Key Points
JAK2, ABL, and MAPK signaling drive adaptive resistance to FLT3 inhibitors.
Polypharmacological targeting of FLT3, JAK2, ABL, and MAPK signaling provides a durable response in AML.
Abstract
Despite significant advancements in developing selective FMS-like tyrosine kinase 3 (FLT3) inhibitors, resistance to treatment is common even on continued therapy. Acquisition of on-target mutations or adaptation to MAPK, JAK2, and ABL signaling pathways drive treatment failure and disease relapse. Although combinatorial targeting of all escape routes in preclinical models demonstrated its efficacy, the clinical application is challenging owing to drug-drug interaction and differing pharmacokinetics of the inhibitors. We reasoned that selective polypharmacological targeting could lead to a durable response with reduced toxicity. A cell-based screening was carried out to identify inhibitors targeting FLT3, RAS-MAPK, BCR-ABL, and JAK2 to target the adaptive resistance observed with FLT3 inhibitors. Here, we show that pluripotin is an equipotent inhibitor of FLT3, BCR-ABL, and JAK2 in addition to inhibiting Ras-GAP and extracellular signal-regulated kinase 1 (ERK1). Structural modeling studies revealed that pluripotin is a type II kinase inhibitor that selectively binds with inactive conformations of FLT3, ABL, and JAK2. Pluripotin showed potent inhibitory activity on both mouse and human cells expressing FLT3ITD, including clinically challenging resistant mutations of the gatekeeper residue, F691L. Likewise, pluripotin suppressed the adaptive resistance conferred by the activation of RAS-MAPK pathways, BCR-ABL, and JAK2 signaling. Treatment with pluripotin curbed the progression of acute myeloid leukemia (AML) in multiple in vivo models including patient-derived primary AML cells in mouse xenotransplants. As a proof of concept, we demonstrate that targeted polypharmacological inhibition of key signaling nodes driving adaptive resistance can provide a durable response.
Introduction
Kinase activating mutations in the FMS-like tyrosine kinase 3 (FLT3) gene represent the most frequent molecular lesion in acute myeloid leukemia (AML).1-3 Approximately one-third of patients with AML harbor internal tandem duplication (ITD), which is associated with poor treatment outcomes and overall survival even after stem cell transplantation.4,5 In addition, a significant number of patients harbor kinase activating mutations from the activation loop (D835) with an unknown prognosis.6,7 Several small-molecule FLT3 tyrosine kinase inhibitors (TKIs) have been evaluated in the last 2 decades that resulted in the approval of midostaurin and gilteritinib for the treatment of AML.
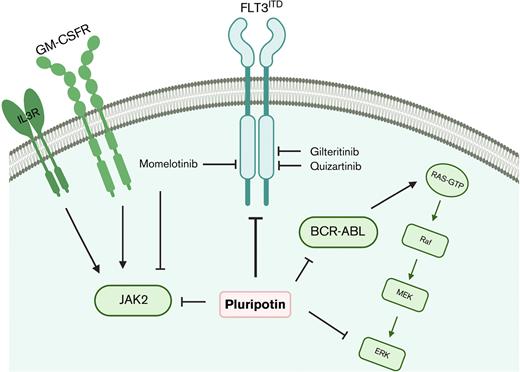
Despite significant advancements in developing FLT3 inhibitors, treatment failure is common owing to the emergence of resistance. Most patients developed resistance within a short duration even on continued TKI therapy. Both type I (gilteritinib and crenolanib) and type II (quizartinib and sorafenib) FLT3 inhibitors have been evaluated in patients. Regardless of their selectivity and mode of inhibition, resistance emerged. Nonetheless, mechanisms driving resistance partly differ between type I and type II inhibitors. For instance, resistance to type II inhibitors is predominately mediated by the on-target selection of resistant mutations in the FLT3 kinase domain, whereas resistance to type I inhibitors is principally driven by off-target activation of RAS-MAPK, BCR-ABL, and JAK2/JAK1 signaling.6,8-10 A minority of patients who showed treatment resistance showed the emergence of mutations in genes regulating metabolism and transcription. However, its relevance in conferring resistance has not been evaluated. Besides, like other TKI-treated malignancies, gatekeeper mutation (F691L) conferred resistance to both type I and type II FLT3 inhibitors and poses a significant clinical challenge. It is not clear why on-target–acquired resistance is more frequent with type II inhibitors compared with type I inhibitors in which adaptive resistance to compensatory signaling is more common. Possibly, the stabilization of active kinase conformation by type I inhibitor is, nonetheless, enzymatically in-active, but its nonenzymatic function (signaling scaffold) drives the adaptation to alternate survival pathways. Nevertheless, these studies informed that FLT3 resistant variants could be suppressed by switching to next-generation FLT3 inhibitors or dose escalation as demonstrated in managing the TKI resistance in chronic myeloid leukemia (CML). However, unlike CML, resistance conferred by off-target activation of MAPKs, BCR-ABL, and JAKs in AML remains a serious challenge.
Besides, FLT3ITD expressing leukemic stem cells (LSCs) residing in bone marrow (BM), like LSCs in CML, are refractory to TKI inhibitors, which serve as a reservoir to develop resistance.3,11 Hematopoietic cytokine–mediated activation of JAK2 and MAPK signaling by FLT3 ligand (FL),12 CXCR4, FGF2,13 interleukin 3 (IL-3), and granulocyte-macrophage colony-stimulating factor (GM-CSF)14 have been described in conferring TKI resistance.14,15 Patients with AML who achieved complete remission lacking FLT3ITD clones showed better overall survival than patients with minimal residual disease (MRD), suggesting that eradicating the FLT3ITD clones will have a durable response with better overall survival. The persistence of residual disease serves as a reservoir to develop resistance that eventually leads to disease relapse. Strategies aimed at greater front line disease eradication and suppression of resistance are needed, most of which depends on further research into combination chemotherapy or to develop polypharmacological agents targeting FLT3, its resistant variants, BCR-ABL, JAK2, and MAPKs. To address this, we performed a cell-based screening to identify the small-molecule inhibitor active against FLT3, BCR-ABL, JAK2, and MAPKs. Here we show that pluripotin (SC-1),16 an inhibitor of RasGAP, and MAPK3,16 potently inhibits the kinase activity of FLT3, BCR-ABL, and JAK2. Structural modeling studies revealed that it binds with inactive conformations of FLT3, JAK2, and ABL. It is an equipotent inhibitor to both FLT3ITD and its most vexing resistant variant, the gatekeeper mutant F691L. Expectedly, treatment with pluripotin efficiently suppressed the adaptive resistance conferred by MAPK, BCR-ABL, and JAK2 signaling. As a proof of concept, we provide evidence that the unique polypharmacology of pluripotin, targeting FLT3, BCR-ABL, JAK2, and MAPK efficiently suppressed the leukemic progression in multiple preclinical mouse models and in mice engrafted with primary AML cells. Our preclinical data suggest that upfront targeting of key signaling nodes driving adaptive resistance by polypharmacological agents provides durable response, which is not achieved by currently used FLT3 inhibitors. The future of drug design should focus on developing polypharmacological agents targeting these signaling nodes to achieve a durable response for AML.
Methods
Plasmids and inhibitors
Retroviral plasmids expressing oncogenic kinases (Jak2, Jak2V617F, Jak2V617F/L983F, BCR-ABL, BCR-ABLT315I, FLT3, and FLT3ITD) and their drug-resistant variants were described earlier.15,17,18 Lentiviruses expressing wild-type NRAS, pHAGE-NRAS and pHAGE-NRAS-Q61K were purchased from Addgene (Watertown, MA). pHAGE-NRAS was mutagenized to obtain pHAGE-NRAS-G12V by site-directed mutagenesis using lightning, quick-change mutagenesis kit from Agilent (Santa CLara, CA) as described earlier.17,19 JAK2 inhibitors, ruxolitinib, fedratinib, and momelotinib were purchased from Chemietech Inc (Indianapolis, IN). ABL inhibitors, imatinib, and nilotinib were obtained from LC Chemicals (Woburn, MA), whereas FLT3 inhibitors and MAPK pathway inhibitors were purchased from AdooQ Biosciences (Irvine, CA).
