Key Points
MAPK alterations are a hallmark of ECD and RDD.
The MEK inhibitor trametinib is active in non-LCHs, including those without BRAF V600E mutations.
Abstract
Erdheim-Chester disease (ECD) and Rosai-Dorfman disease (RDD) are rare non-Langerhans cell histiocytoses (non-LCHs), for which therapeutic options are limited. MAPK pathway activation through BRAFV600E mutation or other genomic alterations is a histiocytosis hallmark and correlates with a favorable response to BRAF inhibitors and the MEK inhibitor cobimetinib. However, there has been no systematic evaluation of alternative MEK inhibitors. To assess the efficacy and safety of the MEK inhibitor trametinib, we retrospectively analyzed the outcomes of 26 adult patients (17 with ECD, 5 with ECD/RDD, 3 with RDD, and 1 with ECD/LCH) treated with orally administered trametinib at 4 major US care centers. The most common treatment-related toxicity was rash (27% of patients). In most patients, the disease was effectively managed at low doses (0.5-1.0 mg trametinib daily). The response rate of the 17 evaluable patients was 71% (73% [8/11] without a detectable BRAFV600E achieving response). At a median follow-up of 23 months, treatment effects were durable, with a median time-to-treatment failure of 37 months, whereas the median progression-free and overall survival were not reached (at 3 years, 90.1% of patients were alive). Most patients harbored mutations in BRAF (either classic BRAFV600E or other BRAF alterations) or alterations in other genes involved in the MAPK pathway, eg, MAP2K, NF1, GNAS, or RAS. Most patients required lower than standard doses of trametinib but were responsive to lower doses. Our data suggest that the MEK inhibitor trametinib is an effective treatment for ECD and RDD, including those without the BRAFV600E mutation.
Introduction
Erdheim-Chester disease (ECD) and Rosai-Dorfman disease (RDD) are non-Langerhans cell histiocytoses (non-LCHs).1 ECD is a CD68-postive, CD1a– L-group non-LCH with multiorgan involvement, characterized by the activation of the MAPK pathway, which commonly occurs because of BRAFV600E mutations.
ECD is a rare cancer that was initially described in a 1930 report by pathologists Jakob Erdheim and William Chester and was first included in the World Health Organization classification of hematopoietic tumors in 2016.2 The putative cell of origin of ECD is a CD34+ hematopoietic stem cell in the bone marrow. The characteristic clinical manifestation of ECD is diffuse osteosclerotic lesions, particularly affecting the diaphysis of appendicular long bones, which are present in 80% or 90% of patients and consist of foamy lipid-laden histiocytes.2,3 These are painful in approximately one-third of affected individuals. Other common features include orbital infiltration; lung, kidney, retroperitoneal, and cardiac involvement; xanthomas; and diabetes insipidus as well as other endocrinopathies (due to the infiltration of the adrenal and pituitary glands).4,5 The central nervous system is affected in up to half of the patients.6 Neurological involvement is associated with a worse overall prognosis irrespective of symptom burden and can cause a myriad of symptoms, including weakness, ataxia, and dysarthria.
RDD, also known as R group histiocytosis or sinus histiocytosis with massive lymphadenopathy, is a non-LCH, occurring most commonly in children and young adults of African descent. The 2022 World Health Organization classification of hematolymphoid tumors recognizes RDD (along with ECD) as a subtype of plasmacytoid dendritic cell neoplasms.7 RDD commonly presents with bulky cervical lymphadenopathy in children, whereas extranodal manifestations, including skin involvement, are most common in adults.
ECD and RDD have been treated with a wide variety of therapies, including interferon-alpha, anakinra (an interleukin-1 receptor antagonist), cladribine, and imatinib, with variable success.2,8-16 Approximately half of patients with ECD have the BRAFV600E somatic mutation, which results in constitutive activation of the MAPK pathway.17-19 These patients have been shown to respond to BRAF inhibitors, such as dabrafenib or vemurafenib; the latter treatment was approved for patients with ECD by the US Food and Drug Administration (FDA) on the basis of an open-label, multiple-cohort, single-arm trial.20
Translational studies and clinical reports have revealed that patients with ECD or RDD who do not have the BRAFV600E mutation tend to have other molecular alterations in the MAPK pathway, including in the KRAS, NRAS, and MAP2K1 genes, and may therefore respond to MEK inhibitors.21,22 Interestingly, 1 case study demonstrated remarkable activity of single-agent cobimetinib (a second-generation MEK inhibitor) in a patient with RDD and a KRAS mutation.22 Cobimetinib has also been shown to be efficacious in the treatment of ECD and other histiocytosis, producing durable responses. Diamond et al23 demonstrated an overall response rate (per investigators’ defined positron emission tomography [PET] response criteria in solid tumors) of 89% in a cohort of 18 patients, which resulted in FDA approval. In addition, a retrospective study of cobimetinib in RDD demonstrated an overall response rate of 63%.24 Furthermore, cobimetinib was found efficacious in a case study of RDD in the context of RAS-associated autoimmune leukoproliferative disorder with malignant transformation.25 Moreoever, RASopathies, which are inherited disorders due to alterations in the RAS pathway, may also respond to MEK inhibitors.26
MEK inhibitors, as a class, may represent an attractive treatment option for ECD and RDD, given that patients without the BRAFV600E mutation typically have downstream mutations in the MAPK pathway. The MEK inhibitor trametinib is of particular interest, given its selectivity and potency, and we hypothesize that it could provide another effective option for targeted therapy. Herein, we evaluated the safety and efficacy of targeting the MAPK pathway using trametinib as a therapeutic strategy for patients with ECD and RDD.
