Key Points
Engineering CAR T cells to express SLC7A5/SLC7A11 or downstream arginase enzymes enhances CAR T-cell activity in the cancer environment.
Abstract
Cancer cells take up amino acids from the extracellular space to drive cell proliferation and viability. Similar mechanisms are applied by immune cells, resulting in the competition between conventional T cells, or indeed chimeric antigen receptor (CAR) T cells and tumor cells, for the limited availability of amino acids within the environment. We demonstrate that T cells can be re-engineered to express SLC7A5 or SLC7A11 transmembrane amino acid transporters alongside CARs. Transporter modifications increase CAR T-cell proliferation under low tryptophan or cystine conditions with no loss of CAR cytotoxicity or increased exhaustion. Transcriptomic and phenotypic analysis reveals that downstream, SLC7A5/SLC7A11–modified CAR T cells upregulate intracellular arginase expression and activity. In turn, we engineer and phenotype a further generation of CAR T cells that express functional arginase 1/arginase 2 enzymes and have enhanced CAR T-cell proliferation and antitumor activity. Thus, CAR T cells can be adapted to the amino acid metabolic microenvironment of cancer, a hitherto recognized but unaddressed barrier for successful CAR T-cell therapy.
Introduction
Chimeric antigen receptor (CAR) T cells are being increasingly used in clinical practice for relapsed B-cell malignancies. However, significant numbers of patients still relapse after CAR T-cell therapy.1 In acute myeloid leukemia (AML) or solid cancers, meaningful clinical activity is rarer.2 The expansion of CAR T cells is often poor, with CAR T cells becoming rapidly undetectable.3-6 Thus, to optimize these therapies to adapt and be functional in patients remains a major and exciting challenge.7
One of the critical factors regulating T-cell proliferation and phenotype is the access to sufficient nutrients within the environment.8,9 Amino acids are key components for metabolic, signaling, and protein synthesis pathways in multiple cells. Uptake by cancer cells or myeloid-derived suppressor cells mean CAR T cells must compete for the limited availability of semiessential or essential amino acids. We investigated how re-engineering CAR T-cell amino acid uptake or catabolism can improve function.
Methods
Vectors & CAR T-cell constructs
T cells expressing CAR for GD2 or CD33 were generated as previously described in the study by Fultang et al.10 Modification of the 3’ end of the base CAR expression cassette was performed to include the full coding sequence (CDS) of either human SLC7A5 or SLC7A11 or arginase 1 (Arg1) or arginase 2 (Arg2). The resulting codon-optimized CDSs were synthesized and subcloned downstream of and within the same open reading frame of the base CAR expression cassettes (GenScript), with each CDS separated by a self-cleaving P2A peptide. All the sequences were validated by restriction enzyme analysis and DNA sequencing.
Results and discussion
We and others have previously shown that AML provides a model of the amino acid–depleted immunosuppressive environment for testing T-cell immunotherapies.11,12 Neutral amino acids such as tryptophan are transported into cells through the expression of SLC7A5 whereas cystine, the oxidized form of cysteine, is transported through SLC7A11.13,14 Transcriptomic profiling of 562 patient samples identified the expression of SLC7A5 or SLC7A11 by AML blasts (supplemental Figure 1A), which we reconfirmed at the protein level (supplemental Figure 1B).15 Culture in tryptophan or cystine-depleted conditions reduced the AML viability (supplemental Figure 1C). The inhibition of SCL7A5 (supplemental Figure 1D) or SLC7A11 (supplemental Figure 1E) led to a decreased AML viability confirming blasts consumed tryptophan or cystine from the microenvironment.16,17
The examination of T cells from patients with AML shows that both CD4+ and CD8+ T cells also express SLC7A5 or SLC7A11 (supplemental Figure 1F-G).18 The absence of tryptophan or cystine impairs conventional T cell and CAR T-cell proliferation (Figure 1A-B). We hypothesized that enhancing amino acid import would improve CAR T-cell function. A third generation of CAR T cells expressing SLC7A5 or SLC7A11 alongside an anti-CD33 adaptor were engineered (supplemental Figure 2A-E) with an enhanced uptake of tryptophan or cystine respectively (supplemental Figure 2F-G).10,14,19 Modified CAR T cells retained antigen-specific cytotoxicity (Figure 1C) and the activation-induced interferon-γ (IFN-γ) release (Figure 1D). The activation marker CD69 was more highly expressed in transporter-modified CAR T cells over time (supplemental Figure 3A-B). No significant changes in exhaustion markers LAG3, PD-1, and TIM3 were observed (supplemental Figure 3C-H). A significant reduction in tumor necrosis factor-α (TNF-α) was observed in transporter-modified CAR T cells (supplemental Figure 4A-F).
