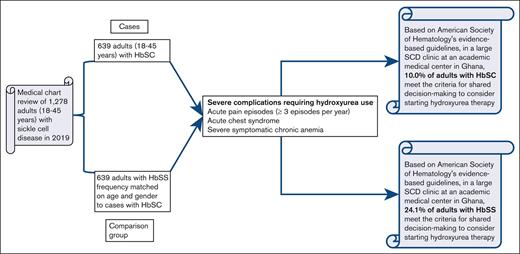
Key Points
A total of 10% and 24% of adolescents and adults with HbSC and HbSS, respectively, in Ghana have severe SCD.
Less than 1% and 3% of adolescents and adults with severe HbSC and HbSS, respectively, have been treated with hydroxyurea in Ghana.
Abstract
Sickle cell hemoglobin SC (HbSC) disease is the second most frequent sickle cell disease (SCD) genotype after sickle cell anemia (HbSS). Globally, ∼55 000 newborns with HbSC are delivered annually, with the highest HbC gene frequency in West Africa. In Ghana, 40% of adults visiting the Ghana Institute of Clinical Genetics SCD clinic have HbSC. Unlike HbSS, hydroxyurea use is not routinely recommended for individuals with HbSC because of the perceived high-risk to benefit ratio. To test the hypothesis that at least 5% of adults with HbSC will meet the American Society of Hematology criteria for severe disease, we conducted a retrospective descriptive cohort study of all individuals with HbSC (≥18 years) who visited the clinic in 2019. Adults with HbSC aged from 18 to 45 years were selected. We identified a comparison group of 639 individuals with HbSS and matched the frequency based on the age and sex of individuals with HbSC. Severe disease was defined as a history of ≥3 SCD-associated moderate or severe pain episodes per year, history of acute chest syndrome, and severe symptomatic chronic anemia that interferes with daily activities or quality of life. The study end points were the proportion of individuals with SCD who met the definition of severe disease and were eligible for hydroxyurea. In total, 64 of 639 (10.0%) individuals with HbSC met the eligibility criteria for hydroxyurea therapy compared with 154 of 639 (24.1%) individuals with HbSS. Less than 1% and 3% of individuals with severe HbSC and HbSS, respectively, were routinely prescribed with hydroxyurea in this tertiary care medical center.
Introduction
Sickle cell hemoglobin SC (HbSC) disease is the second most frequent hemoglobinopathy after sickle cell anemia (HbSS). Globally, ∼55 000 newborns with HbSC disease are delivered yearly, with the highest hemoglobin C (HbC) gene frequency in West Africa.1-3 A retrospective hospital-based study conducted in Ghana reported that of ∼5 500 adolescents and adults with SCD who were examined over 2 years, ∼40% (2159/5452) had HbSC disease.4 Among individuals with HbSC, rates of maternal-fetal morbidity, retinopathy, avascular necrosis (AVN) of the hip, priapism, and chronic kidney disease are increased compared with historical controls.5,6 Although rates of albuminuria are lower in HbSC compared with HbSS, albuminuria is prominent in HbSC occurring in >23% of the adult population.7 In addition, thrombosis, silent cerebral infarction, sensorineural hearing loss, and pulmonary hypertension may be higher than previously suspected.8 The disease pathogenesis among individuals with HbSC is reported to be “modulated by interactions between HbS, HbC, and red blood cell (RBC) dehydration from altered membrane transporter function”9 Studies by Nagel et al10 and Gualandro et al11 reported HbSC RBCs as having equal amounts of HbS and HbC with low HbF levels.9 “The presence of HbSC may lead to an acceleration of HbC crystallization, RBC dehydration, HbS polymerization, and subsequently RBC deformability.”12 The low levels of HbF in individuals with SCD are disadvantageous because they do not impede HbC crystallization and HbS polymerization,10 2 significant processes that result in SCD complications.
Unlike HbSS, hydroxyurea therapy is not routinely recommended or used for individuals with HbSC, partly because HbSC is generally believed to be milder, and the toxicity profile of hydroxyurea has not been formally evaluated in a randomized controlled trial in this population. Generally, individuals with HbSC are excluded from most randomized controlled trials focused on decreasing vaso-occlusive events in SCD. However, recent clinical trials leading to the approval of newer disease-modifying drugs, such as voxelotor and crizanlizumab, included individuals with HbSC disease.13,14
Given the high proportion of adults with HbSC who may benefit from hydroxyurea therapy in a large clinic for adults with SCD in Ghana, we tested the hypothesis that at least 5% of adults with HbSC will meet the American Society of Hematology (ASH) criteria for severe disease and treatment with hydroxyurea.
Methodology
Study design
This was a 1-year retrospective descriptive cohort study, starting from the date of the first clinic visit in 2019. The study end points were the proportion of individuals with SCD (both HbSC and HbSS) who met the definition of severe disease and were thus eligible for hydroxyurea therapy. We obtained approval from the institutional review board of Korle-Bu Teaching Hospital (KBTH-ADM/0769/2021). This study was conducted in accordance with the Declaration of Helsinki.
