Key Points
Presence of posttransplant MRD has an adverse prognostic impact on the outcome irrespective of pretransplant MRD in patients with AML/MDS.
Acquisition of FDTC is associated with improved survival and low rates of posttransplant MRD positivity.
Abstract
Allogeneic stem-cell transplant allows for the delivery of curative graft-versus-leukemia (GVL) in patients with acute myeloid leukemia/myelodysplasia (AML/MDS). Surveillance of T-cell chimerism, measurable residual disease (MRD) and blast HLA-DR expression may inform whether GVL effectiveness is reduced. We report here the prognostic impact of these biomarkers in patients allografted for AML/MDS. One hundred eighty-seven patients from FIGARO, a randomized trial of reduced-intensity conditioning regimens in AML/MDS, were alive and relapse-free at the first MRD time-point and provided monitoring samples for flow cytometric MRD and T-cell chimerism, requested to month+12. Twenty-nine (15.5%) patients had at least 1 MRD-positive result posttransplant. MRD-positivity was associated with reduced overall survival (OS) (hazard ratio [HR], 2.18; P = .0028) as a time-varying Cox variable and remained significant irrespective of pretransplant MRD status in multivariate analyses (P < .001). Ninety-four patients had sequential MRD with T-cell chimerism results at months+3/+6. Patients with full donor T-cell chimerism (FDTC) had an improved OS as compared with patients with mixed donor T-cell chimerism (MDTC) (adjusted HR=0.4; P = .0019). In patients with MDTC (month+3 or +6), MRD-positivity was associated with a decreased 2-year OS (34.3%) vs MRD-negativity (71.4%) (P = .001). In contrast, in the group with FDTC, MRD was infrequent and did not affect the outcome. Among patients with posttransplant MRD-positivity, decreased HLA-DR expression on blasts significantly reduced OS, supporting this as a mechanism for GVL escape. In conclusion, posttransplant MRD is an important predictor of the outcome in patients allografted for AML/MDS and is most informative when combined with T-cell chimerism results, underlining the importance of a GVL effect in AML/MDS.
Introduction
Allogeneic stem-cell transplant (allo-SCT) is an important curative strategy in acute myeloid leukemia (AML) because of a reduction in relapse risk irrespective of cytogenetic risk.1,2 This is partly due to a potent graft-versus-leukemia (GVL) effect, which can be manipulated, for example, through alterations in immunosuppression.3,4 A spectrum of mechanisms may lead to failure of the GVL response and, therefore, contribute to disease relapse; these include HLA haplotype loss,5 downregulation of HLA-DR, and deregulation of inhibitory molecules on the surfaces of leukemic blasts.6,7 These mechanisms have, to date, been studied in the context of patients with relapsed disease, and their value in predicting relapse has not been prospectively evaluated. Donor-host chimerism serves as a biomarker of GVL, and mixed donor T-cell chimerism reflects the presence of bidirectional tolerance.8 There are conflicting reports as to the prognostic significance of mixed donor chimerism in earlier studies involving both myeloid and lymphoid subtypes.9 Therefore, although in AML and MDS, mixed donor T-cell chimerism at 3 months may be associated with an increased risk of disease relapse,10 it is not sufficient alone to identify patients with a high likelihood of impending clinical relapse. Additional monitoring strategies are required to appropriately target interventions that may reduce the risk of disease relapse which remains the most common reason for transplant failure. Measurable residual disease (MRD) provides a means of dynamic risk assessment in AML at different treatment stages.11 Sequential MRD tests have now increasingly been used to guide interventions aimed at reducing overt relapse. However, evidence supporting this strategy is predominantly from AML subtypes that have leukemic-specific polymerase chain reaction (PCR) targets, such as core-binding factor or NPM1-mutated AML and from small series or cases in clinical practice, often incorporating MRD-informed interventions that may influence the outcome.11,12 Flow cytometry allows for more patients to be monitored, but its implementation after transplant has been restricted by concerns that include sensitivity (10−4 compared with 10−5 to 10−6 for PCR) and insufficient published data on serial flow cytometric assessments of AML in MRD in this setting. There is also uncertainty with regard to the clinical interpretation of posttransplant MRD results in the context of donor-host chimerism status. Therefore, there remains a need to evaluate the predictive value of posttransplant-MRD monitoring for patients with AML and high risk MDS as well as the relationship of MRD with serial donor chimerism status.
With this study, to the best of our knowledge, we present the first prospective correlation of the prognostic impact of posttransplant T-cell chimerism and MRD in patients who received allografts for AML/MDS. This was performed as part of the FIGARO trial, a randomized controlled trial of reduced-intensity conditioning (RIC) regimens. We recently reported the primary outcome of this study alongside the prognostic value of pretransplant MRD.10 Here, we report the dynamics of posttransplant MRD assessed via flow cytometry up to 12 months after transplant and the interaction of MRD with potential modulators of GVL–T-cell chimerism, and HLA-DR expression on leukemic blasts.
Methods
Study design
Serial samples for MRD analysis were prospectively collected pre and posttransplant as part of the FIGARO study of RIC regimens. This was a phase 2, randomized, controlled trial (2013-2017) in which patients were assigned to either fludarabine [Flu] + cytarabine + amsacrine + Busulphan [Bu] + anti-thymocyte globulin (ATG) or a control arm of the investigator’s choice of control arm regimen (Flu/Bu/ATG; Flu/Bu/alemtuzumab; or Flu/Melphalan/alemtuzumab).10 Patients were eligible for trial entry if they had AML or high-risk MDS (defined as patients with an international prognostic scoring system score of intermediate-1, with > 5% blasts, or intermediate-2 or high-risk, who had < 5% blasts at the time of random assignment). All patients with AML were either in complete remissions or had primary refractory AML. The cytogenetic risk was defined as previously described.10,13 Patients received either peripheral blood– or bone marrow (BM) stem cells from an HLA identical (HLA-A/-B/-C/-DRbeta1) –matched sibling or ≥ 7/8 HLA-A/-B/-C/-DRbeta1 adult–unrelated donor. The FIGARO trial protocol including MRD monitoring (EudraCT 2012-005538-12) was approved by the UK research ethics service, National Research Ethics Service. The study was conducted in accordance with the Declaration of Helsinki.