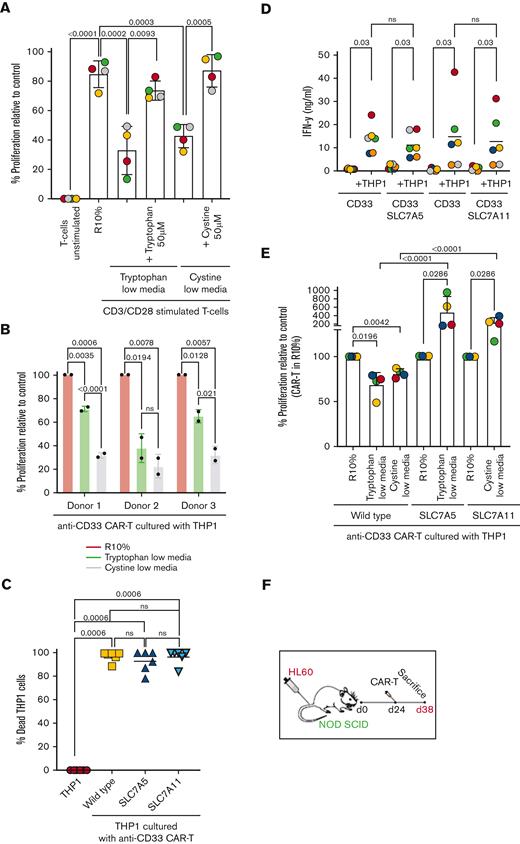
Insertion of SLC7A5 or SLC7A11 amino acid transporters upregulates arginase expression in CAR T cells. (A) The proliferation of T cells is significantly impaired by tryptophan or cystine-free culture conditions, in vitro as measured by CSFE dilution using flow cytometry after 72 hours. (B) The proliferation of anti–CD33-CAR T cells in response to CD33+ THP1 leukemia cells is significantly reduced by tryptophan or cystine-free conditions in vitro as measured by flow cytometry after 72 hours. Anti–CD33-CAR T cells produced from 3 individual human donors are shown. (C) Expression of SLC7A5 or SLC7A11 has no detrimental effect on the cytotoxicity of CAR T cells against target CD33+ THP1 in vitro. The percentage of dead THP1 cells measured by flow cytometry after 72 hours. (D) Activation–induced IFN-γ release remains unchanged by the expression of SLC7A5 or SLC7A11 after CAR T-cell culture with target CD33+ THP1 in vitro. IFN-γ concentration was measured by bead immunoassay in cell culture supernatants after 72 hours. (E) SLC7A5 or SLC7A11–modified anti-CD33 CAR T cells have enhanced proliferation under tryptophan- or cystine-low (75% free) culture conditions respectively, compared with unmodified CAR T cells, in vitro. CAR T-cell proliferation was measured by flow cytometry after 72 hours. (F) Schematic showing NOD-SCID mice engrafted with CD33+ HL60 AML. Following confirmed engraftment mice were injected with modified or control CAR T cells from 3 human donors. (G) The proliferation of unmodified or SLC7A5- or SLC7A11–modified anti-CD33 CAR T cells in NOD-SCID mice with established engraftment of CD33+ AML blasts (HL60). Data from the day of euthanization. CAR T-cell frequency was determined by qPCR. Pooled data from 3 human donors. (H) SLC7A5- or SLC7A11–modified anti-CD33 CAR T cells reduce the expansion of CD33+ HL60 in the bone marrow of murine xenografts, compared with those treated with unmodified CAR T cells. Fold change in AML on the day of euthanization from the day of CAR T administration. Pooled data from 3 human donors. (I) Heatmap of the differential gene expression analysis comparing SLC7A5 or SLC7A11–modified anti-CD33 CAR T cells with unmodified anti-CD33 CAR T cells from n = 5 human donors. Top 500 genes shown. The red arrow indicates the ARG2 gene. (J) Intracellular Arg1 enzyme expression is significantly increased in SLC7A5- or SLC7A11–modified anti-CD33 CAR T cells compared with unmodified CAR T cells cultured with CD33+ THP1, under R10%, tryptophan-low (75% free), or cystine-low (75% free) conditions. Intracellular ARG1 staining as measured by flow cytometry in CAR T cells from n = 3 human donors. (K) Intracellular Arg2 enzyme expression is significantly increased in SLC7A5- or SLC7A11–modified anti-CD33 CAR T cells compared with unmodified CAR T cells cultured with CD33+ THP1, under tryptophan-low (75% free) or cystine-low (75% free) conditions. Intracellular Arg2 staining as measured by flow cytometry in CAR T cells from n = 3 human donors. (L) Arginase enzyme activity, measured by catabolism of arginine into ornithine and urea, is increased in SLC7A5- or SLC7A11–modified anti-CD33 CAR T cells compared with unmodified CAR T cells. CAR T cells were cultured with CD33+ THP1 for 48 hours, before sorting and enzyme activity analysis. Data from n = 4 human donors is shown. CFSE, carboxyfluorescein succinimidyl ester; qPCR, quantitative polymerase chain reaction.