Cell lines
BaF3 cell line, an IL-3 dependent hematopoietic pro–B cell line, was transduced for stable expression of oncogenic kinases Jak2, Jak2V617F, BCR-ABL, and FLT3ITD. Retroviral production and transduction were performed as described earlier.11,18 Human AML cell lines Molm13, MV4-11, KG-1, ME-1, Kasumi, TF-1, NB4, HL60, THP-1, and MO7-E were grown in RPMI 1640 supplemented with 10% fetal calf serum (FCS) except for the TF-1 growth media, which was supplemented with 10 ng/mL human IL-3 as described earlier.15
Cell viability and western blotting assays
The efficacy of inhibitors for kinase selectivity was determined by dose-dependent cell proliferation assays using BaF3 expressing either WT (FLT3 or Jak2 or BCR-ABL) or oncogenic kinases (Jak2V617F or FLT3ITD and its resistant variants). Ten thousand cells (human AML cell linesor IL-3 independent BaF3 cells stably expressing Jak2V617F, FLT3WT, FLT3ITD, and its drug-resistant variants) were plated in triplicate in 96-well plates in 0.1 mL RPMI 1640 medium containing 10% FCS with varying inhibitor concentrations. Similarly, 10 000 human primary AML cells were plated in triplicate in 96-well plates in 0.1 mL Iscove modified Dulbecco medium with 10% FCS containing either 3 cytokines (100 ng/mL stem cell factor [SCF], 50 ng/mL thyroid peroxidase [TPO], and 20 ng/mL IL-6) or 6 cytokines (100 ng/mL SCF, 50 ng/mL TPO, 20 ng/mL IL-6, 50 ng/mL FLT3LG, 50 ng/mL GM-CSF, and 20 ng/mL IL-3). After 60 hours, cell viability was assessed using WST-1 reagent (Takara Bio USA, Inc, San Jose, CA). Absorbance (A = 450 nM) was averaged and plotted against inhibitor concentration as a best-fit sigmoidal curve, using the Prism software version 9.0 (GraphPad, LaJolla, CA). The concentration resulting in 50% maximal inhibition was reported as the cellular 50% inhibitory concentration (IC50). Combination index (CIIC50) was calculated using the formula combination index CIIC50 = (D1)c/(D1)a + (D2)c/(D2)a. (D1)a and (D2)a represent the concentrations of each drug alone at IC50, whereas (D1)c and (D2)c were the concentrations of drugs in combination at IC50. CI IC50 <1, =1, and >1 indicated synergism, additive, and antagonism, respectively.
Four to 6 million leukemic cells were suspended in lysis buffer followed by 3 short bursts of sonication. The composition of lysis buffer has been described earlier. Lysates were separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to supported nitrocellulose membrane (Bio-Rad, Hercules, CA) and probed with the following antibodies from Cell Signaling Technology: anti–phospho-FLT3 (pFLT3) (monoclonal antibody #4577), FLT3 (monoclonal antibody #3462), pMEK1/2 (Ser217/221) (#9121), phospho–extracellular signal-regulated kinase 1/2 (pErk1/2) (Thr202/Tyr204) (#9106), pStat5 (Tyr694) (C11C5) (#9359), MEK1/2 (47E6) (#9126), Erk1/2 (#9102), Stat5 (#9363). All primary antibodies were used at dilutions as recommended by the manufacturer. Anti-mouse or anti-rabbit immunoglobulin G horseradish peroxidase--conjugate secondary antibodies (GE Healthcare, Chicago, IL) were used at a 1:5000 dilution. Horseradish peroxidase–conjugated antibodies β-actin (13E5) (#5125) from Cell Signaling Technology, Inc was used. Immunoblots were developed using SuperSignal West Dura Extended Duration Substrate (Thermo Fisher Scientific, Waltham, MA) followed by scanning on ChemiDoc Touch Imaging System (Bio-Rad). All western blots were replicated twice.
Structural modeling, inhibitor docking, and representation
SwissDock20,21 was used to dock pluripotin at the adenosine triphosphate (ATP) site of active and inactive conformations of ABL (PDB:2GQ2 and PDB:3CS9), Jak2 (PDB:6VGL and PDB: 3UGC), and FLT3 kinases (PDB:6JQR and PDB: 4XUF). Free energies for ligand binding are estimated on a grid22 and ranked using Chemistry at Harvard Macromolecular Mechanics. Structural modules with the most favorable energies are clustered,23 and the most favorable clusters are contained in the output PDB file. For each cluster, binding modules with the lowest free energies are selected for further verification. Figures were generated using PyMol.
Apoptosis assay
Briefly, 1 × 106 cells were seeded into 6-well plates with varying pluripotin concentrations (0-100 nM). Cells were collected 24 hours after drug incubation and stained with annexin V and propidium iodide (PI) for 15 minutes according to the manufacturer’s protocol (BD Biosciences, Franklin Lakes, NJ). Flow cytometry analysis was done on FACSAria (BD Biosciences). Results were analyzed using FlowJo software.
Isolation of Lin− and CD34+ cells, ex vivo culture, and apoptotic assay
Mouse BM from the WT and Rosa21CReERT2;Flt3ITD/Tet2fl/fl was collected from the femurs and tibias after euthanasia. Lineage negative (Lin−) cells were purified using magnetic beads (Miltenyi Biotec, North Rhine-Westphalia, Germany) according to the manufacturer’s instructions. The isolated cells were plated in 24-well plates in serum-free expansion medium (SFEM) in triplicate containing 50 ng/mL of TPO, SCF, and FLT3LG, and 10 ng/mL IL-3 with 0.1 μM and 1 μM of pluripotin and gilteritinib, respectively after incubation at 37°C for 6 days. On day 6, cells were stained for annexin V, PI, and cell-surface markers (Sca-1 and c-Kit) as described earlier. Labeled cells were analyzed by flow cytometry (FACSAria, BD Biosciences). Likewise, human CD34+ cells from normal and AML donor were isolated using magnetic beads as described earlier. A hundred thousand CD34+ cells from both normal and leukemic samples were seeded with and without FLT3 inhibitors, described above, in SFEM media reconstituted with 100 ng/mL of SCF, FLT3LG, TPO, and GM-CSF and 10 ng/mL of IL-3 and IL-6. On day 6, cells were harvested and stained for annexin V and PI along with CD34 and CD38 cell-surface markers as described earlier. Labeled cells were analyzed by fluorescence-activated cell sorter (FACS) and results were analyzed using FlowJo. Five thousand CD34 cells were seeded with and without kinase inhibitors in methocult (STEMCELL Technology) in triplicate. Colonies were enumerated at day 14 and presented as percent relative to vehicle treatment (considered as 100%).
In vivo efficacy of pluripotin
Human AML cell lines with FLT3ITD mutations (Molm13 and MV4-11) were transduced to express firefly luciferase and cherry fluorescent proteins for in vivo imaging and tracking the disease progression, respectively.15,18 Virus production and transduction were performed as described above. One million cherry+ cells were injected into the tail veins of immunocompromised NSG-SGM3 (NSGS) mice (Jackson Laboratories, Bar Harbor, ME). We did not use NSG mice for in vivo validation studies as described in previous studies24 because they lack key human hematopoietic cytokines implicated in conferring resistance to TKI treatment. For instance, hematopoietic cytokines, IL-3, GM-CSF, and FLT3LG were shown to abrogate TKI response in AML by activating JAK2 and RAS-MAPK signaling.14,15 The triple transgenic NSGS mice expressing human IL-3, GM-CSF, and SCF, although not perfect, closely represent clinical condition. Besides, NSGS mice allow superior engraftment of diverse hematopoietic lineages and primary AML samples than the NSG mice. Two days after the transplantation, mice were intraperitoneally administered with gilteritinib (30 mg/kg daily) or pluripotin (15 mg/kg daily). The leukemic burden was determined weekly using peripheral blood (PB). All experiments involving mice were performed according to the National Institutes of Health Guide for the care and use of laboratory animals and were approved by the Cincinnati Children’s Hospital Institutional Animal Care and Use Committee.
In vivo efficacy of pluripotin in murine AML model
One million BM cells from the Rosa21:CReERT2/Flt3ITD/ITD/Tet2fl/flmice mixed with 1 million BoyJ mice (CD45.1) were transplanted in lethally irradiated recipient BoyJ mice. After 2 weeks of transplantation, Tet2 was deleted by tamoxifen injection after drug treatments as described in the preceding section. Leukemic burdens were monitored weekly using PB by FACS and hemacytometer.
In vivo xenograft of AML and treatment
Three million primary human AML cells were transplanted in sublethally irradiated NSGS mice. After 2 to 4 weeks, leukemic engraftments were analyzed by determining the levels of human CD45 using FACS. Drug treatments, as described above, were started after 3 weeks of transplantation and continued up to 16 weeks. Leukemic burdens were analyzed weekly by determining the levels of CD45.
Statistical analysis
Statistical analyses were conducted using Prism software version 9.0 (GraphPad). The median survival was calculated by a log-rank test. For in vitro studies, statistical significance was determined by the 2-tailed unpaired Student t test. A P value of <.05 was considered statistically significant. For all figures, not significant P values, ∗P ≤ .05, ∗∗P ≤ .01, ∗∗∗P ≤ .001, ∗∗∗∗P ≤ .0001. Unless otherwise indicated, all data represent the mean ± standard deviation (SD) from the 3 technical replicates.
