In this issue of Blood Advances, the study by Ghunney et al1 sheds light on the prevalence and severity of HbSC disease, the second most common variant of sickle cell disease (SCD), in a large cohort of patients with SCD at the Ghana Institute of Clinical Genetics SCD Clinic. The authors find that about 10.5% and 24.1% of individuals with HbSC and HbSS, respectively, were eligible for initiation of hydroxyurea based on disease severity; unfortunately, of those, less than 3% and 1%, respectively, were prescribed the drug. These numbers underscore the following 2 major health disparities: 1 in access to hydroxyurea in a sub-Saharan Country as compared to US and European settings where hydroxyurea uptake is much higher, and 1 within the SCD population itself, where patients with HbSC receive substandard care even when compared to their counterparts with HbSS.
Historically, HbSC disease has been viewed as a more benign form of SCD that presents with clinical events similar in quality to those of HbSS, albeit with decreased frequency (except for retinopathy). The pathogenesis of HbSC disease also incompletely overlaps with that of its more common variant, in that hyperviscosity, rather than anemia and hemolysis, plays a disproportionate role in its morbidity. Because of these considerations, HbSC has been excluded from many seminal trials for SCD. The most egregious example of the overlooking of HbSC by the medical and research community is epitomized by the tentative approach to hydroxyurea use for this variant. The results of the landmark Multicenter Study of Hydroxyurea (MSH) in SCD trial, showing that hydroxyurea significantly reduced the rate of vaso-occlusive crises and other complications in patients with HbSS and HbS/beta zero thalassemia (another severe variant), firmly established it as the mainstay of treatment for SCD.2 Many subsequent trials and observational studies confirmed its benefits across ethnically and geographically diverse populations across the world. Yet, its potential role in HbSC has been mostly extrapolated from the experience in HbSS disease. Landmark clinical trials on the heels of MSH, such as BABY-HUG (testing the effect of hydroxyurea in young children)3 and REACH (the first to demonstrate the efficacy of hydroxyurea in Africa)4 have failed to address the knowledge gap on the benefit of the drug in HbSC disease because of the persistent exclusion of patients with this SCD variant. The handful of studies aimed at assessing the effect of hydroxyurea on HbSC disease have been observational,5-8 and as such, there has been a poor consensus among providers regarding the role of hydroxyurea in HbSC despite current guidelines recommending its use for all variants. Fortunately, the inclusion of a few participants with HbSC in the recent phase III trials of the newer disease–modifying therapies, such as voxelotor and crizanlizumab, has started to reverse this trend, but much remains to be done to bridge the knowledge gap on the effectiveness of hydroxyurea in HbSC disease.
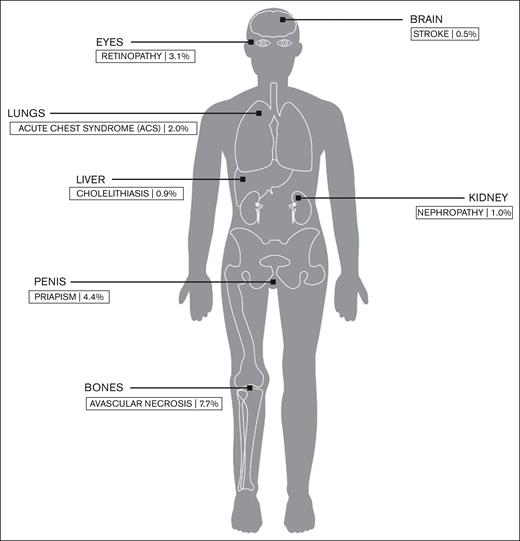
Ghunney et al1 also show us that the prevalence of HbSC disease is higher in Ghana (40%) than in the US (30%), which further underscores the urgency of addressing important questions on the options for disease-modifying therapy for this large population of patients. Interestingly, the prevalence of some HbSC comorbidities in the study by Ghunney et al (see figure) is lower than reported in prior studies in higher-income countries9; this could be a result of survival bias because many children with severe complications die before reaching adulthood in Ghana owing to a lack of early diagnosis and adoption of preventive interventions. As newborn screening and early intervention programs are progressively being implemented,10 individuals with HbSC will be expected to survive to late adulthood, which will expose them to conventional cardiovascular and age-related morbidity and mortality risk factors in addition to SCD-specific morbidity; the resulting public health challenge in tackling the health care needs of this population may appear daunting, but compelling, nonetheless.
Prevalence of common SCD complications within the patient population with HbSC disease at the Ghana Institute of Clinical Genetics.
Prevalence of common SCD complications within the patient population with HbSC disease at the Ghana Institute of Clinical Genetics.
There are many barriers to the accessibility of hydroxyurea and other disease-modifying treatments for SCD in Africa. As highlighted in this study, hydroxyurea is not affordable to patients in low-income settings such as Ghana and Nigeria where the disease is most prevalent. Financial barriers will similarly place other widely more expensive treatments, such as stem cell transplantation and gene therapy, beyond reach for all but a few patients in sub-Saharan Africa, which implies that hydroxyurea will likely remain the only option to treat SCD in these settings for years to come. Health care institutions and governments should negotiate more favorable and equitable hydroxyurea pricing with pharmaceutical companies in these settings. Additional barriers such as lack of provider training in prescribing hydroxyurea should also be addressed as these are likely to significantly impact the management of patients living in rural and underserved areas where hematology subspecialists are scarce. Of note, these barriers are not exclusive to sub-Saharan Africa and are keenly experienced by patients living in rural and remote areas in the United States as well. Common approaches such as education of primary care providers and other health care professionals and community workers should be explored. In addition, community efforts aimed at minimizing the stigma of SCD, increasing awareness of hydroxyurea, and dispelling unfounded myths about the drug can also prove helpful.
Despite ongoing efforts to make hydroxyurea more accessible, such as its inclusion among the medications covered by the National Health Insurance Scheme in Ghana and other countries, and promotion of local manufacturing, hydroxyurea uptake will continue to be sluggish if there are no definitive studies on its effectiveness in individuals with HbSC. In general, urgent effort across the world is needed into ascertaining the role of existing and future therapies for the management of HbSC disease as well as interventions that address patient, provider, and systemic barriers to hydroxyurea use.
Conflict-of-interest disclosure: The authors declare no competing financial interests.