Insertion of SLC7A5 or SLC7A11 amino acid transporters upregulates arginase expression in CAR T cells. (A) The proliferation of T cells is significantly impaired by tryptophan or cystine-free culture conditions, in vitro as measured by CSFE dilution using flow cytometry after 72 hours. (B) The proliferation of anti–CD33-CAR T cells in response to CD33+ THP1 leukemia cells is significantly reduced by tryptophan or cystine-free conditions in vitro as measured by flow cytometry after 72 hours. Anti–CD33-CAR T cells produced from 3 individual human donors are shown. (C) Expression of SLC7A5 or SLC7A11 has no detrimental effect on the cytotoxicity of CAR T cells against target CD33+ THP1 in vitro. The percentage of dead THP1 cells measured by flow cytometry after 72 hours. (D) Activation–induced IFN-γ release remains unchanged by the expression of SLC7A5 or SLC7A11 after CAR T-cell culture with target CD33+ THP1 in vitro. IFN-γ concentration was measured by bead immunoassay in cell culture supernatants after 72 hours. (E) SLC7A5 or SLC7A11–modified anti-CD33 CAR T cells have enhanced proliferation under tryptophan- or cystine-low (75% free) culture conditions respectively, compared with unmodified CAR T cells, in vitro. CAR T-cell proliferation was measured by flow cytometry after 72 hours. (F) Schematic showing NOD-SCID mice engrafted with CD33+ HL60 AML. Following confirmed engraftment mice were injected with modified or control CAR T cells from 3 human donors. (G) The proliferation of unmodified or SLC7A5- or SLC7A11–modified anti-CD33 CAR T cells in NOD-SCID mice with established engraftment of CD33+ AML blasts (HL60). Data from the day of euthanization. CAR T-cell frequency was determined by qPCR. Pooled data from 3 human donors. (H) SLC7A5- or SLC7A11–modified anti-CD33 CAR T cells reduce the expansion of CD33+ HL60 in the bone marrow of murine xenografts, compared with those treated with unmodified CAR T cells. Fold change in AML on the day of euthanization from the day of CAR T administration. Pooled data from 3 human donors. (I) Heatmap of the differential gene expression analysis comparing SLC7A5 or SLC7A11–modified anti-CD33 CAR T cells with unmodified anti-CD33 CAR T cells from n = 5 human donors. Top 500 genes shown. The red arrow indicates the ARG2 gene. (J) Intracellular Arg1 enzyme expression is significantly increased in SLC7A5- or SLC7A11–modified anti-CD33 CAR T cells compared with unmodified CAR T cells cultured with CD33+ THP1, under R10%, tryptophan-low (75% free), or cystine-low (75% free) conditions. Intracellular ARG1 staining as measured by flow cytometry in CAR T cells from n = 3 human donors. (K) Intracellular Arg2 enzyme expression is significantly increased in SLC7A5- or SLC7A11–modified anti-CD33 CAR T cells compared with unmodified CAR T