Key Points
R-COMP is a curative option for older patients with DLBCL and intermediate- to high-risk EPI, even in the presence of a baseline cardiopathy.
R-CHOP is confirmed as the standard therapy for patients at low risk based on the EPI.
Abstract
Rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) is the most commonly used regimen for the upfront treatment of diffuse large B-cell lymphoma (DLBCL). However, it is associated with cardiotoxicity, especially in older patients. Substituting doxorubicin with non-PEGylated liposomal doxorubicin (R-COMP) may reduce the risk of cardiac events, but its efficacy has never been demonstrated in prospective trials. We describe the characteristics and outcome of patients with DLBCL aged ≥65 years prospectively enrolled in the Elderly Project by the Fondazione Italiana Linfomi and treated with full doses of R-CHOP or R-COMP per local practice. Starting from 1163 patients, 383 (55%) were treated with R-CHOP and 308 (45%) with R-COMP. Patients treated with R-COMP were older (median age, 76 vs 71 years), less frequently fit at simplified geriatric assessment (61% vs 88%; P < .001), and had a more frequent baseline cardiac disorders (grade >1, 32% vs 8%; P < .001). Three-year progression-free survival (PFS) was similar between R-CHOP and R-COMP (70% and 64%); 3-year overall survival was 77%, and 71% respectively. R-CHOP was associated with better PFS vs R-COMP only in the Elderly Prognostic Index (EPI) low-risk group. The two groups had similar rates of treatment interruptions due to toxicities or of cardiac events (P = 1.00). We suggest R-COMP is a potentially curative treatment for older patients with intermediate- or high-risk EPI, even in the presence of a baseline cardiopathy. R-CHOP is confirmed as the standard therapy for low risk patients.
Introduction
Anthracyclines are fundamental in the therapy of aggressive lymphomas. The combination of monoclonal anti-CD20 antibody (rituximab) and cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP), R-CHOP, has long been considered the standard frontline treatment for diffuse large B-cell lymphoma (DLBCL).1
Unfortunately, anthracyclines are associated with dose-dependent cardiotoxicity, particularly at cumulative doses of >450 mg/m2, with early clinical and subclinical evidence even at doses of >200 mg/m2.2-4
Age is a significant risk factor for cardiotoxicity.3-8 In the baseline cardiovascular risk stratification recently proposed in the position paper by the Cardio-Oncology Study Group of the Heart Failure Association of the European Society of Cardiology, age ≥65 years results in a medium risk score, and age ≥80 years results in a high-risk score.9 Older patients often have additional cardiovascular risk factors such as diabetes and hypertension, and the administration of a full-dose CHOP regimen can be challenging.
Reducing doses is one of the strategies to mitigate toxicity in older patients with DLBCL. The R-miniCHOP regimen, as initially proposed by Peyrade et al,10 is a valid option in patients ≥80 years because it has a better safety profile, but it may decrease treatment efficacy because of the dose reduction of all active agents. The use of non-PEGylated liposomal doxorubicin (NPLD) has already demonstrated a lower risk of cardiotoxicity in a large randomized study in women with metastatic breast cancer.11 The use of R-COMP, which substitutes conventional doxorubicin with NPLD, has been described by several small, mostly retrospective studies on patients with DLBCL.12-15 Rigacci et al16 recently published an extensive retrospective analysis that describes the outcome of 946 patients with lymphoma treated with R-COMP because of age being >65 years and/or presence of a baseline cardiopathy. This study confirmed the efficacy of the R-COMP regimen (3-year overall survival [OS], 70%), apparently similar to historical R-CHOP data, with a low rate of cardiotoxicity (grade 3-4 in 5% of cases).
One single-arm17 and 2 randomized18,19 prospective trials have reported that R-COMP and R-CHOP have similar efficacy, but they could not demonstrate a clear reduction in the drop in the left ventricular ejection fraction (LVEF) after treatment in patients without comorbidities. Finally, a prospective phase 2 study of 50 patients with DLBCL with preexisting cardiac comorbidity found that the use of R-COMP was a feasible option.20
The Fondazione Italiana Linfomi (FIL) has recently published a multicenter prospective observational study, the “Elderly Project” (EP), on the outcome of a large series of older patients (age ≥65 years) diagnosed with DLBCL and classified as fit, unfit, or frail per a simplified geriatric assessment (sGA) based on age (≤80 or >80 years), cumulative illness rating scale for geriatrics (CIRS-G), activities of daily living (ADL), and instrumental ADL (IADL).21 The sGA groups, international prognostic index (IPI) score, and hemoglobin (Hb) levels were independent predictors of OS and were used to build the new Elderly Prognostic Index (EPI). In the EP, the treatment choice was left to the discretion of the treating physician and could include the use of NPLD. This report describes the characteristics and outcomes of patients enrolled in the EP, comparing patients treated with NPLD with those who received conventional doxorubicin.
Methods
To conduct this analysis, firstly, we used the data set of the EP study, which analyzed 1163 patients of the 1353 who were prospectively enrolled from December 2013 to December 2017. The main aim of this study was to compare outcomes of older patients with DLBCL treated using R-CHOP with those treated using R-COMP. The EP included all consecutive patients with histologic confirmation of DLBCL aged ≥65 years for whom sGA results were available and who provided signed informed consent. Patients with a diagnosis other than DLBCL, including follicular lymphoma grade 3b or high-grade lymphoma, were excluded. Treatment decision was independent of sGA results and was left up to the treating physician. For the purpose of this substudy, we included all patients treated with full doses of R-CHOP or with the same regimen but with NPLD (R-COMP). The use of NPLD (50 mg/m2) is allowed according to Italian law n. 648/96.
