TO THE EDITOR:
Marginal zone lymphoma (MZL) is an indolent B-cell non-Hodgkin lymphoma that comprises 7% of all non-Hodgkin lymphomas.1-3 The optimal frontline management of MZL is not well defined, and the current treatment recommendations are adapted mainly from follicular lymphoma. With the advent of novel agents, the outcomes of MZL have improved significantly in the past decade.4 Ibrutinib, the first-in-class covalent Bruton tyrosine kinase inhibitor was approved for patients with relapsed/refractory (R/R) MZL based on the results of a phase 2 clinical trial wherein the overall response rate (ORR) was 48%.5 In the recently updated long-term follow-up of this study, the ORR was 58% with a median duration of response of 27.6 months.6 However, the outcomes of patients who progress on ibrutinib are largely unknown. Hence, we sought to evaluate the outcomes of patients with R/R MZL who experienced progression on ibrutinib therapy.
This was a multicenter retrospective cohort study and included adult patients (18 years or older) with R/R MZL who received ibrutinib between 2010 and 2019 at 25 US medical centers. The study was approved by the institutional review boards at all the participating sites and performed in compliance with the Declaration of Helsinki. To be eligible for the analysis, patients must have received ibrutinib monotherapy in the R/R setting and progressed on it.
The primary objective of the study was to evaluate the overall survival (OS) after the progression or relapse on ibrutinib. The secondary objectives were response rates and progression-free survival (PFS) to first salvage therapy after the progression on ibrutinib. The patients who achieved a complete response (CR) or partial response (PR) to ibrutinib as their best response were categorized as “ibrutinib responders,” whereas those with the progression of the disease as their best response to ibrutinib were categorized as primary progressors (PPs). Patients who initially responded or had stable disease (SD) and then progressed on ibrutinib were categorized as secondary progressors (SPs). PFS was defined as the time from the start of the first salvage therapy (after ibrutinib progression) to progression/relapse on the first salvage therapy or death from any cause. Postrelapse OS was defined as the time from progression/relapse on ibrutinib therapy to the date of death or last follow-up. There was no central imaging review, and tumor assessment was performed according to individual center practices.
Demographic and disease characteristics were summarized using descriptive statistics, such as median and range for continuous variables and frequency and percentage for categorical variables, compared among study groups using the Kruskal-Wallis test or Fisher exact test. Postrelapse OS and PFS were estimated using the Kaplan-Meier method and compared between groups via the log-rank test. Cox proportional hazard regression models were used to estimate the hazard ratios for risk of progression or death. Analyses were performed using Stata software (version 16; StataCorp, College Station, TX). All statistical tests were 2 sided with a type I error of 0.05.
Among the 119 patients who received ibrutinib monotherapy in R/R MZL, 47 patients progressed. Of these, 15 (32%) and 32 (68%) were PPs and SPs, respectively (supplemental Figure 1). The baseline characteristics of all patients stratified by primary and secondary progression are provided in supplemental Table 1. The 2 groups (PPs and SPs) were well balanced regarding the salient baseline characteristics.
Of the 47 patients who progressed on ibrutinib, follow-up data were not available in 4 patients (all in the PP group), leaving 43 patients for the assessment of postibrutinib relapse outcomes. Among the remaining 43 patients, the best response to ibrutinib before progression included 20 (46%) with CR/PR, 12 (28%) with SD, and 11 (26%) with primary progression. Among the 43 patients, 17 (40%) received ibrutinib in second-line therapy, 17 (40%) received ibrutinib in third-line therapy, and 9 (20%) received ibrutinib in fourth-line therapy and beyond. Among the PPs (n = 11), 36% (n = 4) received ibrutinib in second-line therapy, 36% (n = 4) in third-line therapy, and 27% (n = 3) in fourth-line therapy and beyond. Among SPs (n = 32), 41% (n = 13) received ibrutinib in second-line therapy, 41% (n = 13) in third-line therapy, and 19% (n = 6) in fourth-line therapy and beyond. The median follow-up postibrutinib progression was 9.9 months, 9.8 months, and 10 months in all patients (n = 43), PP, and SP cohorts, respectively.