Among the 187 patients providing posttransplant-MRD data, all patients received pretransplant serotherapy, 147 received pretransplant ATG (5 mg/kg over 2-3 days), and 40 received alemutuzumab. All patients received cyclosporin as graft-versus-host disease (GVHD) prophylaxis commencing from day −1 to +60 with the aim of achieving cyclosporine levels from 150 to 200 ug/L.
During the first 12 months after the transplant, MRD monitoring (day +42, months +3, +6, +9, or +12) and peripheral blood samples for T-cell chimerism (every 3 months during the first year) were collected from all patients who were alive and relapse-free, as specified in the trial protocol. T-cell chimerism was analyzed in local laboratories (supplemental Methods). Flow cytometric MRD results were an exploratory objective of the trial and, therefore, not made available to treating clinicians.
Acquisition of full donor T-cell chimerism (≥ 95%) was similar in control and experimental arms and was not affected by pretransplant MRD status.10 As directed in the protocol, donor lymphocyte infusions (DLIs) were administered for mixed donor chimerism but not influenced by MRD (as MRD results not reported). The schedule of DLIs administered for mixed donor chimerism was delivered as per the protocol (supplemental Methods).10 There was no difference in the frequency of DLI administered between patients who received ATG compared with patients who received alemtuzumab (51% vs 49%, respectively).
MRD quantification
MRD was assessed using flow cytometry, as previously described, in a central reference laboratory.10,14 Sample logistics, processing, and analysis strategy are provided in the supplemental Methods. Seven hundred and seventy-eight adequate BM samples were received after the transplant from 187 patients. The assay limit of detection was ∼0.05% of leukocytes. To exclude variability arising from the subjective interpretation, flow cytometry standard files from MRD testing were analyzed using a previously validated unsupervised approach.10,15 Blast cells (CD117+/CD34+) from test samples were clustered together with a 40 or 50 control BM reference set using the RPhenograph clustering algorithm.16 Threshold values defining the limits of normal antigen expression for each cluster were then calculated from the 10th/90th percentiles of control blast fluorescence intensity values and applied to identify different from normal blast subpopulations with antigen over/underexpressions relative to those in control blasts. Assay limits were calculated from the reference set of control BMs for each antibody combination of the MRD panel. MRD test positivity required the detection of aberrant blasts in at least 2 of the 3 antibody combinations or positivity by high specificity aberrancy markers (CD7 and CD56). In order to evaluate detectable MRD blasts for potentially decreased HLA Class II expression, the aberrant blast clusters in MRD-positive (MRD+) samples were screened for HLA-DR–negative blasts (further details in supplemental Methods).
Statistical analysis and outcomes
Categorical data were tabulated with percentages and compared using χ2 tests. Overall survival (OS), event-free survival, cumulative incidence of relapse (CIR), and transplant-related mortality were assessed throughout the analysis using Kaplan-Meier curves or cumulative incidence curves, as appropriate. One- and 2-year estimates and medians are presented alongside 95% confidence intervals, as appropriate. OS and event-free survival are calculated in months from the relevant time point to death or the first of relapse or death; data of patients who do not experience an event were censored at the date of their last follow-up visit. CIR was calculated in months from the relevant time point to the date of relapse. Death without relapse was treated as a competing event at the date of the patient’s death, and data of patients who remain alive and relapse-free were censored at the date of their last follow-up visit. Transplant-related mortality is calculated in months from the relevant time point to death from a transplant-related cause. Death from any other cause was treated as a competing event at the date of the patient’s death, and data of patients who remained alive were censored at the date of their last follow-up visit. Comparisons between treatment arms were made using log-rank tests or Gray test for outcomes that involved a competing risk. Multivariate analysis was conducted using a Cox proportional hazard model to assess the treatment effect after adjusting for appropriate factors. Time-varying Cox models were applied for both MRD and GVHD assessments in which sample outcomes varied over time; these models were appropriately adjusted for relevant factors. Hazard ratios (HRs) with 95% confidence interval (CI) and P values are presented for all Cox models.
The median follow-up of the study was 49.7 (41, 58.6) months.
Results
Posttransplant MRD and patient characteristics
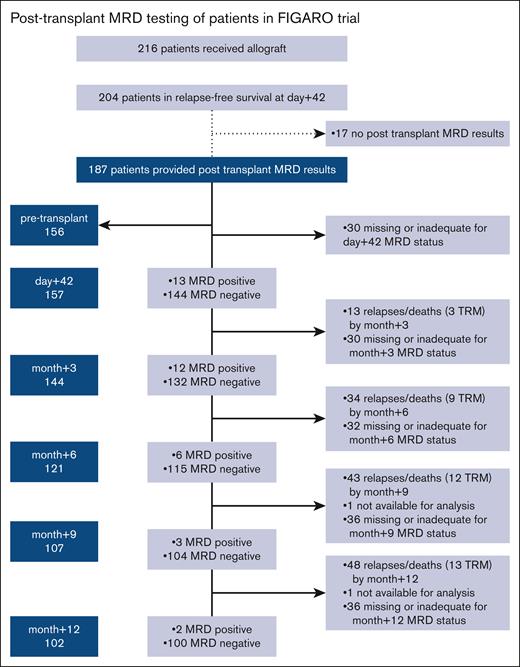
Two hundred and four of the 216 patients who underwent the transplant in the FIGARO trial between 2014 and 17 were alive and relapse-free on day +42. Of these, 187 patients provided BM samples for posttransplant-MRD monitoring, requested by trial protocol from day +42 up to month +12 after the allo-SCT (Figure 1). Twenty-nine out of 187 (16%) patients had 1 or more MRD+ samples during the 12 months after allo-SCT. The highest frequency of MRD positivity in available samples in the first year after the transplant occurred on day +42 or month +3 and decreased over the subsequent 12 months (Figure 1).
