Abstract
Human telomeres are tandem arrays that are predominantly composed of 5′-TTAGGG-3′ nucleotide sequences at the terminal ends of chromosomes. These sequences serve 2 primary functions: they preserve genomic integrity by protecting the ends of chromosomes, preventing inappropriate degradation by DNA repair mechanisms, and they prevent loss of genetic information during cellular division. When telomeres shorten to reach a critical length, termed the Hayflick limit, cell senescence or death is triggered. Telomerase is a key enzyme involved in synthesizing and maintaining the length of telomeres within rapidly dividing cells and is upregulated across nearly all malignant cells. Accordingly, targeting telomerase to inhibit uncontrolled cell growth has been an area of great interest for decades. In this review, we summarize telomere and telomerase biology because it relates to both physiologic and malignant cells. We discuss the development of telomere- and telomerase-targeted therapeutic candidates within the realm of myeloid malignancies. We overview all mechanisms of targeting telomerase that are currently in development, with a particular focus on imetelstat, an oligonucleotide with direct telomerase inhibitory properties that has advanced the furthest in clinical development and has demonstrated promising data in multiple myeloid malignancies.
Telomere biology
Human telomeres are tandem arrays that are predominantly composed of 5′-TTAGGG-3′nucleotide sequences at the terminal ends of chromosomes.1,2 These sequences are thought to serve 2 primary functions: they preserve genomic integrity by protecting or capping the ends of chromosomes, preventing inappropriate degradation by DNA repair mechanisms, and they prevent the loss of genetic information during cellular division. With each division, telomeres shorten by ∼50 to 200 base pairs because of the inherent inability of DNA polymerase and associated cellular machinery to replicate the terminal end of the lagging strand of chromosomal DNA.3 When telomere shortening reaches a critical threshold, termed the Hayflick limit, cell signaling cascades are triggered leading to senescence or apoptosis.4,5
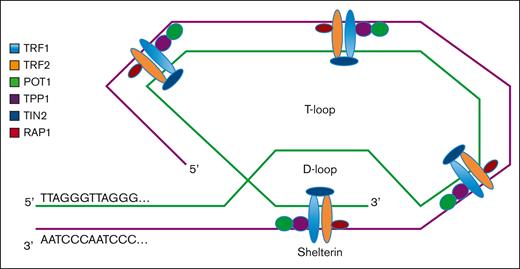
To prevent senescence or apoptosis, there are a group of telomere-associated proteins involved in stabilization and maintenance of telomere structure and length. Shelterin is a multiprotein complex of 6 subunits (ie, telomeric repeat-binding factors 1 and 2 [TRF1 and TRF2], TRF2-interacting protein 1, protection of telomerase 1 [POT1], TRF1-interacting nuclear factor 2 [TIN2], and TIN2-interacting protein 1 [TPP1]) that binds to telomeres and is involved in the formation of a T-loop/D-loop structure (Figure 1), which stabilizes and protects the 3′ overhang single-stranded terminal sequence from DNA damage surveillance enzymes, and facilitates the physiologic and pathologic lengthening of telomeres.6,7 TRF1 and TRF2 bind double-stranded telomeric DNA via their C-terminal MYB domains, stabilizing the T-loop/D-loop structure and preventing the recognition and degradation of telomeres by DNA repair proteins. Loss of TRF2 results in the activation of the ataxia telangiectasia and mutated kinase checkpoint and p53-dependent cell cycle arrest, leading to cellular apoptosis because of effective loss of telomere function.8 Expression of TRF1 is upregulated in pluripotent stem cells, and the loss of TRF1 leads to cellular senescence.9,10 TRF2-interacting protein 1 is recruited via its interaction with TRF2 and binds DNA at the double-strand–single-strand junction of the telomere. POT1 forms a heterodimer with TPP1 that binds directly to the telomeric single-stranded DNA and protects it from inappropriate ataxia telangiectasia and Rad3–related protein–mediated DNA repair response.11,12 Finally, TIN2 interacts with TRF1, TRF2, and POT1/TPP1, acting as an essential hub for the assembly of the entire shelterin complex. Purification of shelterin subcomplexes and reconstitution of the entire complex recently revealed fully dimeric stoichiometry with unusual, extensive conformational variability, facilitating shelterin’s diverse functional mechanisms at telomeres.13 In summary, shelterin subunits function together to protect the structure of telomere DNA and prevent inappropriate DNA damage responses. Loss of shelterin proteins results in uncapped telomeres, which ultimately leads to apoptosis.
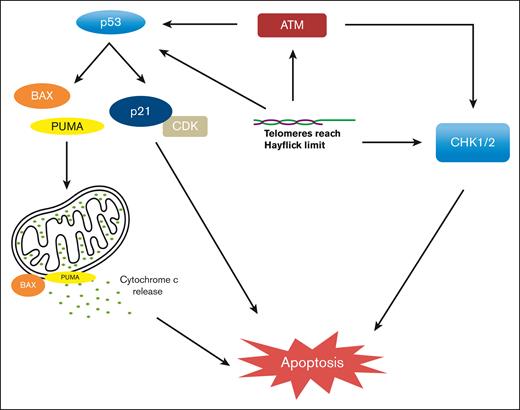
Although the exact mechanisms of replicative senescence and apoptosis remain incompletely understood, several studies suggest that cell cycle arrest relies on activation of DNA damage response proteins and checkpoint inhibition in both p53-dependent and p53-independent pathways. Uncapped or unprotected telomeres result in spontaneous telomere fusion and recognition by ataxia telangiectasia and mutated kinase, leading to the activation of p53-mediated G1 cell cycle arrest.14,15 In addition, shortened or fused telomeres activate proapoptotic BCL2-Associated X Protein (BAX) and p53-upregulatd modulator of apoptosis (PUMA), leading to the release of cytochrome c, resulting in apoptosis.16 Telomere dysfunction can also lead to apoptosis in TP53-incompetent cells, thereby activating p53-independent pathways, at least in part, via the activation of the CHK1/2 pathways leading to senescence or apoptosis (Figure 2).11,17,18
p53-dependent and p53-independent mechanisms of apoptosis when TL reaches Hayflick limit.
p53-dependent and p53-independent mechanisms of apoptosis when TL reaches Hayflick limit.