Methods
Patients
This retrospective, observational study evaluated clinical data from adult patients with progressive non-LCH, specifically ECD and RDD, who were treated with trametinib at the Department of Investigational Cancer Therapeutics of The University of Texas MD Anderson Cancer Center, the Rebecca and John Moores Cancer Center at University of California San Diego Health, Memorial Sloan Kettering Cancer Center, or Mayo Clinic from 2015 to 2021. The study was conducted in accordance with institutional review board guidelines of MD Anderson Cancer Center (RCR04-567), Moores Cancer Center, Memorial Sloan Kettering (institutional review board approval for data analysis with waiver of consent for data analysis only), Mayo Clinic, and any investigational therapies for which patients gave consent (PREDICT protocol; #NCT02478931). Demographic data, including age, sex, and ethnicity were recorded using patient records and clinical notes. Clinical data, such as treatment regimens, response, toxic effects, outcomes, symptom burden at baseline and follow-up intervals, and sites of histiocytosis involvement at diagnosis were also obtained.
Treatment and response assessment
Trametinib was orally administered daily. The administered dose was titrated to patient tolerance, ranging from 0.5 mg to the FDA-approved dose of 2 mg daily. The evaluation of response was based on the treating physicians’ assessment of fluorodeoxyglucose (FDG)-PET and computed tomography data, with complete response indicating complete resolution of lesions and fluorodeoxyglucose (FDG) uptake, partial response indicating partial resolution of lesions and FDG uptake, stable disease indicating no new lesions, and progressive disease indicating progressive or new lesions. Overall, RECIST 1.1 and PET response criteria were considered to be the general framework, although ECD often presents with diffuse disease, which is not always evaluable for target lesions.23 Adverse events and symptoms were assessed clinically. Responses were assessed via imaging every 2 months.
Molecular testing
We obtained formalin-fixed, paraffin-embedded tumor tissue samples collected during routine therapeutic or diagnostic procedures from the specimen repositories of the participating institutions. DNA was extracted from microdissected, paraffin-embedded tumor sections and analyzed for the presence of the BRAFV600E mutation using a polymerase chain reaction–based DNA sequencing method and/or targeted next-generation sequencing (NGS), as previously reported.27 Variants of unknown significance were excluded.
Outcome and statistical analysis
Time-to-treatment failure (TTF) was defined as the interval between the initiation and discontinuation of therapy for any reason (including side effects of therapy without progression). Progression-free survival (PFS) was defined as the interval between the initiation of therapy and disease progression or death from any cause. Overall survival (OS) was defined as the time from the date of therapy initiation to either the date of death or the date of the last known follow-up. Only patients with at least 1 treatment assessment and adequate follow-up were considered evaluable. Using Kaplan-Meier analysis, estimates of TTF, PFS, and OS were calculated. Evaluation of patients who were still progression-free or alive at the last evaluation were censored on that date for PFS or OS, respectively. There are no prospectively validated response criteria for ECD. However, FDG-PET–computed tomography is considered to be the optimal modality for ECD response assessment, and investigator/physician-defined modified PET response criteria in solid tumors are used.23 Statistical analyses were conducted using IBM SPSS Statistics version 26.0 for Windows and the survival (v3.2.7) package in R software (version 4.0.4) and plotted using SPSS and the survminer (version 0.4.9) package in R.
Results
Patients
Twenty-six patients with non-LCH were included in this study: 17 with ECD, 3 with RDD, 5 with RDD/ECD overlap, and 1 with ECD/LCH overlap. The mean age of the patients at diagnosis was 49.3 years, and most patients (n = 18; 69%) were men (Table 1). Seven patients (27%) had central nervous system involvement. The median number of lines of prior therapy was 2.