Posttransplant MRD testing among patients in FIGARO trial. MRD results are depicted for remission marrows.
Posttransplant MRD testing among patients in FIGARO trial. MRD results are depicted for remission marrows.
Baseline transplant and disease characteristics were similar between patients who had at least 1 MRD+ result and patients who remained with MRD-negative results over the 12 months of monitoring (Table 1). In multivariate analysis, the only factor associated with the presence of detectable MRD after the transplant was the presence of MRD before the transplant (P = .03).
Baseline characteristics of patients who underwent the transplant in the FIGARO trial grouped based on the posttransplant MRD status
| . | Posttransplant MRD status . | Overall N (%) . | P . | ||
|---|---|---|---|---|---|
| Positive at any time point N (%) . | Negative only N (%) . | Missing throughout∗ N (%) . | |||
| Treatment arm | |||||
| FLAMSA-BU | 10 (34) | 77 (49) | 21 (72) | 108 (50) | .21 |
| Flu/Bu/ATG | 9 (31) | 51 (32) | 3 (10) | 63 (29) | — |
| Flu/melphalan/alemtuzumab | 6 (21) | 21 (13) | 3 (10) | 30 (14) | — |
| Flu/Bu/alemtuzumab | 4 (14) | 9 (6) | 2 (7) | 15 (7) | — |
| Age | |||||
| <=60 y | 19 (66) | 93 (59) | 14 (48) | 126 (58) | .61 |
| >60 y | 10 (34) | 65 (41) | 15 (52) | 90 (42) | — |
| Sex | |||||
| Female | 11 (38) | 68 (43) | 12 (41) | 91 (42) | .97 |
| Male | 18 (62) | 90 (57) | 17 (59) | 125 (58) | — |
| Underlying disease | |||||
| AML | 20 (69) | 101 (64) | 23 (79) | 144 (67) | .44 |
| MDS | 9 (31) | 57 (36) | 6 (21) | 72 (33) | — |
| Patients with cytogenetic risk–AML | |||||
| Adverse risk | 9 (45) | 23 (23) | 12 (52) | 44 (31) | .11 |
| Intermediate risk | 9 (45) | 72 (71) | 11 (48) | 92 (64) | — |
| Favorable risk | 2 (10) | 5 (5) | 7 (5) | — | |
| Unknown | 1 (1) | 1 (1) | — | ||
| Disease status (AML only) | |||||
| CR1/CR2 | 18 (90) | 98 (97) | 22 (96) | 138 (96) | .38 |
| Primary refractory | 2 (10) | 3 (3) | 1 (4) | 6 (4) | — |
| FLT3 | |||||
| Absent | 11 (38) | 62 (39) | 14 (48) | 87 (40) | .76 |
| Present | 7 (24) | 28 (18) | 2 (7) | 37 (17) | — |
| Unknown | 11 (38) | 68 (43) | 13 (45) | 92 (43) | — |
| NPM1 | |||||
| Absent | 15 (52) | 61 (39) | 12 (41) | 88 (41) | .90 |
| Present | 3 (10) | 28 (18) | 4 (14) | 35 (16) | — |
| Unknown | 11 (38) | 69 (44) | 13 (45) | 93 (43) | — |
| IPSS (MDS only) | |||||
| Standard risk (<=2) | 8 (100) | 48 (96) | 4 (100) | 60 (97) | 1 |
| High risk (>2) | 2 (4) | 2 (3) | — | ||
| Donor type | |||||
| Sibling | 8 (28) | 31 (20) | 6 (21) | 45 (21) | .82 |
| Unrelated | 21 (72) | 127 (80) | 23 (79) | 171 (79) | — |
| Stem-cell source | |||||
| Peripheral blood | 29 (100) | 151 (96) | 28 (97) | 208 (96) | .86 |
| BM | 7 (4) | 1 (3) | 8 (4) | — | |
| Pretransplant MRD | |||||
| Positive | 10 (34) | 31 (20) | 2 (7) | 43 (20) | .03 |
| Negative | 12 (41) | 89 (56) | 12 (41) | 113 (52) | — |
| Missing | 7 (24) | 38 (24) | 15 (52) | 60 (28) | — |
| DLI | |||||
| Number receiving DLI (before relapse) | 6 (21) | 22 (14) | 2 (7) | 30 (14) | .32 |
| GVHD | |||||
| Acute GVHD grade 2-4 | 7 (24) | 65 (41) | 10 (34) | 82 (38) | .36 |
| Chronic GVHD | 6 (21) | 58 (37) | 3 (10) | 67 (31) | .02 |
| . | Posttransplant MRD status . | Overall N (%) . | P . | ||
|---|---|---|---|---|---|
| Positive at any time point N (%) . | Negative only N (%) . | Missing throughout∗ N (%) . | |||
| Treatment arm | |||||
| FLAMSA-BU | 10 (34) | 77 (49) | 21 (72) | 108 (50) | .21 |
| Flu/Bu/ATG | 9 (31) | 51 (32) | 3 (10) | 63 (29) | — |
| Flu/melphalan/alemtuzumab | 6 (21) | 21 (13) | 3 (10) | 30 (14) | — |
| Flu/Bu/alemtuzumab | 4 (14) | 9 (6) | 2 (7) | 15 (7) | — |
| Age | |||||
| <=60 y | 19 (66) | 93 (59) | 14 (48) | 126 (58) | .61 |