Moreover, there is increasing evidence supporting in vivo functions of the secondary structures of telomeres, particularly G-quadruplexes. These structures involve the interaction of 4 guanine bases in a square planar arrangement stabilized by central cations that are formed from 1, 2, or 4 strands of telomeric DNA in a parallel or antiparallel orientation.19-21 Telomeric G-quadruplexes have been shown to directly regulate telomere protection, transcription, translation, and splicing.22-24
Although shelterin protects the telomere structure from inappropriate degradation and recognition by DNA repair enzymes, the key regulator of telomere length (TL) is telomerase.25 The telomerase holoenzyme is a ribonucleoprotein complex composed of the enzymatic core (telomerase reverse transcriptase [TERT]/telomere RNA component [TERC]) and the H/ACA core (TCAB1, DKC1, NHP2, NOP10, and GAR1), as evidenced in the recently described high-resolution cryogenic electron microscopy structure of human telomerase.12,26 The TERC forms the RNA template that is complementary to telomeric sequences, allowing for efficient binding, and the human TERT (hTERT) functions as the catalytically active component to directly extend telomeres.27,28 TERT, a 130 kDA protein, contains 4 key functional domains, which include the telomerase essential N-terminal domain, RNA binding domain, reverse transcriptase domain, and C-terminal extension domain. TERT activity is highly regulated through transcriptional, posttranscriptional, and posttranslational mechanisms.29
Telomerase activity is upregulated in ∼85% to 90% of malignancies, circumventing cellular signaling that would otherwise lead to replicative senescence or cell death and facilitating immortalized cellular division.30-32 Activity of hTERT is silenced in most adult somatic cell subtypes, with few exceptions, including transient expression in hematopoietic stem cells (HSCs) and activated lymphocytes to promote self-renewal (division without differentiation) and survival.33,34 A small but important subset of malignant cells extends their telomeres by telomerase-independent mechanisms (ie, alternative lengthening of telomeres pathway).35 This is usually observed in mesenchymal tumors originating from bone, soft tissue, and the nervous system.36 In hematologic malignancies, the shortening of TL is accelerated with increased proliferation pressure and is maintained at an extremely short length.37-42 Significantly shortened telomeres, activation of telomerase, and modulation of the expression of telomere-associated proteins are correlated with faster disease progression, reduced response to chemotherapy, and poor prognosis.43-45 The continual expression of hTERT in malignant cells vs transient expression and relative silencing in nonmalignant somatic cells make telomerase an attractive therapeutic target.
Telomeres and telomerase dysfunction are also implicated in chronic inflammatory conditions and aging. Senescent cells, regardless of a stimulus, adopt a senescence-associated secretory phenotype in which the cells remain metabolically active and secrete chemokines and inflammatory cytokines that both prime the microenvironment and contribute to senescence and tumorigenesis as well as accelerate related diseases that are linked to chronic inflammatory states.46 The elaboration is that reactive oxygen species as a consequence of chronic inflammation can directly alter telomeres and, thereby, trigger DNA damage response pathways that promote senescence.47 Interestingly, NF-κB, the master regulator of inflammation, can transcriptionally upregulate telomerase expression, andtelomerase can, in turn, bind to the p65 subunit of NF-κB and regulate the expression of inflammatory gene sets.48 Therefore, telomere/telomerase dysfunction has been identified in chronic lung disease, diabetes, cardiovascular disease, autoimmune disease, and neurologic disorders, to name a few.46
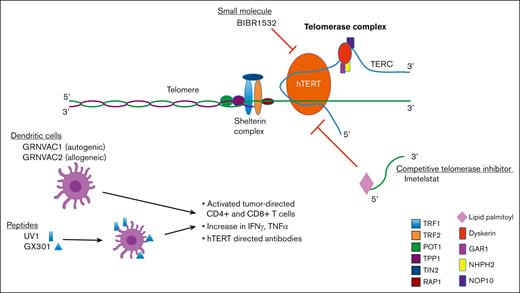
Given the importance of telomerase activity (TA) for malignant cell survival, and lack of activity of hTERT in most somatic cells, there have been significant efforts to develop therapies targeting telomerase via several mechanisms (Figure 3). The therapeutic potential of targeting telomerase has been demonstrated in a number of preclinical studies by transfecting malignant cell types with a catalytically inactive mutant form of hTERT. Nonfunctional hTERT causes the shortening of TL with each division and subsequent cellular senescence or apoptosis in both solid tumor and hematologic malignancy cell types.49,50 In this review, we discuss the data that led to the development of an array of therapeutics that are aimed at targeting telomerase (Table 1). We discuss small-molecule inhibitors, peptide vaccines, TERT-directed dendritic cells, and oligonucleotides, all of which target TA. We summarize the preclinical and clinical data, primarily focusing on drug candidates in myeloid malignancies.
