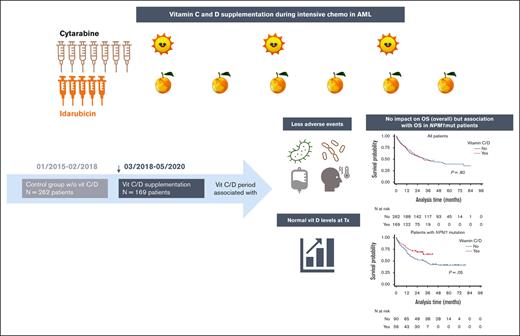
Key Points
Vitamin C/D treatment was associated with less complications during chemotherapy and restores the vitamin D level before allogeneic hematopoietic cell transplantation in patients with AML.
Vitamin C/D treatment was not associated with better OS except in patients with AML with NPM1 mutations.
Abstract
Recent studies have highlighted the role of vitamin C and D in acute myeloid leukemia (AML). In 2018, we changed our practices to add both vitamins to the supportive care for all consecutive patients with AML undergoing intensive chemotherapy. In this study, we compared the outcomes of patients treated before and after this change in practice. From 2015 to 2020, 431 patients were included, 262 of whom received no supplementation and 169 of whom received vitamin supplementation. Vitamin C and vitamin D was administered from day 10 of chemotherapy until hematologic recovery from induction and consolidation. Most patients presented at diagnosis with low levels of vitamin C and D. Upon recovery from induction, vitamin D levels among the vitamin C/D group significantly increased compared with those at diagnosis, and pretransplant levels were significantly higher in the vitamin C/D group compared with the control group (median of 33 vs 19 ng/mL; P < .0001). During induction, the rates of bacterial or fungal infection, hemorrhage, or macrophage activation syndrome were lower in the vitamin C/D group, whereas there was no difference in response rate, relapse incidence, and overall survival (OS). However, the multivariate analysis for OS showed a significant interaction between vitamin C/D and NPM1 mutation, meaning that vitamin C/D supplementation was significantly and independently associated with better OS in patients with NPM1 mutations (hazard ratio [HR], 0.52; 95% confidence interval [CI], 0.30-0.90; P = .019) compared with patients with wild-type NPM1 (HR, 1.01; 95% CI, 0.68-1.51; P = .95). In conclusion, vitamin C/D supplementation is safe and could influence the outcomes of patients with AML undergoing intensive chemotherapy.
Introduction
Acute myeloid leukemia (AML) is a severe myeloid malignancy induced by the oncogenic transformation of hematopoietic progenitors in the bone marrow (BM).1 Considerable progress has been made in understanding leukemogenesis, with the recent discovery of recurrent gene mutations that affect transcription factors, cell signaling, nucleophosmin, epigenetics, DNA methylation, and RNA splicing or the cohesin complex.2 Dysregulation of these pathways interact to produce the main hallmarks of cancer and transform hematopoietic progenitors into AML cells.3 These mutational events can affect enzymes such as ten-eleven translocation (TET) 2 dioxygenase or isocitrate dehydrogenases (IDH), the function of which depends on metabolic cofactors.4-6 Moreover, these alterations, which have a major impact on the cellular transcriptional state, result in the inhibition of expression of genes crucial for myeloid differentiation. In this context, new data have highlighted or reemphasized the role of both vitamin C and D in the pathophysiology and prognosis of AML.
Two major studies have recently demonstrated the role of vitamin C in normal hematopoiesis and leukemogenesis.7,8 Normal hematopoietic stem cells (HSCs) express higher levels of the vitamin C transporter Slc23a2 and consequently higher intracellular levels of vitamin C in HSCs and multipotent progenitor cells compared with in more mature cells. Vitamin C acts as a cofactor of TET proteins, promotes TET2 enzymatic activity, and regulates self-renewal, differentiation, and cell survival of HSCs/multipotent progenitor cells. Conversely, vitamin C depletion accelerates leukemogenesis in a TET2-mutated/FLT3-ITD murine model, which is reversed through vitamin C treatment. The product of IDH1/2 mutations, 2-hydroxyglutarate, also inhibits TET2 activity.9 AML with TET2 mutations displays an overlapping hypermethylation signature with IDH1/2-mutated AML and mutual exclusivity between these 2 mutations is observed in patients with AML.10 Accordingly, a recent study demonstrated that vitamin C induced an epigenetic remodeling in AML with IDH1 mutations, leading to cell proliferation inhibition and induction of cell differentiation.11 Lastly, vitamin C acts in synergy with a hypomethylating agent by increasing a viral mimicry response in a TET2-dependent manner.12 In clinic, most patients present with low vitamin C levels at diagnosis and a case of complete remission (CR) after supplementation with vitamin C as a single treatment has been reported in a patient with DNMT3A, TET2, WT1, and NPM1 mutations with refractory disease.12-14 A small study showed that the addition of vitamin C to decitabine/cytarabine/aclarubicin/granulocyte colony-stimulating factor (G-CSF) improved outcomes compared with decitabine/cytarabine/aclarubicin/G-CSF with no vitamin C.15
The role of vitamin D in inducing myeloid progenitor differentiation into monocytes is long established.16,17 Vitamin D activity depends on the expression of its receptor (VDR), the level of which may differ in patients with AML, with those with myelomonocytic or monocytic differentiation having a higher level than those with minimal differentiation, without maturation, or with maturation per the French-American-British system. A recent study showed that the use of VDR agonists could impair leukemic stem cell activity in a FLT3-internal tandem duplication (ITD)/NPM1-mutated murine model.18 Moreover, our group has shown that the gene expression of the VDR pathway is significantly increased in IDH1-mutated AML compared with wild-type IDH1. IDH mutations and 2-hydroxyglutarate activate the VDR pathway in a CEBPA-dependent manner, priming IDH-mutant AML cells to vitamin D–induced myeloid differentiation.19 Most patients with AML display insufficient or deficient vitamin D levels at diagnosis.20 Moreover, up to 87% of patients undergoing allogeneic stem cell transplantation have a vitamin D deficiency before transplantation, which could have a significant impact, given the role of vitamin D in immune response and inflammation.21 Lower vitamin D levels were significantly associated with poorer overall survival (OS) in AML, both at diagnosis and before allogeneic stem cell transplantation.20-22 However, therapeutic interventions with vitamin D analogs have resulted in inconclusive data because of adverse events related to systemic hypercalcemia.23,24
Based on these recent data, we modified our practices in 2018 by adding both vitamin C and D to the supportive care of all consecutive patients with AML undergoing intensive chemotherapy. In this study, we compared the outcomes of patients treated with intensive chemotherapy before and after this change in practice.
Patients and methods
The study population included all patients aged ≥18 years with newly diagnosed AML (excluding acute promyelocytic leukemia) being treated with intensive chemotherapy and registered in the Toulouse DATAML registry from January 2015 to May 2020. Patients received induction chemotherapy, which included idarubicin at a daily dose of 8 to 9 mg/m2 for 5 days or daunorubicin at a daily dose of 60 to 90 mg/m2 for 3 days, together with continuous IV infusion of cytarabine at a daily dose of 100 to 200 mg/m2 for 7 days. Lomustine (200 mg/m2 orally on day 1) was added for patients aged <60 years. Postremission therapy was adapted based on the risk of relapse and whether an HLA-identical donor had been identified. Younger patients received up to 3 cycles of intermediate-dose (1.5 g/m2 every 12 hours, 3 days) or high-dose (3 g/m2 every 12 hours, 3 days) cytarabine. Patients aged >60 years received up to 6 cycles of idarubicin (8 mg/m2 per day; IV) on day 1 and cytarabine (50 mg/m2 every 12 hours per day) subcutaneously on days 1 to 5. Patients at low risk of relapse (ie, patients with a core binding factor AML or NPM1 mutation without FLT3-ITD allelic ratio of >0.5 and a good molecular response, or patients with a biallelic CEBPA mutation) just received chemotherapy as the postremission therapy. All other patients with an HLA-matched donor underwent allogeneic stem cell transplantation, whereas those without such a donor or those who declined transplantation received chemotherapy. Patients with therapy-related AML or AML with myelodysplasia-related changes could receive CPX-351 from the date of its registration in France.
