | 7 | 3 | ||||
| MDS MLD | 0 | 27 | |||||||||
| LB MDS | 22 | 0 | 86 | 31 | 37 | 19 | 8 | 31 | |||
| MDS EB1 | 29 | 14 | 10 | 23 | 30 | 26 | |||||
| MDS EB2 | 35 | 98 | 31 | 30 | 31 | 32 | |||||
| MDS-EB | 32 | 64 | 112 | 45 | 85 | 41 | 47 | 53 | 61 | 58 | |
| Unspecified MDS | 1 | 10 | 19 | ||||||||
| AML | 37 | 55 | 4 | 11 | 10 | 17 | 5 | 41 | |||
| CMML | 7 | 18 | 11 | 16 | 8 | 11 | 6 | 18 | 11 | ||
| Reported responses | |||||||||||
| CR | 7 | 22 | 30 | 6 | 26 | 13 | 17 | 8 | 32 | 16 | 13 |
| PR | 16 | 0 | 21 | 5 | 0 | 7 | 1 | 7 | 1 | ||
| mCR | 11 | 36 | 22 | 15 | 10 | 23 | |||||
| HI | 37 | 13 | 87/177 | 73/151 | 59/177 | 50 | 18 | 12 | 13 | 18 | 19 |
| HI-E | 13 | 62/157 | 58/130 | 7 | 35/97 | ||||||
| HI-P | 20 | 46/141 | 23/54 | 7 | 30/69 | ||||||
| HI-N | 16 | 25/131 | 17/70 | 3 | 20/53 | ||||||
| RBC TI | 29/65 | 50/111 | 40/71 | 38/88 | 22/66 | ||||||
| OS | 20 mo | 15 mo | 24.5 mo | 23.8 mo | 19.4 mo | 23.5 mo | 19 mo | 10.1mo | 17.7mo | ||
| Trial . | CALGB 9221 . | NCT01522976 . | NCT00071799 . | NCT00102687 . | NCT02158936 . | NCT01751867 . | NCT00260065 . | NCT00043381 . | NCT00067808 . | NCT00043134 . | NCT01041846 . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Drug | Azacitidine | Azacitidine | Azacitidine | Azacitidine | Azacitidine | Decitabine | Decitabine | Decitabine | Decitabine | Decitabine | Decitabine |
| N | 99 | 92 | 179 | 151 | 177 | 132 | 99 | 89 | 95 | 119 | 103 |
| IPSS | |||||||||||
| Low | 2 | 3 | 0 | 1 | |||||||
| INT-1 | 21 | 25 | 5 | 61 | 57 | 52 | 28 | 19 | 8 | 52 | |
| INT-2 | 9 | 40 | 76 | 83 | 58 | 23 | 38 | 26 | 64 | 35 | |
| High | 7 | 20 | 82 | 33 | 20 | 23 | 23 | 11 | 46 | 13 | |
| Cytogenetic risk | |||||||||||
| Good | 29 | 83 | 81 | 49 | 38 | 65 | |||||
| Intermediate | 16 | 37 | 22 | 15 | 9 | 15 | |||||
| Poor | 33 | 50 | 32 | 29 | 57 | 19 | |||||
| MDS subtype | |||||||||||
| MDS SLD | 17 | 0 | 65 | 27 | 20 | 12 | 5 | 4 | |||
| MDS RS | 5 | 0 | 21 | 4 | 17 | 7 | 3 | ||||
| MDS MLD | 0 | 27 | |||||||||
| LB MDS | 22 | 0 | 86 | 31 | 37 | 19 | 8 | 31 | |||
| MDS EB1 | 29 | 14 | 10 | 23 | 30 | 26 | |||||
| MDS EB2 | 35 | 98 | 31 | 30 | 31 | 32 | |||||
| MDS-EB | 32 | 64 | 112 | 45 | 85 | 41 | 47 | 53 | 61 | 58 | |
| Unspecified MDS | 1 | 10 | 19 | ||||||||
| AML | 37 | 55 | 4 | 11 | 10 | 17 | 5 | 41 | |||
| CMML | 7 | 18 | 11 | 16 | 8 | 11 | 6 | 18 | 11 | ||
| Reported responses | |||||||||||
| CR | 7 | 22 | 30 | 6 | 26 | 13 | 17 | 8 | 32 | 16 | 13 |
| PR | 16 | 0 | 21 | 5 | 0 | 7 | 1 | 7 | 1 | ||
| mCR | 11 | 36 | 22 | 15 | 10 | 23 | |||||
| HI | 37 | 13 | 87/177 | 73/151 | 59/177 | 50 | 18 | 12 | 13 | 18 | 19 |
| HI-E | 13 | 62/157 | 58/130 | 7 | 35/97 | ||||||
| HI-P | 20 | 46/141 | 23/54 | 7 | 30/69 | ||||||
| HI-N | 16 | 25/131 | 17/70 | 3 | 20/53 | ||||||
| RBC TI | 29/65 | 50/111 | 40/71 | 38/88 | 22/66 | ||||||
| OS | 20 mo | 15 mo | 24.5 mo | 23.8 mo | 19.4 mo | 23.5 mo | 19 mo | 10.1mo | 17.7mo | ||
HI-E, Hematologic improvement - erythroid; HI-N, hematologic improvement - neutrophils; HI-P, hematologic improvement - platelets; MLD, multilineage dysplasia; RBC TI, red blood cell transfusion independence; RS, ring sideroblasts; SLD, single lineage dysplasia.
Disease risk according to the IPSS was low (n = 14, 1%), intermediate-1 (n = 382, 29%), intermediate-2 (n = 573, 43%), and high (n = 355, 27%). Reported cytogenetic risk (as reported by each study) was good in 448 patients (46% of reported), intermediate in 180 patients (18%), and poor in 351 patients (36%). MDS subtype (n = 1334) included 387 patients with low-blast count MDS (29% of all patients; single lineage dysplasia n = 169, ring sideroblasts n = 67, MDS with Del5q n = 3, multilineage dysplasia n = 60) and 947 patients with high blast count MDS (50%, refractory anemia with EB1 [RAEB1] n = 186, RAEB2 n = 363, not further specified n = 209). An additional 193 patients had an unspecified MDS diagnosis (10%), whereas 311 had AML (RAEB in transformation or AML, 16%) and 146 patients had chronic myelomonocytic leukemia (CMML; 8%).