Patient characteristics (n = 26)
| Mean age (range, y) | 49.3 (23-77) |
| Sex, n (%) | |
| Male | 18 (69) |
| Female | 8 (31) |
| Disease subtype, n (%) | |
| ECD | 17 (65) |
| RDD | 3 (12) |
| ECD/RDD | 5 (20) |
| ECD/LCH | 1 (3) |
| Genotype | |
| BRAFV600E mutation | 9 |
| Other BRAF alteration (including fusion) | 4 |
| MAP2K1 alteration | 6 |
| NRAS alteration | 2 |
| KRAS alteration | 2 |
| ASXL1 alteration | 3 |
| NF1 alteration | 3 |
| MCL1 amplification | 1 |
| RB1 alteration | 1 |
| ERBB2 amplification | 1 |
| RAF1 amplification | 1 |
| APC alteration | 1 |
| CCNE1 alteration | 1 |
| IDH2 alteration | 1 |
| No mutations identified (either BRAF wild-type, not sequenced, or mutation-negative) | 4 |
| Median number of lines of prior therapy (range) | 2 (1-4) |
| Mean age (range, y) | 49.3 (23-77) |
| Sex, n (%) | |
| Male | 18 (69) |
| Female | 8 (31) |
| Disease subtype, n (%) | |
| ECD | 17 (65) |
| RDD | 3 (12) |
| ECD/RDD | 5 (20) |
| ECD/LCH | 1 (3) |
| Genotype | |
| BRAFV600E mutation | 9 |
| Other BRAF alteration (including fusion) | 4 |
| MAP2K1 alteration | 6 |
| NRAS alteration | 2 |
| KRAS alteration | 2 |
| ASXL1 alteration | 3 |
| NF1 alteration | 3 |
| MCL1 amplification | 1 |
| RB1 alteration | 1 |
| ERBB2 amplification | 1 |
| RAF1 amplification | 1 |
| APC alteration | 1 |
| CCNE1 alteration | 1 |
| IDH2 alteration | 1 |
| No mutations identified (either BRAF wild-type, not sequenced, or mutation-negative) | 4 |
| Median number of lines of prior therapy (range) | 2 (1-4) |
Genomic alterations
The most common genetic alteration identified was the BRAFV600E mutation, observed in 9 (35%) patients, all of whom had ECD only, except for 1 patient with ECD/RDD overlap (Table 1). Two patients with BRAFV600E mutations also had NF1 mutations, upon performing tissue-targeted NGS, and 2 patients had concomitant ASXL1 mutations; 1 additional patient had an ASXL1 mutation without BRAFV600E; comorbid myeloid disorders were not observed in the records reviewed. Two patients with BRAFV600E also had other BRAF mutations, 1 with BRAFV471 and 1 with BRAFL485W. Six (23%) of the 26 patients, all of whom had wild-type BRAF tumors, showed MAP2K1 alterations upon performing tissue-targeted NGS. Additionally, 2 patients without BRAFV600E had BRAF fusions (1 with CAPZA2-BRAF fusion and 1 with ANP32A-BRAF fusion). Interestingly, 1 patient had ERBB2 amplification.
Treatment and efficacy
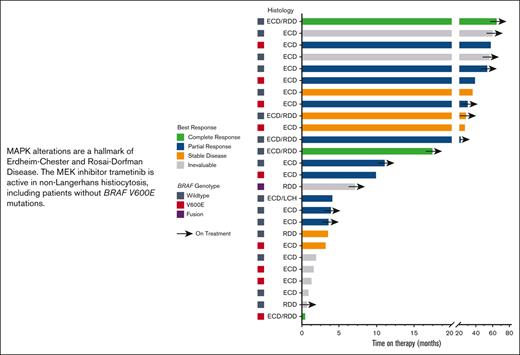
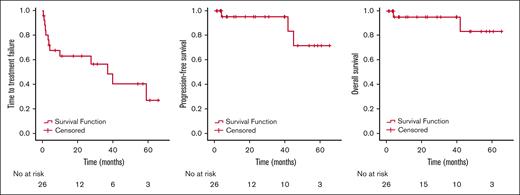
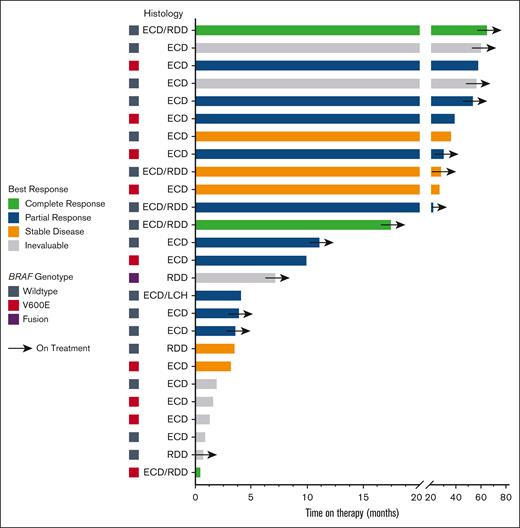
The largest number (n = 9; 35%) of patients were treated with a starting trametinib dosage of 1 mg orally daily (Table 2). The most frequent dosage at the conclusion of treatment was 0.5 mg daily (n = 9; 35%). The median number of lines of prior therapy was 2. Trametinib was used in combination therapy with a BRAF inhibitor among 3 patients (12%): 1 with dabrafenib/prednisone/anakinra, 1 with dabrafenib, and 1 with vemurafenib. Of the 26 patients, 17 were evaluable for response (details of the reasons for not being evaluable are shown in Table 2). Overall, of the 17 evaluable patients, 12 (71%) showed a response. The best response, as determined by the treating clinician, was a partial response in 10 (59%) patients and a complete response in 2 (12%) patients. The remaining evaluable patients showed stable disease as the best response. Of the 12 responding patients, 7 had wild-type BRAF, and all but 1 of these 7 had an alteration elsewhere in the MAPK pathway (Table 2). Altogether, 8 of the 11 patients (73%) who did not harbor a detectable BRAF V600E alteration achieved an objective response to trametinib (1 of these patients had a BRAF fusion). At the median follow-up of 23 months, the median TTF was 37.0 months (95% CI, 19.3-54.7 months; Figure 1). The median OS was not reached (Figure 1); 2 patients with ECD died (1 of suspected myocardial infarction, believed to be unrelated to ECD or treatment, while on therapy and 1 of the complications of valvular disease from previous ECD involvement, 5 months after treatment discontinuation). The median PFS was also not reached (Figure 1). At 1 year, 94.1% of the patients were alive without disease progression, and at 3 years, 90.1% of the patients were alive (Figure 1). The response to and duration of trametinib treatment for each patient are summarized in Figure 2.
