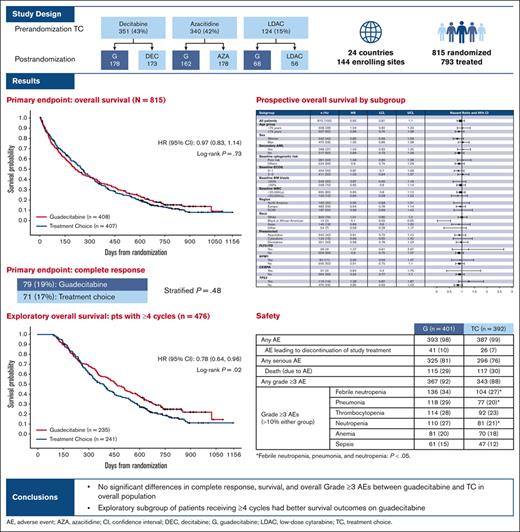
Key Point
No significant differences in complete response, survival, or overall grade ≥3 AEs were observed between guadecitabine and TC.
Abstract
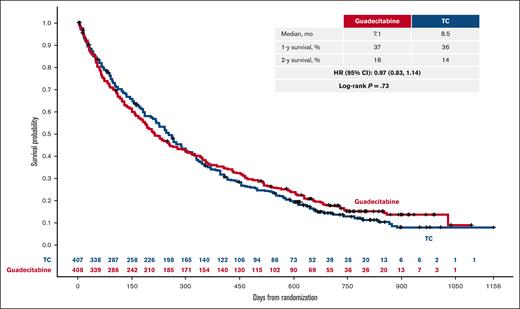
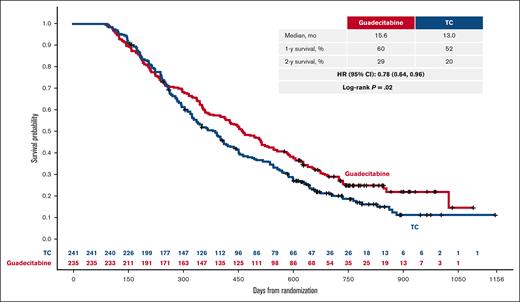
This phase 3 study evaluated the efficacy and safety of the new hypomethylating agent guadecitabine (n = 408) vs a preselected treatment choice (TC; n = 407) of azacitidine, decitabine, or low-dose cytarabine in patients with acute myeloid leukemia unfit to receive intensive induction chemotherapy. Half of the patients (50%) had poor Eastern Cooperative Oncology Group Performance Status (2-3). The coprimary end points were complete remission (19% and 17% of patients for guadecitabine and TC, respectively [stratified P = .48]) and overall survival (median survival 7.1 and 8.5 months for guadecitabine and TC, respectively [hazard ratio, 0.97; 95% confidence interval, 0.83-1.14; stratified log-rank P = .73]). One- and 2-year survival estimates were 37% and 18% for guadecitabine and 36% and 14% for TC, respectively. A large proportion of patients (42%) received <4 cycles of treatment in both the arms. In a post hoc analysis of patients who received ≥4 treatment cycles, guadecitabine was associated with longer median survival vs TC (15.6 vs 13.0 months [hazard ratio, 0.78; 95% confidence interval, 0.64-0.96; log-rank P = .02]). There was no significant difference in the proportion of patients with grade ≥3 adverse events (AEs) between guadecitabine (92%) and TC (88%); however, grade ≥3 AEs of febrile neutropenia, neutropenia, and pneumonia were higher with guadecitabine. In conclusion, no significant difference was observed in the efficacy of guadecitabine and TC in the overall population. This trial was registered at www.clinicaltrials.gov as #NCT02348489.
Introduction
Despite recent progress in the treatment of acute myeloid leukemia (AML), for a long time, the prognosis of older patients unfit to receive intensive chemotherapy is poor. In a recent review of a large population with AML from the National Cancer Institute Surveillance, Epidemiology, and end results data, the median age at diagnosis was 65 years and the 5-year survival rate in patients aged ≥70 years was only 5% between 2010 and 2017.1
The first-generation epigenetically targeted hypomethylating agents (HMAs) azacitidine and decitabine have been approved in Europe as single-agent treatments for adults with AML who are not candidates for intensive induction chemotherapy or hematopoietic cell transplantation based on results from randomized phase 3 trials of these agents vs patient choice or conventional care regimens, with median overall survival (OS) times in the range of 8 to 10 months.2,3 Both agents were recently approved in the United States and Europe for the treatment of unfit patients with AML in combination with the B-cell lymphoma 2 (BCL2) inhibitor venetoclax. This was based on the favorable outcomes of the combination in clinical trials (including phase 3), with a median survival of 14.7 compared with 9.7 months with azacitidine alone.4,5 Further improvement in OS in unfit patients with AML may be brought about by improvements in HMA treatment or a combination of HMA treatment with newer agents, such as isocitrate dehydrogenase (IDH) inhibitors, in patients diagnosed with the targeted mutation6,7 or the CD47 antibody magrolimab.8 Other drug combinations with HMAs, such as histone deacetylase (HDAC) inhibitors9 and targeted fms-like tyrosine kinase-3 (FLT3) inhibitors,10 have failed to show a survival benefit in this difficult-to-treat population.
HMA decitabine requires its incorporation into DNA, making its synthesis phase cycle-dependent.11 Therefore, it is limited by its shorter half-life and exposure time owing to its rapid degradation by cytidine deaminase. Guadecitabine is a new HMA that is a dinucleotide of decitabine and deoxyguanosine linked by a 3'→5' phosphodiester bond and is not metabolized by cytidine deaminase, the enzyme that degrades decitabine.12 Gradual cleavage of this phosphodiesterase bond following subcutaneous injection results in a more sustained formation of decitabine from guadecitabine, prolonging its exposure window.13 This should allow for more incorporation into the DNA of leukemia cells during the synthesis phase of the cell cycle. The prolonged exposure window of decitabine formed after dosing with subcutaneous guadecitabine is the proposed basis for its potentially increased efficacy compared with intravenous decitabine. In addition, the small volume (∼1 mL) of subcutaneous injection offers a more convenient administration route than decitabine 1-hour intravenous infusion. Recently, an oral decitabine/cedazuridine fixed-dose combination was approved by the US Food & Drug Administration based on an equivalent area under the curve to standard-dose IV decitabine, with similar efficacy and safety in patients with myelodysplastic syndromes;14 however, it has not yet been approved for the treatment of AML. Guadecitabine phase 2 data in AML showed that guadecitabine is clinically active as a single-agent HMA, with an overall 53% composite complete remission (CRc) in newly diagnosed AML15 and 30% CRc in relapsed/refractory AML.16 Here, we present the results of the first randomized study of guadecitabine vs treatment choice (TC) in newly diagnosed patients with AML who were unfit for intensive chemotherapy.
