TO THE EDITOR:
Livedoid vasculopathy (LV) is a thrombotic disorder of the dermal vasculature characterized by recurrent, painful leg ulcers. The incidence is 1:100 000 per year, and it predominantly affects women at a ratio of 3:1.1 Its cause is unknown, and although most patients lack medical comorbidities, associations with thrombophilia, autoimmune disease, and venous insufficiency have been reported.2 LV is distinguished from cutaneous vasculitis by the absence of inflammatory cellular infiltrate or vessel wall damage. However, extensive complement (C3) and immunoglobulin (Ig) deposition (IgM > IgG > IgA) has been demonstrated.3,4 It is debated whether this nonspecific deposition provides evidence for an inflammatory etiology.5,6
The possibility that dermal thrombosis in LV may be complement-mediated is of interest. Complement mediates thrombosis and pregnancy loss in antiphospholipid syndrome (APS), a condition associated with LV, and antiphospholipid antibodies have been reported in 18% of patients with LV.7,8 β-2-glycoprotein-I is a regulator of complement, and complement activation leads to increased expression of procoagulant factors and inhibition of fibrinolysis.8,9 There are also histologic similarities between LV and complement-mediated thrombotic microangiopathies (TMAs), such as atypical hemolytic uremic syndrome. Skin biopsies in atypical hemolytic uremic syndrome demonstrate pauci-inflammatory thrombotic vasculopathy with extensive terminal complement deposition.10
The assessment of complement activation has been facilitated by the modified Ham (mHam) assay. The mHam assay uses a nucleated cell line deficient of surface complement inhibitors due to mutations in PIGA. Mean cell viability after exposure to a patient’s serum serves as a proxy for the degree of complement activation in the serum. The mHam assay has been shown to distinguish between complement- and noncomplement-mediated TMAs and predict thrombotic events in patients with APS.11,12
We sought to determine whether patients with LV had evidence of systemic complement dysregulation as measured by the mHam assay.
Participants were identified by querying the Carolina Data Warehouse for Health, a database of electronic medical record information from an academic health system comprising 11 hospitals across North Carolina, for individuals with an International Classification of Disease code associated with LV. The electronic medical record was implemented at the University of North Carolina in April 2014, and the query was conducted in July 2021. Retrieved charts were manually reviewed to confirm record of a skin biopsy consistent with LV as well as clinical documentation supporting the diagnosis. There are no consensus guidelines for the diagnosis of LV; however, supportive histologic features include hyaline thickening of vessel walls in the papillary dermis with focal intraluminal thrombi and minimal inflammatory infiltrate.13 Clinical features include relapsing, painful ulcers over the bilateral lower extremities accompanied by violaceous patches (retiform purpura) and white, stellate scarring (atrophie blanche).2 Participants were included according to our previously published diagnostic approach.14 We excluded those with alternative causes of pauci-inflammatory thrombotic vasculopathy, including embolic and other thrombotic causes (eg, anticoagulant necrosis and cryopathies).
The study was approved by the Institutional Review Board at University of North Carolina and was conducted in accordance with the declaration of Helsinki. Participants were recruited between December 2021 and March 2022, and informed consent was obtained before enrollment. The presence of ulcers was assessed, and serum samples were collected at the time of enrollment.
The mHam test was conducted using participant sera as described previously.11,12 Briefly, PIGA-null TF-1 cells were washed in phosphate-buffered saline and resuspended in a gelatin veronal buffer with calcium and magnesium (B102, Complement Technology Inc) in a 96-well plate at a density of 6700 cells per well. Twenty percent test serum was added to each well in triplicate. Heat-inactivated serum was used as the internal negative control. Shiga Toxin 1 (SML0562, Sigma Aldrich) added to healthy human serum was used as the positive control. Cells were then washed, resuspended in water soluble tetrazolium-1 (WST-1, Roche), a cellular proliferation reagent, and incubated for 2 hours at 37°C. Sample absorbance was calculated at 450 nm using a microplate reader (ELx808, BioTek) at a reference wavelength of 630 nm. The ratio of the absorbance of the sample to that of the heat-inactivated control was subtracted from 1 to determine the percentage of nonviable cells. Values >20% constituted a positive test based on previous experiments.
Soluble C5b-9 (sC5b-9) was determined by enzyme immunoassay, using commercially available antibodies (MicroVue SC5b-9 Plus EIA, Quidel Corporation), and was performed in duplicate for each serum sample. SC5b-9 levels in a healthy cohort have been reported as <300 ng/mL using the same assay.15
Sixteen patients enrolled in the study. Background characteristics and the mHam assay results are outlined in Table 1. Average age of LV onset was 54 years, and 10 patients (62.5%) were female. Seven patients (44%) had ulcers at the time of enrollment, 6 (38%) had laboratory evidence of thrombophilia, and 8 (50%) had venous insufficiency. Serum complements C3 and C4 had been assessed and were within normal limits in 12 patients. Fourteen patients had normal sC5b-9 levels. All patients had received LV-directed therapy: 16 had received antiplatelet agents, 9 had received anticoagulants, 4 had received IV immunoglobulin, and 3 had received rituximab.