The supportive care for intensive chemotherapy, which included posaconazole antifungal prophylaxis, treatment of febrile neutropenia, and disseminated intravascular coagulopathy, as well as blood-product transfusions or prophylactic use of G-CSF, was given per local guidelines, which did not change over the study period. As of March 2018, in the absence of contraindication (renal lithiasis, renal insufficiency, known glucose-6-phosphate dehydrogenase deficiency, or hypercalcemia), vitamin C (1 g × 2 per day, 3 times a week; IV) and vitamin D3 (cholecalciferol, 100 000 IU per week; orally) were administered from day 10 of chemotherapy up to hematologic recovery from induction and consolidation chemotherapy, whatever the baseline level of both vitamins. All patients included in this study who received allo-engraftment received cholecalciferol, 100 000 IU per month and thereafter based on vitamin D levels. Vitamin C was given orally, at the same dose, to patients aged >60 years receiving consolidation cycles as outpatients. Calcemia and albumin levels were measured daily in patients who were hospitalized.
The cytogenetic risk classification was defined according to the UK Medical Research Council classification. A BM assessment was performed in patients treated with intensive chemotherapy after hematologic recovery or, in the case of delayed recovery, between days 35 and 45. Response to treatment, relapse, relapse-free survival (RFS), event-free survival (EFS), cumulative incidence of relapse (CIR), and OS were defined according to the European LeukemiaNet (ELN) 2017 criteria. Measurable residual disease (MRD) levels were determined in the BM and/or blood by serial monitoring of NPM1 mutations, RUNX1-RUNX1T1, or CBFB-MYH11 messenger RNA expression by reverse transcription quantitative polymerase chain reaction after induction chemotherapy (cycle 1) and first consolidation chemotherapy (cycle 2) in younger patients or 2 mini-consolidations in older patients. Informed consent was obtained from all patients in accordance with the Declaration of Helsinki, allowing clinical data to be collected in the anonymized DATAML registry. This study follows the reference methodology in France (MR-004) regarding studies that do not meet the definition of research involving the human person, in particular studies concerning the reuse of data.
Serum dosage of vitamin C and D
Total L-ascorbate (mg/L) was measured using high-performance liquid chromatography (<2.5 mg/L: scorbutic threshold; 2.5-6.2 mg/L: hypovitaminosis; 6.2-10 mg/L: normal level; and >10 mg/L: optimal threshold). 25-Hydroxy vitamin D (ng/mL) was measured using a competitive protein-binding enzyme-linked immunosorbent assay (<12.5 ng/mL: deficiency; 12.5-30: ng/mL insufficiency; and 30-100 ng/mL: optimal value).
Statistical analysis
Statistical analyses were performed using STATA statistical software, release 17.1 (STATA Corp, College Station, TX). All reported P values were 2-sided, and the significance threshold was <.05. We described patient characteristics using numbers and frequencies for qualitative data, and medians, interquartile ranges (IQRs), and ranges (minimum-maximum) for quantitative data. Categorical variables were compared between groups using the χ2 test (or Fisher exact test, when necessary). Student t test was used to compare the distributions of continuous data (Mann-Whitney U test was used when the distribution departed significantly from normality or when homoscedasticity was rejected). For EFS, RFS, and OS, differences in survival functions between groups (ie, between the control group and the vitamin C/D group) were described using Kaplan-Meier curves, together with medians and IQRs, and were tested using the log-rank test. For relapse (CIR), cumulative incidence functions were drawn (considering death as a competing event) and compared using the Gray test. Adjusted hazard ratios (aHRs) and 95% confidence intervals (95% CIs) were assessed using a standard Cox model for EFS, RFS, and OS, and a proportional subdistribution hazard model (an extension of the Cox model) for competing risks for CIR.25 The proportional-hazard assumption was tested for each covariate of the Cox model by the “log-log” plot method curves, and was always met. Differences in response rate were compared using a logistic regression model. Multivariate analyses included groups (ie, control group vs vitamin C/D group) together with potential confounding factors (particularly, differences between groups such as age, Eastern Cooperative Oncology Group [ECOG] status, etc, and known prognostic factors such as NPM1, FLT3-ITD mutations, cytogenetic risk, ELN risk classification, etc) that had a P value of <.20 in the univariate analyses and remained significantly and independently associated with the end point (P < .05), after a backward stepwise selection procedure. Allogeneic stem cell transplantation was evaluated as a time-dependent potential confounder. Interactions between independent covariates and control vs vitamin C/D group were tested in order to identify potential subgroups with significantly different effect of vitamin C/D supplementation. The significance threshold for interaction was <.05, taking into account multiple testing. All interactions were not significant except for the interaction between control vs vitamin C/D group, and NPM1 mutation for OS. Accordingly, analyses were stratified on NPM1 mutation when necessary.
Results
Patients
From 1 January 2015 to 1 May 2020, 431 patients were included, 262 of whom received no supplementation (January 2015-February 2018; control group) and 169 of whom received vitamin C/D supplementation (March 2018-May 2020; vitamin C/D group). Data were updated as of 3 March 2022. The median follow-up was 45.6 months. At diagnosis, patient characteristics were well balanced except for age, ECOG performance status, and serum creatinine level (Table 1). The induction chemotherapy used was idarubicin-cytarabine (n = 177), idarubicin-cytarabine-lomustine (n = 157), daunorubicin-cytarabine (n = 71), CPX-351 (n = 17), or another (n = 9). In AML with FLT3 mutation, midostaurin was added to the chemotherapy program for 1 patient in the control group and for 52 patients in the vitamin C/D groups. An allogeneic stem cell transplantation was performed in 75 (33.9%) patient who were in first CR in the control group and 37 (26.1%) patients in the vitamin C/D groups.