Results
Pluripotin inhibits FLT3, BCR-ABL, and JAK2
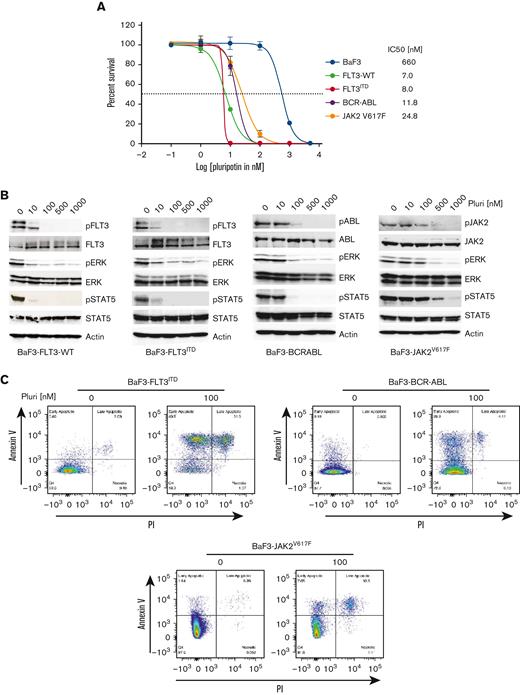
The molecular heterogeneity of AML and the identification of multiple mechanisms of resistance to targeted TKI therapies strongly support the rationale for combinatorial approaches. Although combinatorial treatment strategies have shown effective response in preclinical cancer models, its clinical realization is hampered owing to drug-drug interactions and differing pharmacokinetics. We reasoned that exploiting the polypharmacology of a single drug targeting compensatory survival pathways will be more effective in FLT3-targeted AML. To address this, we performed a cell-based screening of MAPK pathway inhibitors having selectivity for FLT3, BCR-ABL, and JAK2 kinases (supplemental Figure 1A,B). We transduced the Ba/F3 cells, a murine IL-3–dependent hematopoietic cell line, with retroviruses expressing MSCV-FLT3ITD-Ires-cherry, MSCV-BCR-ABL-Ires-venus, and MSCV-Jak2V617F-Ires-GFP. Ba/F3 cells expressing cherry, GFP, and venus were sorted by FACS and selected for IL-3 independence. Expression of constitutively active kinase renders Ba/F3 cells to IL-3 independent growth. A small-molecule library of MAPK pathway inhibitors was screened at 0 μM to 10 μM of drug concentrations for selective inhibition of FLT3, ABL, JAK2, and MAPK (supplemental Figure 1A,B). This screening identified pluripotin as a potent inhibitor of FLT3, BCR-ABL, and JAK2 (Figure 1A). It effectively suppressed the proliferation of BaF3-FLT3WT (IC50, 7 nM), BaF3-FLT3ITD (IC50, 8 nM), BaF3-BCR-ABL (IC50, 12 nM), and BaF3-Jak2V617F (IC50, 25 nM), whereas IC50 for the parental BaF3 is 660 nM, demonstrating a significant therapeutic window. Expectedly, both gilteritinib and quizartinib inhibited the proliferation of BaF3-FLT3ITD but lacked activity against the Ba/F3 cells expressing BCR-ABL and JAK2V617F (supplemental Figure 1C-D). To determine whether the inhibitory effect of pluripotin on cell proliferation is mediated through the on-target inhibition of oncogenic kinase signaling, FLT3, ABL, and Jak2 autophosphorylation as well as phosphorylation of STAT5 and ERK1/2 were evaluated by immunoblotting. Treatment with pluripotin abolished the phosphorylation of autophosphorylated tyrosine residues from the activation loop of FLT3ITD (Y842), BCR-ABL (Y386), and Jak2V617F (Y1002) kinases (Figure 1B). Likewise, treatment with pluripotin inhibited the phosphorylation of STAT5 and ERK1/2 (Figure 1B). Consequently, on-target inhibition of oncogenic kinase signaling resulted in apoptotic cell death determined by annexin V staining (Figure 1C). Altogether, these data demonstrate that pluripotin selectively kills the Ba/F3 cells expressing FLT3ITD, BCR-ABL, and JAK2V617F by blocking their kinase activity and downstream signaling.
Pluripotin potently inhibits the proliferation of Ba/F3 cells expressing FLT3ITD, BCR-ABL, and Jak2V617F. (A) The sigmoidal curve showing the viability of Ba/F3 cells expressing FLT3ITD, BCR-ABL, and Jak2V617F treated with dimethyl sulfoxide or increasing concentrations of pluripotin for 72 hours. IC50 values for each cell line is indicated on the right. (B) Showing pFLT3, pERK1/2, and pSTAT5 levels determined by western blotting using total cells extracts incubated with different concentrations of pluripotin for 2 hours were probed with anti-pFLT3, anti-pSTAT5, anti-pERK1/2, anti-FLT3, anti-ERK1/2, and anti-STAT5 antibodies. (C) Scatter plot showing the extent of apoptosis in Ba/F3 cells expressing FLT3ITD, BCR-ABL, and Jak2V617F after the treatment with 100 nM of pluripotin. The quadrant in each plot show; Q1, early apoptotic cells (annexin V+); Q2, late apoptotic cells (annexin V+ and PI+); Q3, necrotic cells (PI+); and Q4, viable cells (annexin V− and PI−). Representative cell proliferation data is shown from the mean of 2 independent experiments ± SD. Pluri, pluripotin.
Pluripotin potently inhibits the proliferation of Ba/F3 cells expressing FLT3ITD, BCR-ABL, and Jak2V617F. (A) The sigmoidal curve showing the viability of Ba/F3 cells expressing FLT3ITD, BCR-ABL, and Jak2V617F treated with dimethyl sulfoxide or increasing concentrations of pluripotin for 72 hours. IC50 values for each cell line is indicated on the right. (B) Showing pFLT3, pERK1/2, and pSTAT5 levels determined by western blotting using total cells extracts incubated with different concentrations of pluripotin for 2 hours were probed with anti-pFLT3, anti-pSTAT5, anti-pERK1/2, anti-FLT3, anti-ERK1/2, and anti-STAT5 antibodies. (C) Scatter plot showing the extent of apoptosis in Ba/F3 cells expressing FLT3ITD, BCR-ABL, and Jak2V617F after the treatment with 100 nM of pluripotin. The quadrant in each plot show; Q1, early apoptotic cells (annexin V+); Q2, late apoptotic cells (annexin V+ and PI+); Q3, necrotic cells (PI+); and Q4, viable cells (annexin V− and PI−). Representative cell proliferation data is shown from the mean of 2 independent experiments ± SD. Pluri, pluripotin.
Pluripotin abolishes the adaptive resistance conferred by RAS-MAPK, BCR-ABL, and JAK2 signaling
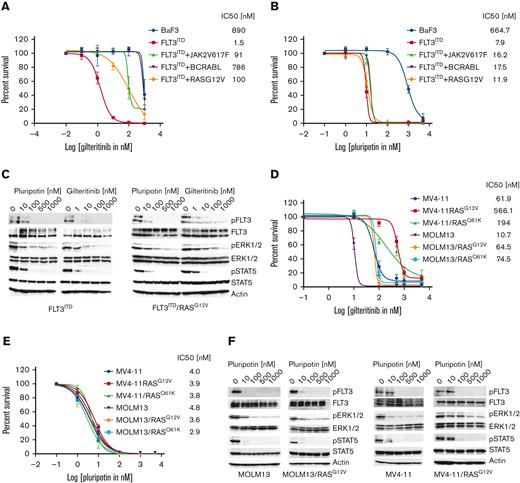
Resistance to type II inhibitors is predominately mediated by on-target resistant mutations from the activation loop and the gatekeeper residues, whereas adaptive resistance is more frequently observed with type I inhibitors. Activation of compensatory survival pathways, such as RAS-MAPK, BCR-ABL, and JAK2, drive adaptive resistance. To determine the effect of these genes in conferring resistance, Ba/F3 cells expressing Ras mutants, BCR-ABL, and JAK2V617F alone or with FLT3ITD were created. Parental BaF3 and BAF3-FLT3ITD cells were transduced with retroviruses expressing NRASG12V-Ires-GFP, NRASQ61K-Ires-GFP, BCR-ABL-Ires-GFP, and JAK2V617F-Ires-GFP. These cell lines were subjected to dose-dependent cell proliferation assays to determine the IC50 values. As expected, BaF3-FLT3ITD cells coexpressing RasG12V, BCR-ABL, and JAK2V617F conferred strong resistance to gilteritinib (Figure 2A; supplemental Figure 2A). Likewise, treatment with trametinib alone failed to suppress the resistance conferred by the RAS mutants or BCR-ABL and JAK2V617F in FLT3ITD-expressing cells (supplemental Figure 2B). As reported earlier,8 we noted a combination of trametinib with gilteritinib suppressed the adaptive resistance conferred by RAS mutants but lacked the therapeutic window (data not shown). To assess the hypothesis that a MAPK inhibitor targeting FLT3, BCR-ABL, and JAK2 would abrogate the adaptive resistance to gilteritinib, we next treated the BaF3-FLT3ITD cells coexpressing RAS variants, BCR-ABL, and JAK2 with increasing pluripotin concentrations. As envisioned, treatment with pluripotin potently suppressed the resistance conferred by FLT3ITD cooperative mutations, RASG12V, RASQ61K, BCR-ABL, and JAK2V617F (Figure 2B; supplemental Figure 2C). A comparative analysis of pFLT3, pERK1/2, and pSTAT5 in BaF3-FLT3ITD and BaF3-FLT3ITD/RASGA12V with gilteritinib and pluripotin inhibition revealed that pluripotin is equally active in suppressing pFLT3 and pSTAT5 in both cell lines, whereas efficacy of gilteritinib was reduced in BaF3-FLT3ITD cells expressing RASG12V (supplemental Figure 2C).