Baseline assessment included age, sex, Ann Arbor stage, bulky sites, B symptoms, IPI, Hb value, fitness status (fit, unfit, or frail) based on the sGA, and the EPI score.
Comorbidities at baseline were reported based on the CIRS-G score, with 5 points of severity for each organ/system. The following baseline cardiovascular risk factors for anthracycline use were considered: age >80 years, previous cardiopathy, chronic kidney disease, hypertension, and diabetes mellitus.9 Cardiac function was assessed before the start of treatment via history review, physical examination, electrocardiogram, and bidimensional echocardiogram, all performed locally at each participating center; the study protocol required no routine assessment of serum cardiac troponin or B-type natriuretic peptide. For patients with heart comorbidity declared as grade 2 to 4 by the treating physician, details were retrieved from medical records regarding each patient’s specific cardiac disease(s), including ischemic cardiopathy, atrial fibrillation, uncontrolled hypertension, left ventricular hypertrophy, mitral or aortic valve disease, ventricular arrhythmia, and/or reduced (<50%) LVEF.
The frequency of efficacy and toxicity assessment was that described in the EP study. Given the observational design of the study, patients were evaluated per the routine clinical practice, that is, after 3 or 4 cycles of treatment and at the end of treatment.
All adverse events were categorized and graded per the National Cancer Institute Common Terminology Criteria for Adverse Events (version 3.0). Major cardiac events during treatment and during follow-up were defined as congestive heart failure, ischemic heart disease, arrhythmia, acute heart attack, and acute pulmonary edema. Periodic echocardiographic monitoring of cardiac function was not required by the study protocol but was, nevertheless, performed per local practice.
The study was approved by the ethics committees of all participating centers.
Statistical analysis
Patient characteristics were reported as absolute and percentage frequencies, and the association between these characteristics and treatment groups was performed using Fisher exact test or χ2 test, when appropriate. The primary study end point was OS, which was calculated from the date of diagnosis to death from any cause or the date of last clinical contact. PFS was the secondary end point and was calculated from diagnosis to the date of progression or death from any cause or last clinical contact for censored patients. Survival was estimated using the Kaplan-Meier method, with 95% confidence interval (95% CI), and the comparison between groups was performed using the log-rank test. The effect of covariates is reported as hazard ratio (HR), with 95% CI estimated using Cox proportional hazard (PH) regression.
Given the absence of randomization and unbalanced patient characteristics in the 2 treatment groups, an inverse probability weight (IPW) analysis on Cox PH regression was conducted using stabilized weights with corrected sandwich variance estimation. The stabilized weights were firstly obtained using a logistic regression to model the probability of treatment (R-CHOP or R-COMP) related to the baseline characteristics (age, sex, bulky disease, B symptoms, IPI, Hb level, ADL, IADL, and comorbidities) and a second logistic regression without potential confounders as the marginal probability of treatment. All reported P values are 2-sided.
Results
Clinical characteristics
Overall, 691 of the 1163 patients enrolled in the EP were treated with R-CHOP (383; 55%) or R-COMP (308; 45%). Patient characteristics are reported in Table 1. The median age was 71 and 76 years for R-CHOP and R-COMP, respectively (P < .001). Patients treated with R-COMP were aged >80 years in 22% of cases, compared with only 2% in the R-CHOP subgroup. Based on sGA, 88%, 11%, and <1% of patients treated with R-CHOP and 61%, 32%, and 6% of patients treated with R-COMP were fit, unfit, and frail, respectively (P < .001). The EPI score was low, intermediate, or high in 39%, 54%, and 8% of patients treated with R-CHOP and in 27%, 49%, and 24% of patients treated with R-COMP, respectively (P < .001). R-COMP and R-CHOP had a similar distribution of cases in different IPI groups.
Patient characteristics
| Characteristics . | R-CHOP . | R-COMP . | Total . | NA . | P value . |
|---|---|---|---|---|---|
| Age (y), median (range) | 71 (65-87) | 76 (65-88) | 73 (64-88) | — | < 001 |
| Age >80 y, n (%) | 8 (2) | 67 (22) | 75 (11) | — | < .001 |
| Male sex, n (%) | 177 (46) | 155 (50) | 332 (48) | — | .285 |
| IPI 3-5, n (%) | 188 (51) | 158 (55) | 346 (53) | 37 | .344 |