cells cultured with CD33+ THP1, under tryptophan-low (75% free) or cystine-low (75% free) conditions. Intracellular Arg2 staining as measured by flow cytometry in CAR T cells from n = 3 human donors. (L) Arginase enzyme activity, measured by catabolism of arginine into ornithine and urea, is increased in SLC7A5- or SLC7A11–modified anti-CD33 CAR T cells compared with unmodified CAR T cells. CAR T cells were cultured with CD33+ THP1 for 48 hours, before sorting and enzyme activity analysis. Data from n = 4 human donors is shown. CFSE, carboxyfluorescein succinimidyl ester; qPCR, quantitative polymerase chain reaction.
The proliferation of modified CAR T cells was compared in low amino acid conditions. Anti-CD33 SLC7A5 or SLC7A11–modified CAR T cells proliferated better in vitro, in low-tryptophan or low-cystine conditions (Figure 1E). In vivo AML-engrafted mice were treated with CAR T cells from 3 human donors (Figure 1F; supplemental Figure 4G). Although CAR T cells can be detected equally in the bone marrow (Figure 1G), transporter-modified CAR T cells significantly increased AML clearance (Figure 1H).
Next, we investigated the intracellular mechanisms through which SLC7A5/SLC7A11 insertions adapt CAR T cells. A Jurkat model system generated through transduction with the same CAR T adaptors was used (supplemental Figure 5A-B).20,21 Basal mitochondrial respiration linked to adenosine diphosphate (ADP) phosphorylation increased in SLC7A11 Jurkat-CAR T cells starved from cystine (supplemental Figure 5C-D), whereas glycolytic proton-efflux rate (glycoPER) was elevated in tryptophan starved SLC7A5 Jurkat-CAR T cells (supplemental Figure 5E). Collectively, these data confirm a significant increase in ATP supply rates within transporter-modified Jurkat-CAR T cells, despite amino acid starvation (supplemental Figure 5F).
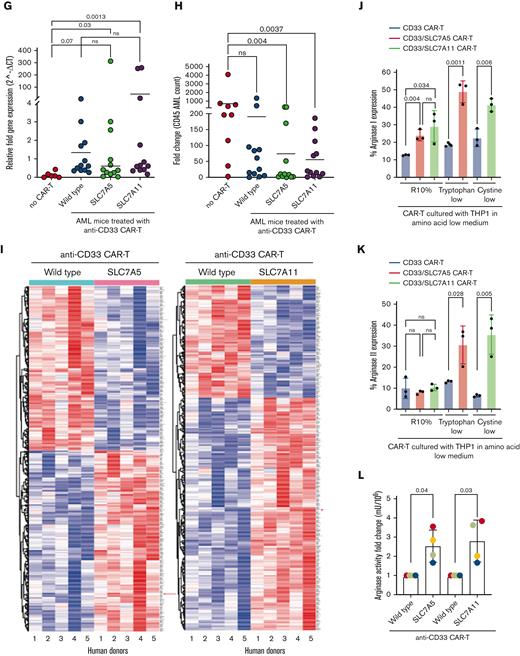
To further examine which intracellular pathways are altered, modified human CAR T cells were cultured with tumor cells in tryptophan/cystine-low media, flow sorted, and subjected to RNA sequencing. Differential gene expression analysis revealed significant changes between standard and transporter-modified CAR T cells (Figure 1I). For both transporter-modified CAR T cells, compared with controls, genes such as E2F2, EPHA4, and SLFN5 were downregulated, consistent with their known mechanisms in regulating T-cell activation. Genes associated with T-cell activation including EGR1, TNFRSF18, DUSP2, SLC3A2 are also upregulated. Interestingly, we identified that both SLC7A5- and SLC7A11-modified CAR T cells upregulated Arg2 expression, an enzyme we have shown to drive cellular proliferation and not regulated by tryptophan/cystine uptake. The analysis of SLC7A5/SLC7A11–modified CAR T cells demonstrated an increased intracellular arginase protein expression (Arg1 and Arg2) following crosstalk with target tumor cells under both complete and amino acid–restricted conditions (Figure 1J-K; supplemental Figure 6A-B). Consistent with this, transporter-modified CAR T cells had increased enzyme activity converting arginine into ornithine and urea (Figure 1L).