| >60 y | 10 (34) | 65 (41) | 15 (52) | 90 (42) | — |
| Sex | |||||
| Female | 11 (38) | 68 (43) | 12 (41) | 91 (42) | .97 |
| Male | 18 (62) | 90 (57) | 17 (59) | 125 (58) | — |
| Underlying disease | |||||
| AML | 20 (69) | 101 (64) | 23 (79) | 144 (67) | .44 |
| MDS | 9 (31) | 57 (36) | 6 (21) | 72 (33) | — |
| Patients with cytogenetic risk–AML | |||||
| Adverse risk | 9 (45) | 23 (23) | 12 (52) | 44 (31) | .11 |
| Intermediate risk | 9 (45) | 72 (71) | 11 (48) | 92 (64) | — |
| Favorable risk | 2 (10) | 5 (5) | 7 (5) | — | |
| Unknown | 1 (1) | 1 (1) | — | ||
| Disease status (AML only) | |||||
| CR1/CR2 | 18 (90) | 98 (97) | 22 (96) | 138 (96) | .38 |
| Primary refractory | 2 (10) | 3 (3) | 1 (4) | 6 (4) | — |
| FLT3 | |||||
| Absent | 11 (38) | 62 (39) | 14 (48) | 87 (40) | .76 |
| Present | 7 (24) | 28 (18) | 2 (7) | 37 (17) | — |
| Unknown | 11 (38) | 68 (43) | 13 (45) | 92 (43) | — |
| NPM1 | |||||
| Absent | 15 (52) | 61 (39) | 12 (41) | 88 (41) | .90 |
| Present | 3 (10) | 28 (18) | 4 (14) | 35 (16) | — |
| Unknown | 11 (38) | 69 (44) | 13 (45) | 93 (43) | — |
| IPSS (MDS only) | |||||
| Standard risk (<=2) | 8 (100) | 48 (96) | 4 (100) | 60 (97) | 1 |
| High risk (>2) | 2 (4) | 2 (3) | — | ||
| Donor type | |||||
| Sibling | 8 (28) | 31 (20) | 6 (21) | 45 (21) | .82 |
| Unrelated | 21 (72) | 127 (80) | 23 (79) | 171 (79) | — |
| Stem-cell source | |||||
| Peripheral blood | 29 (100) | 151 (96) | 28 (97) | 208 (96) | .86 |
| BM | 7 (4) | 1 (3) | 8 (4) | — | |
| Pretransplant MRD | |||||
| Positive | 10 (34) | 31 (20) | 2 (7) | 43 (20) | .03 |
| Negative | 12 (41) | 89 (56) | 12 (41) | 113 (52) | — |
| Missing | 7 (24) | 38 (24) | 15 (52) | 60 (28) | — |
| DLI | |||||
| Number receiving DLI (before relapse) | 6 (21) | 22 (14) | 2 (7) | 30 (14) | .32 |
| GVHD | |||||
| Acute GVHD grade 2-4 | 7 (24) | 65 (41) | 10 (34) | 82 (38) | .36 |
| Chronic GVHD | 6 (21) | 58 (37) | 3 (10) | 67 (31) | .02 |
CR1/2, complete remissions 1 or 2; FLAMSA-BU, Flu + cytarabine + amsacrine + Bu; IPSS, international prognostic scoring system.
Missing throughout for MRD status includes the 12 of 216 patients who underwent transplant and died or relapsed up to day +42.
Presence of MFC MRD after the transplant results in inferior OS and RFS
The presence of MRD positivity at any time point after the transplant was associated with an inferior OS and relapse-free survival (RFS) (Table 2). When posttransplant MRD was treated as a time-dependent variable in a Cox model analysis, there was a significant reduction in both RFS (HR, 5.32 [95% CI, 3.27-8.68]; P < .0001) and OS (HR, 2.18 [95% CI, 1.31-3.62]; P = .0028) for patients who had MRD+ results.
OS, RFS, CIR, and TRM at 2 years from the time of MRD assessment based on the MRD status at each time point
| Outcome . | Time point . | Posttransplant MRD . | ||
|---|---|---|---|---|
| Positive result 2-y estimate (95% CI) . | Negative result 2-y estimate (95% CI) . | P value . | ||
| CIR | D 42 | 92.3 (35.8-99.4) | 22.9 (16.4-30.1) | < .001 |
| Mo 3 | 50.0 (19.2-74.8) | 20.5 (14.0-27.7) | .011 | |
| Mo 6 | 83.3 (8.6-98.7) | 17.4 (11.1-24.9) | < .001 | |
| Mo 9 | 100 (., .) | 14.6 (8.5-22.1) | < .001 | |
| Mo 12 | 50.0 (0.0-96.0) | 14.0 (8.0-21.6) | .19 | |
| OS | D 42 | 30.8 (9.5-55.4) | 66.9 (58.5-74.0) | < .001 |
| Mo 3 | 58.3 (27.0-80.1) | 74.0 (65.5-80.6) | .30 | |
| Mo 6 | 50.0 (11.1-80.4) | 80.6 (72.0-86.8) | < .0001 | |
| Mo 9 | 33.3 (0.9-77.4) | 87.8 (79.5-92.9) | < .0001 | |
| Mo 12 | 50.0 (0.6-91.0) | 94.7 (87.8-97.8) | .18 | |
| RFS | D 42 | 7.7 (0.5-29.2) | 61.0 (52.6-68.5) | < .001 |
| Mo 3 | 50.0 (20.8-73.6) | 67.3 (58.6-74.6) | .13 | |
| Mo 6 | 16.7 (0.8-51.7) | 75.4 (66.3-82.3) | < .001 | |