Study site
This study was conducted at the Ghana Institute of Clinical Genetics, the largest day clinic for adolescents and adults with SCD in Ghana, located on the Korle-Bu Teaching Hospital campus. Approximately 40% of the 3 000 individuals with SCD attending the clinic yearly have HbSC disease.4 Previous data from our study site showed that for the ages from 13 to 44 years, the ratio of individuals with HbSS to HbSC was 2:1. However, this ratio was reversed after the age of 44 years.4
Study population
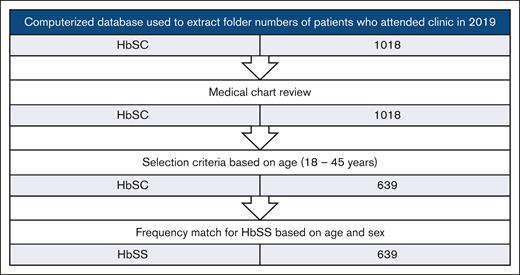
We used a computerized database to extract the folder numbers of all individuals with HbSC who attended the SCD clinic from January 1, 2019, to December 31, 2019 (N = 1018), and a medical chart review was conducted. Adults aged between 18 and - 45 years (n = 639) were selected. We performed a frequency match for individuals with HbSS who attended the clinic within the same period using sex and the age range from 18 to 45 years as the criteria. An upper age limit of 45 years was chosen because fewer individuals with HbSS (deemed a severe phenotype) were available for comparison after this age. Figure 1 demonstrates how the study participants were chosen.
Flowchart showing the selection criteria for HbSC individuals included in this retrospective, descriptive cohort study.
Flowchart showing the selection criteria for HbSC individuals included in this retrospective, descriptive cohort study.
Data collection
Two team members (W.K.G. and J.B.A.-N.) conducted a medical chart review of all the individuals with SCD who met the inclusion criteria. We used an Excel spreadsheet to extract all the selected study participants' SCD-related complications and comorbidities over 1 year in 2019. The SCD-related complications and comorbidities that occurred before 2019 were also documented. Four team members (W.K.G., J.B.A.-N., E.V.A., and E.O.) adjudicated the case files of all individuals with SCD who had severe complications and recorded the number of complications.
Study definitions
We defined severe disease as a history of having ≥3 sickle cell-associated moderate or severe pain episodes per year, history of acute chest syndrome (ACS), and/or severe symptomatic chronic anemia that interferes with daily activities or quality of life.15
An acute pain episode was defined as a new onset of pain that lasts at least 4 hours without any other explanation, except vaso-occlusion, which requires therapy with parenteral opioids in a medical setting.16
Because of the challenges of having a baseline chest radiograph for comparison, a diagnosis of ACS was defined as abnormal findings upon lung examination along with the presence of at least 2 of the following criteria: temperature ≥38.0°C; increased respiratory rate >90th percentile for age or >20 breaths per minute; increased oxygen requirement (Oxygen saturation drop >3% from a documented steady-state value on room air); or a new radiodensity upon chest roentgenography.6,17
Severe symptomatic chronic anemia was defined as a drop in Hb either by ≥2g/dL below the baseline or below 5.0 g/dL with symptoms of anemia.18
We described SCD-associated chronic pain as pain that occurs in a specific tissue or organ is constant, deep, nagging, and achy in nature and lasts more than 3 months.19 For our study, the causes of chronic pain included AVN, active chronic leg ulcers, osteoarthritis, and spondylosis.
Data analysis
Summary statistics for continuous variables are reported as means and standard deviations. Categorical variables and prevalence are reported as counts and percentages. The incidence rates were reported as events per 100 patient-years. To compare the groups, either a χ2 test or Fisher exact test was used for percentages, t test for means, and Poisson regression for incidence rates. Data were analyzed using SPSS version 27 (IBM, Armonk, NY).
Results
Baseline characteristics
The 639 individuals with HbSC had a mean age of 30.7 years and mean baseline Hb of 11.1 g/dL. The demographic characteristics of the participants are presented in Table 1.
