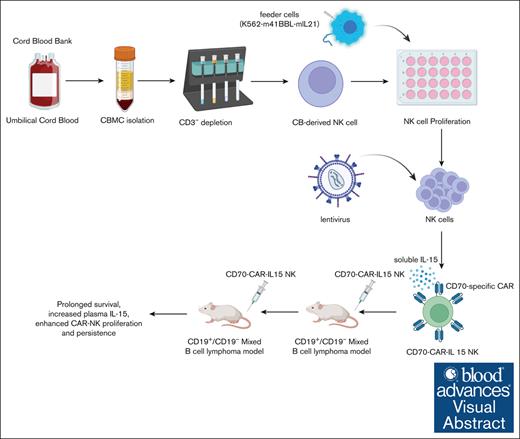
CD70-specific CAR NK cells secrete IL-15 and specifically lyse both CD19-positive and CD19-negative B-cell lymphoma in vitro and in vivo.
Two doses of CD70 CAR IL-15 NK cells increased tumor eradication, plasma IL-15, and CAR NK cell proliferation and persistence.
Visual Abstract
Chimeric antigen receptor (CAR) natural killer (NK) cells can eliminate tumors not only through the ability of the CAR molecule to recognize antigen-expressed cancer cells but also through NK-cell receptors themselves. This overcomes some of the limitations of CAR T cells, paving the way for CAR NK cells for safer and more effective off-the-shelf cellular therapy. In this study, CD70-specific (a pan-target of lymphoma) fourth-generation CAR with 4-1BB costimulatory domain and interleukin-15 (IL-15) was constructed and transduced into cord blood–derived NK cells by Baboon envelope pseudotyped lentiviral vector. CD70-CAR NK cells displayed superior cytotoxic activity in vitro and in vivo against CD19-negative B-cell lymphoma when compared with nontransduced NK cells and CD19-specific CAR NK cells. Importantly, mice that received 2 doses of CD70-CAR NK cells showed effective eradication of tumors, accompanied by increased concentration of plasma IL-15 and enhanced CAR NK cell proliferation and persistence. Our study suggests that repetitive administration-based CAR NK-cell therapy has clinical advantage compared with a single dose of CAR NK cells for the treatment of B-cell lymphoma.
Introduction
Chimeric antigen receptor (CAR) T-cell therapy targeting CD19 is a potentially curative option for the patients with acute lymphocytic leukemia1-3 and relapsed or refractory large B-cell lymphoma (R/R LBCL).4-6 Despite its clinical potentials, the wider clinical application of CAR T-cell therapy is limited by (1) difficulties in preparing autologous CAR T-cell products and the requirement for additional genetic modification to make allogeneic T-cell products safe given the possibility of graft-versus-host disease mediated by T-cell receptors7; (2) treatment-related toxicities that include cytokine release syndrome (CRS), immune effector cell–associated neurotoxicity syndrome (ICANS),8 and hemophagocytic lymphohistiocytosis-like toxicity9,10; and (3) relapse of disease due to the loss of CD19 expression in leukemia and lymphoma cells.11-14
Natural killer (NK) cells are innate immune effector cells that play pivotal roles in immune surveillance by targeting tumor cells or virally infected cells. NK cells recognize their targets in a major histocompatibility complex–unrestricted manner, implying a low risk of graft-versus-host disease during allogeneic transfusion.15 In addition, CRS and ICANS are less likely to occur in NK-based immunotherapy, partly because of a different spectrum of the secreted cytokines compared with CAR T-cell therapy.16,17 These unique features grant NK cells significant advantages as an off-the-shelf product for allogeneic transfusion in cancer immunotherapy. CAR NK therapies have already demonstrated promising efficacy in vivo and ex vivo.18 A recent clinical trial with CAR NK cells for patients with CD19-positive (CD19+) lymphoma further demonstrated a well-tolerated outcome with patients not affected by CRS or ICANS.18 However, significant gaps still exist in the application of CAR NK therapy for the treatment of CD19-negative (CD19−) lymphomas.
CD70, a type 2 transmembrane glycoprotein is highly expressed by many tumors including blood cancer cells.19,20 Targeting CD70 using anti-CD70 monoclonal antibodies21 and anti-CD70 CAR T cells22-25 have shown encouraging efficacy in preclinical and in clinical studies. We previously demonstrated that CD70-CAR T cells could effective eradicate CD19− B-cell lymphoma (BCL).26 In addition, patients who suffered from relapsed central nervous system lymphoma achieved long-term complete remission after treatment with CD70-CAR T cells combined with CD19-CAR T cells.27 Collectively, these results suggest that CD70 may be an attractive CAR NK target for BCL.