| Mo 9 | 33.3 (0.9-77.4) | 79.3 (69.7-86.2) | < .001 | |
| Mo 12 | 50.0 (0.6-91.0) | 83.7 (74.4-89.9) | .31 | |
| TRM | D 42 | — | 16.9 (11.2, 23.5) | .31 |
| Mo 3 | — | 11.4 (6.7-17.6) | .38 | |
| Mo 6 | — | 8.0 (3.9-13.9) | .58 | |
| Mo 9 | — | 4.9 (1.8-10.3) | ||
| Mo 12 | — | 2.1 (0.4-6.8) | ||
| Outcome . | Time point . | Posttransplant MRD . | ||
|---|---|---|---|---|
| Positive result 2-y estimate (95% CI) . | Negative result 2-y estimate (95% CI) . | P value . | ||
| CIR | D 42 | 92.3 (35.8-99.4) | 22.9 (16.4-30.1) | < .001 |
| Mo 3 | 50.0 (19.2-74.8) | 20.5 (14.0-27.7) | .011 | |
| Mo 6 | 83.3 (8.6-98.7) | 17.4 (11.1-24.9) | < .001 | |
| Mo 9 | 100 (., .) | 14.6 (8.5-22.1) | < .001 | |
| Mo 12 | 50.0 (0.0-96.0) | 14.0 (8.0-21.6) | .19 | |
| OS | D 42 | 30.8 (9.5-55.4) | 66.9 (58.5-74.0) | < .001 |
| Mo 3 | 58.3 (27.0-80.1) | 74.0 (65.5-80.6) | .30 | |
| Mo 6 | 50.0 (11.1-80.4) | 80.6 (72.0-86.8) | < .0001 | |
| Mo 9 | 33.3 (0.9-77.4) | 87.8 (79.5-92.9) | < .0001 | |
| Mo 12 | 50.0 (0.6-91.0) | 94.7 (87.8-97.8) | .18 | |
| RFS | D 42 | 7.7 (0.5-29.2) | 61.0 (52.6-68.5) | < .001 |
| Mo 3 | 50.0 (20.8-73.6) | 67.3 (58.6-74.6) | .13 | |
| Mo 6 | 16.7 (0.8-51.7) | 75.4 (66.3-82.3) | < .001 | |
| Mo 9 | 33.3 (0.9-77.4) | 79.3 (69.7-86.2) | < .001 | |
| Mo 12 | 50.0 (0.6-91.0) | 83.7 (74.4-89.9) | .31 | |
| TRM | D 42 | — | 16.9 (11.2, 23.5) | .31 |
| Mo 3 | — | 11.4 (6.7-17.6) | .38 | |
| Mo 6 | — | 8.0 (3.9-13.9) | .58 | |
| Mo 9 | — | 4.9 (1.8-10.3) | ||
| Mo 12 | — | 2.1 (0.4-6.8) | ||
TRM, transplant-related mortality; —, null.
Overall, 45% (13/29) of the patients who tested as MRD+ after the transplant had their first MRD+ time point on day +42 after the transplant. In a landmark analysis from the day +42 after the transplant (Table 2), patients who tested as MRD+ had a 2-year OS of 30.8% (95% CI, 9.5-55.4) in comparison with patients who had tested MRD-negative and had a 2-year OS of 66.9% (95% CI, 58.5-74.0; P < .001). This was due to an increased 2-year CIR of 92.3% (95% CI, 35.8-99.4); supplemental Figure 1) in patients who tested MRD+ as compared with 22.9% (95% CI, 16.4-30.1; P < .001) in patients who had tested MRD-negative on day +42. Patients who had tested MRD+ on day +42 had very rapid relapse kinetics, with more than 50% of patients relapsing by 2 months after the MRD assessment time point (the median CIR from the day +42 sample was 1.8 months).
Relapse risk was also significantly increased with MRD positivity compared with MRD negativity in samples at subsequent assessment time points, as assessed via a landmark analysis (Table 2), with the exception of month 12 (only 2 MRD+ results at month 12).
MRD after the transplant results in inferior outcomes irrespective of the pretransplant MRD status
The previously reported adverse prognostic impact of pretransplant MRD on relapse risk was recently confirmed in patients who entered the FIGARO trial.10 Therefore, we examined whether posttransplant-MRD monitoring adds prognostic information for patients with detectable pretransplant MRD. In a multivariate analysis accounting for other important prognostic factors, such as FLT3-ITD; cytogenetic risk; and chronic GVHD, posttransplant-MRD status remained highly prognostic for the outcome, regardless of pretransplant MRD status. Detectable posttransplant MRD was associated with a significantly lower OS (Table 3) and RFS (supplemental Table 1) in patients with pretransplant MRD positivity (for OS, adjusted HR, 2.70 [95% CI, 1.76, 4.15]; P < .001) and in those with pretransplant MRD negativity (for OS, adjusted HR, 2.68 [95% CI, 1.79, 4.03]; P < .001). Adverse cytogenetics was also an independent predictor of OS in patients with pretransplant MRD negativity.