Demographic and clinical characteristics of adults with HbSC disease compared with adults with HbSS disease
| Characteristics . | HbSC (N = 639) . | HbSS (N = 639) . | P value∗ . |
|---|---|---|---|
| Age, mean (standard deviation) | 30.7 (7.8) | 31.0 (7.8) | .450 |
| Female, n (%) | 387 (60.6) | 387 (60.6) | 1.00 |
| Hemoglobin, g/dL, mean (standard deviation) | 11.1 (1.3) | 8.1 (1.2) | < .001 |
| Disease-modifying therapy, n (%) | |||
| Hydroxyurea | 6 (0.9) | 15 (2.3) | .048 |
| Chronic blood transfusion | 0 (0.0) | 0 (0.0) | 1.00 |
| Number of individuals with only 1 severe complication, n (%) | |||
| Acute pain (≥3 episodes per y) | 54 (8.5) | 126 (19.7) | < .001 |
| ACS | 10 (1.6) | 24 (3.8) | .015 |
| Severe symptomatic chronic anemia | 0 (0.0) | 4 (0.6) | .124†‡ |
| Proportion of individuals with SCD eligible for hydroxyurea using only 1 severe complication (ASH 2014 guidelines), n (%)§ | 64 (10.0) | 154 (24.1) | < .001 |
| Proportion of individuals with SCD eligible for hydroxyurea with 1 or more severe complication (ASH 2014 guidelines), n (%)§ | 67 (10.5) | 168 (26.3) | < .001 |
| Acute pain events per y, n (%) | |||
| 0 | 344 (53.8) | 289 (45.2) | < .001 |
| 1-2 | 238 (37.2) | 212 (33.2) | |
| ≥3 episodes | 57 (8.9) | 138 (21.6) | |
| Pain incidence rate (events per 100 patient-y) (95% CI) | 74.6 (67.9-81.3) | 123.0 (114.4-131.6) | < .001† |
| ACS incidence rate (events per 100 patient-y) (95% CI) | 2.3 (1.2-3.5) | 5.6 (3.8-7.5) | .004† |
| Prevalence of complications among patients, n (%) | |||
| Severe symptomatic chronic anemia, n (%) | 0 (0.0) | 10 (1.6) | .001‡ |
| AVN, n (%) | 49 (7.7) | 61 (9.5) | .231 |
| Cerebrovascular accident/ stroke, n (%) | 3 (0.5) | 7 (1.1) | .204 |
| Priapism, n (%)‖ | 11 (4.4) | 26 (10.3) | .011 |
| Nephropathy, n (%) | 7 (1.1) | 54 (8.5) | < .001 |
| Albuminuria | 0 (0.0) | 54 (100.0) | |
| Painless gross hematuria | 7 (100.0) | 0 (0.0) | |
| Renal papillary necrosis | 7 (100.0) | 0 (0.0) | |
| Proliferative SCD retinopathy, n (%) | 20 (3.1) | 4 (0.6) | < .001 |
| Active chronic leg ulcer, n (%) | 0 (0.0) | 53 (8.3) | < .001 |
| Systemic hypertension, n (%) | 23 (3.6) | 7 (1.1) | .003 |
| Chronic pain, n (%) | 110 (17.2) | 156 (24.4) | .002 |
| Characteristics . | HbSC (N = 639) . | HbSS (N = 639) . | P value∗ . |
|---|---|---|---|
| Age, mean (standard deviation) | 30.7 (7.8) | 31.0 (7.8) | .450 |
| Female, n (%) | 387 (60.6) | 387 (60.6) | 1.00 |
| Hemoglobin, g/dL, mean (standard deviation) | 11.1 (1.3) | 8.1 (1.2) | < .001 |
| Disease-modifying therapy, n (%) | |||
| Hydroxyurea | 6 (0.9) | 15 (2.3) | .048 |
| Chronic blood transfusion | 0 (0.0) | 0 (0.0) | 1.00 |
| Number of individuals with only 1 severe complication, n (%) | |||
| Acute pain (≥3 episodes per y) | 54 (8.5) | 126 (19.7) | < .001 |
| ACS | 10 (1.6) | 24 (3.8) | .015 |
| Severe symptomatic chronic anemia | 0 (0.0) | 4 (0.6) | .124†‡ |
| Proportion of individuals with SCD eligible for hydroxyurea using only 1 severe complication (ASH 2014 guidelines), n (%)§ | 64 (10.0) | 154 (24.1) | < .001 |
| Proportion of individuals with SCD eligible for hydroxyurea with 1 or more severe complication (ASH 2014 guidelines), n (%)§ | 67 (10.5) | 168 (26.3) | < .001 |
| Acute pain events per y, n (%) | |||
| 0 | 344 (53.8) | 289 (45.2) | < .001 |
| 1-2 | 238 (37.2) | 212 (33.2) | |
| ≥3 episodes | 57 (8.9) | 138 (21.6) | |
| Pain incidence rate (events per 100 patient-y) (95% CI) | 74.6 (67.9-81.3) | 123.0 (114.4-131.6) | < .001† |
| ACS incidence rate (events per 100 patient-y) (95% CI) | 2.3 (1.2-3.5) | 5.6 (3.8-7.5) | .004† |
| Prevalence of complications among patients, n (%) | |||
| Severe symptomatic chronic anemia, n (%) | 0 (0.0) | 10 (1.6) | .001‡ |
| AVN, n (%) | 49 (7.7) | 61 (9.5) | .231 |
| Cerebrovascular accident/ stroke, n (%) | 3 (0.5) | 7 (1.1) | .204 |
| Priapism, n (%)‖ | 11 (4.4) | 26 (10.3) | .011 |
| Nephropathy, n (%) | 7 (1.1) | 54 (8.5) | < .001 |
| Albuminuria | 0 (0.0) | 54 (100.0) | |
| Painless gross hematuria | 7 (100.0) | 0 (0.0) | |
| Renal papillary necrosis | 7 (100.0) | 0 (0.0) | |
| Proliferative SCD retinopathy, n (%) | 20 (3.1) | 4 (0.6) | < .001 |
| Active chronic leg ulcer, n (%) | 0 (0.0) | 53 (8.3) | < .001 |
| Systemic hypertension, n (%) | 23 (3.6) | 7 (1.1) | .003 |
| Chronic pain, n (%) | 110 (17.2) | 156 (24.4) | .002 |
Severe symptomatic chronic anemia was defined as a drop in the hemoglobin ≥2g/dL below the steady state.