Telomere-targeted therapies in development
| Compound . | Mechanism . | Current study phase . | Indication(s) . |
|---|---|---|---|
| BIBR1532 | Small-molecule inhibitor of hTERT | Preclinical | Solid and hematologic malignancies |
| UV1 | hTERT peptide vaccine | Phase 2 | Melanoma and NSCLC |
| GX301 | hTERT peptide vaccine | Phase 2 | Prostate cancer |
| AST-1 | hTERT-directed dendritic cell | Phase 1 | AML and prostate cancer |
| VAC2 | hTERT-directed dendritic cell | Phase 2 | NSCLC |
| IME | Oligonucleotide complementary to hTERT template | Phase 3 | MDS and MF |
| Compound . | Mechanism . | Current study phase . | Indication(s) . |
|---|---|---|---|
| BIBR1532 | Small-molecule inhibitor of hTERT | Preclinical | Solid and hematologic malignancies |
| UV1 | hTERT peptide vaccine | Phase 2 | Melanoma and NSCLC |
| GX301 | hTERT peptide vaccine | Phase 2 | Prostate cancer |
| AST-1 | hTERT-directed dendritic cell | Phase 1 | AML and prostate cancer |
| VAC2 | hTERT-directed dendritic cell | Phase 2 | NSCLC |
| IME | Oligonucleotide complementary to hTERT template | Phase 3 | MDS and MF |
Telomerase-targeted therapies
Small molecules
The structure of the telomerase holoenzyme was poorly characterized until recently, limiting the design of small-molecule inhibitors.51 A single compound, BIBR1532, is a small-molecule inhibitor of telomerase and is thought to function by binding to hTERT, disrupting interaction with TERC, thereby inhibiting its function as a complex.52 In vitro, BIBR1532 leads to TL shortening in multiple solid tumor cell lines,53 and induction of p53-mediated cell death in an acute myeloid leukemia (AML) cell line but not in nonmalignant hematopoietic cells.54 In other studies, BIBR1532 suppressed hTERT expression in primary chronic myelogenous leukemia (CML) cells and pre–B-cell acute lymphoblastic leukemia cells.55,56 Reduced hTERT expression was shown to remove the repression of hTERT upon cytochrome c release, reducing mitochondrial potential and stimulating the translocation of Bax to mitochondria, which results in apoptosis.57 In addition, BIBR1532 was shown to upregulate the expression of proapoptotic proteins, such as Bax, Bid, Bad, p21, p53, caspases, and cytochrome C proteins while downregulating the expression of antiapoptotic factors, such as Bcl-2.58 BIBR1532 also increased epigenetic markers such as DNA methyltransferases and ten-eleven translocation 2 expression in AML cells. These findings collectively implicate BIBR1532-mediated telomerase inhibition in noncanonical cell death pathways. Further development of this agent has been limited by poor bioavailability and pharmacokinetic profile. Currently, to our knowledge, there are no clinical data evaluating small-molecule inhibitors of telomerase in any cancer type; however, given the promising in vitro activity, telomerase inhibition by bioavailable small molecules is a viable and rational endeavor, particularly for hematologic malignancies.
A more recently developed small-molecule telomerase substrate approach induces telomerase-mediated targeted telomere uncapping, specifically in cancer cells with telomerase, with minimal effects in healthy cells without telomerase. This approach is based on the nucleoside analog 6-thio-2′-deoxyguanosine (6-thio-dG) that is recognized by telomerase and is incorporated into de novo synthesized telomeres, leading to telomere dysfunction.59,60 This approach would have distinct advantages over direct telomerase inhibition in that uncapping agents may exert a more rapid effect on cancer cells by acting independently of TL and sparing healthy hematopoietic cells. In addition, the presence of tumor DNA in the cytosol activates stimulator of interferon (IFN) genes and ultimately induces downstream type 1 IFN signaling.59,60 This innate tumor-sensing pathway activates plasmacytoid dendritic cell–driven tumor-specific T-cell response. In xenograft-immune competent humanized models of solid cancers that are resistant to PD-1 pathway inhibition, 6-thio-dG was able to restore an antitumor immune response, providing a preclinical rationale for the combination of 6-thio-dG and the immune checkpoint blockade.60 Based on compelling preclinical studies in non–small cell lung cancer (NSCLC), sequencing of 6-thio-dG followed by the PD-1 inhibitor cemiplimab is actively being evaluated in a phase 1 trial enrolling patients with advanced NSCLC after PD-1 inhibitor failure (NCT05208944).
TERT-peptide vaccines
Four TERT-peptide vaccines have been developed in attempts to elicit adaptive immune responses to the enzymatic component of telomerase, hTERT. These vaccines have had preclinical success, eliciting immunologic responses, but have failed to demonstrate efficacy in clinical trials.61 However, with the development of immune checkpoint inhibitors there has been a renewed interest and shift in the development of these peptide vaccines, with the hypothesis that intrinsic immunologic resistance of tumors can now be overcome with combination therapy. UV1 (Ultimovacs) is the only telomerase-directed peptide vaccine that is being developed in the United States. UV1, an hTERT peptide first evaluated in patients with metastatic prostate cancer, demonstrated a CD3/CD8+ T-cell immunologic response but failed to demonstrate efficacy in the primary clinical outcome, progression-free survival (PFS).62 Subsequently, it demonstrated safety, tolerability, and UV-1 specific T-cell response in advanced NSCLC.63 It is now being evaluated in combination with ipilimumab/nivolumab for treatment of metastatic melanoma in the phase 2 INITIUM trial (NCT04382664).
The safety and immunologic response of GX301 (Genovax) was evaluated in a phase 2 trial (NCT02293707) in patients with metastatic castrate-resistant prostate cancer. The vaccine was well tolerated with no grade ≥3 treatment-emergent adverse events. Vaccine-specific immunologic response was evaluated by 3 assays at 2 time points (90 days and 180 days after first immunization). Immunologic assays included a peptide-specific enzyme-linked immunospot assay, intracellular staining, and flow cytometry, all evaluating IFN-γ–secreting T lymphocytes, and a peptide-specific cytotoxicity assay. Immunologic response, defined as a positive result in 1 of 6 of the assays was noted in 95% of patients. There was no significant difference in clinical outcomes, including PFS or overall survival (OS), between immunologic responders and nonresponders.64 Telomerase peptide vaccines have not been evaluated in hematologic malignancies; however, given high expression of telomerase across many hematologic malignancies,43,65 further investigation in this area should be considered.