Characteristics of the 431 patients with AML per vitamin C/D supplementation
| . | Vitamin C/D supplementation . | P value . | Total . | |
|---|---|---|---|---|
| No . | Yes . | |||
| 262 (60.8%) . | 169 (39.2%) . | 431 (100.0) . | ||
| Age, y | ||||
| Median | 60.00 | 65.59 | .002 | 62.08 |
| IQR | 50.50-66.73 | 53.49-70.90 | 51.37-68.73 | |
| Min; max | 18.10; 78.81 | 19.13; 79.80 | 18.10; 79.80 | |
| Age (y), n (%) | .004 | |||
| ≤60 | 131 (50.0) | 61 (36.1) | 192 (44.5) | |
| >60 | 131 (50.0) | 108 (63.9) | 239 (55.5) | |
| Sex, n (%) | .399 | |||
| Male | 138 (52.7) | 82 (48.5) | 220 (51.0) | |
| Female | 124 (47.3) | 87 (51.5) | 211 (49.0) | |
| ECOG, n (%) | .011 | |||
| 0-1 | 179 (69.4) | 135 (80.4) | 314 (73.7) | |
| 2-3-4 | 79 (30.6) | 33 (19.6) | 112 (26.3) | |
| WBC (109/L) | ||||
| Median | 9.71 | 7.60 | 8.90 | |
| IQR | 2.90-46.06 | 2.50-33.50 | .152 | 2.70-41.30 |
| Min; max | 0.60; 403.30 | 0.42; 272.50 | 0.42; 403.30 | |
| WBC (109/L), n (%) | .387 | |||
| <50 | 200 (76.3) | 135 (79.9) | 335 (77.7) | |
| ≥50 | 62 (23.7) | 34 (20.1) | 96 (22.3) | |
| AML status n (%) | .545 | |||
| De novo | 199 (76.0) | 124 (73.4) | 323 (74.9) | |
| Secondary | 63 (24.0) | 45 (26.6) | 108 (25.1) | |
| Platelet count (109/L) | ||||
| Median | 64.00 | 63.00 | 64.00 | |
| IQR | 39.00-104.00 | 36.00-130.00 | .653 | 38.00-117.00 |
| Min; max | 5.00; 748.00 | 5.00; 848.00 | 5.00; 848.00 | |
| Creatinine μM/L (1N), n (%) | .003 | |||
| ≤84 μM/L | 147 (56.1) | 118 (70.2) | 265 (61.6) | |
| >84 μM/L | 115 (43.9) | 50 (29.8) | 165 (38.4) | |
| Albumin (g/L), n (%) | .783 | |||
| <30 | 50 (19.2) | 33 (20.2) | 83 (19.6) | |
| ≥30 | 211 (80.8) | 130 (79.8) | 341 (80.4) | |
| LDH (UI/L) (2N), n (%) | .346 | |||
| ≤468 UI/L | 149 (57.1) | 103 (61.7) | 252 (58.9) | |
| >468 UI/L | 112 (42.9) | 64 (38.3) | 176 (41.1) | |
| Serum ferritin (μg/L) (2N), n (%) | .531 | |||
| ≤613.6 μg/L | 126 (49.0) | 84 (52.2) | 210 (50.2) | |
| >613.6 μg/L | 131 (51.0) | 77 (47.8) | 208 (49.8) | |
| Corrected calcemia (mmol/L) | ||||
| Median | 2.34 | 2.33 | .741 | 2.33 |
| IQR | 2.25-2.44 | 2.26-2.40 | 2.25-2.43 | |
| Min; max | 1.96; 2.88 | 2.02; 2.81 | 1.96; 2.88 | |
| Cytogenetic risk, n (%) | .934 | |||
| Favorable | 27 (10.3) | 16 (9.6) | 43 (10.0) | |
| Intermediate | 177 (67.8) | 116 (69.5) | 293 (68.5) | |
| Adverse | 57 (21.8) | 35 (21.0) | 92 (21.5) | |
| ELN 2017, n (%) | .469 | |||
| Favorable | 92 (39.3) | 62 (37.8) | 154 (38.7) | |
| Intermediate | 75 (32.1) | 46 (28.0) | 121 (30.4) | |
| Adverse | 67 (28.6) | 56 (34.1) | 123 (30.9) | |
| FLT3-ITD mutation, n (%) | .130 | |||
| No | 141 (71.6) | 128 (78.5) | 269 (74.7) | |
| Yes | 56 (28.4) | 35 (21.5) | 91 (25.3) | |
| FLT3-TKD mutation, n (%) | .112 | |||
| No | 163 (91.6) | 144 (86.2) | 307 (89.0) | |
| Yes | 15 (8.4) | 23 (13.8) | 38 (11.0) | |
| NPM1 mutation, n (%) | .076 | |||
| No | 122 (57.5) | 111 (66.5) | 233 (61.5) | |
| Yes | 90 (42.5) | 56 (33.5) | 146 (38.5) | |
| CEBPA mutation, n (%) | .462 | |||
| No | 141 (93.4) | 144 (91.1) | 285 (92.2) | |
| Yes | 10 (6.6) | 14 (8.9) | 24 (7.8) | |
| Monoallelic | 6 (60.0) | 7 (50.0) | .696 | 13 (54.2) |
| Biallelic | 4 (40.0) | 7 (50.0) | 11 (45.8) | |
| IDH1-R132 mutation, n (%) | .181 | |||
| No | 206 (91.6) | 146 (87.4) | 352 (89.8) | |
| Yes | 19 (8.4) | 21 (12.6) | 40 (10.2) | |
| IDH2-R140 mutation, n (%) | .572 | |||
| No | 198 (88.0) | 150 (89.8) | 348 (88.8) | |
| Yes | 27 (12.0) | 17 (10.2) | 44 (11.2) | |
| IDH2-R172 mutation, n (%) | .060 | |||
| No | 222 (98.7) | 159 (95.2) | 381 (97.2) | |
| Yes | 3 (1.3) | 8 (4.8) | 11 (2.8) | |
| DNMT3A mutation, n (%) | .565 | |||
| No | 122 (75.8) | 131 (78.4) | 253 (77.1) | |
| Yes | 39 (24.2) | 36 (21.6) | 75 (22.9) | |
| TET2 mutation, n (%) | .653 | |||
| No | 39 (86.7) | 40 (83.3) | 79 (84.9) | |
| Yes | 6 (13.3) | 8 (16.7) | 14 (15.1) | |
| RUNX1 mutation, n (%) | .655 | |||
| No | 37 (86.0) | 43 (82.7) | 80 (84.2) | |
| Yes | 6 (14.0) | 9 (17.3) | 15 (15.8) | |
| ASXL1 mutation, n (%) | .361 | |||
| No | 49 (87.5) | 153 (91.6) | 202 (90.6) | |
| Yes | 7 (12.5) | 14 (8.4) | 21 (9.4) | |
| TP53 mutation, n (%) | .044 | |||
| No | 46 (97.9) | 58 (86.6) | 104 (91.2) | |
| Yes | 1 (2.1) | 9 (13.4) | 10 (8.8) | |
| At least 1 mutation in DNMT3A/TET2/ASXL1, n (%) | .354 | |||
| No | 22 (31.0) | 32 (38.1) | 54 (34.8) | |
| Yes | 49 (69.0) | 52 (61.9) | 101 (65.2) | |
| Chemotherapy regimen, n (%) | <.0001 | |||
| Daunorubicin-cytarabine | 58 (22.1) | 13 (7.7) | 71 (16.5) | |
| Idarubicin-cytarabine | 99 (37.8) | 78 (46.2) | 177 (41.1) | |
| Idarubicin-cytarabine-lomustine | 98 (37.4) | 59 (34.9) | 157 (36.4) | |
| Other | 7 (2.7) | 2 (1.2) | 9 (2.1) | |
| CPX-351 | 0 (0.0) | 17 (10.1) | 17 (3.9) | |
| Allogeneic SCT in CR1, n (%) | .112 | |||
| No | 146 (66.1) | 105 (73.9) | 251 (69.1) | |
| Yes | 75 (33.9) | 37 (26.1) | 112 (30.9) | |
| Median follow-up, mo (IQR) | 58.2 (50.5-67.0) | 28.7 (24.2-35.4) | <.0001 | 45.6 (29.8-61.5) |
| . | Vitamin C/D supplementation . | P value . | Total . | |