Hypertensive heart disease was defined as systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg after repeated examinations. Patients with documented evidence of taking antihypertensives for at least 2 months were included; however, patients with sickle cell nephropathy who were solely on antihypertensives for their nephropathy were excluded.20
The causes of chronic pain include AVN, osteoarthritis of the knee and elbow joints, spondylosis (both lumbar and cervical), and active chronic leg ulcers.
χ2test for counts and t test for means, unless otherwise noted.
Poisson regression.
Fisher exact test.
Proportion of patients with SCD eligible for hydroxyurea using at least 1 severe complication was considered for patients with multiple severe complications.
The prevalence rate for priapism was calculated using the denominator N = 252 because priapism only occurs in males.
The proportion of individuals with HbSC or HbSS with severe complications who met the criteria for hydroxyurea use
Approximately 54 of 639 (8.5%) individuals with HbSC had ≥3 moderate or severe acute pain episodes per year as a severe complication, whereas 10 of 639 (1.6%) had only ACS as a severe complication. In total, 64 of 639 (10.0%) individuals with HbSC disease had only 1 severe complication and met the eligibility criteria for hydroxyurea therapy. Approximately one-tenth of the individuals with HbSC had 1 or more severe complications and were eligible for hydroxyurea use (67/639 [10.5%]; Table 1).
Comparatively, 154 of 639 (24.1%) individuals with HbSS met the eligibility criteria for hydroxyurea therapy (P < .001). Approximately a quarter of these individuals had 1 or more severe complications and were eligible for hydroxyurea use (168/639 [26.3%]; Table 1).
The pain and ACS rates
Acute pain episodes occurred in 295 of 639 (46.2%) and 350 of 639 (54.8%) individuals with HbSC and HbSS, respectively. ACS episodes occurred in 13 of 639 (2.0%) and 36 of 639 (5.6%) individuals with HbSC and HbSS, respectively. The pain and ACS incidence rates among individuals with HbSC were 74.6 (95% confidence interval [CI], 67.9-81.3) and 2.3 (95% CI, 1.2-3.5) events per 100 patient-years, respectively (Table 1). The pain and ACS incidence rates among individuals with HbSS were 123.0 (95% CI, 114.4-131.6) and 5.6 (95% CI, 3.8-7.5) events per 100 patient-years, respectively (Table 1). There was a difference in the pain and ACS rates between individuals with HbSC and HbSS (P < .001 and P = .004, respectively).
HbSC disease is associated with other severe SCD-related complications similar to HbSS
Approximately 7 of 639 (1%), 49 of 639 (7.7%), 3 of 639 (0.5%), and 11 of 252 (4.4%) individuals with HbSC had SCD nephropathy, AVN, stroke, and priapism, respectively, whereas 54 of 639 (8.5%), 61 of 639 (9.5%), 7 of 639 (1.1%), and 26 of 252 (10.3%) individuals with HbSS had SCD nephropathy, AVN, stroke, and priapism, respectively (Table 1). Although there was a difference in the prevalence of nephropathy and priapism between the individuals with HbSC and HbSS (P < .001 and P = .011, respectively), there was no difference in the prevalence of AVN and stroke between the individuals with HbSC and HbSS (P = .231 and P = .204, respectively; Table 1).
Only 7 of 639 (1%) of individuals with HbSC who had SCD nephropathy presented with painless gross hematuria and were diagnosed with renal papillary necrosis. They had no documented evidence of albuminuria (micro or macro). These individuals with HbSC had a mean age of 26.9 years. Comparatively, 54 of 639 (8.5%) individuals with HbSS with SCD nephropathy had documented persistent proteinuria of ≥2+ and had a mean age of 31.2 years (Tables 1 and 2. Only 1 of 7 (14.3%) individuals with HbSC and 3 of 54 (5.6%) individuals with HbSS with SCD nephropathy had systemic hypertensive disease.