Summary of treatment toxicity and response
| Age at diagnosis (y)/sex . | Histology . | BRAF status . | Other relevant molecular alterations . | Involvement (sites of biopsies in bold) . | Trametinib line of treatment . | Clinically significant treatment-related toxicity . | Best response † . | Trametinib starting dosage . | Trametinib ending dosage . | Comments . |
|---|---|---|---|---|---|---|---|---|---|---|
| 25/F | ECD/RDD | Wild-type ‡ | MAP2K1K57N, ASXL1R965∗ | Skin, bone, heart valve, periaortic | 1 | CR | 0.5 mg daily | 0.5 mg daily | ||
| 66/M | ECD/RDD | Wild-type ‡ | NRASQ61R | Bone, skin, lymph nodes, lung, dura, and orbit | 3 | Congestive heart failure | CR | 1.5 mg daily | 1 mg daily | |
| 36/M | ECD/RDD | Wild-type ‡ | ANP32A-BRAF fusion | Bone, omentum, mesentery, and retroperitoneum | 3 | SD | 1.5 mg daily | 0.5 mg daily | ||
| 53/M | ECD/RDD | Wild-type ‡ | MAP2K1F53L | Bone, pleura, and retroperitoneum | 1 | Fatigue | PR | 0.5 mg daily | 0.5 mg daily | |
| 69/M ∗ | ECD/LCH | Wild-type ‡ | MAP2K1C121S | Brain, bones, retroperitoneum, and periaortic | 2 | Fatigue | PR | 1.5 mg daily | 0.5 mg daily | |
| 49/M ∗ | ECD | Wild-type ‡ | MAP2K1Q56P | Heart, retroperitoneum, pituitary, bones, and skin | 4 | Rash | SD | 1 mg daily | 0.5 mg daily | |
| 55/M | ECD | BRAFV600E | None identified | CNS, adrenal gland, aorta, and perinephric | 2 | Facial acne (rash), chills, rigors, and drug-induced hepatitis | PR | 2 mg daily | 2 mg daily | |
| 38/F | ECD | BRAFV600E§,‡ | BRAFV471F-NF1 splice site mutations, MCL1 amplification | CNS, soft tissue, and bone | 4 | Paronychia and xerosis | SD | 2 mg daily | 2 mg daily | |
| 44/M | ECD/RDD | BRAFV600E§,‖ | MAP2K1 | Eye, vocal cord, periaortic, bone, and sinuses | 3 | Facial acne (rash) | IE | 2 mg daily | 2 mg daily | IE for response because of treatment discontinuation before the first on-treatment assessment |
| 59/M | ECD | Wild-type | None identified | Perinephric, bone, and aorta | 3 | Rash and diarrhea | IE | 2 mg daily | 2 mg daily | IE because of treatment discontinuation before the first on-treatment assessment |
| 36/M | ECD | Wild-type | None identified | Bone and skin | 3 | IE | 1 mg daily | 1 mg daily | IE for response because of lack of measurable disease | |
| 41/M | ECD | Wild-type | None identified | Eyelid | 3 | IE | 2 mg daily | 1.5 mg daily | IE for response because of lack of measurable disease | |
| 60/M | ECD | BRAFV600E‡,‖ | ASXL1E635fs∗15 and CCNE1P396L | Pericardial, perinephric, bone, retroperitoneal, and lymph node | 1 | PR | 1 mg daily | 1 mg daily | ||
| 49/M | ECD | BRAFV600E‡,‖ | ASXL1G646fs∗12, BRAFL485W, and ERBB2 amplification | Bone, kidney, abdomen, and lung | From 1 to 5 (varied with and without dabrafenib and anakinra) | Pneumonia | PR | 1 mg daily | 1 mg daily | |
| 40/F | ECD | Wild-type | No additional molecular testing performed | Bone and brain | 1 | Nausea | PR | 1 mg daily | 1.5 mg daily | |
| 23/F | RDD | CAPZA2-BRAF fusion ‡,‖ | IDH2A47V and RAF1 amplification | Brain | 1 | Mucositis and rash | IE | 2 mg every other day | 2 mg daily | IE for response because of loss to follow-up |
| 28/F | RDD | Wild-type ‖ | APCE1157fs | Breast and thigh | 1 | Facial acne (rash) | IE | 1 mg daily | 1 mg daily | IE for response because of loss to follow-up |
| 69/F | RDD | Wild-type ‖ | GNASR201C | Ear and eye | 1 | SD | 1 mg daily | 1.5 mg daily | ||
| 66/M | ECD | Wild-type ‖ | STK11 (splice site SNV) | Bone, aorta, and peritoneum | 3 | IE | 1 mg daily | IE for response because of loss follow-up | ||