Pluripotin abolishes the adaptive resistance conferred by RASmutants, BCR-ABL, and Jak2V617. Dose-response sigmoidal curve showing the proliferation of Ba/F3 cells expressing FLT3ITD and cooperating mutants BCR-ABL, RASG12V, and Jak2V617F implicated in adaptive resistance. A dose-dependent cell proliferation showing resistance to gilteritinib (A) although treatment with pluripotin fully inhibited their proliferation (B). (C) A comparative immunoblot analysis showing inhibition of FLT3, ERK1/2, and STAT5. Total cell extracts from the BaF3-FLT3ITD and BaF3-FLT3ITD/RASG12V cells treated with gilteritinib or pluripotin for 2 hours were probed with anti-pFLT3, anti-pSTAT5, anti-pERK1/2, anti-FLT3, anti-ERK1/2, and anti-STAT5 antibodies. The sigmoidal curves showing the proliferation of human AML cell lines, MOLM13 and MV4-11, expressing RasG12V and RASQ61K resistant to gilteritinib (D) whereas pluripotin treatment fully inhibited their proliferation (E). (F) Immunoblot analysis showing inhibition of proliferation is owing to on-target inhibition of FLT3, ERK1/2, and STAT5. Total cell extracts from the AML cells treated with pluripotin for 2 hours were probed with anti-pFLT3, anti-pSTAT5, anti-pERK1/2, anti-FLT3, anti-ERK1/2, and anti-STAT5 antibodies. Representative cell proliferation data and western blottings are shown from 2 independent experiments. Error bars represent ± SD.
Pluripotin abolishes the adaptive resistance conferred by RASmutants, BCR-ABL, and Jak2V617. Dose-response sigmoidal curve showing the proliferation of Ba/F3 cells expressing FLT3ITD and cooperating mutants BCR-ABL, RASG12V, and Jak2V617F implicated in adaptive resistance. A dose-dependent cell proliferation showing resistance to gilteritinib (A) although treatment with pluripotin fully inhibited their proliferation (B). (C) A comparative immunoblot analysis showing inhibition of FLT3, ERK1/2, and STAT5. Total cell extracts from the BaF3-FLT3ITD and BaF3-FLT3ITD/RASG12V cells treated with gilteritinib or pluripotin for 2 hours were probed with anti-pFLT3, anti-pSTAT5, anti-pERK1/2, anti-FLT3, anti-ERK1/2, and anti-STAT5 antibodies. The sigmoidal curves showing the proliferation of human AML cell lines, MOLM13 and MV4-11, expressing RasG12V and RASQ61K resistant to gilteritinib (D) whereas pluripotin treatment fully inhibited their proliferation (E). (F) Immunoblot analysis showing inhibition of proliferation is owing to on-target inhibition of FLT3, ERK1/2, and STAT5. Total cell extracts from the AML cells treated with pluripotin for 2 hours were probed with anti-pFLT3, anti-pSTAT5, anti-pERK1/2, anti-FLT3, anti-ERK1/2, and anti-STAT5 antibodies. Representative cell proliferation data and western blottings are shown from 2 independent experiments. Error bars represent ± SD.
Likewise, human AML cells (MOLM13 and MV4-11) expressing RASG12V and RASQ61K conferred resistance to gilteritinib, whereas treatment with pluripotin fully suppressed the cell proliferation with a concomitant reduction in phosphorylation of FLT3 and its substrates, STAT5 and ERK1/2 (Figure 2D-F). Next, we evaluated the efficacy of pluripotin on non-FLT3–mutated cell lines harboring mutations in MAPK pathways and transcription factors (both point mutations and genefusions, supplemental Table 1). Pluripotin inhibited the proliferation of most non-FLT3–mutated AML cell lines except the ME1, THP1, and MO7-E. Pluripotin sensitivity corelates with the concomitant inhibition of pSTAT5 and pERK1/2, whereas resistant cell lines (ME1, THP1, and MO7-E) show persistent pSTAT5 and pERK levels (supplemental Figure 2F). To test whether the BCL2 inhibitor, venetoclax, can sensitize these resistant cell lines for TKI inhibition as recently shown in an elegant study by Zhu et al,25 a dose-dependent inhibition analysis of venetoclax alone was carried out. It showed that MOLM13, THP1, NB4, and TF1 cell lines are resistant to venetoclax (supplemental Figure 2G). As noted earlier, MOLM13 and non-FLT3–mutated AML cells do not show synergistic response to the combination of venetoclax plus giltertinib.25 Strikingly, venetoclax in combination with pluripotin exhibited synergistic response in both FLT3-mutated and non-FLT3–mutated cells, except for the MOLM13 and MO7-E cells (supplemental Figure 2G). Together, these data provide evidence that the unique polypharmacology of pluripotin effectively suppresses the adaptive resistance observed with treatment with gilteritinib, and perhaps combinatorial targeting of Bcl2 will impart greater efficacy in combating the resistance.
Molecular docking studies reveal that pluripotin binds with inactive conformations of FLT3, ABL, and JAK2
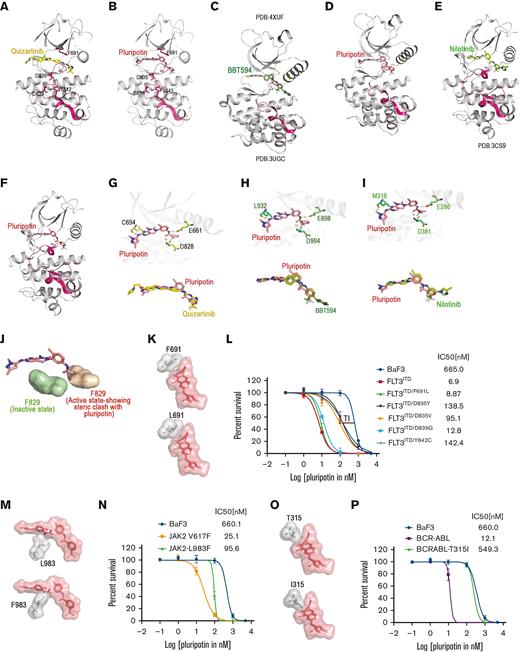
Next, we performed molecular docking studies of pluripotin to understand the structural basis of FLT3, ABL, and JAK2 inhibitions. We used both the inactive and active conformations of FLT3, ABL, and JAK2 for docking studies. Our in silico analysis failed to predict the binding of pluripotin at the ATP site when the active conformations were used for docking. Our docking analysis predicts that pluripotin binds to a closed and enzymatically inactive conformation of FLT3, ABL, and JAK2 in which phenylalanine residue of the DFG motif is displaced to accommodate trifluoro phenyl ring (Figure 3A-F). Pluripotin anchors to the ATP site by coordinating with residues from the kinasehinge (FLT3-Cys 694, ABL-Met 318, and Jak2-Leu 932), helix-C (FLT3-Glu 661, ABL-Glu 286, and Jak2-Glu 898), and catalytic HRD motif (FLT3-Asp 829, ABL-Asp 381, and Jak2-Asp 994) using hydrogen bonds (Figure 3G-I). Active kinase conformation targeted by the type I inhibitors is incompatible for pluripotin binding owing to steric clash with phenylalanine residue of DFG motif adopted in DFG-out conformation (Figure 3J). Likewise, the phenylalanine residue of DFG motifs from the active conformations of ABL and JAK2 kinases rendered steric hindrance to pluripotin binding (not shown). Interestingly, similar to gilteritinib and quizartinib, pluripotin is not in close contact with gatekeeper residue F691. These analyses suggest that mutations activating the kinase enzymatic activity (the activation loop mutants would stabilize the active kinase conformation) will confer resistance to pluripotin, whereas the gatekeeper mutations will be susceptible to inhibition. This analysis also revealed that unlike FLT3, the gatekeeper mutants of ABL will confer resistance to pluripotin because of its close proximity with the gatekeeper residue, threonine 315.