Mean age of HbSC vs HbSS patients with severe complications (standard deviation)
| Characteristics . | HbSC . | HbSS . | P value∗ . |
|---|---|---|---|
| Acute pain (≥ 3 episodes per y) only (n = 180) | 32.8 (8.2) | 30.5 (7.9) | .071 |
| ACS only (n = 34) | 32.7 (6.0) | 31.3 (9.1) | .667 |
| Severe symptomatic chronic anemia only (n = 4) | N/A | 39.8 (8.1) | N/A |
| Symptomatic AVN (n = 110) | 34.8 (6.8) | 32.8 (7.2) | .141 |
| Cerebrovascular accident/stroke (n = 10) | 36.0 (7.6) | 27.9 (10.0) | .247 |
| Priapism (n = 37) | 31.8 (5.8) | 28.5 (6.0) | .252 |
| Nephropathy (n = 61) | 26.9 (5.9) | 31.2 (7.6) | .154 |
| Proliferative SCD Retinopathy (n = 24) | 32.7 (7.3) | 22.3 (4.0) | .012 |
| Active chronic leg ulcer (n = 53) | N/A | 32.7 (7.2) | N/A |
| Systemic hypertension (n = 30) | 38.2 (6.0) | 40.3 (5.1) | .418 |
| Chronic pain (n = 266) | 35.2 (7.4) | 32.7 (7.2) | < .001 |
| Characteristics . | HbSC . | HbSS . | P value∗ . |
|---|---|---|---|
| Acute pain (≥ 3 episodes per y) only (n = 180) | 32.8 (8.2) | 30.5 (7.9) | .071 |
| ACS only (n = 34) | 32.7 (6.0) | 31.3 (9.1) | .667 |
| Severe symptomatic chronic anemia only (n = 4) | N/A | 39.8 (8.1) | N/A |
| Symptomatic AVN (n = 110) | 34.8 (6.8) | 32.8 (7.2) | .141 |
| Cerebrovascular accident/stroke (n = 10) | 36.0 (7.6) | 27.9 (10.0) | .247 |
| Priapism (n = 37) | 31.8 (5.8) | 28.5 (6.0) | .252 |
| Nephropathy (n = 61) | 26.9 (5.9) | 31.2 (7.6) | .154 |
| Proliferative SCD Retinopathy (n = 24) | 32.7 (7.3) | 22.3 (4.0) | .012 |
| Active chronic leg ulcer (n = 53) | N/A | 32.7 (7.2) | N/A |
| Systemic hypertension (n = 30) | 38.2 (6.0) | 40.3 (5.1) | .418 |
| Chronic pain (n = 266) | 35.2 (7.4) | 32.7 (7.2) | < .001 |
The causes of the chronic pain include AVN, osteoarthritis of the knee and elbow joints, spondylosis (both lumbar and cervical), and active chronic leg ulcers.
N/A, not available.
t test.
A total of 49 of 639 (7.7%) individuals with HbSC had symptomatic AVN. Of the 49 individuals with HbSC who had symptomatic AVN, 14 (28.6%) had bilateral symptomatic AVN of the femoral head, and 30 (61.2%) had symptomatic AVN of 1 femoral head, with 20 (66.7%) of these occurring in the right femoral head. Symptomatic AVN of the humeral head occurred in 5 of 49 (10.2%) the individuals with HbSC; 32 of 49 (65.3%) patients were females with a prevalence rate of 8.3%, whereas 17 of 49 (34.7%) were males with a prevalence rate of 6.7%. The mean age of the individuals with HbSC with symptomatic AVN was 34.8 years with 39 of them (79.6%) aged 30 years and above. Approximately one-fifth (11/49 [22.4%]) of the individuals with HbSC with symptomatic AVN had a history of 3 or more acute pain events in a year. Comparatively, 61 of 639 (9.5%) individuals with HbSS had symptomatic AVN. Of the 61 individuals with HbSS who had symptomatic AVN, 22 (36.0%) had bilateral symptomatic AVN of the femoral heads, 33 (54.1%) had symptomatic AVN of 1 femoral head, with 19 (57.6%) of these occurring in the right femoral head. Symptomatic AVN of the humeral head occurred in 6 of 61 (9.8%) individuals with HbSS; 37 of 61 (60.7%) patient were females with a prevalence rate of 9.6%, and 24 of 61 (39.3%) were males with a prevalence rate of 9.5%. The mean age of individuals with HbSS with symptomatic AVN was 32.8 years with 42 of 61 (68.9%) aged 30 years and above. Approximately one-third (21/61 [34.4%]) of the individuals with HbSS and symptomatic AVN had a history of ≥3 acute pain events in a year. There was no difference in the characteristics of symptomatic AVN between the individuals with HbSC and HbSS, Table 3.
