TO THE EDITOR:
Internal tandem duplications (ITDs) of the Fms-like tyrosine kinase 3 (FLT3) gene occur in ∼25% to 30% of acute myeloid leukemia (AML) cases.1,2 The presence of these FLT3-ITDs is associated with adverse outcomes, including higher relapse rates after conventional AML treatment and reduced overall survival.3 In contrast, point mutations occurring within the tyrosine kinase domain (TKD) of FLT3 are less common, accounting for 5% to 10% of AML cases, and have a neutral prognostic impact.4 Recent investigations have provided insight into the potential therapeutic benefits of combining AML chemotherapy with targeted tyrosine kinase inhibitors (TKIs) that specifically target mutated FLT3 receptors. In particular, TKIs such as midostaurin, gilteritinib, and sorafenib have shown promising results at various stages of AML therapy, including first-line treatment, relapse, and maintenance after allogeneic stem cell transplantation.5-9 These benefits are particularly evident in patients harboring FLT3-ITD or TKD mutations. Therefore, the identification of pathogenic FLT3 mutations that can be targeted by TKIs has significant therapeutic implications for the treatment of AML.
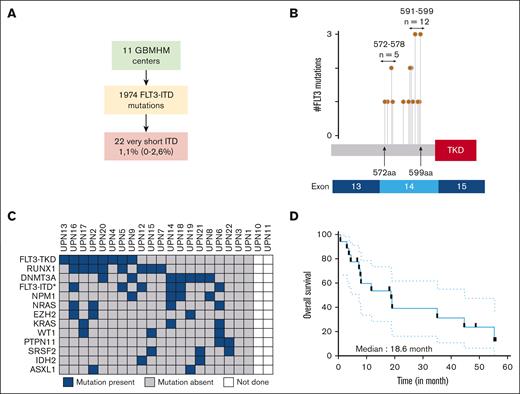
ITDs occur predominantly in the juxtamembrane domain (JMD) of FLT3 and less frequently in the TKD, with the latter being associated with a poorer prognosis.10 Previous investigations have examined the size of ITDs and yielded conflicting results regarding their prognostic significance. Although the structure of the duplicated motif is heterogeneous, these studies consistently reported median ITD sizes ranging from 15 to 54 bp.11-15 We identified 1974 patients with in-frame FLT3 insertions diagnosed at 11 tertiary care centers in France participating in the Groupe des Biologistes Moléculaires des Hémopathies Malignes (GBMHM) network. (Figure 1A; supplemental Table 1).16 Among these patients, 22 (1.1%) were found to have very short in-frame insertions (VSI) within FLT3, resulting in a gain of only 1 or 2 amino acids, a phenomenon that has already been described but not specifically studied (supplemental Table 2).14,15 Interestingly, FLT3-VSIs were mainly observed at amino acid positions 572 to 578 and 591 to 599 in 25% and 60% of the analyzed cases in our cohort, respectively (Figure 1B). Surprisingly, the genomic landscape revealed an unexpected co-occurrence of 8 (40%) RUNX1 mutations, leading to a significant proportion of patients classified as ENL2017 adverse risk (Figure 1C; supplemental Table 3). FLT3-VSI alterations were detected together with FLT3-TKD and/or FLT3-ITD in 12 (54%) cases (supplemental Table 3). The characteristics of these patients are similar to those reported in classic FLT3-ITD cases, including leukocytosis, association with normal karyotype, and mutant or wild-type allele ratio values (supplemental Table 3). Overall survival was poor, with 30% of patients surviving after a median follow-up of 14.9 months, resulting in a median overall survival of 18.6 months (Figure 1D). Interestingly, a nonsignificant trend for decreased survival was observed in patients with simple FLT3 VSIs compared with composite cases with combined VSI and ITD abnormalities (supplemental Figure 1A).