Methods
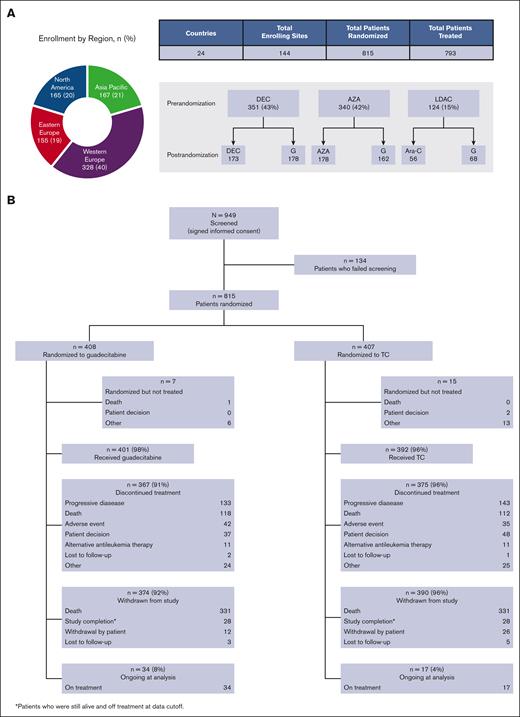
The study was conducted at 144 centers in 24 countries in Asia, Europe, and North America (supplemental Table 1). The trial was approved by the relevant institutional review boards and independent ethics committees and was conducted in accordance with the Declaration of Helsinki. All patients provided written informed consent. The authors had access to all study data, and the analyses were performed by Astex Pharmaceuticals (Pleasanton, CA). Trial information can be found at www.clinicaltrials.gov under identification number NCT02348489.
Patients
Eligible patients were adults with previously untreated AML who were not considered candidates for intensive remission induction chemotherapy, largely based on the criteria of the Italian hematology groups,17 with either age ≥75 or <75 years and ≥1 of the following: poor performance status (Eastern Cooperative Oncology Group Performance Status [ECOG PS] 2 to 3), clinically significant heart or lung comorbidities, liver transaminases >3 times the upper limit of normal, contraindications to anthracycline therapy, and other comorbidities incompatible with intensive remission induction chemotherapy. For eligibility, AML diagnosis was cytologically or histologically confirmed by the investigator according to the 2008 World Health Organization classification (with bone marrow [BM] or peripheral blood [PB] blast counts ≥20%). Creatinine clearance (as estimated by the Cockcroft-Gault or other medically acceptable formulas) must be ≥30 mL/min.
Patients were excluded if they had extramedullary central nervous system AML, a second malignancy requiring active therapy, or prior treatment with decitabine or azacitidine.
Study design and treatment
This was an international, phase 3, multicenter, randomized, parallel-group, open-label, active-controlled study to compare the efficacy and safety of guadecitabine and TC in adults with previously untreated AML who were unfit for intensive remission induction chemotherapy. Randomization was 1:1 and stratified by age, ECOG PS, study-center region, and secondary AML or poor-risk cytogenetics (as determined by the National Comprehensive Cancer Network 2014 criteria). Guadecitabine was administered as 60 mg/m2 per day subcutaneous on days 1 to 5 every 28 days. The treatment options were azacitidine, decitabine, or low-dose cytarabine (LDAC) given as follows: azacitidine 75 mg/m2 per day IV infusion or sc on days 1 to 7 every 28 days; decitabine 20 mg/m2 per day given as a 1-hour IV infusion on days 1 to 5 every 28 days; and LDAC 20 mg sc twice daily on days 1 to 10 every 28 days. Selection of 1 of the TC options was made by the investigator before randomization. All treatments were allowed to be given as outpatient treatment. Other TC treatment parameters, such as dose adjustment guidelines and concomitant supportive care treatment, followed locally approved prescription information and institutional standard practices. The treatment was continued until disease progression, unacceptable toxicity, patient decision, or death occurred.
An independent data monitoring committee was established to provide an independent analysis of accumulating safety and efficacy data and to make recommendations to the sponsor and study steering committee, as needed, to modify or discontinue the trial. Randomized data summaries by treatment arm were provided to the data monitoring committee by an independent statistician.
Efficacy end points
The study had 2 primary end points: complete remission (CR) and OS. Other secondary end points included CRc (CR + CR with incomplete platelet recovery [CRp] and/or CR with incomplete neutrophil recovery regardless of platelets [CRi]), duration of response, progression-free survival, incidence and severity of adverse events (AEs), and 30- and 60-day all-cause mortality. Prospective subgroup analyses of OS were performed based on the following baseline characteristics: age (<75 years vs ≥75 years), sex (female vs male), secondary AML (yes vs no), cytogenetic risk (poor vs other), ECOG PS (0-1 vs 2-3), BM blasts (≤30% vs >30%), total white blood cell counts (WBCs; <20 000/μL vs ≥20 000/μL), geographic region (North America vs Europe vs rest of the world), race (White vs Black or African-American vs Asian vs other), preselected TC (azacitidine vs decitabine vs LDAC), and genetic mutations that influence prognostic risk based on the 2010 European Leukemia Net classification (presence vs absence of nucleophosmin-1 [NPM1], CCAAT enhancer binding protein-α [CEBPA], and FLT3-internal tandem duplication [FLT3-ITD]).18 Tumor protein 53 [TP53] was added as another genetic marker during the study.