| 49/F | ECD | BRAFV600E‡ | NF1H1494Y | Bone, abdomen, and kidney | 3 | Rash and dizziness | IE | 0.5 mg daily (1 week) | 1 mg daily | IE because of treatment discontinuation before the first on-treatment assessment; trametinib used with dabrafenib |
| 56/M | ECD | BRAFV600E | No additional molecular testing performed | Bone and peritoneum | 4 | Renal toxicity | PR | 0.5 mg daily | 0.5 mg daily | Trametinib used with vemurafenib |
| 52/M | ECD | BRAFV600E‡ | NF1S1407R, NRASG60R, KRASA59T | Bone, kidney, abdomen, and lung | 2 | Uveitis | SD | 0.5 mg daily | 0.5 mg every other day | |
| 29/F | ECD | Wild-type ‡ | MAP2K1Q65P | Bone and pericardium | 3 | PR | 0.5 mg daily | 0.5 mg daily | ||
| 77/M | ECD | BRAFV600E | None identified | Bone, aorta, and peritoneum | 2 | Congestive heart failure | IE | 2 mg daily | 2 mg daily | IE because of treatment discontinuation before the first on-treatment assessment; trametinib used with dabrafenib |
| 53/M | ECD | Wild-type ‡ | GNASR201S | Bone and lung | 1 | Mucositis and infection | PR | 1 mg daily | 0.5 mg daily | |
| 59/M | ECD | Wild-type ‡ | KRASA146P, RB1S249, and GNASQ227E | Bone, kidney, abdomen, lung | 1 | PR | 0.5 mg daily | 0.5 mg daily |
| Age at diagnosis (y)/sex . | Histology . | BRAF status . | Other relevant molecular alterations . | Involvement (sites of biopsies in bold) . | Trametinib line of treatment . | Clinically significant treatment-related toxicity . | Best response † . | Trametinib starting dosage . | Trametinib ending dosage . | Comments . |
|---|---|---|---|---|---|---|---|---|---|---|
| 25/F | ECD/RDD | Wild-type ‡ | MAP2K1K57N, ASXL1R965∗ | Skin, bone, heart valve, periaortic | 1 | CR | 0.5 mg daily | 0.5 mg daily | ||
| 66/M | ECD/RDD | Wild-type ‡ | NRASQ61R | Bone, skin, lymph nodes, lung, dura, and orbit | 3 | Congestive heart failure | CR | 1.5 mg daily | 1 mg daily | |
| 36/M | ECD/RDD | Wild-type ‡ | ANP32A-BRAF fusion | Bone, omentum, mesentery, and retroperitoneum | 3 | SD | 1.5 mg daily | 0.5 mg daily | ||
| 53/M | ECD/RDD | Wild-type ‡ | MAP2K1F53L | Bone, pleura, and retroperitoneum | 1 | Fatigue | PR | 0.5 mg daily | 0.5 mg daily | |
| 69/M ∗ | ECD/LCH | Wild-type ‡ | MAP2K1C121S | Brain, bones, retroperitoneum, and periaortic | 2 | Fatigue | PR | 1.5 mg daily | 0.5 mg daily | |
| 49/M ∗ | ECD | Wild-type ‡ | MAP2K1Q56P | Heart, retroperitoneum, pituitary, bones, and skin | 4 | Rash | SD | 1 mg daily | 0.5 mg daily | |
| 55/M | ECD | BRAFV600E | None identified | CNS, adrenal gland, aorta, and perinephric | 2 | Facial acne (rash), chills, rigors, and drug-induced hepatitis | PR | 2 mg daily | 2 mg daily | |
| 38/F | ECD | BRAFV600E§,‡ | BRAFV471F-NF1 splice site mutations, MCL1 amplification | CNS, soft tissue, and bone | 4 | Paronychia and xerosis | SD | 2 mg daily | 2 mg daily | |
| 44/M | ECD/RDD | BRAFV600E§,‖ | MAP2K1 | Eye, vocal cord, periaortic, bone, and sinuses | 3 | Facial acne (rash) | IE | 2 mg daily | 2 mg daily | IE for response because of treatment discontinuation before the first on-treatment assessment |
| 59/M | ECD | Wild-type | None identified | Perinephric, bone, and aorta | 3 | Rash and diarrhea | IE | 2 mg daily | 2 mg daily | IE because of treatment discontinuation before the first on-treatment assessment |
| 36/M | ECD | Wild-type | None identified | Bone and skin | 3 | IE | 1 mg daily | 1 mg daily | IE for response because of lack of measurable disease | |
| 41/M | ECD | Wild-type | None identified | Eyelid | 3 | IE | 2 mg daily | 1.5 mg daily | IE for response because of lack of measurable disease | |