Background characteristics and mHam assay results
| Patient . | Age . | Sex . | Hypercoagulable state . | Autoimmune comorbidity . | Chronic venous insufficiency . | Current ulcers . | mHam assay result . | Mean soluble C5b-9 ± SEM (ng/mL) . |
|---|---|---|---|---|---|---|---|---|
| P1 | 70 | F | None known | Levamisole vasculitis | No | No | Negative | 35.7 ± 8.3 |
| P2 | 79 | F | Triple antiphospholipid antibody positive | Antiphospholipid syndrome | Yes | No | Negative | 54.0 ± 23.3 |
| P3 | 78 | F | None known | None known | Yes | Yes | Negative | 12.3 ± 1.7 |
| P4 | 53 | F | Elevated homocysteine | None known | No | Yes | Negative | 22.3 ± 1.7 |
| P5 | 61 | F | None known | None known | Yes | No | Negative | 24.0 ± 6.7 |
| P6 | 64 | M | History of multiple venous thromboembolic events | None known | Yes | No | Negative | 14.0 ± 3.3 |
| P7 | 41 | M | Plasminogen activator inhibitor mutation | None known | No | Yes | Negative | 4.0 ± 10.0 |
| P8 | 89 | M | Positive anticardiolipin and anti-β-2-GP-I | None known | Yes | Yes | Positive | 34.0 ± 6.7 |
| P9 | 73 | M | None known | Diffuse connective tissue disease | No | No | Negative | 25.7 ± 21.7 |
| P10 | 78 | F | None known | None known | Yes | No | Negative | 44.0 ± 6.7 |
| P11 | 65 | M | Elevated homocysteine | None known | No | Yes | Negative | 29.0 ± 11.7 |
| P12 | 72 | F | None known | None known | No | No | Negative | 2689 ± 1232 |
| P13 | 56 | M | None known | None known | Yes | Yes | Negative | 537.3 ± 483.3 |
| P14 | 68 | F | None known | None known | No | Yes | Negative | 40.7 ± 3.3 |
| P15 | 60 | F | Heterozygous factor V Leiden | None known | Yes | No | Negative | 22.3 ± 1.7 |
| P16 | 65 | F | None known | None known | No | No | Negative | 77.3 ± 36.7 |
| Patient . | Age . | Sex . | Hypercoagulable state . | Autoimmune comorbidity . | Chronic venous insufficiency . | Current ulcers . | mHam assay result . | Mean soluble C5b-9 ± SEM (ng/mL) . |
|---|---|---|---|---|---|---|---|---|
| P1 | 70 | F | None known | Levamisole vasculitis | No | No | Negative | 35.7 ± 8.3 |
| P2 | 79 | F | Triple antiphospholipid antibody positive | Antiphospholipid syndrome | Yes | No | Negative | 54.0 ± 23.3 |
| P3 | 78 | F | None known | None known | Yes | Yes | Negative | 12.3 ± 1.7 |
| P4 | 53 | F | Elevated homocysteine | None known | No | Yes | Negative | 22.3 ± 1.7 |
| P5 | 61 | F | None known | None known | Yes | No | Negative | 24.0 ± 6.7 |
| P6 | 64 | M | History of multiple venous thromboembolic events | None known | Yes | No | Negative | 14.0 ± 3.3 |
| P7 | 41 | M | Plasminogen activator inhibitor mutation | None known | No | Yes | Negative | 4.0 ± 10.0 |
| P8 | 89 | M | Positive anticardiolipin and anti-β-2-GP-I | None known | Yes | Yes | Positive | 34.0 ± 6.7 |
| P9 | 73 | M | None known | Diffuse connective tissue disease | No | No | Negative | 25.7 ± 21.7 |
| P10 | 78 | F | None known | None known | Yes | No | Negative | 44.0 ± 6.7 |
| P11 | 65 | M | Elevated homocysteine | None known | No | Yes | Negative | 29.0 ± 11.7 |
| P12 | 72 | F | None known | None known | No | No | Negative | 2689 ± 1232 |
| P13 | 56 | M | None known | None known | Yes | Yes | Negative | 537.3 ± 483.3 |
| P14 | 68 | F | None known | None known | No | Yes | Negative | 40.7 ± 3.3 |
| P15 | 60 | F | Heterozygous factor V Leiden | None known | Yes | No | Negative | 22.3 ± 1.7 |
| P16 | 65 | F | None known | None known | No | No | Negative | 77.3 ± 36.7 |
Anti-β-2-GP-I, anti–β-2-glycoprotein I antibody; F, female; M, male; SEM, standard error of the mean.