Historical outcomes for DNA methyltransferase inhibitors used as monotherapy
Of the 1908 patients enrolled on clinical trials that reported a marrow response, overall response rate (ORR; CR, PR, mCR) was 24% (n = 464; 95% confidence interval [CI], 0.22-0.26; random effects model 22%; 95% CI, 0.17-0.27). A total of 267 patients had a reported CR, or 14%, whereas an additional 68 achieved (4%) and 129 mCR (7%). Reporting of hematologic response and the number of patients eligible for hematologic response assessment varied across studies; trials reporting HI enrolled a total of 1604 patients. Of these, a total of 476 patients (30%; 95% CI, 0.27-0.32) reported hematologic response, irrespective of concurrent marrow response (random effects model 25%; 95% CI, 0.19-0.33).
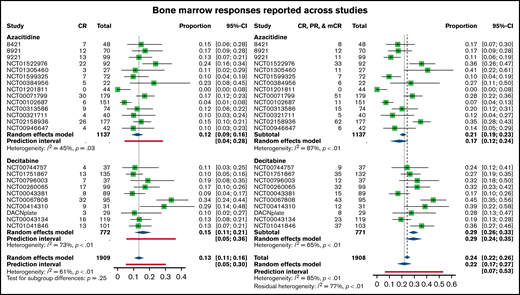
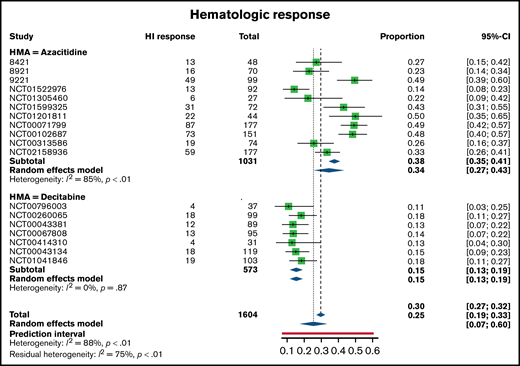
There were small differences in response rates between patients treated with azacitidine monotherapy and those enrolled on studies with decitabine monotherapy. The marrow ORR was 21% with azacitidine (95% CI, 0.19-0.23) and 29% with decitabine (95% CI, 0.26-0.33). Of this, a total of 145 patients treated with azacitidine had a CR (13%), whereas 122 patients treated with decitabine had a CR (16%) (Figure 1). There was a higher proportion of mCR responses among decitabine-treated patients (75, 10%) than in the azacitidine patients (54, 5%) and similar PR responses (azacitidine n = 39, 3%; decitabine n = 29, 4%). With respect to hematologic responses, the pattern was slightly different. More patients treated with azacitidine achieved HI (38%; 95% CI, 0.35-0.41) compared with decitabine (15%; 95% CI, 0.13-0.19) (Figure 2).
Bone marrow response rates across trials, separated between azacitidine (top) and decitabine (bottom) monotherapy. Shown are complete remissions across trials (CR, left forest plot) as well as previously reported combined responses (CR, PR, and mCR, right forest plot).
Bone marrow response rates across trials, separated between azacitidine (top) and decitabine (bottom) monotherapy. Shown are complete remissions across trials (CR, left forest plot) as well as previously reported combined responses (CR, PR, and mCR, right forest plot).
Hematologic response rates as reported across trials, separated between azacitidine (top) and decitabine (bottom) monotherapy.
Hematologic response rates as reported across trials, separated between azacitidine (top) and decitabine (bottom) monotherapy.
We evaluated study demographic factors associated with marrow ORR according to treatment with azacitidine or decitabine monotherapy. There was no significant difference in median baseline ANC (P = .08), hemoglobin (P = .23), or platelet count (P = .8) between azacitidine and decitabine studies. Among trials using azacitidine, there was an improvement in ORR when more patients on the trial had low baseline blast counts (P < .001), whereas in decitabine trials increased ORR was associated with having more patients on a trial with higher blast counts (P = .001). Trials that had more patients with good cytogenetic risk had higher ORR for both azacitidine (P = .09) and decitabine (P < .001) studies. Azacitidine and decitabine studies reported similar OS (P = .36), progression free survival (P = .11), and duration of response (P = .48).
Actively enrolling trials using DNMTIs in MDS
We identified 49 actively enrolling clinical trials that included azacitidine or decitabine as front-line therapy and were enrolling patients with MDS. Of these, a total of 10 studies had a randomization arm as part of the trial design (Table 3). Only 5 studies were phase 3; the rest were phase 1 (16), phase 1 and 2 (9), phase 2 (18), and 1 was a pilot study. The anticipated enrollment of all currently enrolling studies was 5099 patients; 2138 of those anticipated enrollments were for randomized studies (42%). Many studies, however, are not exclusive to MDS; of the 10 randomized studies, 4 also were enrolling patients with AML and/or CMML.