Characteristics of symptomatic AVN in adults with HbSC disease compared with adults with HbSS disease
| Characteristics . | HbSC (N = 49) . | HbSS (N = 61) . | P value . |
|---|---|---|---|
| Sex | .616 | ||
| Male, n (%) | 17 (34.7) | 24 (39.3) | |
| Female, n (%) | 32 (65.3) | 37 (60.7) | |
| Age, mean (standard deviation) | 34.8 (6.8) | 32.8 (7.2) | .141 |
| Number of patients with symptomatic AVN aged 30 y and above, n (%) | 39 (79.6) | 42 (68.9) | .204 |
| Symptomatic AVN of the femoral head(s), n (%) | 44 (89.8) | 55 (90.1) | .949 |
| Bilateral symptomatic AVN of the femoral heads, n (%) | 14 (28.6) | 22 (36.0) | .405 |
| Unilateral symptomatic AVN of the femoral head, n (%) | 30 (61.2) | 33 (54.1) | .453 |
| Symptomatic AVN of right femoral head, n (%)∗ | 20 (66.7) | 19 (57.6) | .292 |
| Symptomatic AVN of the humeral head(s), n (%) | 5 (10.2) | 6 (9.8) | .949 |
| Symptomatic AVN with a history of 3 or more acute pain events per y, n (%) | 11 (22.4) | 21 (34.4) | .169 |
| Characteristics . | HbSC (N = 49) . | HbSS (N = 61) . | P value . |
|---|---|---|---|
| Sex | .616 | ||
| Male, n (%) | 17 (34.7) | 24 (39.3) | |
| Female, n (%) | 32 (65.3) | 37 (60.7) | |
| Age, mean (standard deviation) | 34.8 (6.8) | 32.8 (7.2) | .141 |
| Number of patients with symptomatic AVN aged 30 y and above, n (%) | 39 (79.6) | 42 (68.9) | .204 |
| Symptomatic AVN of the femoral head(s), n (%) | 44 (89.8) | 55 (90.1) | .949 |
| Bilateral symptomatic AVN of the femoral heads, n (%) | 14 (28.6) | 22 (36.0) | .405 |
| Unilateral symptomatic AVN of the femoral head, n (%) | 30 (61.2) | 33 (54.1) | .453 |
| Symptomatic AVN of right femoral head, n (%)∗ | 20 (66.7) | 19 (57.6) | .292 |
| Symptomatic AVN of the humeral head(s), n (%) | 5 (10.2) | 6 (9.8) | .949 |
| Symptomatic AVN with a history of 3 or more acute pain events per y, n (%) | 11 (22.4) | 21 (34.4) | .169 |
The denominator for individuals with HbSC who had symptomatic AVN of the right femoral head was n = 30, whereas that for individuals with HbSS was n = 33.
A small number of individuals with HbSC, 3 of 639 (0.5%), had a history of overt stroke. All 3 individuals with HbSC with a history of stroke were female with a mean age of 36.0 years and a mean Hb of 11.1 g/dL. These strokes occurred before the study period. All 3 individuals had associated medical comorbidities: 2 had systemic hypertension and 1 had valvular heart disease with valve replacement before stroke. Two individuals had infarcted lesions in the basal ganglia. Comparatively, 7 of 639 (1.1%) individuals with HbSS had a history of overt stroke. Of these, 4 of 7 (57.1%) were females and 3 of 7 (42.9%) were males. The 7 individuals with HbSS with a history of stroke had a mean age of 27.9 years (Table 2).
None of the individuals with HbSC had an active chronic leg ulcer, whereas 53 of 639 (8.3%) individuals with HbSS had an active chronic leg ulcer at the time of the study (Table 1).
Other SCD-related complications
Cholelithiasis
A very small percentage, 0.9% (6/639) of individuals with HbSC had a history of cholelithiasis compared with 9.2% (59/639) of individuals with HbSS.
Proliferative SCD retinopathy
Proliferative SCD retinopathy was more common in individuals with HbSC (3.1%; 20/639) than in individuals with HbSS (0.6%; 4/639; P < .001). Among those with proliferative SCD retinopathy, individuals with HbSC were older than those with HbSS (mean age, 32.7 vs 22.3 years, respectively; P = .012; Tables 1 and 2).
Chronic pain
The causes of chronic pain included AVN, osteoarthritis of the knee and elbow joints, spondylosis (both lumbar and cervical), and active chronic leg ulcers. Overall, 110/ of 639 (17.2%) individuals with HbSC had a history of chronic pain. Comparatively, 156 of 639 (24.4%) individuals with HbSS had a history of chronic pain. The mean age of individuals with SCD who had chronic pain was 33.7 years. The individuals with HbSC who had chronic pain had a mean age of 35.2 years, whereas those with HbSS who had chronic pain had a mean age of 32.7 years (range, 18-45 years). Only 1 of 639 (0.2%) and 3 of 639 (0.5%) individuals with HbSC and HbSS who had chronic pain, respectively, were on hydroxyurea at the time of the study.