TERT-directed dendritic cell therapies
Dendritic cells have been engineered to target hTERT by transfecting patient-derived mature dendritic cells with hTERT-coding messenger RNA.66 Logistics of developing these cellular therapies are complicated and expensive, requiring patient leukopheresis and ex vivo reengineering of mature dendritic cells to express and present hTERT epitopes to T cells in vivo to elicit a malignant cell–directed adaptive immune response. AST-1, previously GRNVAC1, was evaluated in a phase 1 clinical trial involving patients with AML after induction chemotherapy in first or second complete remission. The cellular therapy was generally well tolerated, although 1 patient developed immune thrombocytopenic purpura, and hTERT-specific immune response to malignant cells was demonstrated via an enzyme-linked immunospot assay in 11 of 19 (58%) evaluable subjects.66 AST-1 was also evaluated in metastatic prostate cancer, and similarly demonstrated tolerability and immunogenicity.67 There are currently no ongoing trials evaluating this therapy. VAC2 (Lineage Cell Therapeutics), previously GRNVAC2, is an allogeneic dendritic cell derived from embryonic stem cells that is being evaluated in a phase 2 trial in advanced NSCLC (NCT03371485). There are no ongoing trials evaluating hTERT dendritic cell therapies in hematologic malignancies at this time.
Competitive telomerase inhibitor
Of the telomerase inhibitors being developed, imetelstat (IME; Geron Inc) has advanced the furthest in clinical trials. IME is a 13-mer N3′–P5′ thio-phosphoramidate oligonucleotide with a covalently bound 5′ palmitoyl (C16) lipid group to enhance cell permeability. IME is complementary to the TERT RNA template, thereby competitively inhibiting its function and preventing the lengthening and maintenance of telomeres.68 In vitro, IME has been shown to inhibit the proliferation of a variety of tumor cell lines69,70 and deplete CD34+ myelofibrosis (MF) HSCs/progenitor cells (HSPCs) by disrupting normal megakaryopoiesis and selectively inhibiting the production of progrogenic cytokines in these cells,71 prompting investigation in both solid and hematologic malignancies.
In an early phase clinical trial of pediatric patients with solid tumors, IME had significant dose-limiting hematologic toxicities.72 A subsequent phase 2 trial evaluating the efficacy of IME as a maintenance therapy in patients with advanced NSCLC after platinum-based chemotherapy demonstrated no benefit in the primary outcome of PFS.73 Interestingly, this study used TL as a biomarker and demonstrated a trend toward improved PFS in those with shorter baseline TL, suggesting on-target effect. One of the predominant toxicities seen in the solid tumor studies was thrombocytopenia. Accordingly, there was a shift in focus toward evaluation of IME in hematologic indications. The clinical development of this first-in-class oligonucleotide has been studied for the treatment of essential thrombocythemia (ET) and multiple myeloma and has progressed to phase 3 trials for the treatment of MF and myelodysplastic syndrome (MDS) (Table 2). IME has also been extensively studied in AML preclinically, with ongoing plans for clinical evaluation. Here, we focus on the indications in which IME has been studied in myeloid malignancies (Table 3).
Ongoing phase 3 trials
| Trial name . | Study arms . | Population . | Primary end point . |
|---|---|---|---|
| IMpactMF (NCT045761) | 9.4 mg/kg IV every 3 weeks vs BAT∗ | DIPSS intermediate-2 + MF, refractory to JAKi | OS |
| IMerge (NCT02598661) | 7.5 mg/kg IV every 4 weeks vs placebo | IPSS low- to intermediate-risk MDS, transfusion dependence refractory to ESA | TI |
| Trial name . | Study arms . | Population . | Primary end point . |
|---|---|---|---|
| IMpactMF (NCT045761) | 9.4 mg/kg IV every 3 weeks vs BAT∗ | DIPSS intermediate-2 + MF, refractory to JAKi | OS |
| IMerge (NCT02598661) | 7.5 mg/kg IV every 4 weeks vs placebo | IPSS low- to intermediate-risk MDS, transfusion dependence refractory to ESA | TI |
Hydroxyurea, thalidomide or analog, IFN, danazol, HMA, or chemotherapy; excludes JAKi.
IME indications and phase of development
| Indication . | Phase of development . | Ongoing trials? . |
|---|---|---|
| MDS | Phase 2 (high risk), phase 3 (low risk) | Yes |
| MF | Phase 3 | Yes |
| ET | Phase 2 | No |
| MM | Phase 2, limited by clinical hold | No |
| AML | Phase 2 | Yes |
| Lymphoid malignancies | Planned | No |
| Indication . | Phase of development . | Ongoing trials? . |
|---|---|---|
| MDS | Phase 2 (high risk), phase 3 (low risk) | Yes |
| MF | Phase 3 | Yes |
| ET | Phase 2 | No |
| MM | Phase 2, limited by clinical hold | No |
| AML | Phase 2 | Yes |
| Lymphoid malignancies | Planned | No |
MM, multiple myeloma.
IME preclinical and clinical data in myeloid malignancies
Chronic myelogenous leukemia
CML is a BCR-ABL1–driven myeloproliferative neoplasm (MPN), the pathobiologic understanding of which contributed to the development of small molecular tyrosine kinase inhibitors (TKIs) that have transformed the management and outcomes of patients. However, there still are patients with CML who develop a blast phase (BP) disease, which is associated with a dismal outcome, and the only curative option is HSC transplantation (HSCT). The leukemic stem cell that drives TKI-resistant CML-BP is associated with the upregulation of the Wnt/β-catenin self-renewal pathway and increased expression of telomerase.74 In vitro treatment with the combination of dasatinib and IME resulted in the selective depletion of the leukemic stem cell population through the downregulation of β-catenin in patient CML-BP cells.74,75 Treatment of a CML-BP xenograft mouse with IME or mismatched control resulted in reduced engraftment of the TKI-resistant myeloid cells and reduction in both β-catenin transcript and protein levels.74 These data support the clinical evaluation of IME in patients with advanced phase CML or those who have developed TKI-resistant disease.
ET
ET is an indolent form of MPN, characterized by thrombocytosis and associated with an increased risk of thrombotic complications, hemorrhagic complications, or both.76,77 The main treatment goal of ET is the reduction in the risk of thrombohemorrhagic complications with the use of aspirin in patients at lower risk (aged <60 years, and/or absence of previous thrombosis) and cytoreductive therapy with hydroxyurea, anagrelide, or an IFN-α derivative in individuals at higher risk (aged, >60 years and/or history of thrombosis).76 There are no approved targeted therapies for the treatment of ET.