Actively enrolling clinical trials
| NCT No. . | Title . | Drug . | Enrollment . | Randomization . | Study phase . |
|---|---|---|---|---|---|
| NCT03593915 | A Phase 1b/2 Study of Alvocidib Plus Decitabine in Patients With MDS | Decitabine | 49 | None | 1| 2 |
| NCT03045510 | Efficacy and Safety of Ultra Small Dose Decitabine for the Lower Risk MDS Patients With Transfusion Dependent | Decitabine | 50 | None | 2 |
| NCT02781883 | Clinical Trial of BP1001(Liposomal Grb2 Antisense Oligonucleotide) in Combination With Decitabine in AML / High Risk MDS | Decitabine | 108 | None | 2 |
| NCT03855371 | Mutant p53-based Personalized Trial Using Decitabine and Arsenic Trioxide on AML/MDS | Decitabine | 3 | None | 1 |
| NCT02269280 | Phase II Decitabine (DAC) Versus Azacitidine (AZA) in Myelodysplastic Syndrome (MDS) | Decitabine/Azacitidine | 240 | Yes | 2 |
| NCT03066648 | Study of PDR001 and/or MBG453 in Combination With Decitabine in Patients With AML or High Risk MDS | Decitabine/Azacitidine | 235 | None | 1 |
| NCT01211457 | Study of Sapacitabine in Acute Myeloid Leukemia (AML) or Myelodysplastic Syndromes (MDS) | Decitabine | 65 | None | 1|2 |
| NCT01515527 | Cladribine Plus Low Dose Cytarabine (LDAC) Alternating With Decitabine in Patients With Acute Myeloid Leukemia (AML) or High-Risk Myelodysplastic Syndrome (MDS) | Decitabine | 160 | None | 2 |
| NCT03502668 | Phase 1-2 Study of Low Dose ASTX727 (ASTX727 LD) in Lower Risk MDS | Decitabine | 160 | Yes | 1|2 |
| NCT02890329 | Ipilimumab and Decitabine in Treating Patients With Relapsed or Refractory Myelodysplastic Syndrome or Acute Myeloid Leukemia | Decitabine | 48 | None | 1 |
| NCT03358719 | DEC-205/NY-ESO-1 Fusion Protein CDX-1401, Poly ICLC, Decitabine, and Nivolumab in Treating Patients With Myelodysplastic Syndrome or Acute Myeloid Leukemia | Decitabine | 18 | None | 1 |
| NCT03946670 | A Study of MBG453 in Combination With Hypomethylating Agents in Subjects With IPSS-R Intermediate, High or Very High Risk Myelodysplastic Syndrome (MDS). | Decitabine/Azacitidine | 120 | Yes | 2 |
| NCT03404193 | Venetoclax in Combination With Decitabine in r/r AML and MDS | Decitabine | 280 | None | 2 |
| NCT03661307 | Quizartinib, Decitabine, and Venetoclax in Treating Participants With Untreated or Relapsed Acute Myeloid Leukemia or High Risk Myelodysplastic Syndrome | Decitabine/Azacitidine | 52 | None | 1|2 |
| NCT03356080 | DLAAG in the Treatment of Acute Myeloid Leukemia (AML) and Myelodysplastic Syndrome With Blast Excess | Decitabine | 50 | None | 2 |
| NCT04013880 | ASTX727 and FT-2102 in Treating IDH1-Mutated Recurrent/Refractory Myelodysplastic Syndrome or Acute Myeloid Leukemia | Decitabine | 80 | None | 1|2 |
| NCT03906695 | Phase 1 Trial of ASTX727 in Subjects With Lower-risk Myelodysplastic Syndromes | Decitabine | 30 | None | 1 |
| NCT03745716 | APR-246 & Azacitidine for the Treatment of TP53 Mutant Myelodysplastic Syndromes (MDS) | Azacitidine | 156 | Yes | 3 |
| NCT02942290 | A Study Evaluating Venetoclax in Combination With Azacitidine in Subjects With Treatment-Naive Higher-Risk Myelodysplastic Syndromes (MDS) | Azacitidine | 80 | None | 1 |
| NCT02985190 | A Study of Azacitidine in Myelodysplastic Syndrome (MDS) Associated to Systemic Auto-immune and Inflammatory Disorders | Azacitidine | 30 | None | 2 |
| NCT03978364 | A Study of Azacitidine with or without homoharringtonine for Patients With Int/High -Risk MDS and AML-MRC | Azacitidine | 100 | Yes | 3 |
| NCT03113643 | SL-401 in Combination With Azacitidine or Azacitidine/Venetoclax in Acute Myeloid Leukemia (AML) or High-Risk Myelodysplastic Syndrome (MDS) | Azacitidine | 56 | None | 1 |
| NCT02530463 | Nivolumab and Ipilimumab With 5-azacitidine in Patients With Myelodysplastic Syndromes (MDS) | Azacitidine | 120 | None | 2 |
| NCT03564873 | Omacetaxine + Azacitidine in Untreated Patients With High Grade MDS | Azacitidine | 51 | None | 1|2 |
| NCT03094637 | Azacitidine and Pembrolizumab in Treating Patients With Myelodysplastic Syndrome | Azacitidine | 40 | None | 2 |
| NCT03338348 | Study of Vosaroxin With Azacitidine in Patients With Newly Diagnosed Acute Myeloid Leukemia or Myelodysplastic Syndrome With Excess Blasts-2 | Azacitidine | 168 | None | 2 |
| NCT02750995 | Peptide Vaccination in Combination With Azacitidine for Patients With MDS and AML | Azacitidine | 15 | None | 1 |
| NCT03217903 | High Risk Myelodysplasia Treated by Azacytidine: Genetic and Epigenetic (MYRAGE) | Azacitidine | 32 | None | Not applicable |
| NCT02719574 | Open-label Study of FT-2102 With or Without Azacitidine or Cytarabine in Patients With AML or MDS With an IDH1 Mutation | Azacitidine | 500 | Yes | 1|2 |
| NCT02319369 | Safety, Tolerability and Pharmacokinetics of Milademetan Alone and With 5-Azacitidine (AZA) in Acute Myelogenous Leukemia (AML) or High-Risk Myelodysplastic Syndrome (MDS) | Azacitidine | 156 | None | 1 |
| NCT03268954 | Pevonedistat Plus Azacitidine Versus Single-Agent Azacitidine as First-Line Treatment for Participants With Higher-Risk Myelodysplastic Syndromes (HR MDS), Chronic Myelomonocytic Leukemia (CMML), or Low-Blast Acute Myelogenous Leukemia (AML) | Azacitidine | 450 | Yes | 3 |
| NCT02807558 | A Biomarker-Directed phase 2 Trial of SY-1425 in Patients With Acute Myeloid Leukemia or Myelodysplastic Syndrome | Azacitidine | 162 | None | 2 |
| NCT03397173 | TET2 Mutations in Myelodysplastic Syndromes and Acute Myeloid Leukemia With Azacitidine + Ascorbic Acid | Azacitidine | 28 | None | 2 |
| NCT02553941 | Ibrutinib and Azacitidine for Treatment of Higher Risk Myelodysplastic Syndrome | Azacitidine | 24 | None | 1 |