Medical comorbidities
Systemic hypertension
At the time of the study, 23 of 639 (3.6%) individuals with HbSC had been diagnosed with systemic hypertension compared with 7 of 639 (1.1%) individuals with HbSS (Table 1).
The proportion of individuals who were on disease-modifying therapy at the time of study
Only 6 of 639 (0.9%; HbSC) and 15 of 639 (2.3%: HbSS) individuals were on hydroxyurea during the study period. None of the individuals with HbSC or HbSS were on regular blood transfusion therapy or any other disease-modifying therapy at the time of the study.
Discussion
A subset of individuals with HbSC disease have manifestations of severe SCD; however, there are limited data to demonstrate the magnitude of morbidity when compared with individuals with HbSS. We demonstrated for the first time, to our knowledge, that ∼10% of adults with HbSC compared with 24.1% of adults with HbSS met the ASH criteria for treatment with hydroxyurea therapy. In both cases, only ∼1% and 2% of adults with HbSC and HbSS, respectively, were on hydroxyurea for 1 year.
Seventy-one (11.1%) of the individuals with HbSC had significant SCD comorbidities, including renal papillary necrosis, stroke, AVN, and priapism. SCD nephropathy is associated with a spectrum of renal diseases ranging from hyposthenuria to end-stage renal disease. Studies have reported that renal disease in individuals with HbSC occurs at a later age and rarely leads to end-stage renal disease.9 The Cooperative Study of SCD had a large sample size (n > 4000 children and adults) with no individual with HbSC having an overt stroke.21 In contrast, Sathi et al reported 1 of 34 (2.9%) individuals having overt strokes and 5 of 34 (14.7%) having silent strokes in their population who were affected with HbSC (n = 34).8 Our study also reported 3 of 639 (0.5%) individuals with HbSC having overt strokes. Lastly, Pecker et al reported that proliferative SCD retinopathy is the most common complication of HbSC disease, with a peak incidence in the third and fourth decades.9 Our study was not different, with proliferative SCD retinopathy being more common in individuals with HbSC (mean age, 32.7 years) than in those with HbSS (mean age, 22.3 years).
The evidence to support hydroxyurea for severe HbSC disease has been limited because of the exclusion of individuals with HbSC in the seminal double-blind, randomized clinical trial.22 Most studies reporting hydroxyurea use in HbSC disease were small observational studies or limited trials with inconclusive evidence.23 Our study demonstrated that ∼10.0% of adults with HbSC disease had severe complications requiring hydroxyurea treatment. This proportion is high when compared with other studies in which a proportion of 5% was reported.23 The major concern regarding hydroxyurea use in the HbSC population is an increase in hemoglobin, which may increase the likelihood of vaso-occlusive events or hyperviscosity syndrome.12,24 These practical and theoretical concerns regarding hydroxyurea for individuals with HbSC can only be assessed in randomized controlled trials with a data safety monitoring board assessing patient safety. Given the decreased frequency of postulated adverse events associated with hydroxyurea, a large trial with significant follow-up is needed to demonstrate its safety.
Optimally, all individuals with HbSS should be offered hydroxyurea based on the NHLBI guidelines; however, in both the United States and Ghana, multiple barriers exist as to why hydroxyurea is not offered or offered and not prescribed. In academic centers in the United States, hydroxyurea is used in ∼60% of adults with HbSS25; however, it has been sparingly used in adults with HbSS and HbSC in low-income settings. In our study, <4% of individuals with severe SCD living in Ghana were treated with hydroxyurea. Three major factors have been documented to contribute to the low hydroxyurea use in Nigeria (a low-middle-income setting) and the United States of America (a high-income setting): health systems-related barriers, provider-related barriers, and patient-related barriers.26-28 In Ghana, hydroxyurea was only approved for use in individuals with SCD by the Ghana Food and Drug Authority in 2018.29 Hydroxyurea is also available in only a few pharmacies in large metropolitan areas. An important barrier to hydroxyurea use in individuals with SCD in Ghana is the financial constraint. The annual cost of hydroxyurea therapy imported from a European pharmaceutical company (including scheduled laboratory investigations) for a 60 kg adult on 1 000 mg daily in Ghana is ∼$360 USD which is close to half the calculated minimum annual wage of ∼$750 USD.30 On June 19, 2021, the government of Ghana announced the provision of hydroxyurea for people with SCD through the National Health Insurance Scheme,31 but while this scheme is being gradually implemented a significant proportion of individuals with SCD or their caregivers still bear the full cost of treatment. Approximately 95% of individuals with SCD at the clinic for adults with SCD at Ghana Institute of Clinical Genetics and Korle-Bu are on an insurance scheme. Because of financial constraints, they cannot afford out-of-pocket treatment costs. Currently, the cost of 2 500 mg capsules of hydroxyurea imported from a European pharmaceutical company in Ghana is ∼6.0 Ghana cedis (equivalent to $0.75 USD). Alternatively, 2 500 mg capsules of hydroxyurea produced by a private pharmaceutical company in Ibadan, Nigeria, cost ∼2.5 Ghana cedis (equivalent to $0.32 USD or 132 Nigerian Naira). The hydroxyurea capsules produced in Nigeria have been used in prior clinical trials in Northern Nigeria,32,33 and by Lagunju over a course of 10 years in children with HbSS and have been proven to be safe and effective.34 In Ghana, given the current birth rate of 28.23 per 1 000 people,35 the estimated number of newborns delivered with SCD each year (2% or ∼18 000 newborns each year),35,36 the urbanization rate of ∼58%,35 and the poverty rate of 25.5%,37 the most viable long-term solution for improving Ghanaian access to hydroxyurea will require local or regional hydroxyurea production at subsidized costs.