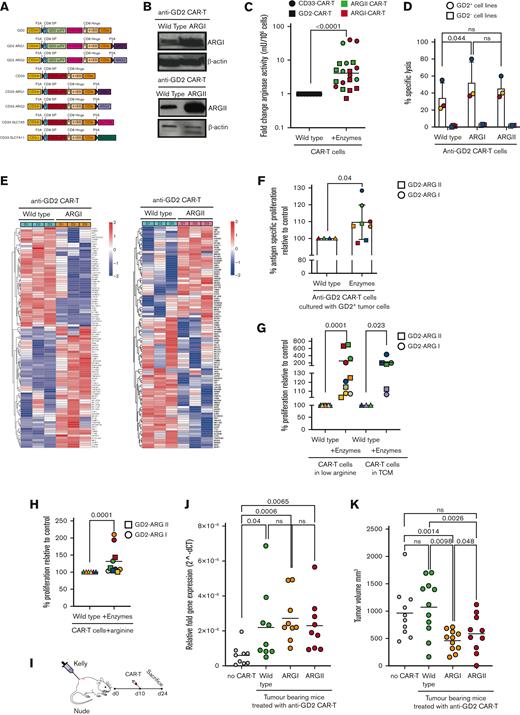
We hypothesized that Arg2 lies downstream of SLC7A5/SLC7A11 function and signaling and may be a central regulator of CAR T-cell activity. To answer this question, a further series of CAR T-cell constructs were generated in which Arg2 or its homolog Arg1 were inserted in anti-CD33 or anti-GD2 CAR T cells (Figure 2A; supplemental Figure 7A). Arg1 or Arg2 proteins were expressed (Figure 2B) and were functional in converting arginine to urea (Figure 2C). No negative effects on cytotoxicity (Figure 2D), exhaustion (supplemental Figure 7B-D), or activation-induced IFN-γ release (supplemental Figure 7E-F) were seen.
Insertion of arginase enzymes enhances CAR T-cell activity in vitro and in vivo. (A) Schematic of CAR T-cell constructs containing the basic anti-CD33-CAR scFv-CD8 hinge-41BB-CD3ζ or the basic anti-GD2-CAR scFv with CH2CH3 spacer-CD8 hinge-41BB-CD3ζ, in conjunction with Arg1 or Arg2. A truncated CD34 is expressed for CAR T identification and purification. (B) Representative expression of Arg1 or Arg2 by anti-GD2 CAR T cells as demonstrated by western blot, with actin loading control. Representative of N = 3 individual donors. (C) Arginase enzyme activity, measured by catabolism of arginine into ornithine and urea, is increased in Arg1- or Arg2–modified anti-GD2 or anti-CD33 CAR T cells compared with unmodified CAR T cells. Data from n = 20 human CAR T-cell donors. (D) Arginase enzyme insertions lead to no detrimental effect on CAR T-cell–specific cytotoxicity against anti-GD2+ tumor cell targets, as measured by chromium release. (E) Heatmap of the differential gene expression analysis comparing Arg1- or Arg2–modified anti-GD2 CAR T-cells with unmodified anti-GD2 CAR T-cells from n = 3 human donors. Top 100 genes shown. (F) Arginase–modified anti-GD2 CAR T-cells have enhanced proliferation in the presence of anti-GD2 tumor target cells, compared with unmodified CAR T cells, in vitro. CAR T-cell proliferation was measured by flow cytometry after 72 hours. (G) Arginase–modified anti-GD2 CAR T cells have enhanced proliferation under low-arginine (75% free) or tumor-conditioned media culture conditions, compared with unmodified CAR T cells, in vitro. CAR T-cell proliferation was measured by 3H-thymidine uptake after 96 hours. (H) Arginase–modified anti-GD2 CAR T cells have enhanced proliferation when culture conditions are supplemented with 100μM arginine, compared with unmodified CAR T cells, in vitro. CAR T-cell proliferation was measured by 3H-thymidine uptake after 96 hours. (I) Diagram illustrating subcutaneous engraftment of GD2+ KELLY cell line in nude mice, before the administration of CAR T cells on Day +10. (J) Proliferation of unmodified control or Arg1- or Arg2–modified anti-GD2 CAR T cells in nude mice with established engraftment of GD2+ tumor cells (KELLY). Data from the day of euthanization. CAR T-cell copies determined by qPCR. (K) Arg1- or Arg2–modified anti-GD2 CAR T cells reduce tumor volume, compared with those treated with unmodified CAR T cells. qPCR, quantitative polymerase chain reaction.