The response was assessed by an independent pathologist blinded to treatment assignment through evaluation of PB, BM, and transfusion data using the 2003 International Working Group AML response criteria pre- and posttreatment,19 with CRp and CRi assessed as separate categories under CRc. The response was assessed on day 1 of each cycle, starting on day 1 of cycle 3. BM samples were collected at screening and at the end of cycles 2, 4, and 6 (day 1 of cycles 3, 5, and 7) unless PB showed persistence of leukemic blasts that excluded the possibility of a marrow response. After cycle 6 (ie, starting with day 1 of cycle 7), BM aspirate/biopsy was repeated every 3 months for the first year of the study and then every 6 months thereafter until PB or BM assessment showed disease progression or relapse. All patients were followed-up for survival. The duration of CR was assessed from the time of first observation until relapse. Progression-free survival was assessed as the time from the date of randomization until the earliest relapse, progression by either the investigator or central reading center, start of alternative treatment, or death.
Cytogenetics and genetic biomarker assessment
Cytogenetics were recorded at screening using a local center assessment and classified using the National Comprehensive Cancer Network version 2.2014 criteria.20 BM and blood samples were collected within 28 days before starting the study treatment to evaluate gene mutations: NPM1, CEBPA, FLT3-ITD, and TP53. Molecular genetic analyses were performed centrally at Ulm University Hospital, Ulm, Germany using next-generation sequencing, where applicable, as previously described.21,22
Safety
Safety assessments were performed for all patients who received ≥1 dose of the study treatment. All treatment-emergent AEs (AEs that occurred after the start of the study treatment until 30 days after the last dose of treatment or until starting a new antileukemic treatment) were recorded, regardless of the causal relationship with the study treatment. AEs were reported and graded using the Common Terminology Criteria for Adverse Events v4.03.
Statistical analyses
By trial design, the overall (2-sided) α level of 0.05 was split between the 2 primary end points of CR (0.04) and OS (0.01). If statistical significance was achieved for CR hierarchically, the final analysis of OS was conducted at an overall 2-sided 0.05 α level. If the CR analysis was not significant (P > .04), survival analysis was conducted at the 0.01 α level. Assuming a CR rate of ∼20% for patients treated in the TC group and 30% in the guadecitabine group, 800 patients (400 per treatment group) would provide ∼89% power to detect an overall difference of 0.10 when using a 2-sided Cochran Mantel-Haenszel test with a 2-sided α level of 0.04. For OS, an analysis of 670 death events would provide 90% power to detect a hazard ratio (HR) of ∼0.78 (a difference in median survival between 7 months in the TC group and 9 months in the guadecitabine group), when using a 2-sided stratified log-rank test at a 0.05 α level. Hence, the assessment of OS required approximately the same sample size of 800 patients.
All efficacy analyses were performed on an intent-to-treat basis (all randomized patients; efficacy analysis data set). Safety analyses were performed for all the treated patients (safety data set). The CR rate was compared between the 2 treatment groups using the Cochran Mantel-Haenszel test at an α level of 0.04 stratified by the stratification factors used at randomization: age (<75 years vs ≥75 years), ECOG PS (0-1 vs 2-3), study-center region (North America vs Europe vs rest of the world), and presence of either secondary AML or poor-risk cytogenetics (secondary AML or known poor-risk cytogenetics vs de novo AML with no known poor-risk cytogenetics). Survival curves were estimated using the Kaplan-Meier method and formally compared between the 2 treatment groups using a 2-sided stratified log-rank test stratified by the same stratification factors described for the CR rate.
For subgroup analysis of OS, the HR of guadecitabine vs TC and associated 95% confidence intervals (CIs) were provided for subgroups prospectively defined in the protocol, as well as for post hoc exploratory subgroups of patients who received ≥4 or ≥6 cycles. All log-rank P values for exploratory analyses were nominal.
All statistical tests and CIs created were 2-sided with an α value of 0.05 unless otherwise specified. SAS 9.4 (SAS Institute Inc, Cary, NC) was used for the analyses.
Results
Patient disposition
Patients were randomized in the study between 19 March 2015, and 25 November 2016. The median follow-up was 25.5 months (interquartile range, 22.4-29.9), with a data cutoff in May 2018. Of the 949 patients screened for the study, 815 were randomized to either guadecitabine (n = 408) or TC (n = 407), and 793 actually received treatment (guadecitabine: n = 401; TC: n = 392). Most screening failures were either because the patients did not conform to the strict eligibility criteria of unfitness to intensive chemotherapy or withdrawal of consent before randomization. The preselected TC assignment before randomization, randomization assignment, and patient disposition are shown in Figure 1.
Patient Assignments and Disposition. Pre- and postrandomization assignments (A) and overall patient disposition (B). DEC, decitabine; G, guadecitabine AZA, azacitidine, LDAC, low dose Ara-C.
Patient Assignments and Disposition. Pre- and postrandomization assignments (A) and overall patient disposition (B). DEC, decitabine; G, guadecitabine AZA, azacitidine, LDAC, low dose Ara-C.
Most patients (85%) were preselected for a TC of HMA equally split between azacitidine (42%) and decitabine (43%). At the time of data cutoff, 94% of the treated patients discontinued treatment. The most common reasons for treatment discontinuation were progressive disease (35%), death (29%), patient decision (11%), and AE (10%). The proportion of patients who discontined treatment by reason was similar in the guadecitabine and TC groups.
Baseline characteristics
Table 1 summarizes the patient and disease characteristics at baseline for all those randomized (efficacy analysis set). The demographic characteristics were well balanced between the treatment groups. The overall median age of the patients was 76 years (range 56-94). Half of the patients (50%) had a poor ECOG PS of 2 to 3, including 10% with a PS of 3; approximately one-third (35%) had poor-risk cytogenetics. Proliferative disease (defined as a baseline total WBC ≥20 000/μL) was present in 15% of patients. Mutations in TP53 and NPM1 were found in 14% and 11%, respectively. FLT3-ITD and CEBPA were found in 4% of patients.