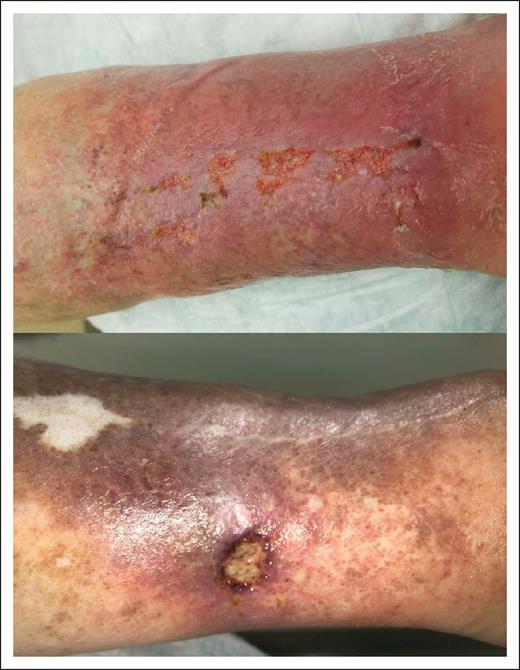
One patient had a positive mHam assay result. He had developed recurrent bilateral lower extremity ulceration 7 years before enrollment. His antinuclear antibody was positive at a titer of ≥1:640, and anti–double-stranded DNA antibodies were positive as well. Antiphospholipid antibodies were tested on 2 occasions separated by >12 weeks. Anticardiolipin IgM antibody levels were 12 and 14 IgM phospholipid units, with a reference range from 0 to 11 IgM phospholipid units. Anti–β-2-glycoprotein 1 IgG antibody levels were 34 and 45 units per mL, with a reference range from 0 to 20 units per mL. There was no evidence of a lupus anticoagulant. C3, C4, and sC5b-9 levels were normal. No history of additional thrombotic events or systemic autoinflammatory disease was obtained, but he had ultrasonographic evidence of chronic venous insufficiency in the left leg. Representative images of his ulcerations are shown in Figure 1.
Clinical features of LV in a patient (P8) with a positive mHam test result. (Top) Active ulcers on a background of purple, discolored skin. (Bottom) Healing ulcer with evidence of atrophie blanche and postinflammatory hyperpigmentation.
Clinical features of LV in a patient (P8) with a positive mHam test result. (Top) Active ulcers on a background of purple, discolored skin. (Bottom) Healing ulcer with evidence of atrophie blanche and postinflammatory hyperpigmentation.
Most patients with LV do not have evidence of complement dysregulation, as measured by the mHam assay. A single positive mHam result was found for a patient with antiphospholipid antibodies. Positive mHam results are associated with the presence of antiphospholipid antibodies and predict initial or recurrent thrombosis in this population.12 Two patients had elevated sC5b-9 levels; however, elevations in serum complement byproducts are unreliable for predicting thrombosis in APS or distinguishing between complement and non-complement-mediated TMA.15,16 Whether these findings are incidental or reflective of complement being 1 of several pathways that lead to thrombosis in LV remains to be determined.
Despite our findings, a central role for complement in the pathogenesis of LV is impossible to exclude. It is possible that complement dysregulation in LV may occur at a local, rather than systemic level. It is also possible that complement activation may be correlated with disease activity, and more than half of patients did not have ulcers at time of enrollment. Recruitment of an ideal cohort of patients with active lesions was limited by the low incidence of the disease, and a future multicenter study may be the most feasible approach. Further research could include a longitudinal study to determine whether complement activity is associated with active disease, a study examining other serum complement biomarkers or complement spatial regulatory gene expression in skin biopsy specimens, as well as a study investigating complement dysregulation in the subgroup of patients with LV with antiphospholipid antibodies.
Acknowledgment: This study was funded by a North Carolina Translational and Clinical Sciences Institute pilot grant.
Contribution: H.E., S.M., S.C., and R.A.B. designed the research; H.E., G.F.G., and X.-Z.P. performed the research and collected data; H.E. wrote the manuscript; and all authors analyzed the data and reviewed and approved the final version of the manuscript.
Conflict-of-interest disclosure: R.A.B. has performed consulting work for and received research support from Alexion Pharmaceuticals and provided consulting work for Apellis Pharmaceuticals. S.C. has served on advisory boards for Alexion Pharmaceuticals, Sanofi Genzyme, Sobi, and Takeda. S.C.'s institution has received research support on her behalf from Takeda. G.F.G. has served on advisory boards for Apellis Pharmaceuticals and Alexion Pharmaceuticals; contributed to the Merck Manual for which she received an honorarium; and their spouse is an employee of and holds stock in Pfizer. The remaining authors declare no competing financial interests.
Correspondence: Harish Eswaran, Division of Hematology, Department of Medicine, University of North Carolina, 102 Mason Farm Rd, Chapel Hill, NC 27514-4617; e-mail: harish.eswaran@unchealth.unc.edu.
References
Author notes
Data are available on request from the corresponding author, Harish Eswaran (harish.eswaran@unchealth.unc.edu).