| Hb, median (range) | 12.5 (5.8-17.2) | 12.4 (7.1-17.5) | 12.5 (5.8-17.5) | 14 | .228 |
| Hb <12 g/dL, n (%) | 131 (34) | 129 (43) | 260 (38) | 14 | .021 |
| B symptoms, n (%) | 85 (22) | 92 (30) | 177 (26) | — | .023 |
| Bulky yes, n (%) | 122 (32) | 85 (28) | 207 (30) | 11 | .315 |
| sGA | — | < .001 | |||
| FIT | 338 (88) | 189 (61) | 527 (76) | ||
| UNFIT | 44 (11) | 100 (32) | 144 (21) | ||
| FRAIL | 1 (<1) | 19 (6) | 20 (6) | ||
| ADL | — | .003 | |||
| 6 | 353 (92) | 259 (84) | 612 (89) | ||
| 5 | 18 (5) | 34 (11) | 52 (7) | ||
| 0-4 | 12 (3) | 15 (5) | 27 (4) | ||
| IADL | — | < .001 | |||
| 8 | 332 (87) | 230 (75) | 562 (81) | ||
| 6-7 | 36 (9) | 57 (18) | 93 (13) | ||
| 0-5 | 15 (4) | 21 (7) | 36 (5) | ||
| Comorbidities, CIRS-G | |||||
| Heart > 1, n (%) | 32 (8) | 99 (32) | 131 (19) | — | < .001 |
| Liver > 1, n (%) | 11 (3) | 14 (5) | 25 (4) | — | .306 |
| Hypertension > 1, n (%) | 80 (21) | 117 (38) | 197 (29) | — | < .001 |
| Genitourinary > 1, n (%) | 28 (7) | 22 (7) | 50 (7) | — | 1.00 |
| Kidney > 1, n (%) | 5 (1) | 13 (4) | 18 (3) | — | .028 |
| Endocrine > 1, n (%) | 47 (12) | 65 (21) | 112 (17) | — | .020 |
| Musculoskeletal > 1, n (%) | 23 (6) | 28 (9) | 51 (7) | — | .143 |
| Nervous system> 1, n (%) | 9 (2) | 10 (3) | 19 (3) | — | .492 |
| Psychiatric>1, n (%) | 15 (4) | 17 (6) | 32 (5) | — | .364 |
| Risk factors for anthracycline use | < .001 | ||||
| None | 137 (36) | 38 (12) | 175 (25) | — | |
| At least one | 246 (64) | 270 (88) | 516 (75) | ||
| EPI score | 43 | < .001 | |||
| Low risk | 141 (39) | 76 (27) | 217 (33) | ||
| Intermediate risk | 196 (54) | 139 (49) | 335 (52) | ||
| High risk | 28 (8) | 68 (24) | 96 (15) |
| Characteristics . | R-CHOP . | R-COMP . | Total . | NA . | P value . |
|---|---|---|---|---|---|
| Age (y), median (range) | 71 (65-87) | 76 (65-88) | 73 (64-88) | — | < 001 |
| Age >80 y, n (%) | 8 (2) | 67 (22) | 75 (11) | — | < .001 |
| Male sex, n (%) | 177 (46) | 155 (50) | 332 (48) | — | .285 |
| IPI 3-5, n (%) | 188 (51) | 158 (55) | 346 (53) | 37 | .344 |
| Hb, median (range) | 12.5 (5.8-17.2) | 12.4 (7.1-17.5) | 12.5 (5.8-17.5) | 14 | .228 |
| Hb <12 g/dL, n (%) | 131 (34) | 129 (43) | 260 (38) | 14 | .021 |
| B symptoms, n (%) | 85 (22) | 92 (30) | 177 (26) | — | .023 |
| Bulky yes, n (%) | 122 (32) | 85 (28) | 207 (30) | 11 | .315 |
| sGA | — | < .001 | |||
| FIT | 338 (88) | 189 (61) | 527 (76) | ||
| UNFIT | 44 (11) | 100 (32) | 144 (21) | ||
| FRAIL | 1 (<1) | 19 (6) | 20 (6) | ||
| ADL | — | .003 | |||
| 6 | 353 (92) | 259 (84) | 612 (89) | ||
| 5 | 18 (5) | 34 (11) | 52 (7) | ||
| 0-4 | 12 (3) | 15 (5) | 27 (4) | ||
| IADL | — | < .001 | |||
| 8 | 332 (87) | 230 (75) | 562 (81) | ||
| 6-7 | 36 (9) | 57 (18) | 93 (13) | ||
| 0-5 | 15 (4) | 21 (7) | 36 (5) | ||
| Comorbidities, CIRS-G | |||||
| Heart > 1, n (%) | 32 (8) | 99 (32) | 131 (19) | — | < .001 |
| Liver > 1, n (%) | 11 (3) | 14 (5) | 25 (4) | — | .306 |
| Hypertension > 1, n (%) | 80 (21) | 117 (38) | 197 (29) | — | < .001 |
| Genitourinary > 1, n (%) | 28 (7) | 22 (7) | 50 (7) | — | 1.00 |
| Kidney > 1, n (%) | 5 (1) | 13 (4) | 18 (3) | — | .028 |
| Endocrine > 1, n (%) | 47 (12) | 65 (21) | 112 (17) | — | .020 |
| Musculoskeletal > 1, n (%) | 23 (6) | 28 (9) | 51 (7) | — | .143 |
| Nervous system> 1, n (%) | 9 (2) | 10 (3) | 19 (3) | — | .492 |
| Psychiatric>1, n (%) | 15 (4) | 17 (6) | 32 (5) | — | .364 |
| Risk factors for anthracycline use | < .001 | ||||
| None | 137 (36) | 38 (12) | 175 (25) | — | |
| At least one | 246 (64) | 270 (88) | 516 (75) | ||
| EPI score | 43 | < .001 | |||
| Low risk | 141 (39) | 76 (27) | 217 (33) | ||
| Intermediate risk | 196 (54) | 139 (49) | 335 (52) | ||
| High risk | 28 (8) | 68 (24) | 96 (15) |
Continuous covariate: Mann-Whitney test; categorical: Fisher exact test or χ2 test.
NA, not assessed.
Patients treated with R-COMP had a significantly higher frequency of baseline cardiopathy (grade >1, 32% vs 8%; P < .001) and a higher frequency of hypertension (grade >1, 38% vs 21%; P < .001), chronic kidney disease (grade >1, 4% vs 1%; P = .028), and endocrine disorders (mainly diabetes mellitus) (grade >1, 21% vs 12%; P = .02). We did not observe any differences in the other comorbidities reported in the CIRS-G assessment at diagnosis. Considering that multiple cardiovascular risk factors may exist, especially in older patients, we analyzed the sum of the main risk parameters suggested in the position article by Lyon et al9: age >80 years, previous cardiopathy, hypertension, chronic kidney disease, and diabetes. There were significantly more cases with at least 1 adverse risk factor (1-5) in the R-COMP group than in the R-CHOP group (270 [88%] vs 246 [64%]; P < .001).