Among the 43 patients, only 25 received subsequent therapy, with bendamustine-based therapies being the most common (n = 6), followed by non–bendamustine-based alkylator therapies (n = 5), lenalidomide and rituximab (R2, n = 4), phosphatidylinositol-3-kinase inhibitors (n = 3), and ibrutinib and anti-CD20 monoclonal antibodies (n = 3). Table 1 shows the breakdown of the first salvage therapy as well as their response rates after progression on ibrutinib. All patients (n = 4) receiving R2 therapy after ibrutinib failure/progression responded (CR = 1, PR = 3).
First-line salvage therapy after ibrutinib progression/relapse
| . | N = 25 (%)∗ . | CR . | PR . | SD . | PD . |
|---|---|---|---|---|---|
| BR and BO | 6 (24) | 4 | 0 | 0 | 2 |
| R2 | 4 (16) | 1 | 3 | 0 | 0 |
| Phosphatidylinositol-3-kinase inhibitor | 3 (12) | 0 | 1 | 2 | 0 |
| IR or IO | 3 (12) | 0 | 1 | 1 | 1 |
| Others† | 9 (36) | 1 | 2 | 4 | 2 |
| . | N = 25 (%)∗ . | CR . | PR . | SD . | PD . |
|---|---|---|---|---|---|
| BR and BO | 6 (24) | 4 | 0 | 0 | 2 |
| R2 | 4 (16) | 1 | 3 | 0 | 0 |
| Phosphatidylinositol-3-kinase inhibitor | 3 (12) | 0 | 1 | 2 | 0 |
| IR or IO | 3 (12) | 0 | 1 | 1 | 1 |
| Others† | 9 (36) | 1 | 2 | 4 | 2 |
BO, bendamustine and obinutuzumab; BR, bendamustine and rituximab; IO, ibrutinib and obinutuzumab; IR, ibrutinib and rituximab; R2, rituximab and lenalidomide (revlimid).
Among the 43 patients who progressed/relapsed after ibrutinib, only 25 received subsequent therapy.
Others: 5 included alkylator-based (nonbendamustine) therapies, 1 platinum-based, 2 anti-CD20 monoclonal antibodies, and 1 BCL-2 inhibitor.
The median PFS among the recipients of the first salvage therapy (n = 25) was 18.2 months (supplemental Figure 2). The PFS between the different salvage therapies is shown in supplemental Figure 3, and the PFS based on the best response to ibrutinib before progression is shown in supplemental Figure 4. Owing to low numbers, formal statistical comparisons were not conducted.
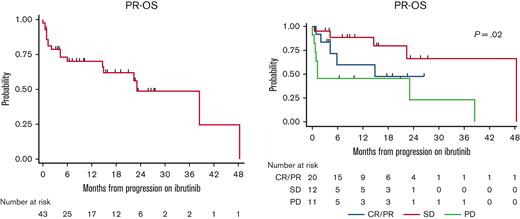
The median postrelapse OS (n = 43) was 23.1 months (95% confidence interval, 14.6-not reached) (Figure 1A). On stratifying the postrelapse OS based on the best response to ibrutinib before progression, patients who achieved CR/PR or SD to ibrutinib before progression had a median postrelapse OS of 48.4 months and 14.9 months, respectively, whereas those who had primary progression, had a median postrelapse OS of 1.2 months (Figure 1B). Although the median postrelapse OS was 38.5 months vs 23.1 months vs 4.3 months in those who received ibrutinib in second-line therapy vs third-line therapy vs fourth-line therapy, respectively, this did not reach statistical significance (P = .22) (supplemental Figure 5).
Postrelapse OS. (A) Postrelapse OS (PR-OS) among all evaluable patients. (B) Postrelapse OS based on their ibrutinib response before progression.
Postrelapse OS. (A) Postrelapse OS (PR-OS) among all evaluable patients. (B) Postrelapse OS based on their ibrutinib response before progression.