An in vitro study of IME-treated mononuclear cells isolated from both healthy donors and patients with ET demonstrated an inhibition of the spontaneous proliferation of megakaryocyte cell colonies in mononuclear cells derived from patients with ET; however, IME did not inhibit cytokine-induced megakaryocyte colony formation in cells from healthy donors.78 These data suggest an increased IME-sensitivity of ET megakaryocytes compared with that of cells from healthy donors. Further laboratory investigation of the effects of IME on MPN megakaryocytes revealed that IME delays normal megakaryocyte precursor cell maturation while affecting multiple points of maturation of MPN megakaryocytes as well as selectively inhibiting the production of fibrogenic cytokines from these cells.79 These observations support the clinical effects on the MPN disease state but also provide mechanistic insights into the treatment-related thrombocytopenia that is observed with IME therapy. This informed a phase 2 trial evaluating the safety and efficacy of IME in patients with ET who were refractory to, or intolerant of, conventional therapies. Of 18 patients treated with IME, 16 (89%) achieved complete hematologic response, defined by the platelet response component of the 2009 European LeukemiaNet response criteria. Molecular responses, defined as a ≥50% reduction in variant allele frequency (VAF) from baseline value in patients with <50% VAF at baseline, or a reduction of ≥25% from baseline in those with >50% VAF at baseline, were seen in 88% (7 of 8) of patients who harbored JAK2V617F.80 A more detailed analysis of clonal dynamics in response to IME treatment demonstrated that most clones with additional myeloid-associated mutations decreased with the driver mutation, including adverse risk mutations ASXL1, EZH2, and U2AF1.81 Notably, TP53, DNMT3a, and SF3B1 mutations were associated with worse molecular response and, although molecular response was similar in those with and without additional mutations, driver mutation reduction was deeper in clones lacking subclonal mutations and correlated with longer treatment response with IME.
Grade ≥3 neutropenia and anemia occurred in 10 of 18 (56%) and 3 of 18 (17%) treated patients, respectively. There were no cases of grade ≥3 thrombocytopenia. In total, 8 of 18 (44%) patients discontinued treatment before the primary end point assessment. This study demonstrated rapid and durable hematologic and molecular responses in ET. A clinical hold was placed on the IME development in 2014 because of reports of liver enzyme elevations in patients enrolled in the MF pilot study, in which elevations of aspartate aminotransferase (27%), alkaline phosphatase (21%), and bilirubin (12%) were noted.82 All liver enzyme abnormalities were reversible and without clinical effect.83 This hold was lifted in November 2014 after elevated transaminases were determined to be reversible and without clinical significance. However, further development in ET has been paused because of concern for appropriate balance between clinical benefit and therapy-related toxicity in this typically indolent disease.
MF
MF is an MPN characterized by bone marrow fibrosis, cytopenias, constitutional symptoms, and splenomegaly related to extramedullary hematopoiesis, with a propensity to transform to AML. Preclinical data evaluating IME in MF HSPCs in vitro demonstrated selective sensitivity in MF HSPCs but not cord blood HSPCs.71 IME treatment of immunodeficient mice that received transplantation with MF CD34+ cells resulted in a significant reduction in MF CD34+ chimerism.71 In addition, a phase 2 pilot study demonstrated disease-modifying clinical activity in patients with MF and potential to cause notable myelosuppression. Patients with Dynamic International Prognostic Scoring System-plus (DIPSS-plus) intermediate-2 or high-risk MF were treated with 9.4 mg/kg IME IV every 3 weeks (group A) or weekly for 4 weeks, followed by every 3 weeks (group B). Of the 33 patients enrolled, 79% had received prior therapy and 48% had received JAK inhibitor (JAKi) treatment. Seven of 33 patients (21%) attained a complete or partial response (CR/PR) based on the International Working Group consensus criteria for treatment response in MF. Of the 4 patients who achieved a CR, 3 (75%) were in dosing Group A. Bone marrow fibrosis was reversed in all patients who achieved CR (4 of 4). Although not statistically significant because of small trial population, all responders harbored a JAK2 driver mutation. Seven of 26 (27%) patients with JAK2 mutations, 0 of 11 (0%) of those with ASXL1 mutations, and 0 of 3 (0%) of those with IDH1/2 mutations attained CR/PR. Four of 11 (38%) patients with spliceosome mutations (U2AF1, SF3B1, and SRSF2) achieved CR. The most notable adverse event was reversible myelosuppression that led to dose reductions in 67% of patients enrolled. Grade 3/4 myelosuppression occurred in 88% of treated patients, with 1 death in the weekly dosed Group B cohort, attributed to treatment-related cytopenias.83 Because of higher toxicity, the weekly Group B dosing schedule was no longer evaluated. Laboratory correlative studies did not confirm any predictive value of baseline TL on response, nor was there a significant change in TL in posttreatment samples.83 This raises the possibility of unrecognized off-target or noncanonical effects of IME. Telomere-independent functions of telomerase have been described in cell proliferation and signaling, such as Wnt/β-catenin and NF-κB signaling.48,84,85 Moreover, telomerase has been implicated in mitochondrial RNA processing,86 ribosomal DNA transcription,87 and DNA damage repair.88 In addition to these noncanonical telomerase functions that IME may suppress by reducing TA and TERT expression, potential off-target mechanisms of action of IME have been postulated. Although the activation of toll-like receptor (TLR) signaling has been disputed in an in vitro study,89 IME may alter cellular processes through its G-quadruplexforming activity,90 potentially altering the chromosomal structure and regulating gene expression as well as interfering with nuclear or cytosolic G-quadruplex–binding proteins.91 Nonetheless, because of the disease-modifying activity noted in this pilot study, IME moved into further clinical investigation in MF using the less toxic Group A dosing schedule instead of the Group B dosing schedule.