Insertion of arginase enzymes enhances CAR T-cell activity in vitro and in vivo. (A) Schematic of CAR T-cell constructs containing the basic anti-CD33-CAR scFv-CD8 hinge-41BB-CD3ζ or the basic anti-GD2-CAR scFv with CH2CH3 spacer-CD8 hinge-41BB-CD3ζ, in conjunction with Arg1 or Arg2. A truncated CD34 is expressed for CAR T identification and purification. (B) Representative expression of Arg1 or Arg2 by anti-GD2 CAR T cells as demonstrated by western blot, with actin loading control. Representative of N = 3 individual donors. (C) Arginase enzyme activity, measured by catabolism of arginine into ornithine and urea, is increased in Arg1- or Arg2–modified anti-GD2 or anti-CD33 CAR T cells compared with unmodified CAR T cells. Data from n = 20 human CAR T-cell donors. (D) Arginase enzyme insertions lead to no detrimental effect on CAR T-cell–specific cytotoxicity against anti-GD2+ tumor cell targets, as measured by chromium release. (E) Heatmap of the differential gene expression analysis comparing Arg1- or Arg2–modified anti-GD2 CAR T-cells with unmodified anti-GD2 CAR T-cells from n = 3 human donors. Top 100 genes shown. (F) Arginase–modified anti-GD2 CAR T-cells have enhanced proliferation in the presence of anti-GD2 tumor target cells, compared with unmodified CAR T cells, in vitro. CAR T-cell proliferation was measured by flow cytometry after 72 hours. (G) Arginase–modified anti-GD2 CAR T cells have enhanced proliferation under low-arginine (75% free) or tumor-conditioned media culture conditions, compared with unmodified CAR T cells, in vitro. CAR T-cell proliferation was measured by 3H-thymidine uptake after 96 hours. (H) Arginase–modified anti-GD2 CAR T cells have enhanced proliferation when culture conditions are supplemented with 100μM arginine, compared with unmodified CAR T cells, in vitro. CAR T-cell proliferation was measured by 3H-thymidine uptake after 96 hours. (I) Diagram illustrating subcutaneous engraftment of GD2+ KELLY cell line in nude mice, before the administration of CAR T cells on Day +10. (J) Proliferation of unmodified control or Arg1- or Arg2–modified anti-GD2 CAR T cells in nude mice with established engraftment of GD2+ tumor cells (KELLY). Data from the day of euthanization. CAR T-cell copies determined by qPCR. (K) Arg1- or Arg2–modified anti-GD2 CAR T cells reduce tumor volume, compared with those treated with unmodified CAR T cells. qPCR, quantitative polymerase chain reaction.