Among the 43 patients, 6 patients (14%) had high-grade transformation with 1 in the PP cohort (9%, 1/11) and 5 in the SP cohort (16%, 5/32). A total of 18 patients died, with 8 in the PP cohort and 10 in the SP cohort. The most common cause of death was lymphoma progression (61%). The breakdown of the causes of death stratified by the PP and SP cohorts is provided in supplemental Table 2.
In this multicenter retrospective study, we analyzed the outcomes of patients with R/R MZL who progressed on ibrutinib and made several important observations. First, the postrelapse OS was poor in the PP cohort. Second, the response rates varied among the different salvage therapies after ibrutinib progression; however, the small sample size precluded formal statistical comparison between the various regimens. Finally, although there was no significant difference in postrelapse OS based on the ibrutinib line of therapy before progression, the low numbers preclude definitive conclusions due to insufficient power.
Patients with R/R MZL achieving primary progression on ibrutinib had dismal postrelapse OS. One of the reasons for this finding is that 73% (8/11) of patients with primary progression did not receive subsequent therapy in this study. The median postrelapse OS was significantly shorter in PPs relative to SPs. Hence, identifying effective treatment options for patients with primary progression represents an unmet need. Although attempts have been made to understand the resistance mechanism to ibrutinib in MZL, this has been mainly limited to secondary progression (acquired resistance)7 rather than to primary progression (primary resistance) and needs to be explored further.
Outside of Bruton tyrosine kinase inhibitors, there are other classes of agents that are active in MZL including phosphatidylinositol-3-kinase inhibitors,8-10 immunomodulators (lenalidomide),11,12 and BCL2 inhibitors.13-15 Given the plethora of therapeutic options, the most challenging question currently is how best to sequence these agents. In our study, we saw that patients who experienced disease progression on ibrutinib and subsequently received R2 had a 100% ORR. These results need to be interpreted with caution given the small sample size. Notably, there is a paucity of information on the role of bispecific antibodies in R/R MZL, as all the studies included only transformed MZLs. Although the results with chimeric antigen receptor T-cell therapy in R/R MZL were disappointing (median PFS, 11.8 months),16 the sample size was small (n = 22) to draw definitive conclusions. Ongoing (#NCT04245839)/future studies will provide more information on the role of cellular therapies (chimeric antigen receptor T cell and bispecific antibodies) in R/R MZL.
Our study has inherent limitations of retrospective design including nonuniform selection of salvage therapy, response assessment, and timing of ibrutinib therapy. Although there was no central pathology review, the diagnosis was confirmed by expert hematopathologists at the respective academic institutions. Notably, it is hard to draw any definitive conclusions regarding the rate of transformation between PP and SP cohorts because of the high number of deaths in the PP cohort and the inability to perform competing risk modeling due to low numbers.
In conclusion, to our knowledge, this is the first study that reports the outcomes associated with patients with R/R MZL who progressed on ibrutinib. We show that patients who are PPs are a very high-risk subset with poor outcomes. Efforts need to be made to understand the resistance mechanism in this group to identify appropriate novel therapy combinations to improve outcomes.
Contribution: N.E. conceived and designed the study and prepared first draft of the manuscript; N.E. and Q.Z. analyzed the data; and all authors collected and assembled the data, provided the interpretation, provided critical and insightful comments, and approved the final version of the manuscript.