The details of grade 2 to 4 baseline cardiopathy present before the start of chemotherapy were analyzed for 84 patients and 27 patients treated with R-COMP and R-CHOP, respectively; these details are shown in Table 2.
Baseline cardiac comorbidities and cardiotoxicity after treatment based on R-CHOP and R-COMP
| Comorbidities (n = 691) . | Grade . | R-CHOP . | R-COMP . | Total . | P value . |
|---|---|---|---|---|---|
| . | n (%) . | n (%) . | n (%) . | ||
| Heart CIRS-G | 0-1 | 351 (92) | 209 (68) | 560 (81) | < .001 |
| 2-4 | 32 (8) | 99 (32) | 131 (19) | ||
| Total | 383 | 308 | 691 | ||
| Cardiopathy with CIRS-G 2/4 | R-CHOP | R-COMP | Total | ||
| Ischemic cardiopathy | 5 (19) | 28 (33) | 33 (30) | .225 | |
| Atrial fibrillation | 8 (30) | 31 (37) | 39 (35) | .644 | |
| Hypertension | 3 (11) | 4 (5) | 7 (6) | .358 | |
| Ventricular hypertrophy | 2 (7) | 4 (5) | 6 (5) | .632 | |
| Valvulopathy | 7 (26) | 5 (6) | 12 (11) | .008 | |
| Arrhythmia | 2 (7) | 2 (2) | 4 (4) | .248 | |
| LVEF <50% | — | 3 (4) | 3 (3) | ||
| Other | — | 7 (8) | 7 (6) | ||
| Missing | 6 | 14 | 20 | ||
| CTCAE treatment (n = 175) | Grade | R-CHOP | R-COMP | Total | P value |
| 0 | 55 (86) | 97 (87) | 152 (87) | .484 | |
| 1 | 1 (2) | 5 (4) | 6 (3) | ||
| 2 | 6 (9) | 5 (4) | 11 (6) | ||
| 3-4 | 2 (3) | 4 (4) | 6 (3) | ||
| Total | 64 | 111 | 175 | ||
| Any vs 0 | 9 (14) | 14 (13) | 23 (13) | .819 | |
| 3-4 vs 0-2 | 2 (3) | 4 (4) | 6 (3) | 1.000 |
| Comorbidities (n = 691) . | Grade . | R-CHOP . | R-COMP . | Total . | P value . |
|---|---|---|---|---|---|
| . | n (%) . | n (%) . | n (%) . | ||
| Heart CIRS-G | 0-1 | 351 (92) | 209 (68) | 560 (81) | < .001 |
| 2-4 | 32 (8) | 99 (32) | 131 (19) | ||
| Total | 383 | 308 | 691 | ||
| Cardiopathy with CIRS-G 2/4 | R-CHOP | R-COMP | Total | ||
| Ischemic cardiopathy | 5 (19) | 28 (33) | 33 (30) | .225 | |
| Atrial fibrillation | 8 (30) | 31 (37) | 39 (35) | .644 | |
| Hypertension | 3 (11) | 4 (5) | 7 (6) | .358 | |
| Ventricular hypertrophy | 2 (7) | 4 (5) | 6 (5) | .632 | |
| Valvulopathy | 7 (26) | 5 (6) | 12 (11) | .008 | |
| Arrhythmia | 2 (7) | 2 (2) | 4 (4) | .248 | |
| LVEF <50% | — | 3 (4) | 3 (3) | ||
| Other | — | 7 (8) | 7 (6) | ||
| Missing | 6 | 14 | 20 | ||
| CTCAE treatment (n = 175) | Grade | R-CHOP | R-COMP | Total | P value |
| 0 | 55 (86) | 97 (87) | 152 (87) | .484 | |
| 1 | 1 (2) | 5 (4) | 6 (3) | ||
| 2 | 6 (9) | 5 (4) | 11 (6) | ||
| 3-4 | 2 (3) | 4 (4) | 6 (3) | ||
| Total | 64 | 111 | 175 | ||
| Any vs 0 | 9 (14) | 14 (13) | 23 (13) | .819 | |
| 3-4 vs 0-2 | 2 (3) | 4 (4) | 6 (3) | 1.000 |
Fisher exact test. Type of cardiopathy with CIRS 2 or 4 in 111 cases out of 131 (85%).
CTCAE, common terminology criteria for adverse events.
Efficacy
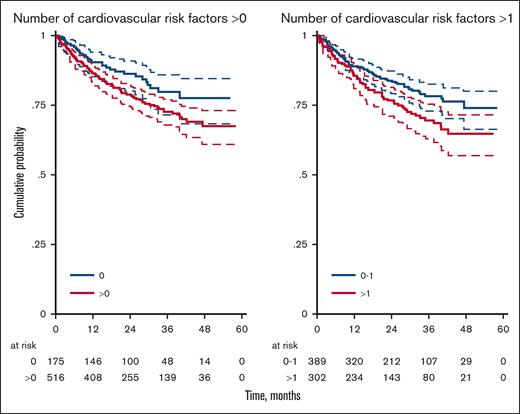
Information on the number of cycles was available for 684 of the 691 patients (99%); incomplete cycles were recorded for 36 (9.4%) and 23 (7.6%) patients in the R-CHOP and R-COMP subgroups, respectively (P = .415). In the whole EP cohort, the OS of patients treated with R-CHOP or R-COMP at full doses was significantly better than that of patients treated with reduced doses or palliation (OS at 3 years, 74% vs 49%; supplemental Figure 1) Regardless of the type of treatment, patients with at least 1 cardiovascular risk factor had a significantly worse 3-year OS (73% vs 80%; P = .045); the difference was even more evident for patients with ≥2 risk factors than for those with 0 or 1 risk factor (70% vs 78%; P = .015; Table 3; Figure 1).