Multivariate Cox model of OS in pretransplant subgroups of patients who tested as MRD+ and MRD-negative
| Pretransplant MRD status . | Variable . | Reference level . | HR (95% CI) . | P . |
|---|---|---|---|---|
| MRD+ | FLT3 status: present | Absent | 1.32 (0.87-2.00) | .19 |
| Cytogenetic risk group: adverse | Favorable/intermediate risk | 1.38 (0.91-2.09) | .13 | |
| Posttransplant MRD status (time-dependent): positive | Negative | 2.70 (1.76-4.15) | < .001 | |
| Chronic GVHD (time-dependent): yes | No | 0.92 (0.53-1.62) | .78 | |
| MRD-negative | FLT3 status: present | Absent | 1.31 (0.88-1.97) | .18 |
| Cytogenetic risk group: adverse | Favorable/intermediate risk | 1.61 (1.08-2.39) | .019 | |
| Posttransplant MRD status (time-dependent): positive | Negative | 2.68 (1.79-4.03) | < .001 | |
| Chronic GVHD (time-dependent): Yes | No | 0.92 (0.54-1.57) | .77 |
| Pretransplant MRD status . | Variable . | Reference level . | HR (95% CI) . | P . |
|---|---|---|---|---|
| MRD+ | FLT3 status: present | Absent | 1.32 (0.87-2.00) | .19 |
| Cytogenetic risk group: adverse | Favorable/intermediate risk | 1.38 (0.91-2.09) | .13 | |
| Posttransplant MRD status (time-dependent): positive | Negative | 2.70 (1.76-4.15) | < .001 | |
| Chronic GVHD (time-dependent): yes | No | 0.92 (0.53-1.62) | .78 | |
| MRD-negative | FLT3 status: present | Absent | 1.31 (0.88-1.97) | .18 |
| Cytogenetic risk group: adverse | Favorable/intermediate risk | 1.61 (1.08-2.39) | .019 | |
| Posttransplant MRD status (time-dependent): positive | Negative | 2.68 (1.79-4.03) | < .001 | |
| Chronic GVHD (time-dependent): Yes | No | 0.92 (0.54-1.57) | .77 |
MRD status and chronic GVHD analyzed as time-dependent variables.
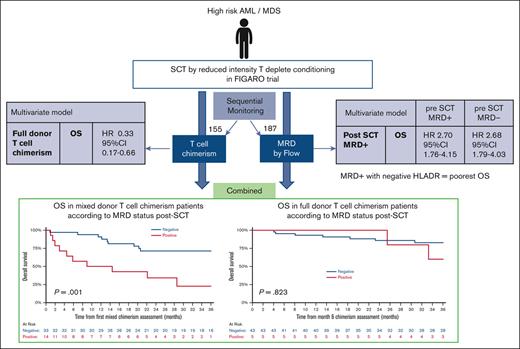
Mixed donor T-cell chimerism results in inferior RFS and OS
In contrast to myeloablative-conditioned transplants, RIC–allo-SCTs are frequently associated with mixed donor chimerism that may persist for months.17 In the FIGARO trial, there was no detectable significant difference in the acquisition of full donor T-cell chimerism at month +3, based on the conditioning regimen10 (supplemental Table 2). Of the 155 patients with sequential chimerism results (supplemental Figure 2A), 52 had mixed donor T-cell chimerism at month +3 while being relapse-free. Only 7 patients converted from full to mixed donor T-cell chimerism, with 5 conversions occurring at month +6. In a comparison of the characteristics of patients with mixed vs full (≥95%) donor T-cell chimerism, there were no patient, disease, or transplant factors that differed significantly between the 2 groups of patients (supplemental Table 2).
The presence of mixed T-cell chimerism significantly reduced both OS and RFS. Treating T-cell chimerism as a time-dependent variable, attaining full donor T-cell chimerism significantly improved OS (HR, 0.33; 95% CI, 0.17-0.66; P = .0018) in adjusted analyses that excluded MRD but accounted for ATG/campath use (Table 4).
Multivariate Cox model analysis of the impact of mixed donor T-cell chimerism on OS; chimerism as a time-dependent variable
| Variable . | Reference level . | HR (95% CI) . | P . |
|---|---|---|---|
| T-cell chimerism status (time-dependent): full | Mixed | 0.33 (0.17-0.66) | .0018 |
| Conditioning regimen: other | FLAMSA-BU | 1.30 (0.69-2.45) | .41 |
| Cytogenetic risk group: adverse | Favorable/intermediate risk | 1.74 (0.94-3.23) | .079 |
| FLT3 status: present | Absent | 1.33 (0.69-2.56) | .40 |
| ATG/campath | ATG | 0.71 (0.31-1.59) | .40 |
| Variable . | Reference level . | HR (95% CI) . | P . |
|---|---|---|---|
| T-cell chimerism status (time-dependent): full | Mixed | 0.33 (0.17-0.66) | .0018 |
| Conditioning regimen: other | FLAMSA-BU | 1.30 (0.69-2.45) | .41 |
| Cytogenetic risk group: adverse | Favorable/intermediate risk | 1.74 (0.94-3.23) | .079 |
| FLT3 status: present | Absent | 1.33 (0.69-2.56) | .40 |
| ATG/campath | ATG | 0.71 (0.31-1.59) | .40 |
Posttransplant MRD is prognostic only in patients with mixed donor T-cell chimerism
Next, we examined whether there was an interaction between T-cell chimerism and posttransplant MRD. We initially focused on the first 6 months after the transplant, including only patients with sufficient T-cell chimerism and posttransplant MRD results up to month 6 (n = 94, supplemental Figure 2B). Among the total patients, 10.6% (5/47) with full donor T-cell chimerism at both months 3 and 6 had detectable MRD. In comparison, 29.8% (14 in 47) patients with mixed donor T-cell chimerism at months 3 or 6, had MRD positivity before or at the time of mixed donor chimerism (χ2, P = .018). When patients had a mixed donor T-cell chimerism result, the presence of prior or concurrent detectable MRD significantly affected the 2-year OS (MRD+, 34.3% [95% CI, 11.6-58.7] vs MRD-negative, 71.4% [95% CI, 52.2-84.0]; P = .001) and RFS (MRD+, 23.1% [95% CI, 5.6-47.5] vs MRD-negative, 63.6% [95% CI, 44.9-77.5]; P = .004) but had no detectable effect on patients with full donor T-cell chimerism (Figure 2).
Outcomes based on the MRD status in patients with mixed T-cell chimerism and full donor T-cell chimerism. Mixed T-cell chimerism ([A] OS [B] RFS) or full donor T-cell chimerism ([C] OS, [D] RFS). Mixed chimerism is defined as <95% donor:host CD3+ T-cell ratio at month >3 or 6; Full chimerism is defined as >95% donor:host CD3+ T-cell ratio at month >3 and 6. MRD status defined from results up to month >6 for patients with full donor chimerism or up to the time point of mixed chimerism result.