| NCT04022785 | PLX51107 and Azacitidine in Treating Patients With Acute Myeloid Leukemia or Myelodysplastic Syndrome | Azacitidine | 32 | None | 1 |
| NCT03814005 | A Study of Pevonedistat in Combination With Azacitidine in Participants With Higher-risk Myelodysplastic Syndromes (HR MDS), Chronic Myelomonocytic Leukemia (CMML), or Acute Myelogenous Leukemia (AML) With Severe Renal Impairment or Mild Hepatic Impairment | Azacitidine | 60 | None | 1 |
| NCT03047993 | Glutaminase Inhibitor CB-839 and Azacitidine in Treating Patients With Advanced Myelodysplastic Syndrome | Azacitidine | 40 | None | 1|2 |
| NCT03248479 | Hu5F9-G4 Monotherapy or Hu5F9-G4 in Combination With Azacitidine in Patients With Hematological Malignancies | Azacitidine | 96 | None | 1 |
| NCT03383575 | Azacitidine and Enasidenib in Treating Patients With IDH2-Mutant Myelodysplastic Syndrome | Azacitidine | 105 | None | 2 |
| NCT03999723 | Combining Active and Passive DNA Hypomethylation (Azacitidine and Vitamin C) in MDS, CMML, AML | Azacitidine | 182 | Yes | 2 |
| NCT03873311 | Azacytidine + HAG Regimen in Elderly Patients With Myeloid Malignancy. | Azacitidine | 120 | None | 3 |
| NCT01812252 | Chemotherapy in Treating Patients With Myelodysplastic Syndrome Before Donor Stem Cell Transplant | Decitabine/Azacitidine | 60 | Yes | 2 |
| NCT01787487 | Ruxolitinib Phosphate and Azacytidine in Treating Patients With Myelofibrosis or Myelodysplastic Syndrome/Myeloproliferative Neoplasm | Azacitidine | 125 | None | 2 |
| NCT02981615 | Phase 3 Multicenter Randomized Double-blind Placebo Controlled Study With Antibacterial Prophylaxis in Azacitidine Treated MDS Patients | Azacitidine | 170 | Yes | 3 |
| NCT03493646 | Evaluating in Vivo AZA Incorporation in Mononuclear Cells Following Vidaza or CC486 | Azacitidine | 60 | None | 2 |
| NCT03326310 | Study of MEK Inhibitor Selumetinib in Combination With Azacitidine in Patients With Higher Risk Chronic Myeloid Neoplasia | Azacitidine | 18 | None | 1 |
| NCT03358719 | DEC-205/NY-ESO-1 Fusion Protein CDX-1401, Poly ICLC, Decitabine, and Nivolumab in Treating Patients With Myelodysplastic Syndrome or Acute Myeloid Leukemia | Azacitidine | 18 | None | 1 |
| NCT03471260 | Ivosidenib and Venetoclax With or Without Azacitidine in Treating Participants With IDH1 Mutated Hematologic Malignancies | Azacitidine | 48 | None | 1|2 |
| NCT02038777 | A Study Of PF-04449913 In Japanese Patients With Select Hematologic Malignancies | Azacitidine | 49 | None | 1 |
| NCT No. . | Title . | Drug . | Enrollment . | Randomization . | Study phase . |
|---|---|---|---|---|---|
| NCT03593915 | A Phase 1b/2 Study of Alvocidib Plus Decitabine in Patients With MDS | Decitabine | 49 | None | 1| 2 |
| NCT03045510 | Efficacy and Safety of Ultra Small Dose Decitabine for the Lower Risk MDS Patients With Transfusion Dependent | Decitabine | 50 | None | 2 |
| NCT02781883 | Clinical Trial of BP1001(Liposomal Grb2 Antisense Oligonucleotide) in Combination With Decitabine in AML / High Risk MDS | Decitabine | 108 | None | 2 |
| NCT03855371 | Mutant p53-based Personalized Trial Using Decitabine and Arsenic Trioxide on AML/MDS | Decitabine | 3 | None | 1 |
| NCT02269280 | Phase II Decitabine (DAC) Versus Azacitidine (AZA) in Myelodysplastic Syndrome (MDS) | Decitabine/Azacitidine | 240 | Yes | 2 |
| NCT03066648 | Study of PDR001 and/or MBG453 in Combination With Decitabine in Patients With AML or High Risk MDS | Decitabine/Azacitidine | 235 | None | 1 |
| NCT01211457 | Study of Sapacitabine in Acute Myeloid Leukemia (AML) or Myelodysplastic Syndromes (MDS) | Decitabine | 65 | None | 1|2 |
| NCT01515527 | Cladribine Plus Low Dose Cytarabine (LDAC) Alternating With Decitabine in Patients With Acute Myeloid Leukemia (AML) or High-Risk Myelodysplastic Syndrome (MDS) | Decitabine | 160 | None | 2 |
| NCT03502668 | Phase 1-2 Study of Low Dose ASTX727 (ASTX727 LD) in Lower Risk MDS | Decitabine | 160 | Yes | 1|2 |
| NCT02890329 | Ipilimumab and Decitabine in Treating Patients With Relapsed or Refractory Myelodysplastic Syndrome or Acute Myeloid Leukemia | Decitabine | 48 | None | 1 |
| NCT03358719 | DEC-205/NY-ESO-1 Fusion Protein CDX-1401, Poly ICLC, Decitabine, and Nivolumab in Treating Patients With Myelodysplastic Syndrome or Acute Myeloid Leukemia | Decitabine | 18 | None | 1 |
| NCT03946670 | A Study of MBG453 in Combination With Hypomethylating Agents in Subjects With IPSS-R Intermediate, High or Very High Risk Myelodysplastic Syndrome (MDS). | Decitabine/Azacitidine | 120 | Yes | 2 |
| NCT03404193 | Venetoclax in Combination With Decitabine in r/r AML and MDS | Decitabine | 280 | None | 2 |
| NCT03661307 | Quizartinib, Decitabine, and Venetoclax in Treating Participants With Untreated or Relapsed Acute Myeloid Leukemia or High Risk Myelodysplastic Syndrome | Decitabine/Azacitidine | 52 | None | 1|2 |
| NCT03356080 | DLAAG in the Treatment of Acute Myeloid Leukemia (AML) and Myelodysplastic Syndrome With Blast Excess | Decitabine | 50 | None | 2 |
| NCT04013880 | ASTX727 and FT-2102 in Treating IDH1-Mutated Recurrent/Refractory Myelodysplastic Syndrome or Acute Myeloid Leukemia | Decitabine | 80 | None | 1|2 |
| NCT03906695 | Phase 1 Trial of ASTX727 in Subjects With Lower-risk Myelodysplastic Syndromes | Decitabine | 30 | None | 1 |
| NCT03745716 | APR-246 & Azacitidine for the Treatment of TP53 Mutant Myelodysplastic Syndromes (MDS) | Azacitidine | 156 | Yes | 3 |
| NCT02942290 | A Study Evaluating Venetoclax in Combination With Azacitidine in Subjects With Treatment-Naive Higher-Risk Myelodysplastic Syndromes (MDS) | Azacitidine | 80 | None | 1 |