OS for patients with 0 vs 1-5 cardiovascular risk factors and for patients with 0-1 vs 2-5 cardiovascular risk factors
| Risk group . | N (%) . | 3-y OS (%) (95% CI) . | HR (95% CI) . | P value . |
|---|---|---|---|---|
| 0 | 175 (25) | 80 (72-86) | 1.00 | |
| 1-5 | 516 (75) | 73 (67-77) | 1.51 (1.01-2.27) | .045 |
| Risk group | N (%) | 3-y OS (%) (95% CI) | HR (95% CI) | P value |
| 0-1 | 389 (56) | 78 (73-83) | 1.00 | |
| 2-5 | 302 (44) | 70 (63-75) | 1.49 (1.08-2.05) | .015 |
| Risk group . | N (%) . | 3-y OS (%) (95% CI) . | HR (95% CI) . | P value . |
|---|---|---|---|---|
| 0 | 175 (25) | 80 (72-86) | 1.00 | |
| 1-5 | 516 (75) | 73 (67-77) | 1.51 (1.01-2.27) | .045 |
| Risk group | N (%) | 3-y OS (%) (95% CI) | HR (95% CI) | P value |
| 0-1 | 389 (56) | 78 (73-83) | 1.00 | |
| 2-5 | 302 (44) | 70 (63-75) | 1.49 (1.08-2.05) | .015 |
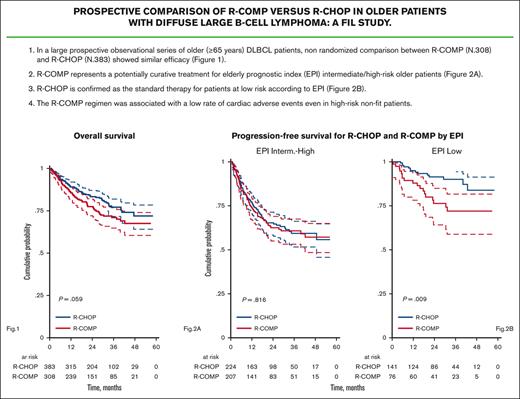
With a median follow-up of 30 months (range, 1-58 months), 204 events of PFS were observed, including 159 progressions. PFS at 3 years was 70% (95% CI, 64-75) for R-CHOP and 64% (95% CI, 57-69) for R-COMP (P = .059; Figure 2A). Overall, 150 deaths were reported, including 106 deaths due to lymphoma progression and 44 unrelated to lymphoma. Causes of death other than lymphoma included toxicity (32 patients: 14 infections, 3 cachexia, 1 kidney failure, and 14 other unspecified nonhematological toxicities), second cancers (3 patients), and other events not specified (9 patients). Among the 32 deaths in patients with cardiac risk factors, 24 were due to progression (75%) and none were due to cardiac complications. OS at 3 years was 77% (95% CI, 72-82) for R-CHOP and 71% (95% CI, 65-76) for R-COMP (P = .059; Figure 2B).
PFS and OS by treatment. (A) PFS for R-CHOP and R-COMP. (B) OS for R-CHOP and R-COMP.
PFS and OS by treatment. (A) PFS for R-CHOP and R-COMP. (B) OS for R-CHOP and R-COMP.
After adjustment in Cox PH regression based on sGA (HR, 1.12; 95% CI, 0.49-1.58; P = .514), EPI (HR, 1.10; 95% CI, 0.78-1.56; P = .582), and cardiovascular risk factors (HR, 1.26; 95% CI, 0.90-1.75; P = .175), the risk of death associated with R-COMP was comparable with that of R-CHOP. Moreover, no advantage of R-CHOP over R-COMP emerged either in OS (HR, 1.06; 95% CI, 0.74-1.52; P = .753) or in PFS (HR, 1.11; 95% CI, 0.82-1.50; P = .512), as reported in supplemental Table 1 (supplemental Data).
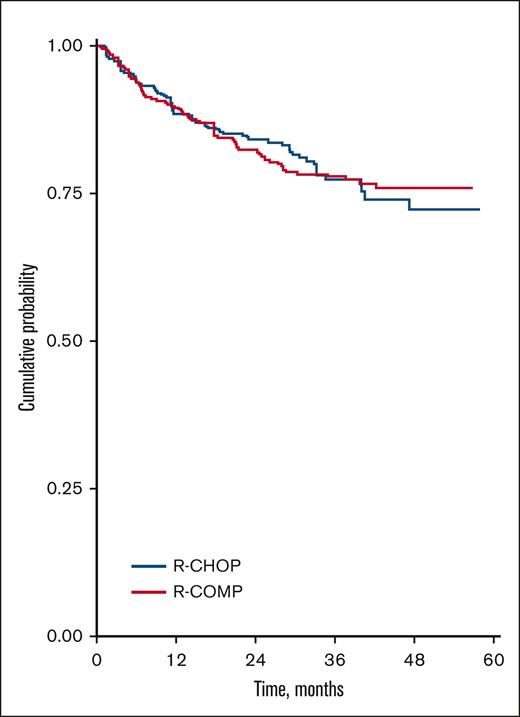
An IPW analysis was conducted in 643 cases and confirmed no significant differences between R-CHOP and R-COMP in terms of OS (HR, 1.01; 95% CI, 0.68-1.50; P = .948; supplemental Table 2; supplemental Data). The IPW analysis also showed that the OS curves of R-CHOP and R-COMP tended to overlap (Figure 3) because of a good balance of covariates between treatments (supplemental Figure 2; supplemental Data).