|---|---|---|---|---|
| No . | Yes . | |||
| 262 (60.8%) . | 169 (39.2%) . | 431 (100.0) . | ||
| Age, y | ||||
| Median | 60.00 | 65.59 | .002 | 62.08 |
| IQR | 50.50-66.73 | 53.49-70.90 | 51.37-68.73 | |
| Min; max | 18.10; 78.81 | 19.13; 79.80 | 18.10; 79.80 | |
| Age (y), n (%) | .004 | |||
| ≤60 | 131 (50.0) | 61 (36.1) | 192 (44.5) | |
| >60 | 131 (50.0) | 108 (63.9) | 239 (55.5) | |
| Sex, n (%) | .399 | |||
| Male | 138 (52.7) | 82 (48.5) | 220 (51.0) | |
| Female | 124 (47.3) | 87 (51.5) | 211 (49.0) | |
| ECOG, n (%) | .011 | |||
| 0-1 | 179 (69.4) | 135 (80.4) | 314 (73.7) | |
| 2-3-4 | 79 (30.6) | 33 (19.6) | 112 (26.3) | |
| WBC (109/L) | ||||
| Median | 9.71 | 7.60 | 8.90 | |
| IQR | 2.90-46.06 | 2.50-33.50 | .152 | 2.70-41.30 |
| Min; max | 0.60; 403.30 | 0.42; 272.50 | 0.42; 403.30 | |
| WBC (109/L), n (%) | .387 | |||
| <50 | 200 (76.3) | 135 (79.9) | 335 (77.7) | |
| ≥50 | 62 (23.7) | 34 (20.1) | 96 (22.3) | |
| AML status n (%) | .545 | |||
| De novo | 199 (76.0) | 124 (73.4) | 323 (74.9) | |
| Secondary | 63 (24.0) | 45 (26.6) | 108 (25.1) | |
| Platelet count (109/L) | ||||
| Median | 64.00 | 63.00 | 64.00 | |
| IQR | 39.00-104.00 | 36.00-130.00 | .653 | 38.00-117.00 |
| Min; max | 5.00; 748.00 | 5.00; 848.00 | 5.00; 848.00 | |
| Creatinine μM/L (1N), n (%) | .003 | |||
| ≤84 μM/L | 147 (56.1) | 118 (70.2) | 265 (61.6) | |
| >84 μM/L | 115 (43.9) | 50 (29.8) | 165 (38.4) | |
| Albumin (g/L), n (%) | .783 | |||
| <30 | 50 (19.2) | 33 (20.2) | 83 (19.6) | |
| ≥30 | 211 (80.8) | 130 (79.8) | 341 (80.4) | |
| LDH (UI/L) (2N), n (%) | .346 | |||
| ≤468 UI/L | 149 (57.1) | 103 (61.7) | 252 (58.9) | |
| >468 UI/L | 112 (42.9) | 64 (38.3) | 176 (41.1) | |
| Serum ferritin (μg/L) (2N), n (%) | .531 | |||
| ≤613.6 μg/L | 126 (49.0) | 84 (52.2) | 210 (50.2) | |
| >613.6 μg/L | 131 (51.0) | 77 (47.8) | 208 (49.8) | |
| Corrected calcemia (mmol/L) | ||||
| Median | 2.34 | 2.33 | .741 | 2.33 |
| IQR | 2.25-2.44 | 2.26-2.40 | 2.25-2.43 | |
| Min; max | 1.96; 2.88 | 2.02; 2.81 | 1.96; 2.88 | |
| Cytogenetic risk, n (%) | .934 | |||
| Favorable | 27 (10.3) | 16 (9.6) | 43 (10.0) | |
| Intermediate | 177 (67.8) | 116 (69.5) | 293 (68.5) | |
| Adverse | 57 (21.8) | 35 (21.0) | 92 (21.5) | |
| ELN 2017, n (%) | .469 | |||
| Favorable | 92 (39.3) | 62 (37.8) | 154 (38.7) | |
| Intermediate | 75 (32.1) | 46 (28.0) | 121 (30.4) | |
| Adverse | 67 (28.6) | 56 (34.1) | 123 (30.9) | |
| FLT3-ITD mutation, n (%) | .130 | |||
| No | 141 (71.6) | 128 (78.5) | 269 (74.7) | |
| Yes | 56 (28.4) | 35 (21.5) | 91 (25.3) | |
| FLT3-TKD mutation, n (%) | .112 | |||
| No | 163 (91.6) | 144 (86.2) | 307 (89.0) | |
| Yes | 15 (8.4) | 23 (13.8) | 38 (11.0) | |
| NPM1 mutation, n (%) | .076 | |||
| No | 122 (57.5) | 111 (66.5) | 233 (61.5) | |
| Yes | 90 (42.5) | 56 (33.5) | 146 (38.5) | |
| CEBPA mutation, n (%) | .462 | |||
| No | 141 (93.4) | 144 (91.1) | 285 (92.2) | |
| Yes | 10 (6.6) | 14 (8.9) | 24 (7.8) | |
| Monoallelic | 6 (60.0) | 7 (50.0) | .696 | 13 (54.2) |
| Biallelic | 4 (40.0) | 7 (50.0) | 11 (45.8) | |
| IDH1-R132 mutation, n (%) | .181 | |||
| No | 206 (91.6) | 146 (87.4) | 352 (89.8) | |
| Yes | 19 (8.4) | 21 (12.6) | 40 (10.2) | |
| IDH2-R140 mutation, n (%) | .572 | |||
| No | 198 (88.0) | 150 (89.8) | 348 (88.8) | |
| Yes | 27 (12.0) | 17 (10.2) | 44 (11.2) | |
| IDH2-R172 mutation, n (%) | .060 | |||
| No | 222 (98.7) | 159 (95.2) | 381 (97.2) | |
| Yes | 3 (1.3) | 8 (4.8) | 11 (2.8) | |
| DNMT3A mutation, n (%) | .565 | |||
| No | 122 (75.8) | 131 (78.4) | 253 (77.1) | |
| Yes | 39 (24.2) | 36 (21.6) | 75 (22.9) | |
| TET2 mutation, n (%) | .653 | |||
| No | 39 (86.7) | 40 (83.3) | 79 (84.9) | |
| Yes | 6 (13.3) | 8 (16.7) | 14 (15.1) | |
| RUNX1 mutation, n (%) | .655 | |||
| No | 37 (86.0) | 43 (82.7) | 80 (84.2) | |
| Yes | 6 (14.0) | 9 (17.3) | 15 (15.8) | |
| ASXL1 mutation, n (%) | .361 | |||
| No | 49 (87.5) | 153 (91.6) | 202 (90.6) | |
| Yes | 7 (12.5) | 14 (8.4) | 21 (9.4) | |
| TP53 mutation, n (%) | .044 | |||
| No | 46 (97.9) | 58 (86.6) | 104 (91.2) | |
| Yes | 1 (2.1) | 9 (13.4) | 10 (8.8) | |
| At least 1 mutation in DNMT3A/TET2/ASXL1, n (%) | .354 | |||
| No | 22 (31.0) | 32 (38.1) | 54 (34.8) | |
| Yes | 49 (69.0) | 52 (61.9) | 101 (65.2) | |
| Chemotherapy regimen, n (%) | <.0001 | |||
| Daunorubicin-cytarabine | 58 (22.1) | 13 (7.7) | 71 (16.5) | |
| Idarubicin-cytarabine | 99 (37.8) | 78 (46.2) | 177 (41.1) | |
| Idarubicin-cytarabine-lomustine | 98 (37.4) | 59 (34.9) | 157 (36.4) | |
| Other | 7 (2.7) | 2 (1.2) | 9 (2.1) | |
| CPX-351 | 0 (0.0) | 17 (10.1) | 17 (3.9) | |
| Allogeneic SCT in CR1, n (%) | .112 | |||
| No | 146 (66.1) | 105 (73.9) | 251 (69.1) | |
| Yes | 75 (33.9) | 37 (26.1) | 112 (30.9) | |
| Median follow-up, mo (IQR) | 58.2 (50.5-67.0) | 28.7 (24.2-35.4) | <.0001 | 45.6 (29.8-61.5) |
1N, upper limit of normal; 2N, 2 times the upper limit of normal; CR1, first CR; max, maximum; Min, minimum; SCT, stem cell transplantation; WBC, white blood cell count.