| NCT02985190 | A Study of Azacitidine in Myelodysplastic Syndrome (MDS) Associated to Systemic Auto-immune and Inflammatory Disorders | Azacitidine | 30 | None | 2 |
| NCT03978364 | A Study of Azacitidine with or without homoharringtonine for Patients With Int/High -Risk MDS and AML-MRC | Azacitidine | 100 | Yes | 3 |
| NCT03113643 | SL-401 in Combination With Azacitidine or Azacitidine/Venetoclax in Acute Myeloid Leukemia (AML) or High-Risk Myelodysplastic Syndrome (MDS) | Azacitidine | 56 | None | 1 |
| NCT02530463 | Nivolumab and Ipilimumab With 5-azacitidine in Patients With Myelodysplastic Syndromes (MDS) | Azacitidine | 120 | None | 2 |
| NCT03564873 | Omacetaxine + Azacitidine in Untreated Patients With High Grade MDS | Azacitidine | 51 | None | 1|2 |
| NCT03094637 | Azacitidine and Pembrolizumab in Treating Patients With Myelodysplastic Syndrome | Azacitidine | 40 | None | 2 |
| NCT03338348 | Study of Vosaroxin With Azacitidine in Patients With Newly Diagnosed Acute Myeloid Leukemia or Myelodysplastic Syndrome With Excess Blasts-2 | Azacitidine | 168 | None | 2 |
| NCT02750995 | Peptide Vaccination in Combination With Azacitidine for Patients With MDS and AML | Azacitidine | 15 | None | 1 |
| NCT03217903 | High Risk Myelodysplasia Treated by Azacytidine: Genetic and Epigenetic (MYRAGE) | Azacitidine | 32 | None | Not applicable |
| NCT02719574 | Open-label Study of FT-2102 With or Without Azacitidine or Cytarabine in Patients With AML or MDS With an IDH1 Mutation | Azacitidine | 500 | Yes | 1|2 |
| NCT02319369 | Safety, Tolerability and Pharmacokinetics of Milademetan Alone and With 5-Azacitidine (AZA) in Acute Myelogenous Leukemia (AML) or High-Risk Myelodysplastic Syndrome (MDS) | Azacitidine | 156 | None | 1 |
| NCT03268954 | Pevonedistat Plus Azacitidine Versus Single-Agent Azacitidine as First-Line Treatment for Participants With Higher-Risk Myelodysplastic Syndromes (HR MDS), Chronic Myelomonocytic Leukemia (CMML), or Low-Blast Acute Myelogenous Leukemia (AML) | Azacitidine | 450 | Yes | 3 |
| NCT02807558 | A Biomarker-Directed phase 2 Trial of SY-1425 in Patients With Acute Myeloid Leukemia or Myelodysplastic Syndrome | Azacitidine | 162 | None | 2 |
| NCT03397173 | TET2 Mutations in Myelodysplastic Syndromes and Acute Myeloid Leukemia With Azacitidine + Ascorbic Acid | Azacitidine | 28 | None | 2 |
| NCT02553941 | Ibrutinib and Azacitidine for Treatment of Higher Risk Myelodysplastic Syndrome | Azacitidine | 24 | None | 1 |
| NCT04022785 | PLX51107 and Azacitidine in Treating Patients With Acute Myeloid Leukemia or Myelodysplastic Syndrome | Azacitidine | 32 | None | 1 |
| NCT03814005 | A Study of Pevonedistat in Combination With Azacitidine in Participants With Higher-risk Myelodysplastic Syndromes (HR MDS), Chronic Myelomonocytic Leukemia (CMML), or Acute Myelogenous Leukemia (AML) With Severe Renal Impairment or Mild Hepatic Impairment | Azacitidine | 60 | None | 1 |
| NCT03047993 | Glutaminase Inhibitor CB-839 and Azacitidine in Treating Patients With Advanced Myelodysplastic Syndrome | Azacitidine | 40 | None | 1|2 |
| NCT03248479 | Hu5F9-G4 Monotherapy or Hu5F9-G4 in Combination With Azacitidine in Patients With Hematological Malignancies | Azacitidine | 96 | None | 1 |
| NCT03383575 | Azacitidine and Enasidenib in Treating Patients With IDH2-Mutant Myelodysplastic Syndrome | Azacitidine | 105 | None | 2 |
| NCT03999723 | Combining Active and Passive DNA Hypomethylation (Azacitidine and Vitamin C) in MDS, CMML, AML | Azacitidine | 182 | Yes | 2 |
| NCT03873311 | Azacytidine + HAG Regimen in Elderly Patients With Myeloid Malignancy. | Azacitidine | 120 | None | 3 |
| NCT01812252 | Chemotherapy in Treating Patients With Myelodysplastic Syndrome Before Donor Stem Cell Transplant | Decitabine/Azacitidine | 60 | Yes | 2 |
| NCT01787487 | Ruxolitinib Phosphate and Azacytidine in Treating Patients With Myelofibrosis or Myelodysplastic Syndrome/Myeloproliferative Neoplasm | Azacitidine | 125 | None | 2 |
| NCT02981615 | Phase 3 Multicenter Randomized Double-blind Placebo Controlled Study With Antibacterial Prophylaxis in Azacitidine Treated MDS Patients | Azacitidine | 170 | Yes | 3 |
| NCT03493646 | Evaluating in Vivo AZA Incorporation in Mononuclear Cells Following Vidaza or CC486 | Azacitidine | 60 | None | 2 |
| NCT03326310 | Study of MEK Inhibitor Selumetinib in Combination With Azacitidine in Patients With Higher Risk Chronic Myeloid Neoplasia | Azacitidine | 18 | None | 1 |
| NCT03358719 | DEC-205/NY-ESO-1 Fusion Protein CDX-1401, Poly ICLC, Decitabine, and Nivolumab in Treating Patients With Myelodysplastic Syndrome or Acute Myeloid Leukemia | Azacitidine | 18 | None | 1 |
| NCT03471260 | Ivosidenib and Venetoclax With or Without Azacitidine in Treating Participants With IDH1 Mutated Hematologic Malignancies | Azacitidine | 48 | None | 1|2 |
| NCT02038777 | A Study Of PF-04449913 In Japanese Patients With Select Hematologic Malignancies | Azacitidine | 49 | None | 1 |
Assessing meaningful response rates compared with historical monotherapy outcomes
Because many studies may not include a comparator arm, particularly in the early phase of a study, it is important to assess the potential efficacy of an DNMTI combination relative to historical outcomes before enrolling patients to large, randomized studies. This is particularly relevant in diseases with few therapeutic options, such as MDS44 ; therapeutic advances have been slow,16 and it is critical that clinical trials seek to advance this care in an efficient manner. This includes the need to prioritize studies likely to improve upon the standard of care.