Pluripotin is a type II inhibitor of FLT3, ABL, and Jak2 and a potent inhibitor of gatekeeper FLT3 mutant, F691L. Ribbon depiction of a structure of FLT3 with quizartinib (A) and with pluripotin (B). Ribbon depiction of Jak2-coordinate with type II inhibitor BBT594 (C) and with pluripotin (D). A ribbon cartoon of ABL-coordinate with type II inhibitor nilotinib (E) and with pluripotin (F). A stick representation of active sites of FLT3 (G), JAK2 (H), and ABL (I) showing pluripotin interaction with residues from the hinge region, catalytic glutamate of helix-C, and the conserved aspartate from the HRD motif. Overlapping binding of pluripotin is shown below. (J) Pluripotin binding is incompatible with active kinase conformation as phenylalanine residue of the DFG motif will block the binding (shown on the red surface) whereas inactive conformation is accessible when Phe will move out (shown on the green surface). (K) A close-up view of the active site of the FLT3 kinase showing the interaction of pluripotin with the gatekeeper, Phe 691 (top). A model showing that the gatekeeper mutation, F691L, will not affect pluripotin binding (bottom). (L) Sigmoidal curve showing that pluripotin is equally active on FLT3ITD and its gatekeeper mutant, although mutants from the activation loop are resistant. Nonetheless, a significant therapeutic index (TI) exists between the activation loop mutants and normal Ba/F3 cells suggesting that dose escalation will suppress these variants. (M) Active site of Jak2 kinase showing the interaction of pluripotin with Leu 983 (top). A phenyl alanine substitution for Leu 983 will confer steric hindrance (bottom). (N) According to prediction, Jak2V617F/L983F mutant conferred resistance to pluripotin. Thus, confirming the model and on-target inhibitory activity of pluripotin. (O) Active site of ABL kinase domain showing the interaction of pluripotin with the gatekeeper residue, Thr 315 (top). An isoleucine substitution for Thr 315 will confer steric hindrance (bottom). (P) As expected from the modeling studies, BCR-ABLT315 mutant conferred resistance to pluripotin. Together, these modeling and in vitro validation data confirm that pluripotin directly binds with inactive confirmations of FLT3, JAK2, and ABL.
Pluripotin is a type II inhibitor of FLT3, ABL, and Jak2 and a potent inhibitor of gatekeeper FLT3 mutant, F691L. Ribbon depiction of a structure of FLT3 with quizartinib (A) and with pluripotin (B). Ribbon depiction of Jak2-coordinate with type II inhibitor BBT594 (C) and with pluripotin (D). A ribbon cartoon of ABL-coordinate with type II inhibitor nilotinib (E) and with pluripotin (F). A stick representation of active sites of FLT3 (G), JAK2 (H), and ABL (I) showing pluripotin interaction with residues from the hinge region, catalytic glutamate of helix-C, and the conserved aspartate from the HRD motif. Overlapping binding of pluripotin is shown below. (J) Pluripotin binding is incompatible with active kinase conformation as phenylalanine residue of the DFG motif will block the binding (shown on the red surface) whereas inactive conformation is accessible when Phe will move out (shown on the green surface). (K) A close-up view of the active site of the FLT3 kinase showing the interaction of pluripotin with the gatekeeper, Phe 691 (top). A model showing that the gatekeeper mutation, F691L, will not affect pluripotin binding (bottom). (L) Sigmoidal curve showing that pluripotin is equally active on FLT3ITD and its gatekeeper mutant, although mutants from the activation loop are resistant. Nonetheless, a significant therapeutic index (TI) exists between the activation loop mutants and normal Ba/F3 cells suggesting that dose escalation will suppress these variants. (M) Active site of Jak2 kinase showing the interaction of pluripotin with Leu 983 (top). A phenyl alanine substitution for Leu 983 will confer steric hindrance (bottom). (N) According to prediction, Jak2V617F/L983F mutant conferred resistance to pluripotin. Thus, confirming the model and on-target inhibitory activity of pluripotin. (O) Active site of ABL kinase domain showing the interaction of pluripotin with the gatekeeper residue, Thr 315 (top). An isoleucine substitution for Thr 315 will confer steric hindrance (bottom). (P) As expected from the modeling studies, BCR-ABLT315 mutant conferred resistance to pluripotin. Together, these modeling and in vitro validation data confirm that pluripotin directly binds with inactive confirmations of FLT3, JAK2, and ABL.
Pluripotin effectively suppresses the FLT3 gatekeeper mutant
To validate our models and the on-target inhibition of FLT3, ABL, and JAK2 by pluripotin, cell proliferation assays with pluripotin were performed using Ba/F3 cells expressing mutant kinases. If our model is correct, we expect that the FLT3-activation loop mutants will confer resistance as resistance to type II FLT3 inhibitors, quizartinib and sorafenib, as reported previously. The gatekeeper F691L mutant conferred cross-resistance to both type II (quizartinib and sorafenib) and type I (gilteritinib and crenolanib) inhibitors, likely causing a steric blockade to inhibitor binding. In contrast, our model suggests that pluripotin binding is unaffected by the substituted leucine at the gatekeeper residue (Figure 3K). Consistent with modeling predictions, activation loop mutants conferred resistance to pluripotin whereas gatekeeper mutant, F691L, was susceptible to inhibition (Figure 3L; supplemental Figure 3). The active site mutant of Jak2L983F conferred cross-resistance to most Jak2 inhibitors.18 Our modeling studies predict that a phenylalanine substitution for Leu 893 will cause a direct steric hindrance to pluripotin binding (Figure 3M). As envisioned, Ba/F3 cells expressing Jak2V617F/L983F conferred resistance to pluripotin (Figure 3N). Likewise, modeling studies with ABL predicted that the gatekeeper mutant, T315I, will block pluripotin binding (Figure 3O). Accordingly, the expression of BCR-ABLT315I conferred resistance to pluripotin (Figure 3P). Altogether, these data clearly demonstrate that pluripotin is a type II inhibitor of FLT3, ABL, and Jak2. Although the activation loop mutants of FLT3 conferred resistance to pluripotin, dose escalation will suppress them, as there is a significant therapeutic window available compared with native Ba/F3 cells (Figure 3).
Pluripotin effectively suppresses murine AML
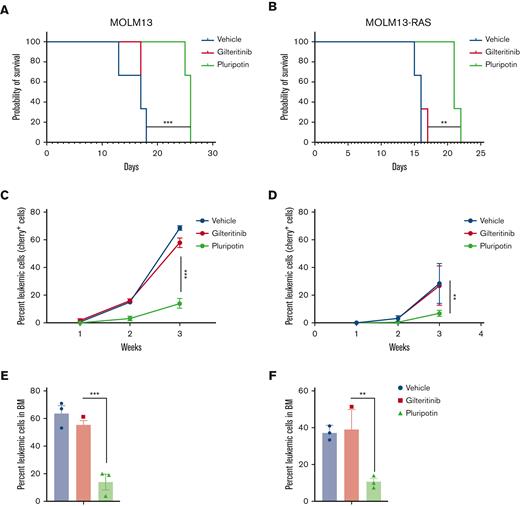
Because hematopoietic cytokines, IL-3, GM-CSF, and FLT3LG have been shown to abrogate TKI response in AML by activating JAK2 and RAS-MAPK signaling,14,15 we used triple transgenic NSGS mice (expressing human IL-3, GM-CSF, and SCF) for accurate in vivo modeling to determine the effect of hematopoietic cytokines in driving the TKI response.11 Prior drug validation studies using NSG mice, nonetheless, showed in vivo efficacy of gilteritinib and quizartinib. However, their antileukemic activity in NSGS mice is lost owing to cytokine-driven resistance. We reasoned that NSGS mice represent more closely the clinical settings for therapeutic validation studies as they express resistant–conferring cytokines. 1 million human AML cells, MOLM13, MOLM13RASG12V, and MV4-11 and MV4-11RASG12V-expressing luciferase and cherry were transplanted into the NSGS mice by injection through the tail vein. Drug treatment (gilteritinib 30 mg/kg and pluripotin 15 mg/kg) was started after 3 days of transplantation. As expected, treatment with gilteritinib did not show any change in suppression of disease progression compared with vehicle-treated mice (Figure 4; supplemental Figure 4). In contrast, treatment with pluripotin extended survival by 8 to 12 days compared with gilteritinib-treated mice (Figure 4; supplemental Figure 4). Likewise, the leukemic burden from the PB and BM, determined by cherry+ cells from deceased or sacked mice, showed a significant reduction only in pluripotin-treated mice.
Pluripotin treatment decreases leukemic burden in vivo and prolongs survival. The survival of NSGS mice transplanted with human AML cell lines MOLM13 (A) and MOLM13-RASG12V (B) is shown. One million MOLM13 and MOLM13-RASG12V cells were transplanted by tail vein injection in NSGS mice. Treatment with gilteritinib (100 mg/kg daily) and pluripotin (15 mg/kg daily) was started after 2 days of transplantation. Control mice were injected with vehicle, phosphate-buffered saline. shown are leukemic burden measured by cherry+ cells in mice recipients of MOLM13 (C) and MOLM13-RASG12V (D). Bar graph showing the percentage of leukemic cells determined by cherry+ cells using FACS in mice transplanted with MOLM13 (E) and MOLM13-RASG12V cell (F). Presented data are from 2 independent experiments (3 mice per group) shown as mean ± SD. ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001.
Pluripotin treatment decreases leukemic burden in vivo and prolongs survival. The survival of NSGS mice transplanted with human AML cell lines MOLM13 (A) and MOLM13-RASG12V (B) is shown. One million MOLM13 and MOLM13-RASG12V cells were transplanted by tail vein injection in NSGS mice. Treatment with gilteritinib (100 mg/kg daily) and pluripotin (15 mg/kg daily) was started after 2 days of transplantation. Control mice were injected with vehicle, phosphate-buffered saline. shown are leukemic burden measured by cherry+ cells in mice recipients of MOLM13 (C) and MOLM13-RASG12V (D). Bar graph showing the percentage of leukemic cells determined by cherry+ cells using FACS in mice transplanted with MOLM13 (E) and MOLM13-RASG12V cell (F). Presented data are from 2 independent experiments (3 mice per group) shown as mean ± SD. ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001.