Baseline characteristics for all randomized patients (efficacy analysis set)
| Patient characteristics∗ . | Guadecitabine (n = 408) . | TC (n = 407) . | Total (N = 815) . |
|---|---|---|---|
| Median age, y (range) | 76 (56-93) | 76 (59-94) | 76 (56-94) |
| Age category, n (%) | |||
| <75 y | 155 (38) | 153 (38) | 308 (38) |
| ≥75 y | 253 (62) | 254 (62) | 507 (62) |
| Sex, n (%) | |||
| Men | 231 (57) | 242 (59) | 473 (58) |
| Women | 177 (43) | 165 (41) | 342 (42) |
| Race, n (%) | |||
| White | 311 (76) | 291 (71) | 602 (74) |
| Black or African-American | 9 (2) | 5 (1) | 14 (2) |
| Asian | 71 (17) | 74 (18) | 145 (18) |
| Other or missing | 17 (4) | 37 (9) | 54 (7) |
| ECOG PS, n (%) | |||
| 0 | 54 (13) | 52 (13) | 106 (13) |
| 1 | 148 (36) | 150 (37) | 298 (37) |
| 2 | 162 (40) | 169 (42) | 331 (41) |
| 3 | 44 (11) | 36 (9) | 80 (10) |
| Secondary AML, n (%)† | |||
| Yes | 148 (36) | 150 (37) | 298 (37) |
| No | 260 (64) | 257 (63) | 517 (63) |
| Cytogenetic risk levels, n (%)‡ | |||
| Better risk | 5 (1) | 12 (3) | 17 (2) |
| Intermediate risk | 239 (59) | 236 (58) | 475 (58) |
| Poor risk | 140 (34) | 141 (35) | 281 (34) |
| Not evaluable or missing | 24 (5) | 18 (4) | 42 (4) |
| Median BM blasts, % | 45 | 41 | 43 |
| WBCs | |||
| ≥20 000/μL, n (%) | 62 (15) | 58 (14) | 120 (15) |
| Mutation, n (%) | |||
| NPM1 | 44 (11) | 46 (11) | 90 (11) |
| CEBPA§ | 13 (3) | 18 (4) | 31 (4) |
| FLT3-ITD | 20 (5) | 16 (4) | 36 (4) |
| TP53 | 60 (15) | 56 (14) | 116 (14) |
| Patient characteristics∗ . | Guadecitabine (n = 408) . | TC (n = 407) . | Total (N = 815) . |
|---|---|---|---|
| Median age, y (range) | 76 (56-93) | 76 (59-94) | 76 (56-94) |
| Age category, n (%) | |||
| <75 y | 155 (38) | 153 (38) | 308 (38) |
| ≥75 y | 253 (62) | 254 (62) | 507 (62) |
| Sex, n (%) | |||
| Men | 231 (57) | 242 (59) | 473 (58) |
| Women | 177 (43) | 165 (41) | 342 (42) |
| Race, n (%) | |||
| White | 311 (76) | 291 (71) | 602 (74) |
| Black or African-American | 9 (2) | 5 (1) | 14 (2) |
| Asian | 71 (17) | 74 (18) | 145 (18) |
| Other or missing | 17 (4) | 37 (9) | 54 (7) |
| ECOG PS, n (%) | |||
| 0 | 54 (13) | 52 (13) | 106 (13) |
| 1 | 148 (36) | 150 (37) | 298 (37) |
| 2 | 162 (40) | 169 (42) | 331 (41) |
| 3 | 44 (11) | 36 (9) | 80 (10) |
| Secondary AML, n (%)† | |||
| Yes | 148 (36) | 150 (37) | 298 (37) |
| No | 260 (64) | 257 (63) | 517 (63) |
| Cytogenetic risk levels, n (%)‡ | |||
| Better risk | 5 (1) | 12 (3) | 17 (2) |
| Intermediate risk | 239 (59) | 236 (58) | 475 (58) |
| Poor risk | 140 (34) | 141 (35) | 281 (34) |
| Not evaluable or missing | 24 (5) | 18 (4) | 42 (4) |
| Median BM blasts, % | 45 | 41 | 43 |
| WBCs | |||
| ≥20 000/μL, n (%) | 62 (15) | 58 (14) | 120 (15) |
| Mutation, n (%) | |||
| NPM1 | 44 (11) | 46 (11) | 90 (11) |
| CEBPA§ | 13 (3) | 18 (4) | 31 (4) |
| FLT3-ITD | 20 (5) | 16 (4) | 36 (4) |
| TP53 | 60 (15) | 56 (14) | 116 (14) |
NCCN, National Comprehensive Cancer Network.
Baseline hematologic characteristics based on local pathologic assessment.
Secondary to myelodysplastic syndromes or other antecedent hematologic disorder and central pathologist AML World Health Organization classification.
Based on local treating center data according to NCCN 2014 classification.
Defined irrespective of presence of bi- or monoallelic mutations in CEBPA.
Treatment exposure and treatment discontinuation
The median number of treatment cycles was 5 for both treatment arms (range 1-38 cycles for guadecitabine and 1-34 for TC). Similar proportions of patients had either dose-reduced cycles (32% and 29% for guadecitabine and TC, respectively) or delayed cycles (46% for guadecitabine and 41% for TC).
Importantly, large but similar proportions of patients (42% and 41% in the guadecitabine and TC arms, respectively) received <4 cycles of treatment. The most common reasons for early treatment discontinuation were death and disease progression (Table 2). Similarly, 54% of the guadecitabine and TC arms received <6 cycles of treatment (supplemental Table 2). There were no significant differences between the 2 treatment arms in the baseline characteristics of the subgroups of patients who received ≥4 or ≥6 cycles.