A phase 2 study entitled IMbark (NCT02426086) was conducted to evaluate the efficacy of IME at 2 doses, 9.4mg/kg and 4.7mg/kg every 3 weeks in patients with MF relapsed/refractory (R/R) to treatment with a JAKi. Of the patients included in this randomized study, 25% were tested as triple negative (JAK2 wild-type, CALR wild-type, and MPL wild-type), and 68% harbored high molecular risk mutations (ASXL1, SRSF2, IDH1/2, and EZH2). Patients treated with the higher dose demonstrated substantial clinical benefit. A reduction in the spleen volume by ≥35% was achieved in 10%, and a reduction in total symptom score by ≥50% was achieved in 32% in the high-dose cohort.92 Notably, 40% attained at least 1 grade reduction in bone marrow fibrosis, and 42% attained a reduction in driver mutation VAF ≥25%.93 These data further suggest that IME targets the underlying malignant MF clone and has disease-modifying effects. In addition, on-target activity was demonstrated with a reduction in baseline TA, and hTERT expression levels. A >50% reduction in TA or a reduction in hTERT expression was considered optimal pharmacodynamic effect, which was correlated with reduced symptom burden, reduced spleen volume, and survival benefit.93 Change in the TL was not reported; however, shorter TL (< median) at baseline was associated with higher rates of spleen and symptom response and a trend to better median OS with IME at 9.4 mg/kg. A key secondary end point, median OS was 28 months in the 9.4 mg/kg–dose group and 20 months in the 4.7 mg/kg–dose cohort. OS at 2 years was 57.5% and 41.8% in the high- and low-dose groups, respectively.92 This suggests a dose-dependent response and compares favorably with the previously reported ∼11 to 14 months median OS in the ruxolitinib-discontinuation population.94-96 Improvement in bone marrow fibrosis by ≥1 grade was associated with a nonsignificant trend toward longer median OS compared with those without improvement in fibrosis (hazard ratio, 0.54; 95% confidence interval, 0.23-1.29). There was also an association between patients who had triple-negative mutation status and longer OS. Median OS was not reached in the triple-negative cohort and was 25 months for patients who were not triple negative.92 In order to elucidate the biological basis supporting clinical benefits observed in patients with triple-negative mutation status, relationships between triple-negative status and baseline TL and hTERT levels were explored. The results showed that triple-negative MF tends to have short TL and high hTERT expression level at baseline, representing a suitable target population for IME.97 This preliminary finding of benefit in this poor-risk subset will be further evaluated in the phase 3 study. The OS of patients enrolled in the IMbark study was compared with a propensity-matched real world data set of patients with MF R/R to JAKi in a retrospective analysis. Median OS of those treated with IME was 29 months vs 12 months in those treated with best available therapy (BAT).98 These reproducible data demonstrate potential survival benefit in patients with MF in the refractory setting, in which expected survival is very limited.
The safety profile of IME in MF in the IMbark trial was most notable for reversible cytopenias. Grade ≥3 neutropenia, anemia, and thrombocytopenia occurred in 10%, 31%, and 23%, respectively, in the low-dose arm, and 32%, 39%, and 41% respectively in the high-dose arm. The majority of cytopenias resolved to grade ≤2 within 4 weeks. Because IME is an oligonucleotide, it was theorized that it may be recognized as a pathogen-associated molecular pattern leading to the activation of TLRs.99 Activation of TLRs leads to activation of innate immune system and proapoptotic response and may result in off-target myelosuppression.99,100 This mechanism was tested and disproven in an in vitro study evaluating IME effect in HEK293 cell lines, indicating that TLR activation does not play a role in IME-induced myelosuppression.89 Dyspnea (13%) and asthenia (10%) were the most frequent grade ≥3 nonhematologic toxicity. There were no reported treatment-related deaths. Adverse events led to the discontinuation of treatment in 25.2% of patients. IME treatment was not associated with treatment-emergent grade ≥3 elevation in liver enzymes or bilirubin in this study.92
Given the evidence of disease modification and association with potential survival benefit coupled with an acceptable toxicity profile, IME is currently being evaluated in the JAKi R/R population. IMpactMF is a controlled phase 3 trial (NCT0457615) that is currently enrolling and plans to randomize the treatment for 320 patients with JAK2 inhibitor refractory MF with either IME at 9.4 mg/kg IV every 3 weeks or BAT. Key eligibility criteria include patients with DIPSS intermediate-2 or high-risk MF refractory to JAKi, measurable spleen size ≥5 cm below costal margin or volume ≥450 cm3 via imaging, symptom burden of ≥5 points on the Myelofibrosis Symptom Assessment Form (version 4.0), and baseline platelet count >75 × 109/L. JAKi-refractory disease was defined as (1) treatment with JAKi for ≥6 months, including ≥2 months at an optimal dose with no decrease in spleen volume, spleen size, or symptoms since the start of JAKi treatment, or a total symptom score of ≥15 at study entry; or (2) treatment with JAKi for ≥3 months with maximal doses and no decrease in spleen volume/size or symptoms.101 The control arm of this registration study will receive the BAT selected by the investigator before randomization and may include treatment with hydroxyurea, thalidomide or an analog, interferon derivatives, danazol, hypomethylating agent, and chemotherapy but, importantly, excludes JAKi. The exclusion of JAKi option in the BAT arm was reasoned by the expectation that an alternative JAKi would unlikely provide survival advantage in a truly JAKi-refractory subpopulation. The primary end point of this trial is OS and key secondary end points include spleen and symptom response rates, PFS, and International Working Group–myeproliferative neoplasms research and treatment (IWG-MRT) response rate. Importantly, to our knowledge, this is the first phase 3 trial to evaluate OS as a primary end point in MF. Exploratory analyses will include the assessment of driver mutation status, baseline TL, and hTERT expression level and correlation with outcomes measures. If this trial demonstrates significant OS benefit, IME may be the first approved agent with a primary goal of extension of life rather than the historical therapeutic goals of spleen size and symptom reduction.