Outcomes based on the MRD status in patients with mixed T-cell chimerism and full donor T-cell chimerism. Mixed T-cell chimerism ([A] OS [B] RFS) or full donor T-cell chimerism ([C] OS, [D] RFS). Mixed chimerism is defined as <95% donor:host CD3+ T-cell ratio at month >3 or 6; Full chimerism is defined as >95% donor:host CD3+ T-cell ratio at month >3 and 6. MRD status defined from results up to month >6 for patients with full donor chimerism or up to the time point of mixed chimerism result.
We extended our analysis by evaluating the relationship between chimerism and MRD up to the first year after the transplant. This remained similar to the pattern observed before 6 months. Only 2 patients converted from full to mixed donor T-cell chimerism after month +6. In patients with sustained full donor T-cell chimerism, MRD positivity remained infrequent (7% [4 of 61] for patients with full donor T-cell chimerism for 2 or more sequential time points, excluding patients with a previous mixed donor T-cell chimerism result or insufficient MRD data). Next, we investigated the frequency and dynamics of leukemia progression from the time of a mixed donor T-cell chimerism result in 24 patients who were MRD negative up to that mixed donor T-cell chimerism result and provided sequential data thereafter. Five (21%) of these 24 patients converted to MRD positivity or relapsed within 3 months; thereafter, only 1 relapse was observed by 2 years.
HLA-DR downregulation on the surface of leukemic blasts refines the prognosis of patients with posttransplant MRD
As the GVL effect may be circumvented by the downregulation of HLA Class II molecules on the surfaces of leukemic blasts, we postulated that patients with posttransplant flow cytometric MRD positivity may be at an increased risk of relapse if the MRD includes HLA-DR downregulation (detected as MRD+/HLA-DR–negative blasts; methods/supplemental Methods). Indeed, all patients with MRD+/HLA-DR–negative results after the transplant (34% of patients who had tested MRD+) relapsed during this study. Interestingly, the presence of HLA-DR–downregulated blasts provided further prognostic discrimination in the posttransplant–MRD+ group; both OS and RFS were significantly reduced (Figure 3; 2-year OS, HLA-DR–negative blasts present 20.0% [95% CI, 3.1-47.5] vs absent 57.9% [95% CI, 33.2-76.3]; P < .001).
Outcomes in patients who tested as MRD+ based on whether patients had MRD with HLA-DR–negative (present) vs MRD without HLA-DR–negative results (absent). (A) OS, (B) RFS since the time of the first MRD+ sample.
Outcomes in patients who tested as MRD+ based on whether patients had MRD with HLA-DR–negative (present) vs MRD without HLA-DR–negative results (absent). (A) OS, (B) RFS since the time of the first MRD+ sample.
Discussion
Our data demonstrate for the first time, to our knowledge, in a prospective cohort that posttransplant-MRD monitoring improves the prediction of OS and RFS, irrespective of pretransplant MRD status in patients who received allografts for AML/MDS using a RIC regimen. We observe an important impact of posttransplant T-cell chimerism on the transplant outcome with, specifically, an interaction between T-cell chimerism and posttransplant MRD for prognosis. Furthermore, HLA-DR expression on blasts appeared to further refine the prognostic impact of MRD positivity after the transplant, suggesting that this may inform novel surveillance approaches for immune evasion from GVL after the allograft.
This study systematically assessed MRD at serial time points for the first 12 months after the transplant, thereby informing clinical practice through the identification of the time points associated with the highest frequency of MRD positivity (occurring at early [day +42 and month +3] time points). The adverse prognoses of posttransplant MRD observed in this study validate recent retrospective studies18-21 and supports the implementation of routine MRD analysis from 1 to 2 months after the transplant as a new standard of care for patients who receive allografts for AML/MDS. Flow cytometric MRD testing extends the availability of posttransplant-MRD monitoring to a much larger proportion of patients than PCR or CD34+ donor chimerism assays. The frequency of MRD positivity after the transplant in our study (16% of trial participants) as assessed using flow cytometry is comparable with that of the next-generation sequencing MRD monitoring studies.20,21 The median time to relapse from MRD positivity allows clinicians to intervene, but strategies additional to immune modulation should be considered in view of the rapid relapse kinetics in some patients.
In our study, performed as part of a randomized, controlled trial, all patients received a RIC regimen. The risk of relapse after RIC allogeneic transplants is higher than that of myeloablative-conditioned allografts, offsetting their lower conditioning-related toxicity;22 thus, MRD monitoring is of particular value in this setting. Studies comparing the impact of conditioning intensity23,24 on posttransplant MRD dynamics provide further clinical insights into the relative importance of conditioning cytoreduction as compared with posttransplant GVL in preventing disease relapse. Lower rates of MRD positivity early after the transplant (days +20-40) have been observed in the myeloablative setting.24 Importantly, in this prospective data set, the presence of posttransplant MRD was not observed to be lower with the use of an intensified chemotherapy–augmented RIC regimen (Flu + cytarabine + amsacrine + Bu) compared with a standard RIC regimen.