Summary of patients who received less than 4 cycles of treatment (all randomized patients)
| Patients, n (%) . | Guadecitabine (n = 408) . | TC (n = 407) . |
|---|---|---|
| <4 cycles | 173 (42) | 166 (41) |
| Primary reason for receiving <4 cycles | ||
| Randomized, but not treated | 7 (2) | 15 (4) |
| AE | 26 (6) | 21 (5) |
| Death | 72 (18) | 64 (16) |
| Progressive disease | 31 (8) | 31 (8) |
| Alternative antileukemia therapy | 3 (<1) | 2 (<1) |
| Patient decision to permanently stop treatment | 23 (6) | 22 (5) |
| Lost to follow-up | 1 (<1) | 0 |
| Other | 10 (2) | 11 (3) |
| Patients, n (%) . | Guadecitabine (n = 408) . | TC (n = 407) . |
|---|---|---|
| <4 cycles | 173 (42) | 166 (41) |
| Primary reason for receiving <4 cycles | ||
| Randomized, but not treated | 7 (2) | 15 (4) |
| AE | 26 (6) | 21 (5) |
| Death | 72 (18) | 64 (16) |
| Progressive disease | 31 (8) | 31 (8) |
| Alternative antileukemia therapy | 3 (<1) | 2 (<1) |
| Patient decision to permanently stop treatment | 23 (6) | 22 (5) |
| Lost to follow-up | 1 (<1) | 0 |
| Other | 10 (2) | 11 (3) |
Efficacy
Clinical response
Clinical response data are summarized in Table 3. The CR rate was 19% in the guadecitabine group and 17% in the TC group (P = .48). The median CR duration was just >7 months in both treatment arms, with no significant difference between the 2 groups. Similar to previous observations with HMA treatment, the time to best response was relatively long, with a median time to best response of ∼4 months, corresponding to ≥4 treatment cycles for both treatment arms. The CR rates were 18% and 19% for the azacitidine and decitabine groups, respectively, in the TC group.
Clinical response∗
| . | Guadecitabine (n = 408) . | TC (n = 407) . | Difference: guadecitabine vs TC (95% CI) . |
|---|---|---|---|
| CR, n (%) | 79 (19) | 71 (17) | 1.92 (−3.67 to 7.50) |
| P value† | .48 | ||
| CRc (CR + CRp and/or CRi) | 93 (23) | 91 (22) | 0.39 (−5.34 to 6.13) |
| Median duration of CR, mo (95% CI) | 7.2 (5.8-8.7) | 7.7 (6.0-9.6) | |
| Median time to best response, mo (range)‡ | 4.2 (1.4-12.4) | 3.9 (1.7-17.0) |
| . | Guadecitabine (n = 408) . | TC (n = 407) . | Difference: guadecitabine vs TC (95% CI) . |
|---|---|---|---|
| CR, n (%) | 79 (19) | 71 (17) | 1.92 (−3.67 to 7.50) |
| P value† | .48 | ||
| CRc (CR + CRp and/or CRi) | 93 (23) | 91 (22) | 0.39 (−5.34 to 6.13) |
| Median duration of CR, mo (95% CI) | 7.2 (5.8-8.7) | 7.7 (6.0-9.6) | |
| Median time to best response, mo (range)‡ | 4.2 (1.4-12.4) | 3.9 (1.7-17.0) |
IWG, International Working Group.
Response assessed based on IWG response criteria for AML by independent pathologist blinded to treatment assignment, with CRp and CRi reported separately
Cochran Mantel-Haenszel method adjusting for stratification factors used at randomization.
Best of CR, CRp, or CRi.
Survival
The median OS was 7.1 months for guadecitabine and 8.5 months for TC: HR 0.97 (95% CI, 0.83-1.14; stratified log-rank P = .73; Figure 2). The survival curves intersected at ∼9 to 10 months after randomization, where Kaplan-Meier estimates of survival were shorter for guadecitabine for the 25th percentile (2.4 vs 3.1 months) and longer for guadecitabine for the 75th percentile (19.5 vs 16.8 months) compared with TC. The 12-month survival rates were 37% for guadecitabine and 36% for TC, and the 24-month survival rates were 18% and 14%, respectively. Median OS values were 8.7 and 8.2 months for the azacitidine and decitabine groups, respectively, in the TC arm. Progression-free survival was similar between guadecitabine and TC (median, 5.3 months [95% CI, 4.5-5.9] and 5.5 months [95% CI, 4.9-5.9], respectively).
Survival analysis (efficacy analysis set). Coprimary end point of OS.
Exploratory survival analyses
Fifty-one patients (34 on guadecitabine and 17 on TC) were alive and continuing treatment at the time of the primary data cutoff after a median follow-up of >2 years; Table 4 summarizes their main characteristics and responses. Compared with the overall population, these patients predominantly had ECOG PS 0 to 1 at baseline (70%), no poor-risk cytogenetics (86%), and nonproliferative disease (total WBCs <20 000/μL; 94%), and none of them had FLT3-ITD or TP53 mutations. None of these patients received hematopoietic stem cell transplantation or intensive chemotherapy as additional or subsequent treatment. Of note, 25% of the long survivors never responded using the International Working Group criteria.