MDS
MDS is a heterogeneous group of clonal hematopoietic disorders characterized by ineffective hematopoiesis and morphologic dysplasia in myeloid lineage hematopoietic cells, resulting in cytopenias and increased risk of transformation to AML.102 Similar to solid tumor cells, TL has been shown to be shorter in leukocytes of patients with MDS compared with those in healthy controls; however, TA is upregulated.103 In the case of del (5q) MDS category, TL has been noted to be shorter than that in age-matched healthy controls and is associated with disease duration and extent of cytopenias. Lenalidomide treatment associated with remarkably high hematologic and cytogenetic responses in this subset of patients with MDS was also associated with increasing TL of peripheral blood cells over time as clonal hematopoiesis was converted to normal polyclonal hematopoiesis.104 Treatment options for patients with MDS are limited and, besides HSCT, do not offer the potential for cure. Existing therapies are focused on alleviating anemia in patients at lower risk, or addressing progressive cytopenias and risk of leukemic transformation in patients at higher risk. As mentioned, the pilot study in patients with MF demonstrated a significantly higher rate of CR in those harboring spliceosome mutations involving SF3B1 or U2AF1 than in patients without these mutations who were treated: 38% vs 4%, respectively.83 Given the high frequency of SF3B1 or U2AF1 mutations in patients with MDS with ringed sideroblasts (RSs), subsequent development in MDS will be focused on this population. In a phase 1 trial, 9 patients with MDS-RS with or without thrombocytosis were enrolled and treated with IME at a dose of 7.5 mg/kg every 4 weeks, reduced from the prior study dose based on the observation of treatment-related myelosuppression. This dosing schedule was well tolerated. Of the 8 patients with red blood cell transfusion dependence at baseline, 3 (38%) achieved transfusion independence (TI) with a median duration of 28 weeks. One patient with baseline splenomegaly achieved a >50% decrease in palpable spleen size. All anemia responders and the patient with a spleen response were found to harbor mutated SF3B1.105 The impact of baseline TA and TL were not described in this trial.
Subsequently, patients with International Prognosis Scoring System (IPSS) lower risk MDS who were transfusion dependent despite treatment with erythropoiesis stimulating agents (ESAs) were treated with IME at 7.5 mg/kg every 4 weeks in a phase 2, open-label, single-arm trial. A total of 57 patients were enrolled, including 38 patients with non-del(5q) MDS. Median baseline transfusion burden was 7 units per 8 weeks in the study population. The primary end point, 8-week TI was achieved in 21 of 57 (37%) of the overall population and 16 of 38 (42%) of the non-del(5q) population. The median time to the onset of red blood cell TI was 8 weeks, and the median duration of TI was 65 weeks in the overall population and 86 weeks in the subset of patients with non-del(5q). TI was independent from baseline transfusion burden. Grade ≥3 neutropenia, anemia, and thrombocytopenia occurred in 60%, 19%, and 54%, respectively, of patients who were treated. Only 1 case of anemia was determined to have a treatment-emergent adverse event. Median duration of grade ≥3 neutropenia and thrombocytopenia was 1.7 and 1.1 weeks, respectively. Ninety percent of neutropenia events and 88% of thrombocytopenia events resolved to grade ≤2 within 4 weeks. To further correlate IME activity, biomarkers including TA levels and hTERT RNA levels were measured in isolated leukocytes of patients with available samples at baseline, on day 8 of each 28-day treatment cycle, and at the time of suspected response or progressive disease. There was a significant reduction (>50%) in the TA noted in 38% of the available samples, and a significant decrease in hTERT RNA detected in 59% of the available samples after initiating IME. Although not statistically significant in the overall population, in the subset population of patients with non-del(5q) MDS, decreases in TA and hTERT levels correlated with anemia response to IME. In patients with SF3B1 mutations, VAF was decreased between 10% and 93% in 10 of 11 patients. There were also decreases in VAF noted in patients with JAK2 and DNMT3A mutations. Partial cytogenetic responses in patients with trisomy 8 occurred in 2 of 3 patients with available posttreatment cytogenetic data; 1 patient had a decrease in trisomy-8 clones from 45% to 5% at 24 weeks, and the other patient had a decrease in trisomy-8 clones from 100% to 5% within 48 weeks. In 4 without trisomy 8, 2 patients with available data had clonal reduction of del(5q).106 These decreases in VAF and clonal reductions via cytogenetic analysis suggest disease-modifying activity and targeting of the malignant clone by IME in MDS. Furthermore, a post hoc analysis of patients with non-del(5q) MDS included within this trial demonstrated a correlation between change in hTERT levels and TA with durable TI rate and OS.107 These data suggest a correlation of on-target pharmacodynamic effects of IME with clinical benefit. Changes in TL with IME therapy were not reported in the primary study or in the posthoc analysis.
Based on the increased TI rate and durability, particularly in the non-del(5q) population in the phase 2 trial, the IMerge trial (NCT02598661) was recently completed. IMerge is a double-blind, randomized (2:1) phase 3 study comparing IME with placebo in patients with non-del(5q) IPSS low- to intermediate-risk MDS with transfusion dependence refractory to ESA. Primary outcome is 8-week TI, secondary end points include improvement in cytopenias, rate of complete remission and partial remission, OS, PFS, and time to transformation to AML. Topline data were recently released indicating that the trial met its primary efficacy end point and the key secondary end points.108 We await final data publication. If this registration trial confirms an effect on the underlying disease process, this would be the first therapy, to our knowledge, for treating low- to intermediate-risk MDS with the potential for disease modification. Given the exciting initial reports in lower risk MDS and the potential to induce molecular responses suggesting anticlonal effects with IME, trials in higher risk MDS are now underway in patients in whom hypomethylating agents have failed and who lack effective therapeutic options (NCT05583552).