Mixed T-cell chimerism after RIC-allo-SCT can persist for several months.17 However, existing data remain inconclusive about the prognostic significance of mixed chimerism after the allograft with respect to relapse risk.17,25,26 Several smaller retrospective studies have identified that the presence of mixed donor chimerism may be associated with an increased risk of disease relapse,27,28 but this observation remains controversial.29,30 In this study, we prospectively demonstrated that the acquisition of full donor T-cell chimerism after a RIC allograft for AML is associated with an improved transplant outcome. Of interest, the acquisition of full donor T-cell chimerism was associated with a low frequency of posttransplant MRD and reduced risk of relapse. Detectable posttransplant MRD was associated with an increased risk of relapse only in patients with mixed donor T-cell chimerism. This observation was held with monitoring up to 12 months after the transplant. Mechanistically, the presence of mixed donor T-cell chimerism may inhibit the activation of GVL via increased donor and host-derived regulatory T-cell population alongside a reduction in the activation of dendritic cells.8 In addition, it can be speculated that AML cells persisting after conditioning may further contribute to hindering donor T-cell activity through an immunosuppressive microenvironment. Therefore, our data support the importance of examining the impact of peritransplant maneuvers, with the potential to optimize the acquisition of full donor T-cell chimerism, such as minimizing posttransplant immunosuppression, T-replete stem-cell dose use, or DLI prophylactic administration. Because more than two-thirds of patients achieved full donor T-cell chimerism in the first 1 or 2 months after RIC regimens, there is a rationale for assessing donor T-cell chimerism before month 3 together with MRD to best inform early post-transplant management.
Our results showing that the decreased expression of HLA-DR on leukemic cells occurs in patients with detectable posttransplant MRD and provides additional prognostic information require further validation. However, these provide the first sequential demonstration that decreased HLA class II expression, a potential mechanism for GVL evasion, may be clinically relevant at MRD levels and was observed in 34% of patients with posttransplant MRD in the FIGARO cohort. The mechanism behind the transcriptional downregulation of major histocompatibility complex class II molecules has been investigated recently,31 and the use of interferon gamma may be of use in reversing this epigenetic silencing.6 It will be important to note in future prospective studies how other inhibitory molecules and changes in the immune microenvironment7,32 may interact with the heightened risk of relapse observed among patients with posttransplant MRD.
In this study, we could not evaluate the impact of MRD below 10-4,which may be detectable via PCR-based assays when patients have a core-binding factor or NPM1-mutated AML. Further prospective studies to examine posttransplant MRD frequency and prognostic impact by genetic subtypes, such as FLT3-ITD, particularly in patients with full donor T-cell chimerism are required, but these will be hampered by the effect of increasing clinical uptake of peritransplant MRD testing to plan interventions. Such interventions may include targeted inhibitors; although these are now increasingly being used as routine posttransplant maintenance therapies.33 Our results also confirm the feasibility of serial flow cytometric MRD for the diagnosis of MRD relapse in the trials of novel preemptive therapy combinations, such as the ALLG AMLM26 INTERCEPT study.34
In summary, this study demonstrates that posttransplant MRD can serve as a paradigm in which GVL mechanisms are of particular importance and may help guide novel therapeutic interventions to restore or potentiate these pathways. Our results provide evidence for the use of combined MRD and T-cell donor chimerism monitoring after RIC allografts, particularly at earlier posttransplant time points.
Acknowledgments
The authors acknowledge the research support and clinical trial funding from CRUK, Bloodwise, and Cure Leukemia.
Authorship
Contribution: All authors assume responsibility for executing the study per the protocol and statistical analysis plan, completeness and integrity of the data, and the decision to submit the manuscript for publication and had access to the primary data and contributed to the manuscript development.
Conflict-of-interest disclosure: C. Craddock has received honoraria from Celgene, Daichi-Sankyo, Novartis, and Pfizer, and research funding from Celgene. J.L. has received travel funding from Novartis and Daichi-Sankyo, and honoraria from Pfizer, Janssen, and Amgen. S.F. has received research funding (to institution) from Bristol Myers Squibb and Jazz, and consulting/honorarium from Novartis. The remaining authors declare no competing financial interests.
Maria Gilleece died on 24 June 2021.
Correspondence: Sylvie Freeman, Clinical Immunology Service, Institute of Immunology and Immunotherapy, College of Medical and Dental Sciences, University of Birmingham, Edgbaston, Birmingham B15 2TT, UK; e-mail: s.freeman@bham.ac.uk.
References
Author notes
∗J.L. and N.M. contributed equally to this work.
Presented in abstract form at the 63rd American Society of Hematology Annual Meeting, Atlanta, GA, 11 December 2021.
Data are available on request from the authors, Sylvie Freeman (s.freeman@bham.ac.uk) and Aimee Jackson (a.e.jackson@bham.ac.uk).
The full-text version of this article contains a data supplement.

![Outcomes based on the MRD status in patients with mixed T-cell chimerism and full donor T-cell chimerism. Mixed T-cell chimerism ([A] OS [B] RFS) or full donor T-cell chimerism ([C] OS, [D] RFS). Mixed chimerism is defined as <95% donor:host CD3+ T-cell ratio at month >3 or 6; Full chimerism is defined as >95% donor:host CD3+ T-cell ratio at month >3 and 6. MRD status defined from results up to month >6 for patients with full donor chimerism or up to the time point of mixed chimerism result.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/7/14/10.1182_bloodadvances.2022009493/2/m_blooda_adv-2022-009493-gr2.jpeg?Expires=1766091580&Signature=uamw-Ktx48WBkc3NS~l0TpQyqkaY1EsXFMj~sJr55EJ-2lHJ0kV9aq-qAOJWfn3aPnOx7Ww~TuGUgJaPsqckv3QB4GdL1FkEMttf4qG2fNvZWUxB2Y9URrt5RiEGJRwQAH3UVJ7uboIPYsGv9xNdTpmM9xa176sQAad68z03D~48y0S6e7KyGCaMmPJOP7o1Z8C-tFGlFOqn8rxHKXsdMEA50FU4KbPVQ7tJ0aDP8kEPcFrwuaOU3kWq5O9GKQL4lyAQUiw8JUO08VkD54~aCX9XCzOLO6EWNyVb-CCaQB0C1NZbBwS-LkGr-GbwAINIlECsU3uwXshQfmaOG-FNSA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