Long survivor∗baseline characteristics and response
| Baseline characteristics . | Guadecitabine (n = 34) . | TC (n = 17) . | Total (N = 51) . |
|---|---|---|---|
| Median age, y (range) | 76.5 (62-93) | 75 (66-82) | 76 (62-93) |
| Sex, n (%) | |||
| Men | 16 (47) | 11 (65) | 27 (53) |
| Women | 18 (53) | 6 (35) | 24 (47) |
| ECOG PS, n (%) | |||
| 0-1 | 26 (76) | 10 (59) | 36 (71) |
| 2-3 | 8 (24) | 7 (41) | 15 (29) |
| Secondary AML, n (%)∗ | 11 (32) | 7 (41) | 18 (35) |
| Poor cytogenetic risk, n (%) | 3 (9) | 4 (23) | 7 (14) |
| Median BM blasts, % (range) | 33 (4-97) | 30 (20-87) | 32 (4-97) |
| WBC <20 000/μL, n (%) | 32 (94) | 16 (94) | 48 (94) |
| Mutation, n (%) | |||
| FLT3- ITD n (%) | 0 | 0 | 0 |
| NPM1 | 5 (15) | 4 (23) | 9 (18) |
| CEBPA | 0 | 0 | 0 |
| TP53 | 0 | 0 | 0 |
| Clinical response n (%) | |||
| CR | 23 (68) | 12 (71) | 35 (69) |
| CRp and/or CRi | 2 (6) | 1 (6) | 3 (6) |
| No response | 9 (26) | 4 (24) | 13 (25) |
| Baseline characteristics . | Guadecitabine (n = 34) . | TC (n = 17) . | Total (N = 51) . |
|---|---|---|---|
| Median age, y (range) | 76.5 (62-93) | 75 (66-82) | 76 (62-93) |
| Sex, n (%) | |||
| Men | 16 (47) | 11 (65) | 27 (53) |
| Women | 18 (53) | 6 (35) | 24 (47) |
| ECOG PS, n (%) | |||
| 0-1 | 26 (76) | 10 (59) | 36 (71) |
| 2-3 | 8 (24) | 7 (41) | 15 (29) |
| Secondary AML, n (%)∗ | 11 (32) | 7 (41) | 18 (35) |
| Poor cytogenetic risk, n (%) | 3 (9) | 4 (23) | 7 (14) |
| Median BM blasts, % (range) | 33 (4-97) | 30 (20-87) | 32 (4-97) |
| WBC <20 000/μL, n (%) | 32 (94) | 16 (94) | 48 (94) |
| Mutation, n (%) | |||
| FLT3- ITD n (%) | 0 | 0 | 0 |
| NPM1 | 5 (15) | 4 (23) | 9 (18) |
| CEBPA | 0 | 0 | 0 |
| TP53 | 0 | 0 | 0 |
| Clinical response n (%) | |||
| CR | 23 (68) | 12 (71) | 35 (69) |
| CRp and/or CRi | 2 (6) | 1 (6) | 3 (6) |
| No response | 9 (26) | 4 (24) | 13 (25) |
Patients who were still alive and on treatment at data cutoff.
Owing to the known delayed response to HMA and the importance of receiving ≥4 treatment cycles to achieve the best response, exploratory survival analyses were conducted in patients who received ≥4 cycles (Figure 3) and ≥6 cycles (supplemental Figure 1). In these subgroups, patients on guadecitabine achieved a longer median survival than those on TC, with similar improvement outcomes for guadecitabine in 1- and 2-year survival estimates. Survival analysis was also conducted in the subgroup of patients who received <4 cycles, which demonstrated no significant difference between the 2 treatment arms.
Prospective subgroup analyses
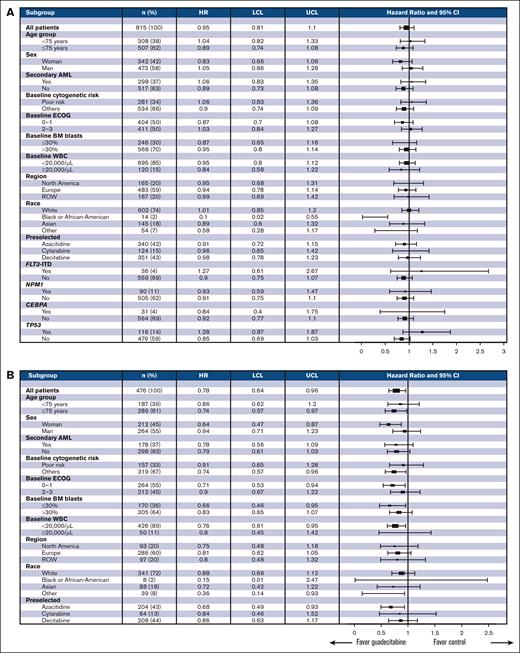
Figure 4A shows no significant differences between guadecitabine and TC in any of the prospectively defined subgroups in the overall population. Notably, there was no difference in survival between guadecitabine and each of the TC drugs. An exploratory forest plot of the prospective subgroups of patients treated with ≥4 cycles showed that survival trends favored guadecitabine in all the subgroups (Figure 4B). Patients who received ≥6 cycles of treatment showed similar findings (supplemental Figure 2).
Forest plot of OS in prospective subgroups (A) and in patients who received ≥4 cycles of treatment (B). LCL, lower confidence limit; ROW, rest of world; UCL, upper confidence limit.
Forest plot of OS in prospective subgroups (A) and in patients who received ≥4 cycles of treatment (B). LCL, lower confidence limit; ROW, rest of world; UCL, upper confidence limit.
Safety
Table 5 shows the safety results, including grade ≥3 AEs that occurred in either group at a rate ≥10%. Most patients in both groups reported grade ≥3 AEs, regardless of causality (92% and 88% in the guadecitabine and TC arms, respectively). The most common grade ≥3 AEs in both groups were hematologic AEs and infections, which were expected in this patient population and with HMA treatment. Patients who received guadecitabine had a significantly higher incidence of febrile neutropenia, neutropenia, and pneumonia. The 30- and 60-day all-cause mortality rates were not significantly different between guadecitabine (11% and 21%, respectively) and TC (10% and 17%, respectively).
Safety results (regardless of causality)
| . | Guadecitabine (n = 401) . | TC (n = 392) . |
|---|---|---|
| Any AE | 393 (98) | 387 (99) |
| AE leading to discontinuation of study treatment | 41 (10) | 26 (7) |
| Any serious AE | 325 (81) | 296 (76) |
| Death (due to AE) | 115 (29) | 117 (30) |
| Any grade ≥3 AE | 367 (92) | 343 (88) |
| Grade ≥3 AEs in >10% of patients (either group) | ||
| Febrile neutropenia | 136 (34) | 104 (27)∗ |
| Pneumonia | 118 (29) | 77 (20)∗ |
| Thrombocytopenia | 114 (28) | 92 (23) |
| Neutropenia | 110 (27) | 81 (21)∗ |
| Anemia | 81 (20) | 70 (18) |
| Sepsis | 61 (15) | 47 (12) |
| . | Guadecitabine (n = 401) . | TC (n = 392) . |
|---|---|---|
| Any AE | 393 (98) | 387 (99) |
| AE leading to discontinuation of study treatment | 41 (10) | 26 (7) |
| Any serious AE | 325 (81) | 296 (76) |
| Death (due to AE) | 115 (29) | 117 (30) |
| Any grade ≥3 AE | 367 (92) | 343 (88) |
| Grade ≥3 AEs in >10% of patients (either group) | ||
| Febrile neutropenia | 136 (34) | 104 (27)∗ |
| Pneumonia | 118 (29) | 77 (20)∗ |
| Thrombocytopenia | 114 (28) | 92 (23) |
| Neutropenia | 110 (27) | 81 (21)∗ |
| Anemia | 81 (20) | 70 (18) |
| Sepsis | 61 (15) | 47 (12) |
Febrile neutropenia, pneumonia, and neutropenia: P < .05.