Identification of very short FLT3 insertions in a multicenter cohort of patients with AML. (A) Flowchart identifying patients with very short FLT3 insertions within the GBMHM network. (B) The genomic localization of the 20 FLT3-VSIs with accessible sequencing data is presented in the context of their respective protein and exon sequences. The term #FLT3 mutations indicates the number of FLT3-VSIs identified at specific amino acid (aa) positions. The number of FLT3-VSIs within the domains spanning aa 572 to 578 and 591 to 599 is given. (C) The genomic landscape of FLT3-VSI in 22 patients from our cohort is shown. The results were obtained during routine diagnostic procedures performed by clinical laboratories at the time of AML diagnosis. Only variants identified at least twice within the cohort are presented in this oncoprint. FLT3-ITD∗ indicates additional ITD mutations. (D) Survival curves for the entire cohort of patients with FLT3-VSI (n = 18 with available data).
Identification of very short FLT3 insertions in a multicenter cohort of patients with AML. (A) Flowchart identifying patients with very short FLT3 insertions within the GBMHM network. (B) The genomic localization of the 20 FLT3-VSIs with accessible sequencing data is presented in the context of their respective protein and exon sequences. The term #FLT3 mutations indicates the number of FLT3-VSIs identified at specific amino acid (aa) positions. The number of FLT3-VSIs within the domains spanning aa 572 to 578 and 591 to 599 is given. (C) The genomic landscape of FLT3-VSI in 22 patients from our cohort is shown. The results were obtained during routine diagnostic procedures performed by clinical laboratories at the time of AML diagnosis. Only variants identified at least twice within the cohort are presented in this oncoprint. FLT3-ITD∗ indicates additional ITD mutations. (D) Survival curves for the entire cohort of patients with FLT3-VSI (n = 18 with available data).
To elucidate their biological relevance, we constructed expression vectors containing 4 FLT3-VSIs representative of the available sequences from 20 cases in our cohort (Figure 2A). These variants were compared with a standard FLT3-ITD derived from the human cell line MOLM-14.17 To evaluate the ability of these different forms to induce growth factor–independent proliferation, we used the following 2 cell lines: the human AML TF-1 cell line and the murine lymphoid Ba/F3 cell line. Notably, TF-1 cells are dependent on granulocyte-macrophage colony-stimulating factor for survival, whereas Ba/F3 cells are dependent on interleukin-3.18 This allowed us to assess the efficacy of the different FLT3 variants in promoting autonomous proliferation (supplemental Figure 1A).
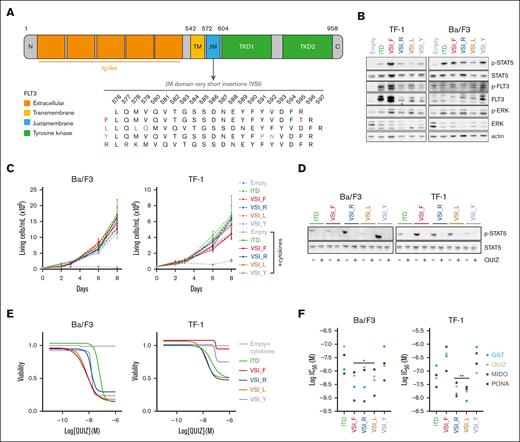
Oncogenic FLT3 signaling triggered by very short FLT3 insertions and their sensitivity to FLT3 inhibitors. (A) A schematic representation of the FLT3 receptor and its different domains is shown. These include the N-terminal (N) and C-terminal (C) regions, immunoglobulin-like (Ig-like) domains, transmembrane (TM) domain, JM domain, and TKD. For a more focused view, an enlarged representation of the aa domain 576 to 597 within the JM domain is provided. This highlights the aa modifications introduced by the 4 FLT3-VSIs (referred to as F, L, Y, and R) selected for modeling. (B) Cells were starved of serum and/or cytokines for 6 hours. Protein extracts were subjected to western blotting with the indicated antibodies. (C) TF-1 and Ba/F3 cells were cultured with or without granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-3 (IL-3), respectively. Cell viability was monitored by trypan blue exclusion assay over a period of 8 days (n = 3). (D) TF-1 or Ba/F3 cells expressing FLT3-VSIs or a classic FLT3-ITD mutations were incubated with either vehicle (−) or 3nM (+) quizartinib (QUIZ) for 48 hours. Western blots were performed using the indicated antibodies. (E-F) TF-1 or Ba/F3 cells transduced with the empty vector were cultured with 10% fetal bovine serum and GM-CSF or IL3, respectively, whereas cells expressing FLT3 variants were cultured without cytokines. Cells were treated with either vehicle (0.1% dimethyl sulfoxide) or various concentrations of QUIZ, gilteritinib (GILT), midostaurin (MIDO), or ponatinib (PONA) for 48 hours. Cell viability was measured using the luminescence-based ATPlite assay (n = 3 biological replicates, each including 3 technical replicates). (E) Cell viability assays performed on Ba/F3 and TF-1 cells exposed to QUIZ were plotted using the log(inhibitor) vs response—variable slope (4 parameters) model implemented in GraphPad (Prism, v9.5.0). (F) Half maximal inhibitory concentrations (IC50) for each TKI and FLT3 variant type were determined in Ba/F3 and TF-1 cell lines using GraphPad. Vertical bars indicate standard deviations. Statistical significance levels are indicated as ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001.