AML
There are emerging preclinical data that demonstrate IME activity in AML. Randomized phase 2–like preclinical trials of IME in patient-derived xenografts (PDXs) from 30 individual patients with AML demonstrated improved survival in PDXs treated with intraperitoneally infused IME at a dose of 15 mg/kg every 48 to 72 hours compared with a control population of PDXs treated with intraperitoneally infused phosphate-buffered saline at equivalent intervals. PDXs with malignant cells transplanted from patients with mutations in NRAS or enriched with oxidative stress–annotated gene expression signatures were more likely to demonstrate responses to IME.91,109-111 In addition, more detailed mechanistic studies uncovered IME-induced ferroptotic AML cell death in AML cell lines and PDXs of AML in vivo,91,112 suggesting a potential mechanism of action of IME that may be independent of telomere shortening and canonical telomerase function in AML. This mechanistic insight was then leveraged to test the sequential administration of standard induction chemotherapy (5 + 3 cytarabine + anthracycline) followed by IME consolidation therapy in an additional randomized phase 2–like preclinical trial involving PDXs of AML.91,110,112 This preclinical trial demonstrated a significantly improved survival and delay of AML relapse in PDXs treated with combination therapy when compared with PDXs treated with either chemotherapy alone or IME monotherapy, suggesting that treatment with IME as consolidation therapy after induction chemotherapy might reduce risk for AML relapse.91,112
An in vitro study of an AML cell line treated with IME in combination with venetoclax, a B-cell lymphoma protein-2 inhibitor, demonstrated an enhanced apoptosis compared with monotherapy with either agent alone. In addition, prolonged survival of an in vivo AML murine model was achieved when IME was combined with venetoclax when compared with monotherapy with either agent alone.113 Subsequently, the combination effect of an hypomethylating agent (HMA) and IME in 2 unique AML cell lines (OCI-AML3 and OCI-AML5) was evaluated and demonstrated possible synergy.114 Together, these data suggest potential benefit of combination therapy with IME in AML, in combination with HMAs and venetoclax or with cytotoxic chemotherapy. With recent widespread adoption of combination HMA and venetoclax therapy in patients with AML who are considered unfit for intensive therapy based on the VIALE-A trial data,115 it may be prudent to evaluate the addition of IME to either venetoclax, HMA, or both. The IMpress trial (NCT05583552) will be the first clinical trial to evaluate IME in AML. This planned phase 2 study is designed to evaluate IME monotherapy in patients with high-risk MDS or AML failing HMA-based therapies. Failure is defined as a lack of CR, PR, or hematologic improvement after at least 2 cycles of HMA + venetoclax, 4 cycles of HMA monotherapy, or relapse at any time after initial CR, PR, or hematologic improvement. In addition, given the strong preclinical data, further exploration of IME as a maintenance therapy for patients with AML after treatment with cytotoxic chemotherapy either before or after allogeneic HSCT may be a strategy worth investigating.
Conclusions and future directions
Telomerase is a promising novel target for a diverse array of indications in myeloid malignancies. Although a number of approaches have been evaluated targeting telomerase, to date, the inhibition of hTERT by oligomer IME has been the most successful and advanced the furthest in trials.
It is remarkable that IME is in late phase testing in 2 related myeloid malignancies focused on different clinical outcome measures in different ends of the disease risk spectrum. In MDS, development is being pursued in patients with non-del(5q) IPSS low- to intermediate-risk MDS with transfusion dependence refractory to ESA.106 In MF, development is focused on the JAKi-refractory population in DIPSS intermediate-2 or high-risk disease.92 Clinical trials in MF typically focus on symptom burden, spleen volume reduction, and reduction in JAK2 VAF. Although these are important end points and are easily measurable within the context of short-term clinical trials, no trials have prospectively evaluated the impact of existing treatments on OS as a primary end point. Long-term follow-up studies suggest OS benefit of commercially available JAK2 therapies; however, the impact is controversial. IME is the first therapy that is seeking approval based on OS benefit in MF. If successful, this has potential to shift the US Food and Drug Administration criteria for approval.
Looking beyond the ongoing studies in MDS and MF, the applications of IME are being explored in a number of other indications. In studies of AML, there are encouraging preclinical data with further improved responses of IME consolidation therapy after induction chemotherapy. The agent is actively being evaluated as a monotherapy in refractory AML. IME may also be evaluated in the near future as a combination therapy partner in AML or as a maintenance therapy option after cytotoxic chemotherapy in certain patient populations. The full promise of telomerase inhibition in malignant disease has not yet been realized; however, emerging data from pivotal registration studies in myeloid malignancies may finally provide a novel therapeutic to our armamentarium.
Authorship
Contribution: J.A.W. wrote and conceptualized the primary draft of the manuscript; C.B. provided meaningful feedback and wrote a significant portion of the scientific background; R.S.K. and C.H.M.J. contributed meaningful edits and contributed to the manuscript; and J.O.M. oversaw the manuscript writing and provided meaningful feedback and edits.
Conflict-of-interest disclosure: R.S.K. is on the speaker bureau of Jazz, Servio, CTI, and Pharma Essential, and serves on the advisory board of or receives honoraria from, Bristol Myers Squibb (BMS), Novartis, AbbVie, Jazz, Servio, Pharma Essential, Taiho, Geron, and CTI. C.H.M.J. is cofounder Aspera Biomedicines. J.O.M. receives clinical research funding paid to the institution from Incyte, Novartis, Roche, Merck, Kartos, CTI Bio, PharmaEssentia, Geron, Promedior, Forbius, and Celgene/BMS, and consulting fees from Incyte, Celgene/BMS, Roche, Novartis, Sierra Oncology, CTI Bio, PharmaEssentia, Constellation, Kartos, and Karyopharm. The remaining authors declare no competing financial interests.
Correspondence: John O. Mascarenhas, Division of Hematology/Oncology, Icahn School of Medicine at Mount Sinai, 1 Gustave L Levy Pl, Box 1079, New York, NY 10029; e-mail: John.Mascarenhas@mssm.edu.