| 60/M | ECD | BRAFV600E‡,‖ | ASXL1E635fs∗15 and CCNE1P396L | Pericardial, perinephric, bone, retroperitoneal, and lymph node | 1 | PR | 1 mg daily | 1 mg daily | ||
| 49/M | ECD | BRAFV600E‡,‖ | ASXL1G646fs∗12, BRAFL485W, and ERBB2 amplification | Bone, kidney, abdomen, and lung | From 1 to 5 (varied with and without dabrafenib and anakinra) | Pneumonia | PR | 1 mg daily | 1 mg daily | |
| 40/F | ECD | Wild-type | No additional molecular testing performed | Bone and brain | 1 | Nausea | PR | 1 mg daily | 1.5 mg daily | |
| 23/F | RDD | CAPZA2-BRAF fusion ‡,‖ | IDH2A47V and RAF1 amplification | Brain | 1 | Mucositis and rash | IE | 2 mg every other day | 2 mg daily | IE for response because of loss to follow-up |
| 28/F | RDD | Wild-type ‖ | APCE1157fs | Breast and thigh | 1 | Facial acne (rash) | IE | 1 mg daily | 1 mg daily | IE for response because of loss to follow-up |
| 69/F | RDD | Wild-type ‖ | GNASR201C | Ear and eye | 1 | SD | 1 mg daily | 1.5 mg daily | ||
| 66/M | ECD | Wild-type ‖ | STK11 (splice site SNV) | Bone, aorta, and peritoneum | 3 | IE | 1 mg daily | IE for response because of loss follow-up | ||
| 49/F | ECD | BRAFV600E‡ | NF1H1494Y | Bone, abdomen, and kidney | 3 | Rash and dizziness | IE | 0.5 mg daily (1 week) | 1 mg daily | IE because of treatment discontinuation before the first on-treatment assessment; trametinib used with dabrafenib |
| 56/M | ECD | BRAFV600E | No additional molecular testing performed | Bone and peritoneum | 4 | Renal toxicity | PR | 0.5 mg daily | 0.5 mg daily | Trametinib used with vemurafenib |
| 52/M | ECD | BRAFV600E‡ | NF1S1407R, NRASG60R, KRASA59T | Bone, kidney, abdomen, and lung | 2 | Uveitis | SD | 0.5 mg daily | 0.5 mg every other day | |
| 29/F | ECD | Wild-type ‡ | MAP2K1Q65P | Bone and pericardium | 3 | PR | 0.5 mg daily | 0.5 mg daily | ||
| 77/M | ECD | BRAFV600E | None identified | Bone, aorta, and peritoneum | 2 | Congestive heart failure | IE | 2 mg daily | 2 mg daily | IE because of treatment discontinuation before the first on-treatment assessment; trametinib used with dabrafenib |
| 53/M | ECD | Wild-type ‡ | GNASR201S | Bone and lung | 1 | Mucositis and infection | PR | 1 mg daily | 0.5 mg daily | |
| 59/M | ECD | Wild-type ‡ | KRASA146P, RB1S249, and GNASQ227E | Bone, kidney, abdomen, lung | 1 | PR | 0.5 mg daily | 0.5 mg daily |
CNS, central nervous system; CR, complete response; F, female; IE, inevaluable; M, male; PD, progressive disease; PR, partial response; SD, stable disease; SNV, single nucleotide variant.
Patient deceased.
CR indicates resolution of lesions, PR indicates improvement but not resolution, SD indicates no change, PD indicates progressive or new lesions, and IE indicates patients in whom response could not be evaluated, and reasons are listed in “Comments.”
Tissue-targeted NGS in CLIA-certified lab, including MSK-IMPACT, MD Anderson Oncomine, Foundation One and others.
Urine cell-free DNA
Plasma-targeted NGS in CLIA-certified lab, including Guardant 360, Foundation Liquid CDx and others. (polymerase chain reaction–based testing for BRAF V600E for others)
Kaplan-Meier curves showing the TTF, PFS, and OS. TTF (left), PFS (middle), and OS (right).
Kaplan-Meier curves showing the TTF, PFS, and OS. TTF (left), PFS (middle), and OS (right).
Swimmer plot showing time on therapy segregated by best response and genotype. Arrows indicate patients still on treatment.
Swimmer plot showing time on therapy segregated by best response and genotype. Arrows indicate patients still on treatment.