Oncogenic FLT3 signaling triggered by very short FLT3 insertions and their sensitivity to FLT3 inhibitors. (A) A schematic representation of the FLT3 receptor and its different domains is shown. These include the N-terminal (N) and C-terminal (C) regions, immunoglobulin-like (Ig-like) domains, transmembrane (TM) domain, JM domain, and TKD. For a more focused view, an enlarged representation of the aa domain 576 to 597 within the JM domain is provided. This highlights the aa modifications introduced by the 4 FLT3-VSIs (referred to as F, L, Y, and R) selected for modeling. (B) Cells were starved of serum and/or cytokines for 6 hours. Protein extracts were subjected to western blotting with the indicated antibodies. (C) TF-1 and Ba/F3 cells were cultured with or without granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-3 (IL-3), respectively. Cell viability was monitored by trypan blue exclusion assay over a period of 8 days (n = 3). (D) TF-1 or Ba/F3 cells expressing FLT3-VSIs or a classic FLT3-ITD mutations were incubated with either vehicle (−) or 3nM (+) quizartinib (QUIZ) for 48 hours. Western blots were performed using the indicated antibodies. (E-F) TF-1 or Ba/F3 cells transduced with the empty vector were cultured with 10% fetal bovine serum and GM-CSF or IL3, respectively, whereas cells expressing FLT3 variants were cultured without cytokines. Cells were treated with either vehicle (0.1% dimethyl sulfoxide) or various concentrations of QUIZ, gilteritinib (GILT), midostaurin (MIDO), or ponatinib (PONA) for 48 hours. Cell viability was measured using the luminescence-based ATPlite assay (n = 3 biological replicates, each including 3 technical replicates). (E) Cell viability assays performed on Ba/F3 and TF-1 cells exposed to QUIZ were plotted using the log(inhibitor) vs response—variable slope (4 parameters) model implemented in GraphPad (Prism, v9.5.0). (F) Half maximal inhibitory concentrations (IC50) for each TKI and FLT3 variant type were determined in Ba/F3 and TF-1 cell lines using GraphPad. Vertical bars indicate standard deviations. Statistical significance levels are indicated as ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001.
Under cytokine deprivation conditions, our observations showed that all FLT3-VSI variants induced constitutive signaling through FLT3. This was evidenced by increased phosphorylation of STAT5 (Tyr-694) and FLT3 (Tyr-591) compared with empty vector transduced cells (Figure 2B).19,20 However, we observed heterogeneity among the different FLT3-VSI, particularly in Ba/F3 cells. Furthermore, all VSI conferred cytokine-independent growth in vitro, comparable to cells transduced with the FLT3-ITD vector (Figure 2C). These results underscore that FLT3-VSIs activate FLT3 signaling and maintain autonomous cell growth at levels similar to that of classical FLT3-ITDs. We then evaluated the efficacy of 4 different small-molecule FLT3-targeting TKIs: quizartinib, gilteritinib, midostaurin, and ponatinib. Notably, all of these TKIs, when used at nanomolar concentrations, exhibited almost complete inhibition of FLT3 signaling in both TF-1 and Ba/F3 cells, as evidenced by modulation of FLT3, STAT5, and ERK phosphorylation levels (Figure 2D; supplemental Figure 1B). In addition, the 4 TKIs induced a dose-dependent reduction in cell viability after 48 hours of incubation. However, the dose-response profile showed a more robust effect in Ba/F3 cells compared with TF-1 cells, indicating a lower degree of oncogenic addiction to FLT3 signaling in the latter (Figure 2E; supplemental Figure 1C). Notably, the cytotoxic effects of TKIs on cell-lines expressing FLT3-VSI were found to be comparable, and in some cases, superior to those observed in cells transduced with a conventional ITD (Figure 2F). These results highlight the potential therapeutic efficacy of TKIs against FLT3-mutant AML harboring very short insertions in the FLT3 gene.