Discussion
The results reported here are the largest randomized trial with a long median follow-up of >2 years of HMA or LDAC in treatment-naïve patients with AML who were unfit to receive intensive induction chemotherapy. The trial did not meet its coprimary end points of improving CR or OS using guadecitabine in the overall population. Although the current standard of care for these patients has moved on to a combination of HMA and venetoclax, it is still important to conduct research for better HMAs, as these agents remain an essential component in combination treatment. It is also important for the treatment of myelodysplastic syndromes, for which single-agent HMAs remain the standard of care.
We applied strict criteria of unfitness for intensive chemotherapy,16 which resulted in a patient population with a worse general condition and prognosis than those enrolled in previous randomized trials of azacitidine and decitabine.2,3 Previous trials excluded patients with ECOG PS 3 and the percentage of patients with PS 2 was 23% to 24% compared with 50% with PS 2 to 3 in the present trial, including 10% with PS 3. In addition, previous trials excluded patients with proliferative disease (WBC >15 000/μL and >40 000/μL in the azacitidine and decitabine trials, respectively), whereas the present trial had no such exclusion criterion, which resulted in 15% of the patients having baseline WBC >20 000/μL. The aforementioned differences probably explain why median survival in the present trial (7.1 and 8.5 months in the guadecitabine and TC arms, respectively) was in the lower range of previous single-arm HMA randomized trials (range, 7.7-10.4 months). This also probably explains the lower CR rate for guadecitabine in this study compared with the phase 2 data.15 The other coprimary end point of CR (19% and 17% for guadecitabine and TC, respectively) in this trial was similar to that in previous trials (∼16%-20%). Owing to the large sample size of the trial, the survival HR and its relatively narrow 95% CI (HR, 0.97 [95% CI, 0.83-1.14]) probably excluded any large survival difference between guadecitabine and TC treatments. We previously published a post hoc comparison between patients treated with azacitidine vs decitabine in this trial, which showed similar CR and survival outcomes in patients treated with these 2 HMA treatments.23
It is now established that the HMAs decitabine and azacitidine at the currently approved doses act, at least in part, through the epigenetic mechanism of DNA hypomethylation, leading to the reexpression of tumor suppressor genes, immune modulatory effects, and cellular differentiation, with less associated cytotoxicity.11,24 Unlike cytotoxic chemotherapy, epigenetic mechanisms of action require multiple cycles to achieve maximal response and, similar to targeted agents, also require prolongation and continuation of treatment following response to achieve maximal response and survival.9,25,26 In addition, patients can achieve long stabilization of the disease and some survival benefit without an objective response.27 Our findings from the long survivors seem to support this observation, as 25% of the long survivors of almost 3 years never had a response based on objective criteria. The recommended minimal number of HMA cycles to achieve a response based on the US Food and Drug Administration and European Medicines Agency prescribing information and the European Society for Medical Oncology and European Leukemia Net guidelines is 4 to 6 cycles.28,29 In the present trial, the time to best response was ∼4 months; however, ∼42% and ∼54% of the patients in each group received <4 and <6 cycles, respectively. In this context, it is difficult to establish the superiority of 1 HMA over another, because approximately half of the patients in this trial did not receive an optimal HMA treatment duration. This was probably because of the poor general condition of the patients enrolled in this trial. To test this hypothesis, exploratory survival analyses were conducted for patients who received ≥4 or ≥6 cycles. The percentages of patients in these subgroups were almost identical between the guadecitabine and TC arms with similar baseline characteristics, making significant selection bias favoring 1 group over the other unlikely. In the subgroup of patients who received ≥4 or ≥6 cycles of HMA treatment, those who received guadecitabine had better survival outcomes. However, these analyses had an inherent bias in that they excluded patients who died early, and there was a nonsignificant trend of early mortality with guadecitabine from the 60-day mortality data. For this reason, an ad-hoc survival analysis was also performed for patients who received <4 cycles, and there was no significant survival difference between the 2 treatment arms in those patients. Although the exploratory comparison was performed in a subgroup that was not prospectively determined at baseline, it may be possible that a better selection of unfit patients who still have good ECOG PS and fewer comorbidities may result in a patient population that is likely to receive ≥4 cycles. Indeed, the prospective subgroup analysis in the overall population showed survival trends favoring guadecitabine in the prospective subgroups that were mainly enrolled because of age ≥75 years (HR, 0.89) or PS 0 to 1 (HR, 0.87). Similarly, the characteristics of long survivors in Table 4 provide the baseline factors that are likely associated with longer treatment and better survival and suggest that CR induction failure should not be a reason to stop HMA treatment, as ∼25% of those patients never achieved an objective response. In addition, patient hospitalization during treatment in the first cycle with prophylactic antimicrobial treatment similar to that implemented in the VIALE-A phase 3 trial of venetoclax with azacitidine5 may prevent some of the early deaths, 1 of the most common reasons for patients not receiving adequate treatment duration.
The proportion of patients with grade ≥3 AEs, regardless of causality, was similar between guadecitabine (92%) and TC (88%). Certain common hematologic and infection grade ≥3 AEs occurred; however, at a statistically higher incidence with guadecitabine (ie, febrile neutropenia, neutropenia, and pneumonia). This observation may be explained by the potent cytotoxic effect of guadecitabine. These events are expected from AML treatment and should be manageable in experienced treatment centers.