Survival rates based on geriatric categories
We analyzed the OS and PFS of patients treated with R-CHOP vs R-COMP among the geriatric categories defined by the sGA and EPI scores.
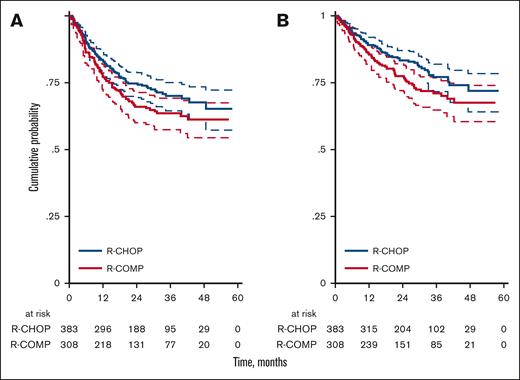
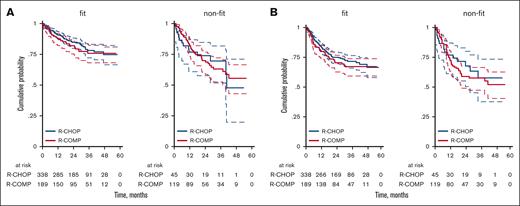
Considering only fit patients according to sGA, the 3-year OS was 78% (95% CI, 72-83) for R-CHOP and 76% (95% CI, 68-82) for R-COMP (P = .731; Figure 4A), whereas the 3-year PFS was 72% (95% CI, 66-77) for R-CHOP and 67% (95% CI, 59-74) for R-COMP (P = .392; Figure 4B). In patients who were not considered fit (including unfit and frail cases) according to sGA, the 3-year OS was 70% (95% CI, 52-82) for R-CHOP and 0.63% (95% CI, 53-72) for R-COMP (HR, 0.97; 95% CI, 0.53-1.79; P = .923; Figure 4A). The 3-year PFS was 58% (95% CI, 38-73) for R-CHOP and 58% (95% CI, 47-66) for R-COMP (HR, 1.16; 95% CI, 0.65-2.07; P = .620; Figure 4B).
OS and PFS by treatment and simplified geriatric assessment (sGA). (A) OS for R-CHOP and R-COMP based on sGA, in patients who are fit and nonfit; (B) PFS for R-CHOP and R-COMP based on sGA, in patients who are fit and nonfit.
OS and PFS by treatment and simplified geriatric assessment (sGA). (A) OS for R-CHOP and R-COMP based on sGA, in patients who are fit and nonfit; (B) PFS for R-CHOP and R-COMP based on sGA, in patients who are fit and nonfit.
With matched propensity score (Mahalanobis matching, caliper 0.3), the HR of R-COMP vs R-CHOP was 0.96 (95% CI, 0.62-1.48; P = .860).
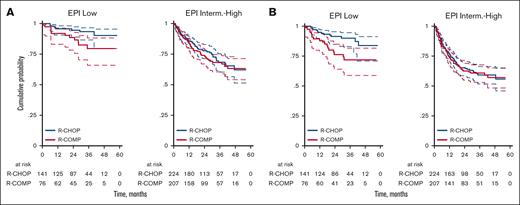
According to the EPI score, we observed a 3-year OS of 93% (95% CI, 87-97) for patients at low risk treated with R-CHOP and 79% (95% CI, 66-88) for patients at low risk treated with R-COMP (P = .074; Figure 5A). The 3-year PFS was 90% (95% CI, 83-94) for patients at low risk treated with R-CHOP and 72% (95% CI, 59-82) for patients at low risk treated with R-COMP (P = .009; Figure 5B). Overall, the outcomes were worse for patients in the intermediate/high-risk category but similar between the R-CHOP and R-COMP groups (3-year OS, 68% vs 68% [P = .997]; 3-year PFS, 59% vs 61% [P = .816]; Figure 5A-B).
OS and PFS by treatment and by EPI risk categories. (A) OS for R-CHOP and R-COMP based on EPI in patients at low and intermediate-high risk (B) PFS for R-CHOP and R-COMP based on EPI in patients at low and intermediate-high risk.
OS and PFS by treatment and by EPI risk categories. (A) OS for R-CHOP and R-COMP based on EPI in patients at low and intermediate-high risk (B) PFS for R-CHOP and R-COMP based on EPI in patients at low and intermediate-high risk.
Safety
There were no differences in treatment interruption owing to toxicities (7% for R-CHOP and 11% for R-COMP; P = .079). The rate of early death (<90 days) was also the same in both groups (2.9%).
We examined cardiotoxicity in the 175 patients for whom the treating physician declared a nonhematological toxicity and/or early interruption of the planned treatment. We observed a toxic cardiac event in 13% of these cases, with 6 (3%) grade 3 to 4 cardiac events, including 3 patients with ischemic disease, 2 with congestive heart failure, and 1 with atrial fibrillation. No cardiac death has been reported so far. Only 4 patients stopped treatment because of cardiotoxicity (all treated with R-COMP). We did not observe any differences between patients treated with R-COMP and R-CHOP in terms of the rate of cardiac events of any grade or for grade 3 to 4 events (P = .819 and P = 1.00, respectively) (Table 2).