Toxicity
Most patients started at a trametinib dosage of 1 mg orally daily (n = 9; 35%), and the most frequent dosage at the conclusion of treatment was 0.5 mg orally daily (n = 9; 35%) (Table 2). Seventeen (65%) of the 26 patients had clinically significant treatment-related toxicities. Seven (41%) of the 17 patients with toxicity had a rash, most commonly an acneiform rash on the face. Of the patients with rash, 1 also had mucositis, 1 had dizziness, 1 had diarrhea, and 1 had concomitant chills, rigors, and drug-induced hepatitis. Of the patients without rash, 2 reported fatigue, 2 had heart failure with reduced ejection fraction, 1 had mucositis and an unspecified infection, 1 had renal dysfunction, 1 had uveitis, 1 had xerosis and paronychia, 1 had nausea, and 1 had pneumonia (Table 2). Trametinib was discontinued for 7 patients because of drug-related side effects: 1 patient with cardiac toxicity, 1 with uveitis, 1 with drug-induced hepatitis, 1 with renal toxicity, and 2 with rash; the seventh patient discontinued therapy because of toxicity issues, but the specific toxic effect was not documented, and the patient was lost to follow-up shortly thereafter. Of the 6 patients with a duration of treatment less than or equal to 4 months, 4 discontinued treatment because of toxicity, 1 died, and 1 was lost to follow-up after moving out of the country.
Discussion
An increased understanding of the molecular landscape of histiocytic disorders has resulted in the introduction of personalized, targeted therapies into the therapeutic armamentarium, which has transformed these often lethal diseases into chronic and relatively manageable conditions.2,20 Based on research indicating that the activation of the MAPK pathway is a hallmark of non-LCH, in the current study, patients with ECD and/or RDD were offered the oral MEK inhibitor trametinib, irrespective of the underlying molecular profile of their disease.21,27 Overall, more than two-thirds (71%) of the evaluable patients responded, which is comparable with previously published data on the MEK inhibitor cobimetinib.23 Treatment effects in our study were durable: the median TTF was 37 months, median PFS was not reached, and 90% of patients were alive at 3 years. Trametinib has a long half-life, which allows for its once-daily administration. In addition, trametinib has a safety profile comparable with that of cobimetinib.23,28,29
This study was a large, multicenter analysis of trametinib therapy for patients with non-LCHs. This further validates the findings previously reported with cobimetinib, indicating that MEK inhibitors may produce durable responses with an acceptable side effect profile, although most of our patients needed dose reductions. The challenge with respect to tolerance has been previously reported among patients with ECD, with therapeutic medications ranging from interferon to BRAF and MEK inhibitors, all appearing to require substantial dose reductions for patients with histiocytic diseases, as compared with patients with other cancers.29 Indeed, the MEK inhibitor cobimetinib required dose reductions for 56% of patients with histiocytic disorders.23 The reason that individuals with ECD and related histiocytic diseases may be particularly prone to toxicity is not known. Fortunately, even with reduced doses (from 25% to 50% of FDA-recommended doses), most individuals with ECD and related histiocytic diseases appear to respond to targeted therapies, such as MEK or BRAF inhibitors, and dose adjustments often effectively prevent discontinuation.
The responsiveness to MEK inhibition may be because most patients harbor mutations in BRAF (either classic BRAFV600E mutations or other BRAF alterations, including fusions) or alterations in genes involved in the MAPK pathway, such as MAP2K1, NF1, GNAS, or RAS (as observed in our patients). Our findings suggest that trametinib may be particularly useful for patients with and without the BRAFV600E mutation or other targetable alterations or with an unknown molecular profile. Specifically, because 8 of 11 patients (73%) who did not have a discernible BRAFV600E alteration attained an objective response, trametinib monotherapy can be considered for patients with wild-type or unknown BRAF status, whereas combination with a BRAF inhibitor can be considered should a BRAFV600E mutation be identified (although the benefit of monotherapy vs doublets remains unclear). The choice of trametinib vs cobimetinib (or even other MEK inhibitors) has not been objectively compared.
Notably, we observed a median TTF of 37 months, and the median PFS was not reached. These data suggest that, unlike common solid tumors, such as melanoma, patients with ECD/RDD treated with trametinib are not prone to the development of adaptive resistance within the first few months or years of therapy; however, the optimal duration of therapy and the risk of recurrence/progression upon treatment discontinuation remain unclear.30 MEK inhibition with trametinib specifically also appears to induce more durable treatment effects in ECD/RDD than in other cancers, such as those studied in adult and pediatric NCI-MATCH.31,32 This may be due to higher dependency on the MAPK signaling pathway and consequently less demand for profound inhibition or the need to target co-driver alterations, as evidenced by the efficacy of low doses in our patient population, although the mechanisms underlying this are not precisely known.