Structurally, the FLT3 receptor tyrosine kinase consists of several domains. Within the extracellular domain, immunoglobulin-like domains are responsible for binding the FLT3 ligand (FL). Upon ligand-induced dimerization, autophosphorylation occurs at specific tyrosine residues in the kinase domain, initiating downstream signaling cascades that regulate cell proliferation, survival, and differentiation.21 Physiologically, JMD interactions maintain FLT3 in an autoinhibited state by spanning the N- and C-terminal lobes of the catalytic domain. FL-induced activation disrupts the inhibitory contacts through autophosphorylation of conserved tyrosine residues in the JMD, whereas ITD impairs this autoinhibition mechanism. Notably, our results demonstrate that even a small insertion of 1 or 2 amino acids within essential JMD residues, mainly at position 592 to 599, significantly induces constitutive oncogenic activation of FLT3 signaling. Interestingly, this level of activation was comparable to that induced by longer insertions, underscoring the remarkable sensitivity of the JMD to even small changes in length.
The identification of FLT3-VSIs expands our understanding of the mutational landscape of FLT3-driven cancers. Although these mutations represent a small proportion (1.1%) of all FLT3 insertions, they may have clinical significance in certain patient populations. Notably, FLT3-VSI alterations were frequently associated with FLT3-TKD (27%) or FLT3-ITD (18%) in this cohort. Although the presence of composite FLT3 mutations is well-documented in patients who relapse after TKI treatment, data regarding the co-occurrence of distinct FLT3 mutations are limited and suggest that FLT3-TKD mutations can be found in >10% of diagnostic samples harboring FLT3-ITD.22,23 Similarly, the correlation between FLT3-VSI and RUNX1 mutations is higher than expected for FLT3-ITD, which may contribute to the unfavorable outcome observed in our study and warrants further investigation in larger cohorts of patients with AML.24
Through experimental analysis using cytokine-dependent hematopoietic cell lines, we found that these very short insertions activated FLT3 signaling, induced cytokine-independent growth, and demonstrated significant sensitivity to FLT3-targeting TKIs. These findings are significant because the validated commercial kit LeukoStrat CDx FLT3 Mutation Assay, currently used as a standard tool to select patients with AML for treatment with midostaurin, is only validated to detect FLT3-ITD between 30 and 279 bp.25 Maintaining this detection threshold could therefore lead to misdiagnosis in a subset of patients with FLT3-VSI AML, a scenario in which TKI treatment has the potential to significantly improve clinical outcomes.
In conclusion, these findings have clinical implications for the diagnosis, prognosis, and therapeutic decision-making of patients with FLT3-driven malignancies.
Acknowledgments: The authors thank the Geneva Reader Assay Development and Screening core facilities and in particular Yves Cambet for technical support and insightful collaboration.
This work was supported by grants from the Copley May, Dr. Henri Dubois-Ferrière, Dinu Lipatti, Medic and Lombard Odier (Fonds Jean Pastré) Foundations, as well as Ligue Genevoise Contre le Cancer.
Contribution: J.T., S.M., C.L., P.S., and O.K. developed the methodology; J.T., S.M., P.S., and O.K. performed formal analysis; N.D., A.B., A.S., P.H., L.R., S.C., M.T., F.F., P.F-G., Y.L.B., A-S.A., L.M., S.T., E.D., and F.D. carried out the investigation; J.T. was responsible for funding acquisition, prepared the original draft of the manuscript, and performed visualization; and J.T., P.S., and O.K. conceptualized and validated the study, were responsible for project administration, supervised the study, and reviewed and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of the members of the French GBMHM group appears in “Appendix.”