Discussion
The EP is a large observational prospective series of older patients (age ≥65 years) with DLBCL treated per local clinical practice in a multicenter setting. This ad hoc analysis suggests that the efficacy of the R-COMP regimen with NPLD instead of conventional doxorubicin is similar to that of the standard R-CHOP regimen and has an acceptable safety profile.
Cardiotoxicity remains an important issue when seeking a curative approach for older patients affected by aggressive lymphoma. Immunochemotherapy with R-CHOP has been the gold standard since the pivotal study by Coiffier et al, which enrolled patients aged 60 to 80 years with normal LVEF at baseline.1 Anthracyclines are the backbone of this therapy but may cause significant cardiotoxicity, which increases with age, particularly in patients aged >80 years.6-8 Moreover, older patients often have multiple cardiovascular risk factors associated with acute and late cardiotoxicity.22 These patients are rarely included in clinical trials, and choosing the best treatment in real life always involves finding a balance between efficacy and tolerability. To reduce cardiotoxicity, an option is to substitute conventional anthracyclines with liposome-encapsulated formulations, which determine a lower peak plasma concentration and a preferential distribution to the liver, spleen, and lymphoid tissue.23 Despite favorable pharmacokinetics and pharmacodynamics, the use of NPLD in clinical practice is still a matter of debate because large randomized clinical trials comparing R-COMP with R-CHOP are lacking and unlikely to be planned in the future. A noninferior efficacy of R-COMP was recently suggested in a metanalysis by Visco et al,24 which included 10 selected studies, with a prospective design only in 6 cases.
The prospective EP study, recently published by the FIL, included all patients with DLBCL aged >64 years and consecutively examined at 55 centers in Italy.21 In this substudy of the original data set of the EP (1207 total eligible cases), the outcome of 383 patients with DLBCL treated with the standard full-dose R-CHOP regimen was compared with that of 308 patients treated with NPLD as part of the R-COMP regimen at the same dose of doxorubicin. Unlike other series investigating the role of NPLD in DLBCL treatment, the EP was unique in that it characterized all enrolled cases using a simplified model of geriatric assessment that combined age (≥ or <80 years), ADL, IADL, and CIRS-G. In this study, we additionally examined the prognostic role of specific cardiovascular risk factors suggested in the position article of the European Society of Cardiology (age >80 years, previous cardiopathy, hypertension, chronic kidney disease, and diabetes),8 with our results confirming a significant detrimental effect on survival for cases with at least one of these conditions.
Because the choice between R-COMP and R-CHOP was per the treating physician’s discretion, without randomization, the 2 treatment groups had different baseline characteristics, as expected. In particular, patients treated with R-COMP were older, with ∼25% of them aged >80 years and less fit (38% were unfit/frail with sGA vs 12% in the R-CHOP group). The EPI score, introduced by the EP study as a new prognostic parameter combining fitness status and high-risk features related to lymphoma, was intermediate/high in 73% of the R-COMP and in 62% of the R-CHOP cases. More than 80% of the patients in the R-COMP subgroup had at least 1 cardiovascular risk factor, and almost one-third of cases had a grade 2 to 4 cardiopathy. We could not examine other lifestyle risk factors such as smoking or obesity.
In our real-life experience, the choice of NPLD was highly dependent on clinical features and not merely on age. A complete evaluation of comorbidities by CIRS-G was planned at screening for all prospectively enrolled patients. This contrasts with prior findings by Rigacci et al,15 which found that the reason for treatment with NPLD was older age in 49% of cases; a preexisting cardiac comorbidity was retrospectively identified for only 32% of patients.
Even if the R-COMP subgroup had more patients who were nonfit and at high-risk based on the EPI and patients with cardiopathies, the outcome, in terms of 3-year PFS and 3-year OS in the whole series, was similar to that of R-CHOP. The survival curves were superimposable after a propensity score analysis balancing the 2 treatment groups. Despite a high percentage of moderate/severe baseline cardiopathy cases, we did not observe any safety concerns (only 4% of patients treated with R-COMP had a grade 3-4 cardiotoxicity). Therefore, although most of these patients would probably not have been treated with conventional doxorubicin, the availability of NPLD provided them with an increased opportunity of receiving treatment with curative intent.
Analyzing survival curves based on the geriatric categories, we confirm that the new EPI score was better than sGA as a predictive parameter. It made it possible to distinguish between older patients with low-risk disease and good fitness status but with excellent OS and PFS and patients with higher risk disease and/or unfitness but with worse outcomes. In patients with a low-risk EPI score (0-1), R-CHOP performed better than R-COMP, in terms of 3-year PFS (90% vs 72%; P = .009), although there was only a trend in favor of R-CHOP in terms of 3-year OS (93% vs 79%; P = .074). In patients with intermediate/high-risk EPI scores (2-8), we did not observe any difference between R-CHOP and R-COMP in terms of 3-year PFS (59% vs 61%; P = .816) or 3-year OS (68% with both treatments). As mentioned previously, a considerable proportion of these patients had cardiovascular risk factors, which represented a contraindication to conventional doxorubicin.
A detailed analysis of acute cardiotoxicity was performed in 175 patients with known nonhematological adverse events and/or early interruption of the planned treatment. Data regarding subclinical or late cardiotoxicity are not reported because the study did not include systematic monitoring of LVEF in the follow-up after immunochemotherapy. With these limitations, and considering the low number of events, we did not observe any difference in terms of cardiotoxicity between R-CHOP and R-COMP. Overall toxicity was mild (13%, all grades and 3%, grade 3-4) and comparable with that reported in a recent meta-analysis of adult patients with non-Hodgkin lymphoma treated with standard doxorubicin.25 The absence of an excess of events in a population at high risk may suggest the role of NPLD in reducing the cardiotoxicity of anthracyclines.