In this study, we generated interleukin-15 (IL-15)–secreting CD70 CAR NK cells derived from the mononuclear cells of cord blood. CD70-CAR NK cells showed robust target-dependent cytotoxicity both in vitro and in vivo. Strikingly, repetitive administration of CD70-CAR NK cells induced durable tumor elimination in animal models of CD19− B-cell lymphomas. Our results demonstrate, to our knowledge, for the first time, the exceptional antilymphoma efficacy of CD70-targeting CAR NK cells and suggest their potential for clinical immunotherapy.
Methods
Ethical statement
All umbilical cord blood from donors used in this study were obtained after receiving informed consent. All animal studies were approved by the institutional animal care and use committee of Zhejiang University. The study was approved by the ethics committee of the Second Affiliated Hospital, College of Medicine, Zhejiang University.
Cell lines
Human lymphoma cell lines Raji (Burkitt lymphoma cell, CCL-86) and Jeko-1 (mantle cell lymphoma cell, CRL-3006) were purchased from the American Type Culture Collection and Procell Life Sciences Limited (Wuhan, China), respectively. Lymphoma cells were cultured in RPMI 1640 (Gibco) supplemented with 10% fetal bovine serum (FBS, Gibco) and 1% penicillin/streptavidin (Gibco). NK92MI cells were purchased from Procell Life Sciences Limited and cultured in minimum (or minimal) essential medium α (MEM-α; Gibco) containing 12.5% FBS (Gibco), 12.5% horse serum (Gibco), 0.2 mM inositol, 0.1 mM mercaptoethanol, and 0.02 mM folic acid. HEK-293T/17 cells purchased from the American Type Culture Collection were maintained in Dulbecco modified Eagle medium (DMEM, Gibco) with 10% FBS and 1% penicillin/streptomycin (Gibco).
We established CD70- or CD19-knockout Raji and Jeko-1 lymphoma cell lines using CRISPR-Cas9 technology. These cells also expressed different fluorescent reporter genes transfected by lentiviral vectors and were named Raji-CD70KO-CBG99#–blue fluorescent protein (BFP) cells, Raji-CD19KO-CBRluc–green fluorescent protein (GFP), Jeko-1-CD70KO-CBRluc-GFP, and Jeko-1-CD19KO-CBG99#-BFP cells, respectively. All the engineered cell lines were sorted by flow cytometry (supplemental Figure 1). The single-guide RNAs were designed and synthesized by GenScript Biotech Co, Ltd. The single-guide RNA sequences are displayed in supplemental Table 1.
Generation of CD70 CAR IL-15 and CD19 CAR IL-15 constructs
CAR NK cells were constructed by synthesized DNA fragments encoding the following components in-frame from the 5′ to the 3′ end: the signal sequence of the CD8α; anti-CD70 single-chain variable (scFv) fragments, which was described in a previous study,26 or anti-CD19 scFv, which was described in a previous study28; followed by the CD8α-based extracellular spacer and transmembrane domain; and the cytoplasmic domains of 4-1BB and CD3ζ. IL-15 gene was followed with the CAR constructs by a P2A peptide. Anti-CD19 scFv and anti-CD70 scFv DNA fragment was human codon optimized, synthesized by GenScript Biotech Co, Ltd, and cloned into a third-generation lentiviral backbone plasmid with seamless cloning technology. The full-term sequence of CD19-CAR-IL-15 and CD-70-CAR IL-15 are displayed in supplemental Table 1.
Lentivirus production
Lentivirus was packaged in HEK-293T/17 cells in 10-cm dishes using Baboon envelope (BaEV) pseudotyped lentiviral vector plasmids, as previously described.29 In brief, 1 × 107 HEK-293T/17 cells were cultured in DMEM containing 10% FBS for 24 hours, and then the medium was replaced with Opti-MEM (Gibco) 2 hours before plasmid transfection. For transfection with plasmid, CD70-CAR vector plasmid and packaging plasmid (total of 10 μg) was mixed with 20 μg polyethyleneimine in 500 μL Opti-MEM medium, incubated at room temperature for 15 minutes, and added dropwise to the HEK-239T/17 cells. Viral supernatants were collected 48 hours after transfection and concentrated by overnight centrifugation at 4000g at 4°C. Lentivirus titration was determined on NK92MI cells (CRL-2408) using serial dilutions.