Correspondence: Jerome Tamburini, Faculty of Medicine, University of Geneva, rue Michel Servet 1, Geneva 1206, Switzerland; email: jerome.tamburinibonnefoy@unige.ch.
Appendix
The GBMHM 2023 members are: Abbou Norman, Addakiri Sara, Adje Missa Louis, Altounian Lucille, Arfeuille Chloé, Bachelot Amélie, Baran-Marszak Fanny, Barrial Kildie, Barthel Anne, Becker Martine, Beillard Emmanuel, Bernier Graziella, Beucher Annaëlle, Blanchet Odile, Blateau Pauline, Bloch Julliane, Boucher Lara, Boulland Marie-Laure, Bouvier Anne, Bouzy Simon, Bracquemart Claire, Bravetti Clotilde, Brouzes Chantal, Brugel Patricia, Carillo Serge, Cassinat Bruno, Cave Hélène, Caye-Eude Aurélie, Cayuela Jean-Michel, Charlot Carole, Chauveau Aurélie, Chikhi Sara, Chollet Lionel, Chomel Jean-Claude, Clappier Emmanuelle, Claudel Alexis, Cornillet Lefebvre Pascale, Cottin Laurane, Coude Marie-Magdelaine, Cussac Vincent, Dadone-Montaudie Bérangère, Davi Frédéric, De Mas Véronique, Debliquis Agathe, Decamp Matthieu, Defasque Sabine, Degaud Michaël, Dejean Marlene, Delabesse Eric, Delacour Hervé, Delfau-Larue Marie-Hélène, Desmares Anne, Divoux Marion, Doumbadze Natacha, Dremaux Julie, Drenou Bernard, Dulucq Stéphanie, Dumont Coraline, Duprat Manon, Duranton-Tanneur Valérie, Estienne Marie-Hélène, Etancelin Pascaline, Faucheux Lisa, Fenwarth Laurène, Fleury Carole, Fournier Elise, Friedrich Chloé, Fund Xavier, Gachard Nathalie, Gaillard Jean-Baptiste, Giot Coralie, Goncalves Valérie, Grardel Nathalie, Gubler Brigitte, Haddad Yacine, Hage Sleiman Mehdi, Hayette Sandrine, Hirschauer Claire, Huet Sarah, Humbert Carole, Ittel Antoine, Kaltenbach Sophie, Lachot Sébastien, Laibe Sophy, Lazarian Grégory, Le Brun Valoris, Le Sciellour Christelle, Lebecque Benjamin, Lemaire Isabelle, Le Mene Melchior, Lespinasse Christine, Lhermitte Ludovic, Lode Laurence, Luque Paz Damien, Maingourd Cyril, Maitre Elsa, Mansier, Olivier, Marceau-Renaut Alice, Marcon Angelique, Martin-Gourier Mélanie, Marzac Christophe, Mauduit Claire, Maurice Lucie, Menard Audrey, Merlio Jean-Philippe, Miguet Laurent Yannick, Mokrane Djamia, Monhoven Nathalie, Mozziconacci Marie-Joelle, Muller Marc, Murati-Zeller Anne, Naguib Dina, Narayanin Rajiv, Nibourel Olivier, Noyel Pauline, Osman Jennifer, Pastoret Cédric, Paubel Agathe, Perdrix Anne, Podvin Benjamin, Quilichini Benoit, Raimbault Anna, Ranaweera Arachchige Thimali, Ravalet Noémie, Renard Julie, Renneville Aline, Renosi Florian, Ribourtout Bénédicte, Rizzo David, Rottier Camille, Ryffel Léa, Sloma Ivan, Soubeyran Brigitte, Sorel Nathalie, Spentchian Marc, Taleb Assia, Tassin Thomas, Tchirkov Andrei, Tueur Giulia, Ugo Valérie, Vallat Laurent, Veronese Lauren, Villarese Patrick, Wiber Margaux, and Zalmaï Loria.
References
Author notes
∗P.S. and O.K. contributed equally to this work.
The data underlying this article are available upon reasonable request to the corresponding author, Jerome Tamburini (jerome.tamburinibonnefoy@unige.ch).
The full-text version of this article contains a data supplement.