Vitamin C and D levels at diagnosis and during treatment
The vitamin C level at diagnosis was significantly associated with sex, white blood cell count, and season (autumn/winter vs spring/summer) (supplemental Table 1). In the vitamin C/D group, the median plasma level of vitamin C was 3.20 mg/L (range, 0.47-15.42) at diagnosis and 4.62 mg/L (range, 0.18-18.1) at the time of hematologic recovery from induction chemotherapy (P = .18) (Figure 1A; Table 2). The level of vitamin C was not routinely measured in the control group, precluding any comparison between both groups. The vitamin D level at diagnosis was significantly associated with sex and season (supplemental Table 2). The median level of vitamin D at diagnosis was 16 ng/mL (range, 4-56) and 18 ng/mL (range, 4-42) in the control and vitamin C/D groups, respectively (P = .51). Upon recovery from induction chemotherapy, vitamin D levels significantly increased compared with levels at diagnosis in the vitamin C/D group (median, 39 ng/mL; range, 5-93; P < .0001; Figure 1B). The median pretransplant vitamin D level was significantly higher in the vitamin C/D group compared with the control group (33 vs 19 ng/mL; P < .0001) whereas day-100 posttransplant levels were similar (41 ng/mL in the vitamin C/D group vs 41.5 ng/mL in the control group; P = .91; Table 2).
Vitamin levels at diagnosis and at end of induction chemotherapy in patients of the vitamin C/D supplementation group. (A) Vitamin C levels in mg/L. (B) Vitamin D levels in ng/mL.
Vitamin levels at diagnosis and at end of induction chemotherapy in patients of the vitamin C/D supplementation group. (A) Vitamin C levels in mg/L. (B) Vitamin D levels in ng/mL.
Vitamin C and vitamin D levels at diagnosis and during treatment
| . | Vitamin C/D supplementation . | P value . | Total . | |
|---|---|---|---|---|
| No . | Yes . | |||
| 262 (60.8) . | 169 (39.2) . | 431 (100.0) . | ||
| Vitamin C, diagnosis (mg/L) | ||||
| n/missing | NA | 145/24 | ||
| Median | 3.20 | |||
| IQR | (1.83-6.43) | |||
| Min; max | 0.47; 15.42 | |||
| <2.5, n (%) | 56 (38.6%) | |||
| ≥2.5, <4, n (%) | 27 (18.6%) | |||
| ≥4, <6.2, n (%) | 25 (17.2%) | |||
| ≥6.2, ≤10, n (%) | 27 (18.6%) | |||
| >10, n (%) | 10 (6.9%) | |||
| Postinduction vitamin C (mg/L) | ||||
| n/missing | NA | 97/72 | ||
| Median | 4.62 | |||
| IQR | (2.53-7.15) | |||
| Min; max | 0.18; 18.09 | |||
| <2.5, n (%) | 23 (23.7%) | |||
| ≥2.5, <4, n (%) | 20 (20.6%) | |||
| ≥4, <6.2, n (%) | 20 (20.6%) | |||
| ≥6.2, ≤10, n (%) | 20 (20.6%) | |||
| >10, n (%) | 14 (14.4%) | |||
| Vitamin D, diagnosis (ng/mL) | ||||
| n/missing | 91/171 | 157/12 | 248/183 | |
| Median | 16.00 | 18.00 | .514 | 17.00 |
| IQR | (10.00-27.00) | (14.00-24.00) | (12.00-24.50) | |
| Min; max | 4.00; 56.00 | 4.00; 42.00 | 4.00; 56.00 | |
| <12.5, n (%) | 33 (36.3%) | 34 (21.7%) | 67 (27.0%) | |
| ≥12.5, <30, n (%) | 37 (40.7%) | 103 (65.6%) | 140 (56.5%) | |
| ≥30, n (%) | 21 (23.1%) | 20 (12.7%) | 41 (16.5%) | |
| Postinduction vitamin D (mg/L) | ||||
| n/missing | NA | 112/57 | ||
| Median | 39.00 | |||
| IQR | (27.50-49.00) | |||
| Min; max | 5.00; 93.00 | |||
| <12.5, n (%) | 5 (4.5%) | |||
| ≥12.5, <30, n (%) | 28 (25%) | |||
| ≥30, n (%) | 79 (70.5%) | |||
| Pretransplant vitamin D (ng/mL) | ||||
| n/missing | 74/1 | 37/0 | 112/0 | |
| Median | 19.00 | 33.00 | <.0001 | 24.50 |
| IQR | (12.00-28.00) | (28.00-40.00) | (16.00-33.50) | |
| Min; max | 6.00; 51.00 | 17.00; 54.00 | 6.00; 54.00 | |
| <12.5, n (%) | 20 (27.0) | 0 (0.0) | <.0001 | 20 (17.9) |
| ≥12.5, <30, n (%) | 39 (52.7) | 11 (28.9) | 50 (44.6) | |
| ≥30, n (%) | 15 (20.3) | 27 (71.1) | 42 (37.5) | |
| Day-100 posttransplant vitamin D (ng/mL) | ||||
| n/missing | 68/7 | 31/6 | 99/13 | |
| Median | 41.50 | 41.00 | .906 | 41.00 |
| IQR | (33.50-48.00) | (33.00-49.00) | (33.00-48.00) | |
| Min; max | 7.00; 128.00 | 28.00; 75.00 | 7.00; 128.00 | |
| <12.5, n (%) | 2 (2.9%) | 0 (0.0%) | .675 | 2 (2.0%) |
| ≥12.5, <30, n (%) | 10 (14.7%) | 3 (9.7%) | 13 (13.1%) | |
| ≥30 ng/mL | 56 (82.4%) | 28 (90.3%) | 84 (84.8%) | |
| EOT vitamin D if no allo-SCT (ng/mL) | ||||
| n/missing | 22/124 | 46/59 | 68/183 | |
| Median | 21.00 | 30.50 | .044 | 29.00 |
| IQR | (12.00-32.00) | (25.00-38.00) | (19.50-35.50) | |
| Min; max | 5.00; 96.00 | 8.00; 64.00 | 5.00; 96.00 | |
| <12.5, n (%) | 6 (27.3) | 2 (4.3) | .012 | 8 (11.8) |
| ≥12.5, <30, n (%) | 9 (40.9) | 18 (39.1) | 27 (39.7) | |
| ≥30, n (%) | 7 (31.8) | 26 (56.5) | 33 (48.5) | |
| . | Vitamin C/D supplementation . | P value . | Total . | |
|---|---|---|---|---|
| No . | Yes . | |||
| 262 (60.8) . | 169 (39.2) . | 431 (100.0) . | ||
| Vitamin C, diagnosis (mg/L) | ||||
| n/missing | NA | 145/24 | ||
| Median | 3.20 | |||
| IQR | (1.83-6.43) | |||
| Min; max | 0.47; 15.42 | |||
| <2.5, n (%) | 56 (38.6%) | |||
| ≥2.5, <4, n (%) | 27 (18.6%) | |||
| ≥4, <6.2, n (%) | 25 (17.2%) | |||
| ≥6.2, ≤10, n (%) | 27 (18.6%) | |||
| >10, n (%) | 10 (6.9%) | |||
| Postinduction vitamin C (mg/L) | ||||
| n/missing | NA | 97/72 | ||
| Median | 4.62 | |||
| IQR | (2.53-7.15) | |||
| Min; max | 0.18; 18.09 | |||
| <2.5, n (%) | 23 (23.7%) | |||
| ≥2.5, <4, n (%) | 20 (20.6%) | |||
| ≥4, <6.2, n (%) | 20 (20.6%) | |||
| ≥6.2, ≤10, n (%) | 20 (20.6%) | |||
| >10, n (%) | 14 (14.4%) | |||
| Vitamin D, diagnosis (ng/mL) | ||||
| n/missing | 91/171 | 157/12 | 248/183 | |
| Median | 16.00 | 18.00 | .514 | 17.00 |
| IQR | (10.00-27.00) | (14.00-24.00) | (12.00-24.50) | |
| Min; max | 4.00; 56.00 | 4.00; 42.00 | 4.00; 56.00 | |
| <12.5, n (%) | 33 (36.3%) | 34 (21.7%) | 67 (27.0%) | |
| ≥12.5, <30, n (%) | 37 (40.7%) | 103 (65.6%) | 140 (56.5%) | |
| ≥30, n (%) | 21 (23.1%) | 20 (12.7%) | 41 (16.5%) | |
| Postinduction vitamin D (mg/L) | ||||
| n/missing | NA | 112/57 | ||
| Median | 39.00 | |||
| IQR | (27.50-49.00) | |||
| Min; max | 5.00; 93.00 | |||
| <12.5, n (%) | 5 (4.5%) | |||
| ≥12.5, <30, n (%) | 28 (25%) | |||
| ≥30, n (%) | 79 (70.5%) | |||
| Pretransplant vitamin D (ng/mL) | ||||
| n/missing | 74/1 | 37/0 | 112/0 | |
| Median | 19.00 | 33.00 | <.0001 | 24.50 |
| IQR | (12.00-28.00) | (28.00-40.00) | (16.00-33.50) | |
| Min; max | 6.00; 51.00 | 17.00; 54.00 | 6.00; 54.00 | |
| <12.5, n (%) | 20 (27.0) | 0 (0.0) | <.0001 | 20 (17.9) |
| ≥12.5, <30, n (%) | 39 (52.7) | 11 (28.9) | 50 (44.6) | |
| ≥30, n (%) | 15 (20.3) | 27 (71.1) | 42 (37.5) | |