The following considerations may be made when assessing DNMTI combination trials to consider whether such agents are likely to improve upon DNMTI monotherapy. First, many trials have limited enrollment to a given treatment dose before expansion; smaller numbers of patients (and the selection bias that may occur with early-phase studies) introduces additional uncertainty once that treatment is expanded to a broader population. We therefore provide estimates based on several patient sample sizes. Second, DNMTI combinations may have varying side effect profiles, some of which may be more toxic than others. A highly active combination therapy may be “exciting” in spite of substantially increased toxicity, whereas a less toxic combination may still be exciting if only moderately improving upon standard of care. As such, we provide estimates for both “moderately improved” and “highly improved” outcomes relative to standard of care, established earlier.
We used an estimated CR rate of 14%, based on the previously mentioned analysis, with an estimated marrow ORR of 24% and expected HI of 30% (in addition to marrow responses). We did not estimate a favorable OS outcome because this is typically not available in early-phase studies. Table 4 shows the number of events that would be needed to yield an improvement over standard DNMTI outcomes, based on trial size (eg, for a study with 10 patients, 6-7 true CR events would suggest a moderate improvement over DNMTI monotherapy [CR 30% or higher], whereas 9 true CRs would be needed to feel comfortable that the true CR rate is 50% or higher).
Minimum number of responses suggested to achieve moderate or highly improved responses compared with azacitidine monotherapy
| No. of patients* . | Moderately improved over DNMTI monotherapy . | Highly improved over DNMTI monotherapy . | Expected DNMTI outcomes . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Goal response | CR | CR+PR+mCR | HI | CR | CR+PR+mCR | HI | CR | CR+PR+mCR | HI |
| Alpha = 0.05 | 30% | 50% | 60% | 50% | 70% | 75% | 14% | 24% | 30% |
| 10 | 7 | 9 | 10 | 9 | 10 | – | |||
| 15 | 9 | 12 | 13 | 12 | 15 | 15 | |||
| 20 | 11 | 15 | 17 | 15 | 19 | 19 | |||
| 25 | 13 | 18 | 20 | 18 | 23 | 24 | |||
| 30 | 15 | 21 | 24 | 21 | 26 | 28 | |||
| 35 | 17 | 24 | 27 | 24 | 30 | 32 | |||
| 40 | 19 | 27 | 31 | 27 | 34 | 36 | |||
| 45 | 20 | 30 | 34 | 30 | 38 | 40 | |||
| 50 | 22 | 33 | 37 | 33 | 42 | 44 | |||
| 55 | 24 | 36 | 41 | 36 | 46 | 48 | |||
| 60 | 26 | 39 | 44 | 39 | 49 | 52 | |||
| 65 | 27 | 41 | 47 | 41 | 53 | 56 | |||
| 70 | 29 | 44 | 51 | 44 | 57 | 60 | |||
| 75 | 30 | 47 | 54 | 47 | 61 | 64 | |||
| 80 | 33 | 50 | 57 | 50 | 65 | 68 | |||
| Goal response | CR | CR+PR+mCR | HI | CR | CR+PR+mCR | HI | CR | CR+PR+mCR | HI |
| Alpha = 0.10 | 30% | 50% | 60% | 50% | 70% | 75% | 14% | 24% | 30% |
| 10 | 6 | 9 | 9 | 9 | 10 | 10 | |||
| 15 | 12 | 12 | 13 | 12 | 14 | 15 | |||
| 20 | 10 | 15 | 16 | 15 | 18 | 19 | |||
| 25 | 12 | 18 | 20 | 18 | 22 | 23 | |||
| 30 | 14 | 20 | 23 | 20 | 26 | 27 | |||
| 35 | 16 | 23 | 27 | 23 | 30 | 31 | |||
| 40 | 17 | 26 | 30 | 26 | 34 | 35 | |||
| 45 | 20 | 29 | 33 | 29 | 37 | 39 | |||
| 50 | 21 | 32 | 37 | 32 | 41 | 43 | |||
| 55 | 23 | 35 | 40 | 35 | 45 | 47 | |||
| 60 | 24 | 37 | 43 | 37 | 49 | 51 | |||
| 65 | 25 | 40 | 46 | 40 | 52 | 55 | |||
| 70 | 27 | 43 | 50 | 43 | 56 | 59 | |||
| 75 | 28 | 46 | 53 | 46 | 60 | 63 | |||
| 80 | 31 | 48 | 56 | 48 | 64 | 67 | |||
| No. of patients* . | Moderately improved over DNMTI monotherapy . | Highly improved over DNMTI monotherapy . | Expected DNMTI outcomes . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Goal response | CR | CR+PR+mCR | HI | CR | CR+PR+mCR | HI | CR | CR+PR+mCR | HI |
| Alpha = 0.05 | 30% | 50% | 60% | 50% | 70% | 75% | 14% | 24% | 30% |
| 10 | 7 | 9 | 10 | 9 | 10 | – | |||
| 15 | 9 | 12 | 13 | 12 | 15 | 15 | |||
| 20 | 11 | 15 | 17 | 15 | 19 | 19 | |||
| 25 | 13 | 18 | 20 | 18 | 23 | 24 | |||
| 30 | 15 | 21 | 24 | 21 | 26 | 28 | |||
| 35 | 17 | 24 | 27 | 24 | 30 | 32 | |||
| 40 | 19 | 27 | 31 | 27 | 34 | 36 | |||
| 45 | 20 | 30 | 34 | 30 | 38 | 40 | |||
| 50 | 22 | 33 | 37 | 33 | 42 | 44 | |||
| 55 | 24 | 36 | 41 | 36 | 46 | 48 | |||
| 60 | 26 | 39 | 44 | 39 | 49 | 52 | |||
| 65 | 27 | 41 | 47 | 41 | 53 | 56 | |||
| 70 | 29 | 44 | 51 | 44 | 57 | 60 | |||
| 75 | 30 | 47 | 54 | 47 | 61 | 64 | |||
| 80 | 33 | 50 | 57 | 50 | 65 | 68 | |||
| Goal response | CR | CR+PR+mCR | HI | CR | CR+PR+mCR | HI | CR | CR+PR+mCR | HI |
| Alpha = 0.10 | 30% | 50% | 60% | 50% | 70% | 75% | 14% | 24% | 30% |
| 10 | 6 | 9 | 9 | 9 | 10 | 10 | |||
| 15 | 12 | 12 | 13 | 12 | 14 | 15 | |||
| 20 | 10 | 15 | 16 | 15 | 18 | 19 | |||
| 25 | 12 | 18 | 20 | 18 | 22 | 23 | |||
| 30 | 14 | 20 | 23 | 20 | 26 | 27 | |||
| 35 | 16 | 23 | 27 | 23 | 30 | 31 | |||
| 40 | 17 | 26 | 30 | 26 | 34 | 35 | |||
| 45 | 20 | 29 | 33 | 29 | 37 | 39 | |||
| 50 | 21 | 32 | 37 | 32 | 41 | 43 | |||
| 55 | 23 | 35 | 40 | 35 | 45 | 47 | |||
| 60 | 24 | 37 | 43 | 37 | 49 | 51 | |||
| 65 | 25 | 40 | 46 | 40 | 52 | 55 | |||
| 70 | 27 | 43 | 50 | 43 | 56 | 59 | |||
| 75 | 28 | 46 | 53 | 46 | 60 | 63 | |||
| 80 | 31 | 48 | 56 | 48 | 64 | 67 | |||
Minimum event size calculation is based on CR rate in that group and the sample size, ranging from 10 to 80 patients, and with alphas of 0.05 (top) and 0.10 (bottom).