Our study had several limitations. Firstly, our study was retrospective/real-world and relied on available data from patient medical records. Real-world data have disadvantages in that they are not controlled, and data can be missing, but they may also be useful, especially for rare or ultrarare diseases, for which controlled trials are difficult to conduct; in addition, real-world data do not generally exclude patients based on strict eligibility criteria or comorbidities.33 Secondly, commonly used criteria for response assessment in cancer have limited use in non-LCH tratment, and therefore physicians often assessed response using nonstandard criteria (some patients did not have measurable disease at baseline or were inevaluable). In addition, similar to other efforts in non-LCH studies, we evaluated only a small number of patients with these ultrarare diseases. Additionally, the real-world nature of the study made the quantitative assessment of disease symptoms and treatment-related toxicities challenging. Four patients discontinued the drug before the first treatment assessment; although reasons for discontinuation were not clear, most of these patients received full dose trametinib initially, which may be too high a dose for patients with histiocytosis to tolerate well.29 Still, the observed OS appeared longer than that in historical data, and despite follow-up of nearly 2 years, medians for PFS and OS were reached. It is not clear whether MEK inhibition is effective only among patients with MAPK pathway molecular alterations; in our patient set, only 1 fully evaluable patient had no MAPK pathway alterations, and the patient achieved partial response. Finally, 12% of patients were treated with an additional drug, such as prednisone, anakinra, or a BRAF inhibitor (upon detection of a BRAF mutation). Although these interventions could have influenced treatment efficacy, they also reflected the standard clinical practice patterns in the treatment of non-LCH. Our data and those in the literature do not yet answer key questions such as the potential for cure (2 of our patients attained long-term complete responses) and off-ramp for therapy in such patients, or next steps (other than dose reductions) for toxicity failures. Even so, our findings suggest that most patients with non-LCH can attain durable clinical responses to MEK inhibition with trametinib, albeit at reduced doses (usually 0.5-1.0 mg po daily starting or ending dose) compatible with long-term tolerability.
Acknowledgments
The authors thank the editing services team at the MD Anderson Research Medical Library.
R.K. was supported in part by National Cancer Institute grant P30 CA023100 and the Joan and Irwin Jacobs Fund. E.L.D. was supported by National Institutes of Health/National Cancer Institute Core grant P30 CA008748, National Cancer Institute grant R37 CA259260-01, the Frame Fund, the Applebaum Foundation, and the Joy Family West Foundation. F.J. was supported by the National Center for Advancing Translational Sciences (UL1 TR000371), National Institutes of Health through MD Anderson’s Cancer Center support grant P30CA016672, National Cancer Institute (CA235620-01A1), Rising Tide Foundation (CR-18-600), Andrew Sabin Family Foundation, and the Edrheim-Chester Disease Global Alliance.
Authorship
Contribution: F.J., E.L.D., and R.K. designed the research; A.A., R.K., E.L.D., F.J., and Z.L. analyzed the data; A.A., R.K., E.L.D., and F.J. wrote the manuscript; and all authors performed the research and reviewed the manuscript.
Conflict-of-interest disclosure: E.L.D. receives unpaid editorial support from Pfizer Inc. and paid advisory board membership with Day One Biopharmaceuticals, Springworks Therapeutics, and Opna Bio, all outside the submitted work. R.K. has received research funding from Biological Dynamics, Boehringer Ingelheim, Debiopharm, Foundation Medicine, Genentech, Grifols, Guardant Health, Incyte Corporation, Konica Minolta, Medimmune, Merck Serono, OmniSeq, Pfizer, Sequenom, Takeda, and TopAlliance; consultantcy, speaker fees, and/or advisory board for Actuate Therapeutics, AstraZeneca, Bicara Therapeutics, Biological Dynamics, Caris, Datar Cancer Genetics, Eisai, EOM Pharmaceuticals, Iylon, Merck, NeoGenomics, Neomed, Pfizer, Prosperdtx, Roche, TD2/Volastra, Turning Point Therapeutics, and XBiotech; has an equity interest in CureMatch, CureMetrix, and IDbyDNA; serves on the board of CureMatch and CureMetrix; and is a cofounder of CureMatch. F.J. reports research support from Astex, Novartis, BioMed Valley Discoveries, Fore Biotherapeutics, Deciphera, Bristol Myers Squibb, Asana, IDEAYA Biosciences, Sanofi, Merck, F-star, JS InnoPharm, BioXcel, Lilly, Bicara Therapeutics, PureTech Health, FUJIFILM Pharmaceuticals, SOTIO, Synlogic, NextCure, and Hutchinson MediPharma; is on the scientific advisory boards of IDEAYA Biosciences, Synlogic, SOTIO, PureTech Health, Deciphera, Crown Bioscience, Asana, Fore Biotherapeutics, Novartis, Bicara Therapeutics, and PegaOne; is a paid consultant for Mersana Therapeutics, Flame Biosciences, Cardiff Oncology, MedinCell, and ImmunoMet Therapeutics; and has an ownership interest in Cardiff Oncology. The remaining authors declare no competing financial interests.
Correspondence: Razelle Kurzrock, Medical College of Wisconsin, 8800 West Doyne Ave, Milwaukee, WI 53226; e-mail: rkurzrock@mcw.edu; Eli L. Diamond, Department of Neurology, Memorial Sloan Kettering Cancer Center, 160 East 53rd St, 2nd Floor, Neurology, New York, NY 10022; e-mail: diamone1@mskcc.org; and Filip Janku, Department of Investigational Cancer Therapeutics, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: fjanku@me.com.
References
Author notes
∗A.A., R.K., E.L.D., and F.J. contributed equally to this work.
Data are available on request from the corresponding authors, Razelle Kurzrock (rkurzrock@mcw.edu), Eli L. Diamond (diamone1@mskcc.org), and Filip Janku (fjanku@me.com).