| Day-100 posttransplant vitamin D (ng/mL) | ||||
| n/missing | 68/7 | 31/6 | 99/13 | |
| Median | 41.50 | 41.00 | .906 | 41.00 |
| IQR | (33.50-48.00) | (33.00-49.00) | (33.00-48.00) | |
| Min; max | 7.00; 128.00 | 28.00; 75.00 | 7.00; 128.00 | |
| <12.5, n (%) | 2 (2.9%) | 0 (0.0%) | .675 | 2 (2.0%) |
| ≥12.5, <30, n (%) | 10 (14.7%) | 3 (9.7%) | 13 (13.1%) | |
| ≥30 ng/mL | 56 (82.4%) | 28 (90.3%) | 84 (84.8%) | |
| EOT vitamin D if no allo-SCT (ng/mL) | ||||
| n/missing | 22/124 | 46/59 | 68/183 | |
| Median | 21.00 | 30.50 | .044 | 29.00 |
| IQR | (12.00-32.00) | (25.00-38.00) | (19.50-35.50) | |
| Min; max | 5.00; 96.00 | 8.00; 64.00 | 5.00; 96.00 | |
| <12.5, n (%) | 6 (27.3) | 2 (4.3) | .012 | 8 (11.8) |
| ≥12.5, <30, n (%) | 9 (40.9) | 18 (39.1) | 27 (39.7) | |
| ≥30, n (%) | 7 (31.8) | 26 (56.5) | 33 (48.5) | |
EOT, end of treatment; N/A, not available; SCT, stem cell transplantation.
Outcome of induction chemotherapy
The rate of CR or CR with incomplete hematologic recovery (CRi) was 84.4% in the control group and 84.0% in vitamin C/D group (P = .93). In the multivariate analysis, vitamin C/D supplementation was not significantly associated with CR/CRi, whereas hydroxyurea, ECOG performance status of 2 to 4, adverse risk cytogenetics, and the presence of at least 1 mutation in the DNMT3A, TET2, or ASXL1 genes were significantly and independently associated with a lower probability of CR/CRi. NPM1 mutation was significantly and independently associated with a higher CR/CRi rate (supplemental Table 3). Among the 160 patients evaluated for MRD, 31 (19.4%) achieved a negative MRD result after induction chemotherapy (cycle 1), 20 (20.6%) of whom were in the control group and 11 (17.5%) of whom were in the vitamin C/D group (P = .621). After the first consolidation (cycle 2), 57 patients achieved a negative MRD result (48.3%), 29 (42.6%) of whom were in the control group and 28 (56.0%) of whom were in the vitamin C/D group (P = .151).
During induction chemotherapy, the rates of grade 3 to 4 bacterial infections (35.1% in the control group vs 27.2% in the vitamin C/D group; P = .086), fungal infections (18.3% in the control group vs 10.1% in the vitamin C/D group; P = .019), hemorrhage (5.7% in the control group vs 1.8% in the vitamin C/D group; P = .045), or macrophage activation syndrome (8.8% in the control group vs 1.8% in the vitamin C/D group; P = .002) were lower in the vitamin C/D group (supplemental Table 4). The median level of day-21 calcemia was higher in the vitamin C/D group (2.48 mmol/L; range, 1.72-2.77) compared with the control group (2.44 mmol/L; range, 1.83-2.91; P = .003). However, grade 2 hypercalcemia was observed in only 1 patient from the control group. The day-30 death rate was 4.2% and 2.4% in the control and vitamin C/D groups respectively (P = .31). The day-60 death rate was 8.0% and 5.9% in the control and vitamin C/D groups, respectively (P = .41). A sensitivity analysis of adverse events excluding patients of the vitamin C/D group who received CPX-351 showed similar results (supplemental Table 5).
Relapse and survival outcome
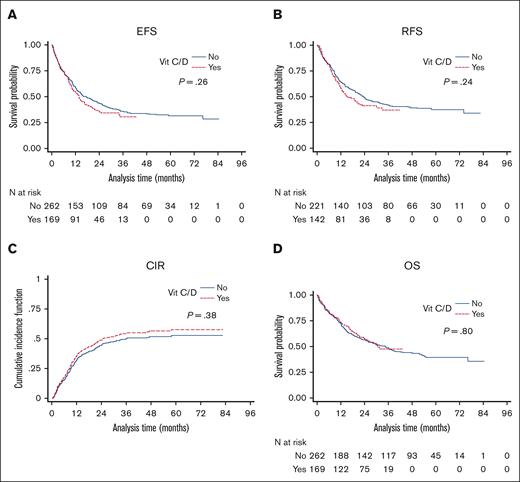
The median follow-up was 58.2 and 28.7 months in the control and vitamin C/D groups, respectively. The median EFS of the whole cohort was 14.9 months (IQR, 5.6-not reached), with no difference between the control group and the vitamin C/D group (17.4 months [IQR, 5.6-not reached] vs 13.9 months [IQR, 5.6-not reached]; P = .26) (Figure 2). The median RFS of the whole cohort was 20.6 months (IQR, 7.6-not reached), with no difference between the control group and the vitamin C/D group (23.5 months [IQR 8.6-not reached] vs 15.5 months [IQR, 7.2-not reached]; P = .24; Figure 2). The 2-year CIR of the whole cohort was 46.4% (95% CI, 41.1-51.5), with no difference between the control group and the vitamin C/D group (46.4% [95% CI, 41.1-51.5] vs 49.2% [95% CI, 43.8-54.4]; P = .38; Figure 2). The median OS of the whole cohort was 34.5 months (IQR, 11.0-not reached), with no difference between the control group and the vitamin C/D group (34.5 months [IQR, 10.7-not reached] vs 30.8 months [IQR, 11.5-not reached]; P = .80; Figure 2). The multivariate analyses for EFS, RFS, and CIR confirmed any significant impact of vitamin C/D supplementation and did not find any interactions between vitamin C/D supplementation and variables of the models (supplemental Table 3). However, the multivariate analysis for OS showed a significant interaction between vitamin C/D supplementation and NPM1 mutation, meaning that vitamin C/D supplementation was significantly and independently associated with better OS in patients with NPM1 mutations (HR, 0.52; 95% CI, 0.30-0.90; P = .019) but not in patients with wild-type NPM1 (HR, 1.01; 95% CI, 0.68-1.51; P = .95; Figure 3; Table 3). Of note, the midostaurin treatment (added to chemotherapy in patients with mutated FLT3) had no significant impact on OS (aHR, 0.79; 95% CI, 0.46-1.34; P = .377). Vitamin C and vitamin D levels at diagnosis (more than median value vs equal to/less than median value) had no prognostic impact on OS (aHR, 0.68; 95% CI, 0.40-1.14; P = .144 for vitamin C; aHR, 0.92; 95% CI, 0.64-1.34; P = .666 for vitamin D). The main characteristics and response to treatment of patients with AML with NPM1 mutations per vitamin C/D supplementation are presented in supplemental Table 6. Their characteristics were well balanced between both groups especially in terms of ELN risk groups and comutations, and there was no significant difference in the frequency of adverse events (supplemental Table 7). The median OS of patients with AML with NPM1 mutations was 37.6 months (IQR, 11.4-not reached), with a significant (P = .05) difference between the control group (31.6 months; IQR, 10.2-not reached) and the vitamin C/D group (not reached, IQR, 13.9-not reached). Of note, the beneficial effect of vitamin C/D in patients with NPM1-mutated AML was neither restricted to cases with M4/M5 morphology (M4/M5 vs non-M4/M5; P = .503) nor to cases with cooccurred IDH1, FLT3-tyrosine kinase domain (TKD), or FLT3-ITD mutations (NPM1-mut and IDH1-mut vs NPM1-mut and wild-type IDH, P = .723; NPM1-mut and FLT3-TKD vs NPM1-mut without FLT3-TKD, P = .065; and NPM1-mut + FLT3-ITD vs NPM1-mut without FLT3-ITD, P = .113).