Discussion
HR-MDS remains a cancer for which there is a desperate need of novel therapeutic strategies. Most recent and ongoing studies seek to improve upon the current standard of care, azacitidine or decitabine, by adding a new agent that may work additively or synergistically to enhance a response rate or improve survival, ideally both. Unfortunately, to date, no such combinations have proven better than DNMTI monotherapy once compared head to head.45 During the past decade, thousands of patients have participated in studies seeking to advance the standard of care and improve outcomes for those yet to be diagnosed, but therapeutic approaches differ little from that 15 years ago.
In this study, we sought to evaluate the outcomes of prior studies of DNMTI monotherapy for HR-MDS to establish “expected” outcomes according to trial characteristics. We surveyed current frontline DNMTI studies in MDS; only ∼20% of these are randomized studies, with nearly one-half of those including other diseases than MDS.
We provide a potential framework here for assessing the likelihood that early data from a combination study will translate to improved outcomes compared with DNMTI monotherapy in a randomized setting. Particularly for smaller trials, it is important to distinguish marrow responses from hematologic responses if comparing with older studies; the number of patient responses in small cohorts generally also needs to be quite high to translate into success in larger studies. It is important to consider that clinically, we often assume ORR corresponds to survival benefit in determining the benefit of a combination therapy in MDS; this is in part because prior studies have shown a survival benefit for patients who achieve a CR with azacitidine monotherapy compared with other responses.46 However, a combination approach with increased toxicity compared with DNMTI monotherapy may demonstrate improvement in response rates without resulting in survival benefit, especially if hematopoiesis does not durably improve, since the most common cause of death in MDS is infection. In this scenario, it may be important to have a higher CR or ORR only in select patients, such as transplant-eligible patients, with bridge to transplant an important goal, whereas other patients may be better served by DNMTI monotherapy or regimen modification after achieving a marrow response.
Importantly, our framework is informative for interpreting several recently reported phase 3 DNMTI combination studies in MDS, particularly around response rate, and emphasize the distinction between using CR rather than combined ORRs when comparing with historical trials. Eprenetapopt (APR246) combined with azacitidine reported 20 of 40 patients achieved a CR in the phase 1b/2 study,47 and powered the phase 3 study for a 50% CR rate.27 However, this analysis would suggest a more conservative goal to reach a 50% CR rate, needing 26 CRs of 40 patients. Nonetheless, the study would have been likely to see a 30% CR rate (more than 19/20 CR in phase 1b/2), which they met in the phase 3 (33.3%). Similarly, the pevonedistat phase 3 study spanned 3 indications (CMML, MDS, AML), similar to prior azacitidine monotherapy studies, after the randomized phase 2 study reported 22/55 remissions.48 This value alone would be unlikely to meet moderate (>30% CR) or highly improved criteria (>50% CR) (Table 4), and, indeed, in the phase 3 study, the CR rate in the experimental group was 28%.49 Our model would have also correlated with other randomized studies, including those assessing DNMTI with or without entinostat, vorinostat, valproic acid, and lenalidomide (Table 5).
