In this issue of Blood Advances, Gustafsson et al examined the effect of an anti-CD45 antibody drug conjugate (ADC) on tissue-resident myeloid cells (TRM) in healthy and atherosclerosis-predisposed mice. The CD45-ADC was able to clear a wide range of specific TRMs and facilitated replacement of these cells by adoptive cell transfer.1 Clonal hematopoiesis (CH), also known as CH of indeterminate potential (CHIP), is a commonly recognized condition in older persons associated with increased risk of cardiovascular- and cancer-associated mortality. In the murine model of CH mutation (Tet2 knockout [KO]) that accelerates atherosclerotic vascular disease, replacement of the Tet2 KO cells with healthy myeloid cells was able to partially reverse this accelerated atherosclerosis. This fascinating work provides a novel take on regenerative medicine strategies to reverse inflammatory diseases, such as atherosclerosis, mediated through effects on TRMs.
TRMs refer to macrophages that reside independently of the blood and bone marrow and include populations such as Kupffer cells within the liver, microglia in the brain, Langerhans cells in the skin, and lung alveolar macrophages. TRMs incorporate a distinct population of macrophages, which differ from monocytes that derive from hematopoietic stem cells, in their embryological origins, developmental trajectories, and functions in health and disease. TRMs often remain independent of the bone marrow throughout their lifespan and in some contexts, are able to self-maintain and proliferate within tissues, without recruitment of monocytes from the blood.2,3 Conventional myeloablative conditioning regimens for hematopoietic stem cell transplants (HSCT) involving cytotoxic chemotherapy and radiotherapy clear myeloid cells from bone marrow and TRM niches, but this comes at the cost of very substantial nondiscriminatory toxicities. More selective conditioning regimens such as CD45- or CD117-directed ADCs offer less-toxic alternatives4-6; however, the extent to which these targeted approaches clear TRMs and thus, whether they have utility in treating macrophage-mediated disease outside the bone marrow has not previously been studied. In this article, Gustafsson et al asked (1) whether administration of an anti-CD45 ADC linked to a cytotoxic payload, pyrrolobenzodiazepine, can effectively deplete hematopoietic cells not just within bone marrow but the TRMs within other tissues and (2) whether this process can be potentially harnessed to alter the disease course of TRM-driven inflammatory conditions, such as CH-related atherosclerosis.
In a series of elegant, systematically designed mouse transplant experiments, Gustafsson et al demonstrate that the anti-CD45 ADC effectively clears wild-type blood and bone marrow hematopoietic cells, allowing almost complete engraftment of transplanted bone marrow cells, including the hierarchy of immature stem cells and precursors. The ADC also effectively cleared TRM and similarly allowed, albeit less complete, replacement by transplanted cells in the order of 70% for lung and liver and 50% in skin. In the brain, microglia were not replaced to a significant extent although brain derived monocytes/macrophages were replaced ∼50%.
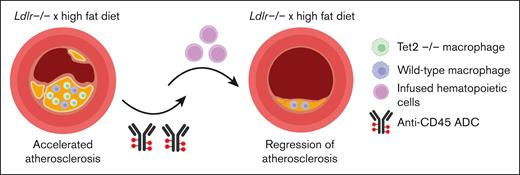
The authors then tested the efficiency of clearance and replacement in a disease context, asking whether the replacement of TRMs might occur in atherosclerotic plaques, in which macrophages accumulate, proliferate, and drive cardiovascular disease. In a mouse model genetically altered to prematurely develop widespread atherosclerotic lesions in response to a high fat diet (the Ldlr KO mice), the same anti-CD45 ADC treatment, followed by transplant with wild-type cells, led to 80% replacement with donor macrophages within the aortic plaques. To test this in the context in which the native TRMs might have a competitive advantage over wild-type cells, the authors used a model of Tet2 KO hematopoietic cells. TET2 loss-of-function mutations are found commonly in CHIP. TET2-mutated CHIP is associated with increased mortality because of an increased risk of cardiovascular disease, mediated by local inflammatory effects within the atheroma.7 Recapitulating these clinical observations and previous reports7 in mice, Tet2 KO cells demonstrated a competitive advantage over wild-type cells when transplanted together into the Ldlr KO mice. Furthermore, Tet2 KO cells exacerbated the atherosclerotic phenotype in Ldlr KO mice, increasing plaque burden. Despite the competitive advantage and larger-sized plaques, subsequent anti-CD45 ADC administration was still able to efficiently clear Tet2 KO cells and allow replacement by transplanted wild-type cells. After 6 weeks, plaque burden was significantly reduced, similar to those mice transplanted with wild-type cells, suggesting a reversal of Tet2 KO-driven atherosclerosis (see figure).
Tet2 KO–/– accelerates atherosclerosis in Ldlr–/– mice maintained on a high fat diet. Conditioning with CD45-ADC and infusion of wild-type hematopoietic cells was able to partially reverse this atherosclerosis. The figure was created with BioRender.com).
Tet2 KO–/– accelerates atherosclerosis in Ldlr–/– mice maintained on a high fat diet. Conditioning with CD45-ADC and infusion of wild-type hematopoietic cells was able to partially reverse this atherosclerosis. The figure was created with BioRender.com).
Targeted conditioning regimens, such as with CD45-ADC conditioning, as an alternative for HSCT in humans, which could substantially reduce morbidity and mortality from HSCT, would certainly broaden its acceptability, particularly in the autologous setting in which conditioning regimens are responsible for most of the toxicity. In the allogeneic or gene-modified setting, the application of CD45-ADC to nonmalignant conditions, for example, storage diseases such as Hurler syndrome (mucopolysaccharidosis) or Gaucher disease, in which HSCT if delivered safely may be curative and obviate the need for life-long, expensive enzyme replacement therapies. For these types of conditions, even partial eradiation of diseased TRMs and replacement with wild type (or even gene-modified hematopoietic cells expressing therapeutic proteins) may exact a disease-modifying effect. The optimal management of CH-related atherosclerosis, beyond standard cardiovascular risk factor management, has not been defined. This work provides provocative data to suggest that the natural history of CH atherosclerosis could be altered by modifying disease-causing TRMs.
There are open questions to this technique and its clinical utility. What level of host TRM replacement is required to exert a disease-modifying effect, in what tissues, and in which diseases? How durable is the engraftment? Would infused cells continue to provide disease modification over prolonged duration? Will these murine studies replicate in the human setting? Nevertheless, indications for HSCT techniques for nonmalignant conditions are expanding, targeted conditioning techniques will undoubtedly make HSCT procedures safer, and infusion of genetically–modified autologous or allogeneic cells for therapeutic purposes are here and now, making this a tantalizing prospect of the not-so-distant future.
Conflict-of-interest disclosure: The authors declare no competing financial interests.