Survival curves in patients of the control and vitamin C/D groups. (A) EFS. Control group: 1-year EFS, 59% (95% CI, 53-65); 3-year EFS, 36% (95% CI, 30-42); 5-year EFS, 32% (95% CI, 26-38). Vit C/D group: 1-year EFS, 57% (95% CI, 53-62); 3-year EFS, 34% (95% CI, 29-38); 5-year EFS, not estimated. (B) RFS. Control group: 1-year RFS, 64% (95% CI, 57-70); 3-year RFS, 41% (95% CI, 35-48); 5-year RFS, 37% (95% CI, 31-44). Vitamin C/D group: 1-year RFS, 58% (95% CI, 50-66); 3-year RFS, 37% (95% CI, 27-46); 5-year RFS, not estimated. (C) CIR. Control group: 1-year CIR, 34% (95% CI, 29-39); 3-year CIR, 52% (95% CI, 47-57); 5-year CIR, 54% (95% CI, 48-60). Vitamin C/D group: 1-year CIR, 34% (95% CI, 29-39); 3-year CIR, 50% (95% CI, 45-56); 5-year CIR, not estimated. (D) OS. Control group: 1-year OS, 72% (95% CI, 66-77); 3-year OS, 49% (95% CI, 43-55); 5-year OS, 39% (95% CI, 33-46). Vitamin C/D group: 1-year OS, 73% (95% CI, 66-79); 3-year OS, 47% (95% CI, 38-56); 5-year OS, not estimated.
Survival curves in patients of the control and vitamin C/D groups. (A) EFS. Control group: 1-year EFS, 59% (95% CI, 53-65); 3-year EFS, 36% (95% CI, 30-42); 5-year EFS, 32% (95% CI, 26-38). Vit C/D group: 1-year EFS, 57% (95% CI, 53-62); 3-year EFS, 34% (95% CI, 29-38); 5-year EFS, not estimated. (B) RFS. Control group: 1-year RFS, 64% (95% CI, 57-70); 3-year RFS, 41% (95% CI, 35-48); 5-year RFS, 37% (95% CI, 31-44). Vitamin C/D group: 1-year RFS, 58% (95% CI, 50-66); 3-year RFS, 37% (95% CI, 27-46); 5-year RFS, not estimated. (C) CIR. Control group: 1-year CIR, 34% (95% CI, 29-39); 3-year CIR, 52% (95% CI, 47-57); 5-year CIR, 54% (95% CI, 48-60). Vitamin C/D group: 1-year CIR, 34% (95% CI, 29-39); 3-year CIR, 50% (95% CI, 45-56); 5-year CIR, not estimated. (D) OS. Control group: 1-year OS, 72% (95% CI, 66-77); 3-year OS, 49% (95% CI, 43-55); 5-year OS, 39% (95% CI, 33-46). Vitamin C/D group: 1-year OS, 73% (95% CI, 66-79); 3-year OS, 47% (95% CI, 38-56); 5-year OS, not estimated.
OS curves in patients of the control and vitamin C/D groups with or without NPM1 mutations. (A) OS in NPM1 mutated patients. (B) OS in NPM1 wild-type patients.
OS curves in patients of the control and vitamin C/D groups with or without NPM1 mutations. (A) OS in NPM1 mutated patients. (B) OS in NPM1 wild-type patients.
Multivariate analyses for OS per NPM1 mutations
| . | OR . | 95% CI . | P value . |
|---|---|---|---|
| NPM1 mutation | |||
| Vitamin C/D | 0.52 | 0.30-0.90 | .019 |
| Hydroxyurea | 1.60 | 1.12-2.26 | .009 |
| Age, >60 y | 1.60 | 1.18-2.18 | .002 |
| Secondary AML | 1.54 | 1.13-2.08 | .006 |
| LDH > 2n | 1.71 | 1.21-2.42 | .002 |
| Serum ferritin > 613.6 μg/L | 1.47 | 1.11-1.95 | .008 |
| Cytogenetic risk | |||
| Intermediate | 2.74 | 1.45-5.18 | .002 |
| Adverse | 3.36 | 1.80-6.35 | <.001 |
| TP53 mutation | 3.01 | 1.41-6.40 | .004 |
| DNMT3A/TET2/ASXL1 mutation | 2.58 | 1.35-4.89 | .004 |
| Allo-SCT∗ | 0.56 | 0.39-0.82 | .003 |
| NPM1 wild type | |||
| Vitamin C/D | 1.01 | 0.68-1.51 | .949 |
| Hydroxyurea | 1.60 | 1.12-2.27 | .009 |
| Age, >60 y | 1.60 | 1.18-2.18 | .002 |
| Secondary AML | 1.54 | 1.13-2.08 | .006 |
| LDH > 2n | 1.71 | 1.21-2.42 | .002 |
| Serum ferritin > 613.6 μg/L | 1.47 | 1.11-1.95 | .008 |
| Cytogenetic risk | |||
| Intermediate | 2.74 | 1.44-5.18 | .002 |
| Adverse | 3.38 | 1.80-6.35 | <.001 |
| TP53 mutation | 3.01 | 1.41-6.40 | .004 |
| DNMT3A/TET2/ASXL1 mutation | 2.58 | 1.36-4.89 | .004 |
| Allo-SCT∗ | 0.56 | 0.39-0.82 | .003 |
| . | OR . | 95% CI . | P value . |
|---|---|---|---|
| NPM1 mutation | |||
| Vitamin C/D | 0.52 | 0.30-0.90 | .019 |
| Hydroxyurea | 1.60 | 1.12-2.26 | .009 |
| Age, >60 y | 1.60 | 1.18-2.18 | .002 |
| Secondary AML | 1.54 | 1.13-2.08 | .006 |
| LDH > 2n | 1.71 | 1.21-2.42 | .002 |
| Serum ferritin > 613.6 μg/L | 1.47 | 1.11-1.95 | .008 |
| Cytogenetic risk | |||
| Intermediate | 2.74 | 1.45-5.18 | .002 |
| Adverse | 3.36 | 1.80-6.35 | <.001 |
| TP53 mutation | 3.01 | 1.41-6.40 | .004 |
| DNMT3A/TET2/ASXL1 mutation | 2.58 | 1.35-4.89 | .004 |
| Allo-SCT∗ | 0.56 | 0.39-0.82 | .003 |
| NPM1 wild type | |||
| Vitamin C/D | 1.01 | 0.68-1.51 | .949 |
| Hydroxyurea | 1.60 | 1.12-2.27 | .009 |
| Age, >60 y | 1.60 | 1.18-2.18 | .002 |
| Secondary AML | 1.54 | 1.13-2.08 | .006 |
| LDH > 2n | 1.71 | 1.21-2.42 | .002 |
| Serum ferritin > 613.6 μg/L | 1.47 | 1.11-1.95 | .008 |
| Cytogenetic risk | |||
| Intermediate | 2.74 | 1.44-5.18 | .002 |
| Adverse | 3.38 | 1.80-6.35 | <.001 |
| TP53 mutation | 3.01 | 1.41-6.40 | .004 |
| DNMT3A/TET2/ASXL1 mutation | 2.58 | 1.36-4.89 | .004 |
| Allo-SCT∗ | 0.56 | 0.39-0.82 | .003 |
2n, 2 times the upper limit of normal; allo-SCT, allogeneic stem cell transplantation.