Single-arm and randomized trial response rates and expected response rates
| Combination . | NCT . | Single-arm response . | Randomized endpoint . | Randomized response . | Expected response (phase 1/2) . |
|---|---|---|---|---|---|
| Azacitidine + entinostat | Phase 1: NCT00101179 Phase 2: NCT00313586 | 3/30 CR 4/30 PR 7/30 HI | TN response rate: 30% vs 16% historical | Aza: 9/74 CR 24/74 TN Aza + entinostat: 8/75 CR 20/75 TN | Unlikely to improve on CR Unlikely to improve on HI |
| Decitabine + valproic acid | Phase 1/2: NCT00075010 Phase 2 randomized: NCT00414310 | 10/53 CR | Response rate | Decitabine: 22/70 CR Decitabine + VPA: 29/79 CR | Unlikely to improve on CR |
| Azacitidine + lenalidomide | Phase 1: NCT00352001 Phase 2: NCT01522976 | 16/36 CR 20/36 HI | ORR including HI | Aza: 24/92 CR 14/92 HI Aza + lenalidomide: 24/93 CR 25/93 HI | Moderately improved CR (>30%, P = .047) Unlikely to have 50% CR |
| Azacitidine + vorinostat | Phase 1: NCT00392353 Phase 2: NCT01522976 | 10/33 CR | ORR including HI | Aza: 24/92 CR Aza + vorinostat: 17/92 CR | Unlikely to improve on CR |
| Azacitidine + eprenetapopt | 20/40 CR | CR rate | Aza: 17/76 22.4% Aza + eprenetapopt: 26/78, 33.3% | Moderately better CR (>30%, P = .006) Unlikely to have 50% CR | |
| Azacitidine + pevonedistat | Phase 2: NCT02610777 Phase 3: NCT03268954 | 22/55 CR EFS and OS NS by ITT | EFS primary endpoint | Did not meet EFS benefit | Unlikely to improve on CR |
| Combination . | NCT . | Single-arm response . | Randomized endpoint . | Randomized response . | Expected response (phase 1/2) . |
|---|---|---|---|---|---|
| Azacitidine + entinostat | Phase 1: NCT00101179 Phase 2: NCT00313586 | 3/30 CR 4/30 PR 7/30 HI | TN response rate: 30% vs 16% historical | Aza: 9/74 CR 24/74 TN Aza + entinostat: 8/75 CR 20/75 TN | Unlikely to improve on CR Unlikely to improve on HI |
| Decitabine + valproic acid | Phase 1/2: NCT00075010 Phase 2 randomized: NCT00414310 | 10/53 CR | Response rate | Decitabine: 22/70 CR Decitabine + VPA: 29/79 CR | Unlikely to improve on CR |
| Azacitidine + lenalidomide | Phase 1: NCT00352001 Phase 2: NCT01522976 | 16/36 CR 20/36 HI | ORR including HI | Aza: 24/92 CR 14/92 HI Aza + lenalidomide: 24/93 CR 25/93 HI | Moderately improved CR (>30%, P = .047) Unlikely to have 50% CR |
| Azacitidine + vorinostat | Phase 1: NCT00392353 Phase 2: NCT01522976 | 10/33 CR | ORR including HI | Aza: 24/92 CR Aza + vorinostat: 17/92 CR | Unlikely to improve on CR |
| Azacitidine + eprenetapopt | 20/40 CR | CR rate | Aza: 17/76 22.4% Aza + eprenetapopt: 26/78, 33.3% | Moderately better CR (>30%, P = .006) Unlikely to have 50% CR | |
| Azacitidine + pevonedistat | Phase 2: NCT02610777 Phase 3: NCT03268954 | 22/55 CR EFS and OS NS by ITT | EFS primary endpoint | Did not meet EFS benefit | Unlikely to improve on CR |
EFS, event-free survival; TN, trilineage; VPA, valproic acid.
In this article, we also show important considerations for study design related to the DNMTI backbone. Indeed, we identified higher marrow response rates with decitabine regimens, whereas there were higher rates of hematologic response with azacitidine regimens. Similarly, decitabine appeared to be slightly more active in trials where more of the patients had excess blasts compared with azacitidine trials, supporting the idea that decitabine in the currently used doses and schedules may be more “intense” or myelosuppressive than azacitidine. With either azacitidine or decitabine, enrolling more patients with high-risk cytogenetics or in high IPSS groups resulted in lower marrow response rates. Because of these associations, it is possible that a given trial may deviate from these “expected” response rates, especially in smaller cohorts, because of higher proportions of patients with certain disease profiles (eg, TP53 mutated). It also remains less clear how historical studies using older WHO definitions of MDS and enrolled based on IPSS risk will compare with modern cohorts of patients with MDS enrolled based on Revised IPSS or newer molecular risk models.4,50 This study is limited by data provided in published manuscripts, and does not include patient-level data; however, these findings are consistent with other analyses.21,51,52 Importantly, patient-level data from modern era trials incorporating a DNMTI monotherapy arm will be very valuable, regardless of the outcome of each study, both to design future trials as well as to understand better which cohorts benefit most in a modern era.53-56
The long time since DNMTI therapy was established remains a challenge in modern MDS trial design because classification and risk stratification tools (and response assessment, including that of the International Working Group) have been revised over time. Many patients with HR-MDS enrolled on DNMTI trials in the 1990s would now be considered to have AML.10 Phase 3 studies often look different in patient composition from early-phase studies, including differential enrollment of high-risk populations and patients with limited performance status. In addition, more recent advances in MDS diagnostics including mutation profiles are generally unavailable for previously published studies. Although mutation-driven therapeutic strategies are emerging in MDS, to date treatment strategies remain largely agnostic of mutation profile for higher risk disease; as such, we believe there is limited impact on our findings in the current treatment environment. Indeed, there is a critical need to share molecularly annotated datasets in both DNMTI monotherapy and DNMTI combinations that will come from recent phase 3 studies; such may help inform future study composition, for instance, the expected response rate in TP53 mutant MDS.
It may also be relevant to distinguish how different endpoints can be valuable at different points in the treatment of patients with MDS. CR and ORR may be relevant early endpoints, particularly if the goal is to proceed to transplant, although such data are less clear in the absence of excess blasts. At the same time, we acknowledge that on their own mCR and PR responses are of less clear value, particularly with the use of increasingly myelosuppressive combinations. We therefore tried to emphasize the importance of looking at CR independently in comparisons because it is CR, not combined survival metrics, that is associated with survival. Other survival endpoints, including event-free survival and OS, are also critical, though take longer to reach and are dependent on the rates of patients undergoing transplant.57
To make real advances in MDS, greater efforts are needed to enroll patients on meaningful clinical trials that have a chance to change the standard of care. This includes trials incorporating transplant into their design given its impact on survival. Early signals are important in DNMTI combination studies, but these need to be fairly strong to translate into clinically meaningful differences.
Authorship
Contribution: A.M.B., G.F., and D.P.S. designed the research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: A.M.B. reports a consulting or advisory role for Agios, Acceleron, BMS/Celgene, Keros Therapeutics, Novartis, Takeda, Taiho Research; funding from Aprea AB (to institution), Celgene (to institution), Bristol Myers Squibb (to institution), Novartis (to institution), Takeda (to institution), Janssen (to institution), GSK (to institution). G.F. reports no competing financial interests. D.P.S. reports employment with Novartis Pharmaceuticals; stock and other ownership interests from Arrowhead Pharmaceuticals, Sage Therapeutics; honoraria from Daiichi Sankyo, Summer Road, Stemline Therapeutics, Celgene, Astex Pharmaceuticals; consulting or advisory role for Pfizer, Janssen Oncology, Agios, Onconova Therapeutics, Geron, Astex Pharmaceuticals; and research funding from Aprea AB (to institution), Celgene (to institution), Bristol Myers Squibb (to institution), H3 Biomedicine (to institution).
Correspondence: Andrew Brunner, Zero Emerson Place, Suite 118, Boston, MA 02114; e-mail: abrunner@mgh.harvard.edu.
References
Author notes
Requests for data sharing may be submitted to Andrew Brunner (abrunner@mgh.harvard.edu).