As expected, limitations are inherent in our retrospective study. With the relatively high mortality rate among individuals with SCD in a low-middle-income country such as Ghana, it is likely that some individuals with severe SCD phenotypes will be lost before their teenage years, thus introducing a survival bias in our study population. However, as shown in this study, individuals with both HbSS and HbSC can have severe SCD phenotypes, and any survival bias will apply to both study groups, although it may be more pronounced in HbSS. Our institute also previously used a hybrid data capture system (demographics were captured electronically, whereas medical information/notes were captured in the medical charts as hardcopies). This made it difficult to trace old medical records to capture exactly when complications occurred before 2019. There was a lack of consistency into the data entry in the medical charts; hence, not all SCD-related clinical outcomes were captured. Because of financial constraints and lack of access to the National Health Insurance Scheme, some individuals with SCD may have managed their acute pain events at home; hence, acute pain events per year are likely to be under-reported. Follow-ups by the public health care staff at the clinic, as well as the encouragement of the population with SCD to subscribe to the National Health Insurance Scheme should be implemented. Owing to nonadherence to the appointment system for follow-up, some individuals with SCD may have been lost to follow-up, which will affect the prevalence of reported chronic complications. Taken together, these limitations most likely underestimate the prevalence of severe SCD in a large clinic for SCD in Ghana.
Conclusions
Based on ASH evidence-based guidelines, in a large SCD clinic at an academic medical center in Ghana, 10.0% (HbSC) and 24.1% (HbSS) of adults met the criteria for shared decision-making to consider starting hydroxyurea therapy. Our results indicate that less than 1% and 3% of adults with severe HbSC and HbSS, respectively, are currently prescribed hydroxyurea at a tertiary care medical center in Ghana. For adults with HbSC, a clinical trial is necessary to assess the relative benefits and risks of hydroxyurea therapy in a low-resource setting. For adults with HbSS and severe disease who are living in Ghana, an urgent unmet humanitarian need requires sufficient resources to prevent premature death, comorbidities, and unremitting vaso-occlusive pain episodes.
Acknowledgments
The authors thank the leadership of the Ghana Institute of Clinical Genetics and Korle-Bu for their administrative support. This study was supported by funding from the American Society of Hematology Global Research Awards.
Authorship
Contribution: E.O., M.R.D., and E.V.A. designed the study; W.K.G., J.B.A.-N., and E.V.A. collected the data; E.O., W.K.G., J.B.A.-N., and E.V.A. adjudicated the case files; W.K.G., E.V.A., J.B.A.-N., E.O., M.R.D., and M.R. performed the analyses; E.O., M.R.D., E.V.A., W.K.G., J.B.A.-N., M.R. and S.A.O. interpreted the results; and all authors participated in writing the manuscript, all authors reviewed and approved the manuscript before submission.
Conflict-of-interest disclosure: E.V.A. received the ASH Global Research Award (2019-2022). M.R.D. and his institution are the sponsors of 2 externally funded research investigator-initiated projects (Global Blood Therapeutics will provide funding for these clinical studies but will not be a cosponsor of either study); he does not receive any compensation for conducting these 2 investigator-initiated observational studies but is a member of the Global Blood Therapeutics advisory board for a proposed randomized controlled trial, for which he receives compensation; he is on the steering committee for a Novartis sponsored phase 2 trial to prevent priapism in men; he was a medical adviser for the development of the CTX001 Early Economic Model; he provided medical input on the economic model as part of an expert reference group for Vertex/CRISPR CTX001 Early Economic Model in 2020; and he provided consultation to the Forma Pharmaceutical company about SCD from 2021 to 2022. The remaining authors declare no completing financial interests.
Correspondence: Edeghonghon Olayemi, Department of Haematology, University of Ghana Medical School, Accra, Ghana; e-mail: edeolayemi@yahoo.com.
References
Author notes
∗W.K.J. and E.V.A. are joint first authors.
All data reported in this article are available on request from the corresponding author, Edeghonghon Olayemi (edeolayemi@yahoo.com).