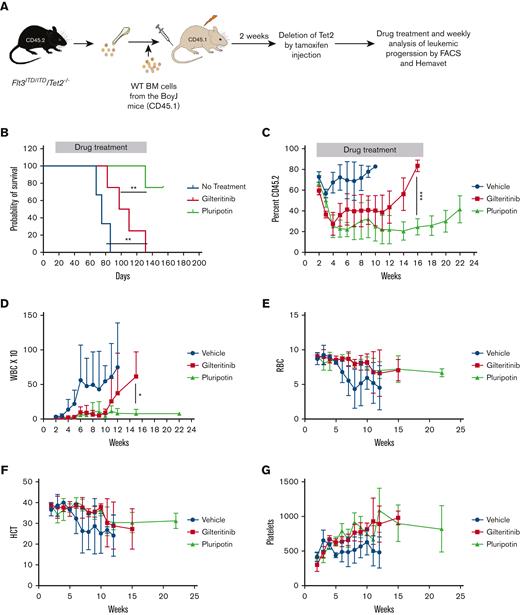
Next, we evaluated the efficacy of pluripotin on primary AML cells using a clinically relevant mouse model of AML (RosaCreERT2: FLT3ITD/Tet2fl/fl).26 BM cells from the RosaCreERT2: FLT3ITD/Tet2fl/fl mice (CD45.2 background) were transplanted into CD45.1+ BoyJ mice (Figure 5A). After 2 weeks of transplantation, tamoxifen was injected to delete the TET2 to start leukemogenesis. All mice developed features consistent with leukemia, elevated WBCs, and anemia (Figure 5B-G). Mice treated with gilteritinib (100 mg/kg) extended the survival (30-40 days) of mice compared with vehicle treatment (Figure 5B). Treatment with gilteritinib suppressed the WBC levels within a week and showed a stable disease up to week 10. However, as observed in patients with AML, all mice developed progressive disease from week 11 and eventually succumbed to leukemia by week 16 (Figure 5B-D). In contrast, mice treated with pluripotin (15 mg/kg daily) showed stable disease, and ∼80% of mice survived (Figure 5B,C). Although, treatment with pluripotin efficiently suppressed the leukemic progression (normal WBC levels and reduced leukemic burden), it is not curative, as treatment discontinuation resulted in leukemic progression after week 16. Similar to gilteritinib, treatment with pluripotin had no significant impact on other blood parameters such as red blood cells, platelet numbers, and hematocrits (Figure 5E-G). Altogether, these data clearly show that pluripotin efficiently suppresses the adaptive resistance to FLT3 TKIs mediated by compensatory survival signaling.
Pluripotin effectively suppresses the TKI-resistant primary AML leukemia induced by FLT3ITD/Tet2-/-. (A) Experimental design of in vivo experiments to test the efficacy of pluripotin in FLT3ITD/Tet2-/-–induced AML using ROSACreERT2: FLT3ITD:Tet2fl/fl mice. (B) Survival curve showing the significant protection of mice with treatment with pluripotin compared with gilteritinib-treated cohort. (C-D) Shown are weekly progression of leukemic cells (CD45.2) and WBC from the PB of mice treated with gilteritinib and pluripotin. Note, pluripotin treatment effectively suppressed the leukemic progression compared with gilteritinib. Weekly blood counts, total red blood cells (RBCs) (E), hematocrit (HCT) (F), and platelets (G) did not show any adverse effect on hematopoiesis suggesting that pluripotin will be safe and effective. Presented data are from 2 independent experiments (5 mice per group) shown as mean ± SD. ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001.
Pluripotin effectively suppresses the TKI-resistant primary AML leukemia induced by FLT3ITD/Tet2-/-. (A) Experimental design of in vivo experiments to test the efficacy of pluripotin in FLT3ITD/Tet2-/-–induced AML using ROSACreERT2: FLT3ITD:Tet2fl/fl mice. (B) Survival curve showing the significant protection of mice with treatment with pluripotin compared with gilteritinib-treated cohort. (C-D) Shown are weekly progression of leukemic cells (CD45.2) and WBC from the PB of mice treated with gilteritinib and pluripotin. Note, pluripotin treatment effectively suppressed the leukemic progression compared with gilteritinib. Weekly blood counts, total red blood cells (RBCs) (E), hematocrit (HCT) (F), and platelets (G) did not show any adverse effect on hematopoiesis suggesting that pluripotin will be safe and effective. Presented data are from 2 independent experiments (5 mice per group) shown as mean ± SD. ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001.
Pluripotin effectively suppresses human AML
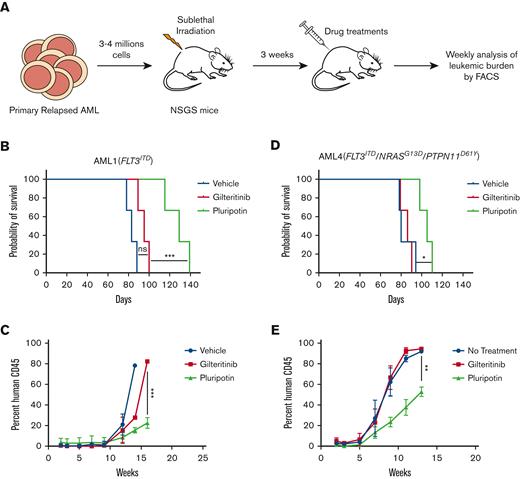
To determine the clinical potential of pluripotin in AML, we performed a comparative cell proliferation assay with increasing concentrations of pluripotin and gilteritinib. Given that the hematopoietic cytokines FL, GM-CSF, and IL-3 abrogate TKI response, we performed cell proliferation assays using a mixture of 3 cytokines (SCF, TPO, and IL-6). We also performed cell proliferation assays with cytokine composition (FL, GM-CSF, IL-3, IL-6, TPO, and SCF) used to propagate both normal hematopoietic stem cells (HSCs) and leukemic cells. Three FLT3ITD+ and 3 non-FLT3–mutated primary AML samples were evaluated (supplemental Figure 5A). Expectedly, gilteritinib efficiently inhibited the proliferation of FLT3ITD AML cells (IC50, 0.6 nM) when assayed in the absence of resistant–conferring cytokines (supplemental Figure 5B). However, gilteritinib was ineffective on leukemic cells harboring WT FLT3 with additional AML-associated mutations. Likewise, the addition of hematopoietic cytokines (FLT3LG, GM-CSF, and IL-3) abrogated the gilteritinib response (supplemental Figure 5C). In contrast, pluripotin inhibited the growth of FLT3ITD AML cells and effectively suppressed the growth factor–mediated resistance suggesting that it will be more effective in vivo (supplemental Figure 5D,E). Interestingly, AML4 (FLT3ITD+ harboring mutations in RAS and PTPN11) that showed primary resistance to gilteritinib is effectively suppressed by pluripotin in both cytokine mixtures (supplemental Figure 5D,E). Next, we evaluated the efficacy of pluripotin in NSGS mouse xenografts transplanted with AML1 (FLT3ITD) and AML4 (FLT3ITD + NRASG13D + PTPN11D61Y) (Figure 6A). Treatment with gilteritinib showed a modest reduction in leukemic burden in mice transplanted with AML1, whereas mice transplanted with AML4 did not respond to treatment. Consequently, treatment with gilteritinib did not show any significant change in survival compared with vehicle-treated mice (Figure 6B-E). Treatment with pluripotin showed a substantial reduction in leukemic burden and extended the survival for 40 and 20 days in mice transplanted with AML 1 and AML 4, respectively (Figure 6B-E). Altogether, these data provide evidence that pluripotin is an effective FLT3 inhibitor and suppresses the adaptive resistance conferred by RAS-MAPK mutations or activation of ABL and JAK2 signaling.
Pluripotin treatment suppresses refractory/relapsed primary human leukemia. (A) Experimental design for the analysis of pluripotin treatment on AML cells from the refractory/relapsed patients. Shown is the survival of NSGS mice transplanted with primary human AML cells from AML1 (B) and AML4 (C). Patient AML1 has only FLT3ITD mutation though AML4 harbors mutation in FLT3ITD, RASG13D, and PTPN11D61V (supplemental Figure 5A). Note, pluripotin treatment with pluripotin significantly prolonged the survival, whereas gilteritinib was ineffective. Shown are leukemic burden measured by hCD45 levels in mice recipients of AML1 (D) and AML4 (E). Presented data are from 2 independent experiments (3 mice per group) shown as mean ± SD. ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001. ns, not significant.