Next, downstream effects on intracellular metabolism were examined. Arg2 modified–Jurkat-CAR T cells (supplemental Figure 8A-C) displayed increased maximal respiratory capacity and consequent spare respiratory capacity under Seahorse Analysis (supplemental Figure 9A-F). Furthermore, intracellular metabolite detection by gas chromatography–mass spectrometry (GC-MS) revealed a significantly increased concentration of the glycolytic end-products pyruvate (Arg1) and lactate (both Arg1/2) within the modified Jurkat-CAR T (supplemental Figure 9G).22 No tricarboxylic acid cycle intermediates were overrepresented, consistent with the observed enhanced basal mitochondrial respiration rates. Moreover, a marked Arg1-linked increase in most amino acid concentrations was recorded; and an increase in tyrosine, tryptophan, glutamine, and glutamate were found in the Arg2–expressing Jurkat-CAR-T cells. This suggests that arginase activity, through the impact on mitochondrial bioenergetics, supports higher levels of intracellular amino acid pools, and thereby, downstream protein synthesis. RNA sequencing of human CAR T cells revealed several significant changes (Figure 2E). Notably, higher expression of genes associated with T-cell activation such as TOB1, KLRB1, or CCR7 were seen (supplemental Figure 9H-I).
To investigate functional benefits, Arg1/Arg2–modified CAR T cells were cultured with target tumor cells in multiple conditions. The antigen-dependent proliferation of modified CAR T cells was improved compared with unmodified CAR T cells (Figure 2F). Notably, modified CAR T cells are more proliferative under low-arginine or tumor-conditioned media cultures (Figure 2G). Consistent with specific enzyme activity supplementation of media with additional arginine-enhanced–modified CAR T-cell proliferation (Figure 2H). Next, CAR T cells from human donors were tested in mice bearing GD2+ tumors (Figure 2I). Compared with control anti-GD2 CAR T cells, increased Arg1- or Arg2-modified CAR T cells were detectable in the tumor tissue of all mice (Figure 2J). Correspondingly, tumors were significantly smaller in mice treated with Arg1/Arg2–modified CAR T cells (Figure 2K). In summary, we suggest that increasing amino acid influx or downstream catabolism is a clinically applicable and technologically advanced process that overcomes the negative impact of the tumor metabolic microenvironment on CAR T-cell function (supplemental Figure 10).
Acknowledgments
The authors thank the patients and parents who contributed samples to the study.
This work was supported by Cancer Research United Kingdom (C17422/A25154) and CRUK Technologies awards, Medical Research Council's Confidence in Concept award, Takeda COCKPI-T Program, Little Princess Trust, Treating Children with Cancer, Birmingham Children’s Hospital Research Fund/Carter the Brave, the Martin family, and the alumni and donors to the University of Birmingham. The authors acknowledge the support and resources of the Birmingham Metabolic Tracer Analysis Core and Cancer Research UK Award C42109/A26982.
Authorship
Contribution: F.M. and C.D.S. designed the study, supervised research, analyzed data, secured funding, and wrote the manuscript; F.M. secured ethical approval; S.P and N.M. designed and performed research; L.F., S.B., L.G., U.S., C.S., A.V., L.V., and C.W. performed the research; Y.P., C.V., and A.B. performed bioinformatic analysis; J.B. performed and supervised Seahorse analyses; D.T. supervised GC-MS experiments; H.E. contributed JPH-203; and all authors contributed to the writing of the manuscript.
Conflict-of-interest disclosure: F.M. and C.D.S. are listed as inventors on the following patents pertaining to this study: WO2019/122936, WO2020260908, GB2018554.2. The remaining authors declare no competing financial interests.
Correspondence: Francis Mussai, Institute of Immunology and Immunotherapy, University of Birmingham, Birmingham, B15 2TT, United Kingdom; e-mail: francis.mussai@nhs.net.
References
Author notes
∗S.P. and N.M. contributed equally to this study.
†C.D.S. and F.M. are joint senior authors.
RNA sequencing data will be deposited and shared according to the University of Birmingham policies. Data are available on request from the corresponding author, Francis Mussai (francis.mussai@nhs.net).
The full-text version of this article contains a data supplement.