Conflict-of-interest disclosure: N.E. is in the speakers bureau of Incyte; receives honoraria, provided consultancy, and serves on the advisory boards for TG Therapeutics, Pharmacyclics, BeiGene, Seattle Genetics, and Novartis; and has received research funding from BeiGene. T.K.M. serves on the advisory board for Seattle Genetics. N.R. has provided consultancy to Kite Pharma, AbbVie, Bristol-Myers Squibb (BMS), and Celgene and received research funding from Genentech and BMS. P.R.G. has provided consultancy to Kite Pharma, Rafael Pharma, Pharmacyclics LLC, and BMS. P.C. receives research funding from ADC Therapeutics and Genentech; serves on the advisory boards for ADC Therapeutics, Genentech, Bayer, Verastem, and Kite Pharma; and is in the speakers bureau of Celgene. P.T. receives consulting fees from ADC Therapeutics, TG Therapeutics, Kura Oncology, and Genentech. S.A. has provided consultancy and serves on the advisory boards for TG Therapeutics, Seattle Genetics, BeiGene, Intellisphere, Fate Therapeutics, and AstraZeneca. R.K. serves on the advisory boards for Celgene Corporation, Gilead Sciences, Juno Therapeutics, Kite Pharma, Janssen, Karyopharm, Pharmacyclics, MorphoSys, Epizyme, Genentech/Roche, EUSA, and Calithera; received grants/research support from Celgene Corporation/Juno Therapeutics/BMS, Takeda, BeiGene, Gilead Sciences/Kite Pharma; and is in the speakers bureau of AstraZeneca, BeiGene, and MorphoSys. S.K. has provided consultancy to Karyopharm and Incyte. Y.S. has received research funding from BMS, Celgene, TG Therapeutics, and BeiGene and has consulted for TG Therapeutics and Epizyme. B.C. has received research funding from Acerta, Celgene, Genentech, Merch, Millennium, MorphoSys, Roche, and Triphase and serves on the advisory boards for Verastem, Seattle Genetics, and AstraZeneca. M.J. has received research funding from Takeda, Fate, and Nektar. A.J.O. has received research funding from Genentech, Spectrum Pharmaceuticals, TG Therapeutics, and Adaptive Biotech. J.B.C. has provided consultancy and serves on the advisory boards for Janssen, BeiGene, AstraZeneca, Loxo/Lilly, Aptitude Health, Kite Pharma/Gilead, and HutchMed and received research funding from AstraZeneca, BMS/Celgene, Genentech, Loxo/Lilly, Takeda, Novartis, HutchMed, and BioInvent. N.P. has received research funding from Genentech and AbbVie. F.T.A. has provided consultancy to Genentech, AstraZeneca, AbbVie, Janssen, Pharmacyclics, Gilead Sciences, Kite Pharma, Celgene, Karyopharm, MEI Pharma, Verastem, Incyte, BeiGene, Johnson and Johnson, Dava Oncology, BMS, Merck, Cardinal Health, ADC Therapeutics, and Epizyme. J.P.A. has received consulting fees and research funding from ADC Therapeutics. S.K.B. has provided consultancy to Monsanto and received honoraria from Atara, Seattle Genetics, Janssen, and Pfizer. N.S.G. has received research funding from Genentech and received honoraria, provided consultancy, and served on the advisory boards for Kite Pharma, ADC Therapeutics, and Novartis. N.G. has provided consultancy and acted in an advisory role for Seattle Genetics, TG Therapeutics, AstraZeneca, Pharmacyclics, BMS, Gilead, BeiGene, Incyte, Karyopharm, Roche/Genentech, Novartis, Loxo/Lilly, Genmab, and Adaptive Biotech. N.L.B. received research funding from ADC Therapeutics, Autolus, BMS, Celgene, Forty Seven, Genentech, Immune Design, Janssen, Merck, Millennium, Pharmacyclics, Affirmed Therapeutics, Dynavax, Gilead, MedImmune, and Novartis and provided consultancy and served on the advisory boards for Kite Pharma, Pfizer, ADC Therapeutics, Roche/Genentech, Seattle Genetics, BTG, and Acerta. G.S. has received honoraria from Kite Pharma and BeiGene. A.F.H. has provided consultancy to BMS, Merck, Gilead, Adaptive Biotech, Seattle Genetics, Karyopharm and received research funding from Merck, Genentech, Gilead, Seattle Genetics, Immune Design, AstraZeneca, Pharmacyclics, and ADCT Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Narendranath Epperla, Division of Hematology, Department of Medicine, The Ohio State University, Columbus, OH 43210; e-mail: narendranath.epperla@osumc.edu.
References
Author notes
Data are available on request from corresponding author, Narendranath Epperla (narendranath.epperla@osumc.edu).
The full-text version of this article contains a data supplement.
A.F.H. and G.S. are joint senior authors.