Pluripotin treatment suppresses refractory/relapsed primary human leukemia. (A) Experimental design for the analysis of pluripotin treatment on AML cells from the refractory/relapsed patients. Shown is the survival of NSGS mice transplanted with primary human AML cells from AML1 (B) and AML4 (C). Patient AML1 has only FLT3ITD mutation though AML4 harbors mutation in FLT3ITD, RASG13D, and PTPN11D61V (supplemental Figure 5A). Note, pluripotin treatment with pluripotin significantly prolonged the survival, whereas gilteritinib was ineffective. Shown are leukemic burden measured by hCD45 levels in mice recipients of AML1 (D) and AML4 (E). Presented data are from 2 independent experiments (3 mice per group) shown as mean ± SD. ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001. ns, not significant.
Pluripotin selectively induced apoptosis in leukemic progenitors
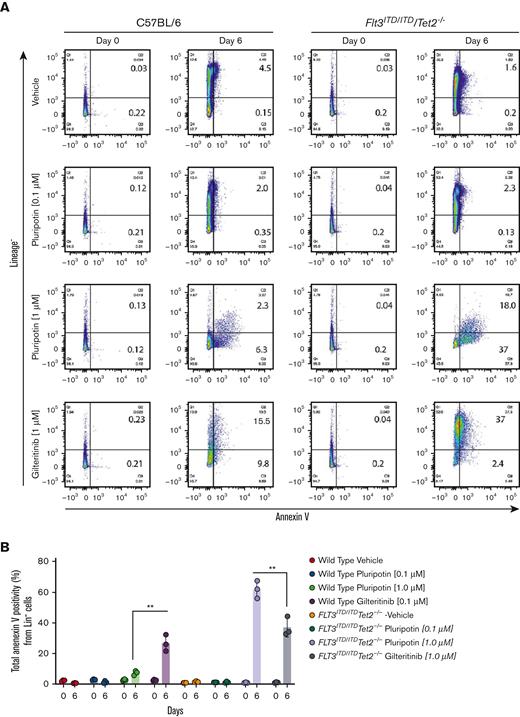
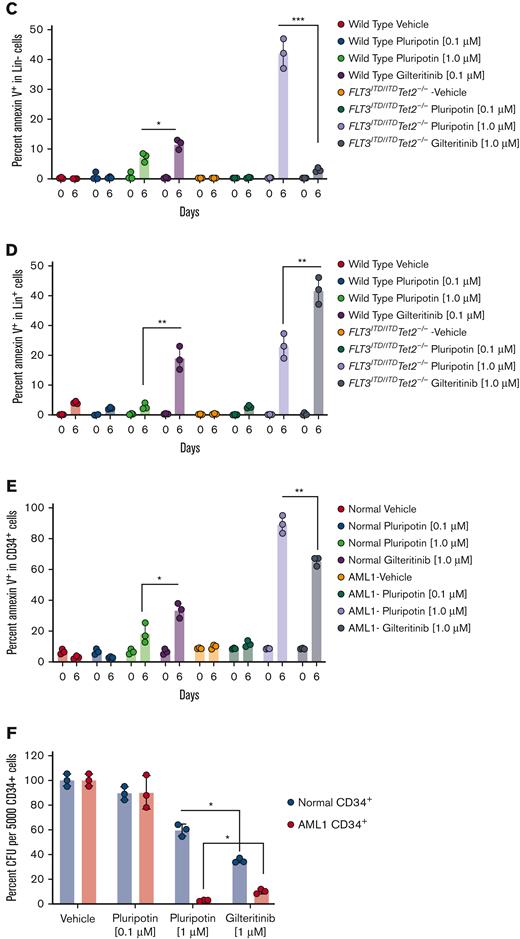
A recent study reported pluripotin supports the ex vivo expansion of normal HSC/progenitor cells,27 which prompted us to determine its selectivity for normal and LSCs. To test this, Lin− cells from the normal and leukemic mice were isolated and grown for 6 days with pluripotin (0.1 μM and 1 μM) and gilteritinib (1 μM) in SFEM media. On day 6, cells were stained with annexin V and stem cell-surface markers (Lin−, Sca1+, and Kit+, collectively called LSK cells) to determine the differentiation and extent of apoptosis in stem progenitor cells. As reported by Turan et al,27 treatment with 1 μM of pluripotin blocked the differentiation of Lin− cells from both leukemic and normal samples but showed the enrichment of LSK cells only from the normal progenitors. In comparison, cells treated with 0.1 μM of pluripotin were ineffective in stopping the differentiation (Figure 7A; supplemental Figure 6A). In contrast, treatment with gilteritinib induced the differentiation of both normal and leukemic progenitors; however, differentiation of leukemic progenitors was significantly higher (∼ 2-fold). Likewise, treatment with gilteritinib showed higher annexin V staining of leukemic cells than normal progenitors (Figure 7A-D). Importantly, it revealed that gilteritinib is minimally active against Lin− leukemic progenitors, although it potently induces apoptosis in differentiated Lin+ cells (Figure 7A-D). In contrast, treatment with pluripotin induced apoptosis in both Lin− and Lin+ cells but with a 6-fold greater selectivity for leukemic progenitors (Figure 7A-D; supplemental Figure 6A-B) that resulted in enrichment of stem/progenitor cells (LSK) only from the normal progenitors (supplemental Figure 6A). We observed that a small population of LSK cells from the pluripotin-treated leukemic progenitors are resistant to treatment, thus phenocopying the in vivo finding in which a small population of cells remain suppressed (cytostatic) during the treatment, which on treatment discontinuation resulted in disease relapse. Likewise, CD34+ cells, the human equivalent of mouse Lin− cells, were isolated and analyzed from the individuals with and without AML. Treatment with pluripotin showed an enhanced apoptotic cell death of leukemic progenitors compared with gilteritinib, though enriching the normal HSC progenitors determined by CD34+/CD38− cell-surface markers (Figure 7E; supplemental Figure 6C). Furthermore, in the colony-forming unit assay (a functional progenitor assay),treatment with pluripotin showed significant protection of normal colony-forming units though efficiently suppressing the emergence of leukemic colonies compared with gilteritinib (Figure 7F). Altogether, these data provide evidence that pluripotin selectively targets leukemic progenitors, though sparing the normal HSC growth.
Pluripotin induces apoptosis in leukemic cells while enriching the normal HSC progenitors. (A) Scatter plot showing the extent of apoptosis in Lin− cells from the WT (left) and leukemic mice (right) on day 0 and after 6 days of drug treatment (vehicle, 0.1 mM and 1 mM pluripotin; 1 mM of gilteritinib). In each density plot quadrant show; Q1, Lin− cells (Lin+ and annexin V−); Q2, apoptosis of differentiated cells (annexin V+ and Lin+); Q3, apoptosis in Lin− cells (Lin− and annexin V+); and Q4, Lin− viable cells (annexin V− and Lin−). (B) Bar plot showing total apoptotic cells from both Lin− and Lin+ cells. (C) Bar plots showing the apoptotic cells from Lin− cells. (D) Bar plot representing apoptotic cells from Lin+ cells. (E) Bar plots showing annexin V+ cells in CD34+ cells from the normal and AML donors before and after 6 days of drug treatment. (F) Bar plot depicting the colony-forming unit (CFU) numbers from the normal and AML CD34 cells treated with vehicle (dimethyl sulfoxide), pluripotin (0.1 mM and 1 mM) and gilteritinib (1 μM). All data presented are shown as mean ± SD collected from 2 independent experiments. ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001.
Pluripotin induces apoptosis in leukemic cells while enriching the normal HSC progenitors. (A) Scatter plot showing the extent of apoptosis in Lin− cells from the WT (left) and leukemic mice (right) on day 0 and after 6 days of drug treatment (vehicle, 0.1 mM and 1 mM pluripotin; 1 mM of gilteritinib). In each density plot quadrant show; Q1, Lin− cells (Lin+ and annexin V−); Q2, apoptosis of differentiated cells (annexin V+ and Lin+); Q3, apoptosis in Lin− cells (Lin− and annexin V+); and Q4, Lin− viable cells (annexin V− and Lin−). (B) Bar plot showing total apoptotic cells from both Lin− and Lin+ cells. (C) Bar plots showing the apoptotic cells from Lin− cells. (D) Bar plot representing apoptotic cells from Lin+ cells. (E) Bar plots showing annexin V+ cells in CD34+ cells from the normal and AML donors before and after 6 days of drug treatment. (F) Bar plot depicting the colony-forming unit (CFU) numbers from the normal and AML CD34 cells treated with vehicle (dimethyl sulfoxide), pluripotin (0.1 mM and 1 mM) and gilteritinib (1 μM). All data presented are shown as mean ± SD collected from 2 independent experiments. ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001.
Discussion
AML is a complex and heterogeneous disease, thus requiring complex therapeutic approaches for effective treatment outcomes. Although targeting multiple lesions using a combinatorial approach is successful in some cases, its utility is limited owing to drug-drug interaction and differing pharmacokinetics. Polypharmacological inhibition of multiple targets using a single agent may overcome the clinical challenges associated with AML treatment. Here, we provide evidence that polypharmacological inhibition of survival pathways associated with acquired and adaptive resistance in AML by pluripotin provides effective treatment outcomes.