Allo-SCT presented as a time-dependent variable.
Discussion
In this study, we showed that vitamin C and D supplementation during intensive chemotherapy for AML was safe, associated with lower rate of grade 3 to 4 adverse events, restores the level of vitamin D to normal before transplantation, and was associated with better OS in patients with an NPM1 mutation.
Vitamin D was administered alongside regular monitoring of blood calcium levels, with weekly doses in the higher range of vitamin D repletion practices for allogeneic stem cell transplantation.26 However, the duration of treatment was in general 3 to 4 weeks during induction chemotherapy and 1 or 2 weeks during consolidation, which is relatively short compared with the duration for patients who receive transplantation.27 This supplementation regimen meant that there was a significant increase in the level of vitamin D with a postinduction median level of 39 ng/mL, and an optimal vitamin D level achieved in most patients (>30 ng/mL), which persisted at time of transplantation. Furthermore, no hypercalcemia was observed in these conditions. In contrast, we failed to correct vitamin C levels using our defined supplementation regimen. This conservative regimen (6 g per week) was chosen based on general supplementation recommendations and to avoid exacerbating potential adverse events, such as acute renal failure or unknown glucose-6-phosphate dehydrogenase deficiency. Extensive clinical experience of administering much higher doses in patients with cancer suggests that the regimen used in our study could be safely improved to restore higher levels of vitamin C. In addition, because oral absorption is very rapidly regulated, it is possible that the oral form used in older patients with AML treated on an outpatient basis at the time of consolidation may have limited the effects of supplementation compared with that in younger patients.27,28 Therefore, we cannot be certain that vitamin C had a therapeutic effect at this dosage. The antileukemic activity of vitamin C is diverse, from a prooxidant effect at high doses to, as shown more recently, an epigenetic modulation at physiological doses.29 Thus, an experimental supplementation regimen for a prospective, randomized clinical trial might include the same vitamin D regimen and a higher frequency and/or dose of vitamin C.
Very few studies have assessed vitamin C and D levels upon diagnosis of AML.14,30,31 We confirmed that most patients newly diagnosed with AML present with low levels of both vitamins. The vitamin C level was significantly associated with leukocytosis and the season. We found no correlation between vitamin C level and mutations. Other studies showed that vitamin C level was correlated with the peripheral blasts percentage but not leukocytosis or ASXL1 mutations.30,31 These discrepancies are probably related to the limited number of patients tested. The vitamin D level was significantly associated with sex and the season. The difference between male and female patients was not previously reported.22 The reasons why women had higher levels than men are not fully understood but could be related to a higher frequency of vitamin D supplementation in women before diagnosis of AML, although we did not collect this information in our database.32
The reduction of some adverse events during induction chemotherapy, such as infections, bleeding, or inflammation, are particularly interesting and could be explained by the properties of both vitamins. Vitamin C helps to regulate the immune system, including macrophage and neutrophil functions, lymphocyte proliferation and differentiation, and the reduction of inflammatory mediators during sepsis.33 Moreover, the antioxidant effect of vitamin C also helps to restore endothelial barrier dysfunction and therefore could limit the risk of bleeding in patients with AML.34 Vitamin D is an immunomodulatory hormone that also regulates several factors in innate and adaptive immunity as well as inflammation and vascular endothelium.35
The interaction between vitamin C/D supplementation and NPM1 mutation in terms of OS is a new, intriguing finding of our study. To our knowledge, there are no studies in the literature specifically assessing activation of the VDR or ascorbic acid pathways in mutated-NPM1 models. However, Paubelle et al showed that the VDR agonist inecalcitol could induce differentiation and control leukemic stem cell homeostasis activity in a NPM1-mutated murine model.18 Another study evaluating a series of VD analogs structurally related to 1,25-dihydroxyvitamin D2 demonstrated that NPM1-mutated samples displayed a higher induction of differentiation than other AML subtypes including FLT3 mutations.36 More studies are needed to fully explore the molecular relationships between vitamin C and D pathways and NPM1 mutations.
It is noteworthy that 2 novel drugs, midostaurin and CPX-351, were introduced during the most recent study period (ie, during the vitamin C/D group study period). However, there was no significant impact on survival. This result must be interpreted with caution. Comparisons with results of large phase 3 studies are problematic, given the different patient characteristics and, above all, the small number of patients in our study.37,38
There are limitations in the analysis of the results because this study is retrospective, monocentric, and included a relatively limited number of patients. Furthermore, the concomitant use of both vitamins precludes dissecting the potential effect of either, or even the value of the combination. However, recent pathophysiological advances, interactions between some driver mutations and the vitamin C and D pathways, and our clinical results are sufficiently encouraging to support prospective clinical trials with carefully designed schemas for administering vitamin C and D to patients with AML.
Acknowledgments
The authors thank the data management unit of Toulouse University Hospital. The authors thank all the members of the Gaël Adolescent Espoir Leucémie association and the Toulouse Cancer Santé Foundation.
Authorship
Contribution: P.L.M. treated patients, and collected and analyzed data; E.B. performed the statistical analysis and wrote the manuscript; S.T., N.G., F.H., and A.H. treated patients; F.V., J.B.R., I.L., L.L., and E.D. performed morphologic, immunophenotypic, cytogenetic, or molecular analyses; A.S. collected data; S.B. and C.R. collected data, treated patients, supervised analyses, and wrote the manuscript; C.R. collected data, treated patients, supervised analyses, and wrote the manuscript; the manuscript was prepared by all the authors with the assistance of a medical writer funded by CHU de Toulouse; and all authors reviewed, provided comments on, and approved the manuscript.
Conflict-of-interest disclosure: C.R. reports receiving research grants (to institution) from AbbVie, Astellas, Bristol Myers Squibb, Jazz Pharmaceuticals, IQVIA; and serves on advisory boards of AbbVie, Astellas, Bristol Myers Squibb, Jazz Pharmaceuticals, Novartis, Servier, Takeda. S.B. serves in an advisory role for AbbVie, Jazz Pharmaceuticals, Daiichi-Sankyo, Sanofi, Astellas, and Bristol Myers Squibb. F.V. received research grants from Pierre Fabre and Roche; and serves as an adviser for Astellas and Amgen. F.H. reports consultancy for Novartis, Pfizer, and Incyte; and received honoraria from Amgen and Servier. I.L. serves in an advisory role for Jazz Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Christian Récher, Service d’Hématologie, Institut Universitaire du Cancer de Toulouse Oncopole, 1 avenue Irène Joliot-Curie, 31059 Toulouse Cedex 9, France; e-mail: recher.christian@iuct-oncopole.fr.
References
Author notes
Data are available on request from the corresponding author, Christian Récher (recher.christian@iuct-oncopole.fr).
The full-text version of this article contains a data supplement.