In conclusion, we conducted the largest global randomized trial to date in patients with AML who were unfit for intensive chemotherapy and were treated with HMAs or LDAC. The trial included more frail patients than previous randomized trials of HMAs, and we could not demonstrate the superiority of the new HMA guadecitabine over TC in the overall population. Further analysis of the genomic landscape of this large cohort treated with HMAs is ongoing and will evaluate the effect of guadecitabine in genetically defined subgroups.
Acknowledgments
The authors thank the patients and their families and all investigators and their support staff at all study centers. Editorial support for this manuscript was provided by BioScience Communications, New York, NY.
The study was funded by Astex Pharmaceuticals Inc.
Authorship
Contribution: P.F., M.G., P.L.K., J.-P.J.I., G.J.R., J.M., J.K., T.R., J.N., W.W.J., X.T., M.O.-U., Y.M., Y.H.M., S.-P. Y., J.B., L.G.-K., J.D., E.G., K.Y., K.D., H. Kantarjian, and H.D. contributed to patient data; all authors had access to data analyses; P.F., M.G., P.L.K., J.-P.J.I., G.J.R., Y.H., M.A., and H. Keer wrote the first draft of manuscript; and all authors reviewed, edited, and approved the manuscript.
Conflict-of-interest disclosure: P.F. has received honoraria and (as Groupe Francophone des Myélodysplasies chairperson) research funds from Astex, AbbVie, Bristol Myers Squibb, Novartis, and Janssen; P.L.K. has received consulting fees from BMS and Takeda; J.-P.J.I. has received clinical research support from Astex, and consulting fees from Ascentage and Daiichi; G.J.R. has consulted for Astex, AbbVie, Actinium, Agios, Amgen, Astellas, Bayer, Bristol Myers Squibb, Celltrion, Celgene, Daiichi, Genentech/Roche, GSK, Helsinn, Janssen, Jasper, Jazz, MedImmune, Mesoblast, Novartis, Otsuka, MEI, Pfizer, Sandoz, and Takeda, and has received research funding from Astex, AbbVie, Agios, Amphivena, Celgene, CTI, Janssen, Karyopharm, MedImmune, MEI, Moffitt Cancer Center, Novartis, Onconova, Sunesis, and Tensha, honoraria from Pfizer, and travel expenses from Astex, AbbVie, Agios, Amgen, Amphivena, Array, Bayer, Celgene, Celltrion, Clovis, Eisai, Genentech/Roche, Janssen, Jazz, Novartis, Pfizer, Sandoz, and Sunesis; J.M. has participated on an advisory board and received honoraria from Astex; T.R. has received grants from and participated on an advisory board for Astex; H. Kantarjian has received grants from AbbVie, Amgen, Ascentage, Bristol Myers Squibb, Daiichi-Sankyo, ImmunoGen, Jazz, and Novartis, and honoraria from AbbVie, Amgen, Amphista, Ascentage, Astellas, Biologix, Curis, Ipsen, KAHRl, Labcorp, Novartis, Pfizer, Shenzhen Target Rx, Stemline, and Takeda; J.N. has consulted for AbbVie, Amgen, Astellas, Novartis, Pfizer, Roche, and Takeda, and received travel expenses from Amgen and Janssen; Y.M. has received honoraria from AbbVie, Amgen, Astellas, BMS, Chugai, Janssen, Kyowa-Kirin, Nippon-Shinyaku, Novartis, Otsuka, Pfizer, Sumitomo, and Takeda, and clinical research funding (to institution) from Chugai; S.-P.Y. has participated on advisory boards for Astex, AbbVie, Amgen, Astellas, and Janssen; J.B. has received honoraria from AbbVie, Amgen, Astellas, Avir, BMS/Celgene, Jazz, Paladin, Pfizer, and Taiho; J.D. has participated in advisory committees for Amgen, Amicus, Angelini, Bristol Myers Squibb, Novartis, Pfizer, and Roche; E.G. has received research funding (to Roswell Park) from Alexion, Astex, Blueprint, Celgene, Celldex, and Genentech, consulting fees from AbbVie, Alexion, Apellis, AstraZeneca, Celgene, CTI, Genentech, Novartis, Taiho, and Takeda, honoraria from AAMDSIF, American Society of Hematology, Highlights of American Society of Hematology, MediCom, Physicians Educational Resource, and Picnic Health, and laboratory material from Imago, and participated in an advisory board for Dresner Foundation; K.Y. has received research funding from Astex, Forma, Geron, Gilead, Janssen, Jazz, Karyopharm, Novartis, Onconova, Roche, and Treadwell, and honoraria from AbbVie, Amgen, and Novartis, and participated in advisory boards for Astellas, BMS/Celgene, GSK, Jazz, Novartis, Pfizer, Roche, Shattuck, Taiho, and Takeda; K.D. has received research funding from Agios, Astellas, Celgene/BMS, and Novartis, and honoraria from Celgene/BMS, Daiichi Sankyo, Jazz, Novartis, and Roche, and participated in advisory boards for AbbVie, Celgene/BMS, Daiichi Sankyo, Jazz, Novartis, and Roche; Y.H., H.K., and M.A. are employees of Astex; H.D. has received honoraria from AbbVie, Agios, Amgen, Astellas, AstraZeneca, Berlin-Chemie, Bristol Myers Squibb, Celgene, GEMoaB, Gilead, Janssen, Jazz, Novartis, Servier, and Syndax, and clinical research funding (to institution) from AbbVie, Agios, Amgen, Astellas, Bristol Myers Squibb, Celgene, Jazz, Kronos, and Novartis. The remaining authors declare no competing financial interests.
Correspondence: Pierre Fenaux, Département (DMU) d’hématologie et immunologie, APHP Nord, Service d'Hématologie Seniors, Hôpital St Louis/Université de Paris, 1 Ave Claude Vellefaux, 75475 Paris, France; e-mail: pierre.fenaux@aphp.fr.
References
Author notes
The clinical data can be requested by any qualified researchers who engage in rigorous independent scientific research. Data are available upon request from https://astx.com/contact/ for review and approval.
The full-text version of this article contains a data supplement.