Frail patients with an absolute contraindication to standard immunochemotherapy with anthracyclines remain an unmet clinical need. The promising results obtained in the relapsed/refractory setting26 have prompted the use of nonchemotherapy-based therapies, such as immunomodulatory agents, and bispecific antibodies even in the first-line treatment of older patients.27-29 If preliminary efficacy data are confirmed in the near future, without any safety concerns, a chemotherapy-free option could become a valid alternative that offers a curative treatment. In the meantime, alternative options to standard R-CHOP (such as R-COMP) that may reduce toxicity or increase the number of patients treated with curative intent are welcome.
In conclusion, with the limitations of a nonrandomized comparison, our data show that in older patients with intermediate/high-risk EPI, R-COMP seems to be a reasonable option, because its efficacy is similar to that of R-CHOP and it offers a curative treatment even in the presence of a baseline cardiopathy or cardiovascular risk factors. In EPI low-risk cases with better prognosis and without cardiovascular issues, R-CHOP remains the standard treatment.
Acknowledgments
The authors and the Fondazione Italiana Linfomi thank the patients, families, caregivers, and principal investigators who participated in the trial. The authors are grateful to Jacqueline M. Costa for the English language editing.
This study was funded by a grant from the GRADE nonprofit foundation and from UniCredit Bank.
Authorship
Contribution: A.A., F.M., and M.S. conceived and designed the study; A.A., L.R., A.T., B.P., S.V.U., F.C., A.F., M.B., S.P., S.L., E.P., V.R.Z., A.M.M., G.M., D.M., R.S., B.B., G.G., M.Z., S.H., G.T., L.F., M.T., A.D.R., M.M., D.V., C.P., L.N., D.D., S.F., E.C., P.B., C.M., V.T., M.S., and F.M. acquired clinical data; L.M. performed statistical analysis; A.A., and F.M. interpreted data and drafted the manuscript; L.R. critically revised the manuscript; and all authors discussed the results and contributed to and approved the final manuscript.
Conflict-of-interest disclosure: A.A. is a member of advisory board for Novartis, Janssen, and AbbVie. A.T. receives honoraria directly received from an entity: Takeda, Kiowa Kyrin, and MSD, and is a member of advisory board for Janssen, Gentili, and Sanofi. F.C. provides consultancy within the past 2 years for AstraZeneca and Roche; receives research funding from Roche; and is a member of advisory board for Roche. A.F. is a member of advisory board for Roche, Janssen, Takeda, Kyowa Kirin, Servier, Incyte, and Gilead. M.B. is a member of advisory board for Roche, Takeda, Kiowa, Gilead, Novartis, Incyte, and Janssen; reports research funding from Roche; and reports membership on another entity's board of directors or its advisory committees for Fondazione Italiana Linfomi (nonprofit foundation). S.L. provides consultancy within the past 2 years for GenMAb, Regeneron, Incyte, Takeda, and is a member of advisory board for Roche, Bristol Myers Squibb (BMS), GenMAb, Janssen, Kite, Gilead, and Regeneron. V.R.Z. receives honoraria from Janssen and is a member of advisory board for Gilead and Novartis. G.M. provides consultancy within the past 2 years for Janssen, Incite, Roche, and AbbVie; received honoraria directly from an entity: Janssen, Incyte, Roche, and AbbVie, and is a member of advisory board for Janssen, Incyte, Roche, and AbbVie. G.G. is a member of advisory board for Roche, Celgene/BMS, Kite/Gilead, Janssen, Takeda, and Servier. S.H. provides consultancy within the past 2 years with Incyte, Ipsen, MSD, Gilead, Novartis, Roche, and Takeda, and receives research funding from Ministero Dell'Istruzione e del Merito (PRIN 2017PPS2X4). M.T. has provided consultancy within the past 2 years for Incyte and is a member of advisory board for Incyte, BeiGene, AbbVie, and Kiowa-Kirin. L.N. provides consultancy for Takeda, Incyte, Kyowa Kirin, Roche, and Janssen. S.F. has provided consultancy within the past 2 years for EusaPharma, Janssen, Sandoz, and AbbVie; receives research funding from Janssen, MorphoSys, and Gilead; receives honoraria directly from an entity: Janssen, EusaPharma, Servier, and Gentili; and is a member of advisory board for EusaPharma, Janssen, Clinigen, Incyte, and Italfarmaco. L.M. has been a scientific consultant within the past 2 years for Sandoz (free of fee). V.T. has provided consultancy within the past 2 years for Sandoz (nonfinancial). M.S. receives honoraria directly from Gilead and Servier, and is a member of advisory board for Gilead, Servier, Novartis, Incyte, and BeiGene. F.M. is a member of the advisory board for Janssen, Gilead, MSD, Takeda, Roche, and Novartis, and has membership on the board of directors or its advisory committees in Fondazione Italiana Linfomi (nonprofit foundation). The remaining authors declare no competing financial interests.
Correspondence: Francesco Merli, Hematology Unit, Azienda Unità Sanitaria Locale–IRCCS di Reggio Emilia, Arcispedale Santa Maria Nuova, Viale Risorgimento 80, 42123 Reggio Emilia, Italy; e-mail: merli.francesco@ausl.re.it.
References
Author notes
In compliance with the domestic ethics guideline and applicable legislation, data sets underlying the results reported in this study can be shared per the approval of each ethics committee up to 5 years after the end of this study.
Additional information regarding the study protocol and statistical analysis plan not provided in the supplemental Data is available on request from the Fondazione Italiana Linfomi operational offices (segreteriadirezione@filinf.it).
The full-text version of this article contains a data supplement.