Kinase inhibitors are classified as type I and type II inhibitors based on their binding to the ATP site.28,29 Inhibitors binding to an inactive conformation are classified as type II inhibitors, although those preferably targeting the active conformation but also binding to inactive conformations are called type I inhibitors.28,30 Both type I (gilteritinib) and type II (quizartinib) inhibitors have been evaluated in AML and they induce superior therapeutic response than the conventional chemotherapy; however, most patients relapsed within months despite continued therapy. Mechanisms driving resistance are partly different for type I and type II inhibitors. For instance, patients treated with quizartinib or sorafenib (type II inhibitors) are prone to select on-target resistant mutations in FLT3 kinase,31,32 although resistance to type I inhibitors (gilteritinib or crenolanib) are mostly driven by the activation of alternate survival signaling (RAS/MAPK, JAK2, and BCR-ABL) because they are equally active to both FLT3ITD and FLT3TKD mutants and are less prone to resistance owing to on-target mutations. Mutations activating the kinase enzymatic activity or destabilizing the inactive conformation are common in patients treated with type II inhibitors.10,17,19 Consequently, the emergence of on-target resistant mutations is more frequent with type II inhibitors because every kinase can adopt multiple inactive conformations.17,19 Therefore, numerous resistance-conferring mutations may emerge to destabilize each inactive conformation, which by default would stabilize the active conformation resulting in enzymatic activation. Therefore, resistant variants selected against type II inhibitors are sensitive to type I inhibitors with few exceptions (gatekeeper variants). Our docking predictions revealed that pluripotin binds to the inactive conformation of FLT3, and its interaction with gatekeeper residue, F691, is minimal. Consequently, it potently inhibits the gatekeeper mutant, F619L, but has reduced activity against the resistant mutants from the activation loop. Nonetheless, given the existing therapeutic index compared with normal Ba/F3 cells, dose increment will be able to suppress the activation loop variants (Figure 3). To test this, we performed in vitro screening using randomly mutagenized FLT3ITD to select for resistant variants, as reported earlier.17 We failed to recover resistant clones at 50 nM of pluripotin, 5-fold higher than the IC50 value (data not shown), whereas quizartinib resistant screening recovered previously-reported mutations from the activation loop and the gatekeeper residue.10 These data support the notion that pluripotin alone will be able to suppress the emergence of on-target resistant mutations at higher concentrations yet retaining a significant therapeutic index.
BCL2 inhibitor, venetoclax, in combination with hypomethylating agents was approved for AML treatment as a front line therapy in older adults.33 However, the efficacy of venetocalx in patients with AML harboring FLT3 mutations thwarted by the expression of MCL1 owing to constitutive FLT3 kinase activity.25,34 Thus, inhibition of FLT3ITD with TKIs restored venetoclax sensitivity as elegantly demonstrated by Zhu et al.25 Importantly, they also noted that the combination of gilteritinib plus venetoclax exerts a synergistic response only in FLT3-mutated AML cells, although non-FLT3–mutated cells were unaffected by this combination. Interestingly, the efficacy of pluripotin in inducing a synergistic response with venetoclax in FLT3- and non-FLT3–mutated AMLs possibly by simultaneously inhibiting the RAS-MAPK and JAK-STAT pathways, highlights the promise of pluripotin in combination with Bcl2/Mcl-1 inhibitors in treating hematologic cancers.
FLT3 TKIs show differential responses to leukemic cells. For instance, FLT3 inhibitors induce apoptosis in circulating blasts but promote differentiation of leukemic blasts from the BM.3 This suggests that the BM niche is a potential source of intrinsic resistance, resulting in leukemic persistence and MRD. These surviving leukemic cells serve as a reservoir to develop resistance.3,11 Hematopoietic cytokines and growth factors (FL and FGF2)12,13 released from the BM stroma/niche abrogate oncogene-dependence resulting in TKI resistance.11 Notably, FL, whose expression is reported to increase during chemotherapy, abrogates TKI response by activating canonical FLT3WT signaling.12,35 However, it is not clear how FLT3WT signaling activation confers resistance. Nonetheless, an altered signaling network engaged by FLT3WT supporting survival during TKI therapy by activating cell-intrinsic inflammatory pathways has been implicated.36,37 Likewise, inflammatory cytokine-induced activation of JAK2 signaling with high levels of pJAK2 is associated with adverse clinical outcomes in AML and therapeutic resistance. Therefore, concomitant targeting of FLT3 and cytokine signaling using JAK2 inhibitor (ruxolitinib) showed an improved response in preclinical mouse models of AML.14,15 Recent studies identified the activation of Ras/MAPK pathways most commonly owing to NRAS or KRAS mutations and less frequently by FLT3 gatekeeper mutations or BCR-ABL1 fusions in driving AML progression.8 We show that polypharmacological targeting of FLT3, ABL, MAPK, and JAK2 by pluripotin effectively suppressed the adaptive resistance, but it is not curative. Treatment withdrawal resulted in disease relapse suggesting the persistence of LSCs during treatment. TKI therapy alone is not sufficient to eliminate LSCs as diverse survival signaling and a higher apoptotic threshold like normal HSCs support their persistence.11 As reported earlier, treatment with gilteritinib induced rapid differentiation of both normal and leukemic Lin− cells38 though differentiation of leukemic progenitors was significantly higher (∼ 2-fold) than normal Lin− cells. In contrast, treatment with pluripotin prevented the differentiation of normal HSC progenitors more strongly than the leukemia cells. Interestingly, unlike gilteritinib, treatment with pluripotin induced apoptosis in Lin− leukemic cells, which provides an explanation for its superior in vivo response. As reported recently by Turan et al,27 we observed the enrichment of normal HSC progenitors with treatment with pluripotin determined by LSK cell-surface markers in mice and CD34+/CD38− for human cells. (supplemental Figure 6). In contrast, pluripotin induced apoptosis in leukemic progenitors in both LSK and CD34+ cells; however, a small fraction of LSK and CD34+/CD38− cells persisted. These persistent cells are intrinsically resistant to TKI treatment, which seemingly constitute MRD in vivo and drive disease relapse after treatment discontinuation. This is in stark contrast to treatment with gilteritinib, which lacks activity in BM-residing leukemic cells in NSGS mice (Figures 4 and 5; supplemental Figure 4). We and others have reported that hematopoietic cytokines abrogate TKI response; therefore, BM-resident leukemic cells do not undergo apoptosis when targeted with FLT3-selective inhibitors.11,14,15 Failure to entirely suppress the MRD and BM-resident leukemic cells are the reasons short remissions with currently used FLT3 TKIs, is inevitable, as disease relapse even on continued treatment. The efficacy of pluripotin in eradicating the bulk of leukemia and suppressing the LSCs in BM in our preclinical models provide evidence that it will exert a durable response like treatment with TKI in CML, which, so far, is not possible with currently used FLT3 TKIs. However, like CML, LSCs will persist, whose eradication is necessary for curative response.
In conclusion, this study demonstrates that pluripotin has several advantages over currently used FLT3 TKIs. Poor efficacy of FLT3 inhibitors in AML is associated with emergence of acquired and adaptive resistance. We demonstrate that pluripotin is a type II inhibitor of FLT3, ABL, JAK2, and RasGAP. It effectively suppressed the resistance conferred by the gatekeeper mutant, F691L. Likewise, it potently suppressed the resistance mediated by the activation of RAS-MAPK, BCR-ABL, and JAK2 signaling in multiple preclinical mouse models of AML. Given that AML is common in older patients, treatment outcome continuously declines with increasing age. Besides, many patients cannot tolerate intensive chemotherapy. We anticipate that the polypharmacological targeting of relevant survival mechanisms in AML using a single agent will be more effective in treating older patients. Altogether, as a proof of concept, we provide evidence that polypharmacological targeting of multiple survival pathways by pluripotin alone induced a durable response by simultaneously suppressing both acquired and adaptive resistance. Besides, as the first example of polypharmacological type II kinase inhibitor, pluripotin may significantly impact future drug design and development of an effective treatment strategy for AML.
Acknowledgments
This study was supported by National Cancer Institute at National Institutes of Health grants RO1CA211594 and RO1CA250516 (M.A.). M.A. is a recipient of the Bridge Grant Award from the American Society of Hematology.
Authorship
Contribution: M. Azhar, Z.K., M.K., J.M., J.S., S.A., A.R., A.A., R.S., A.K., N.S., and N.A. performed the experiments and analyzed the data; M.W. and T.L. provided the primary patient samples; W.S. performed the in silico docking studies; M. Azhar, M.K., Z.K., and M. Azam designed all experiments; and M. Azhar, M.K., and M. Azam wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mohammad Azam, Experimental Hematology and Cancer Biology, Cincinnati Children's Hospital Medical Center, 240 Albert Sabin Way, Room No. S7.601, Cincinnati, OH 45229; e-mail: mohammad.azam@cchmc.org.
References
Author notes
No data have been deposited in the public domain. Materials and other resources are available on request from the corresponding author, Mohammad Azam (mohammad.azam@cchmc.org).
The full-text version of this article contains a data supplement.