NK cells isolation, expansion, activation, and generation of CAR NK cells
Human cord blood was provided by the Zhejiang Cord Blood Bank/Zhejiang Lvkou Biotechnology Co Ltd, China. Cord blood mononuclear cells (CBMCs) were isolated by density-gradient centrifugation. CD3+ T cells were depleted using anti-CD3 microbeads (Miltenyi, no. 130-050-101). To activate human NK cells, CD3− CBMCs were stimulated with feeder cells (irradiated K562-mbIL21-mb41BBL) at a ratio of 1:2 in AIM-V media (Gibco) with 10% FBS (Gibco), 2 mM L-glutamine (Gibco), 1 mM sodium pyruvate (Gibco), 1 mM HEPES (Gibco), 1 mM nonessential amino acids (Gibco), 100 IU/mL penicillin/streptomycin, and 100 IU/mL human IL-2 (Quangang pharmaceutical, Shandong, China). After 5 days, activated NK cells were transduced with BaEV-Rless pseudoenveloped lentivirus carrying CD19-CAR-IL-15 or CD70-CAR-IL-15 at 8 multiplicities of infection in medium containing 10 μg/mL protamine sulfate (Sigma). NK cells were stimulated again at a ratio of 2:1 with feeder cells after 2 days, and cultured for up to 15 days for use of in vitro or in vivo experiments.
Flow cytometry
Cell suspensions were prepared and stained with different antibodies for flow cytometry analyses under an ACEA Novocyte flow cytometer (SCR_019522, ACEA Biosciences, Inc, San Diego, CA), according to standard protocols. In this study, the following antibodies were used: 7-aminoactinomycin D (7-AAD; BioGems, Westlake Village, CA), PB/APC-Cy7–conjugated CD45 (BioLegend, number 304029/304014, HI30), FITC/PE/PE-cy7–conjugated CD56 (BioLegend, number 362546/362508/304628, 5.1H11/5.1H11/MEM-188), FITC/APC/APC-Cy7–conjugated CD3 (BioLegend, number 300406/300412/300426, UCHT1), APC-conjugated CD19 (BioLegend, number 302212, HIB19), and PE/PE-Cy7–conjugated CD70 (BioLegend, number 355104/355112, 113-16).
CD19 CAR NK cells were detected using Alexa Fluor 647–conjugated anti-mouse FMC63 scFv monoclonal antibody (BioSwan Laboratories). CD70 CAR NK cells were first stained with biotinylated recombinant human CD70 protein (Acro, number CDL-H82D7) and secondary stained using PE-SA or APC-SA (BioLegend, number 405204, 405207).
Detection of IL-15 production
The level of IL-15 in supernatants was measured by human IL-15 enzyme-linked immunosorbent assay kit (Excell, Jiangsu, China, number EH057-96), following the manufacturer's instructions. NK92MI cells were infected with different CAR vectors in 200 μL medium in 96-well plates and then cultured for 4 hours. Next, cells were washed and then cultured in 2 mL of fresh medium for 48 hours before collection of supernatants. IL-15 production in CAR NK and nontransduced NK (NT-NK) cells that were cocultured with tumor cells at a ratio of 1:1 was also assessed by enzyme-linked immunosorbent assay.
Cytotoxicity assays
The cytotoxicity of CAR NK cells against BCL was measured by flow cytometry–based assay, as previously described.30 Briefly, control NT-NK and CAR NK cells were cocultured with lymphoma cells at a ratio of 1:2 in 96-well round-bottom plates in 200 μL medium at 37°C and 5% CO2 for 4 hours. And then cells suspensions were mixed and detected after being washed and labeled with CD56-PE-Cy7 (BioLegend, number 304628, MEM-188,) and 7-AAD (BioGems). The percentage of tumor (GFP+/BFP+) and NT-NK/CAR NK cells (CD56+) were then gated on 7-AAD–negative cells by flow cytometry.
CD107a assay
NT-NK or CAR NK cells were cocultured with target cells in the medium containing the GolgiStop protein transport inhibitor (BD Bioscience, number 554724) and CD107a (BioLegend, number 328618, H4A3) antibody at 37°C with 5% CO2 for 5 hours. The surface expression of CD107a in the CAR+ NK cells was determined by flow cytometry. 7-AAD was used to exclude dead cells.
In vivo xenograft mouse studies
Jeko-1-CD19KO and Raji-CD19KO cell xenograft mouse models were established. Using a mixture of target cells (Raj-CD19KO and Raji cells), we also established a novel lymphoma xenografted mouse model. Briefly, 6- to 8-week-old female NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ(NCG) (Research Resource Identifiers: IMSR_JAX:005557) mice (Biocytogen, Beijing, China) were inoculated intravenously with 1 × 104 target cells or mixture cells. Three days later, mice with growing xenografts were randomized to receive single-dose CAR NK cells or 2-dose CAR NK cells. NT-NK cells were used as a control. Lymphoma growth was monitored by bioluminescence imaging using an IVIS lumina II in vivo imaging system (Caliper Biosciences, now PerkinElmer, Waltham, MA) after intraperitoneal D-luciferin salt (3 mg per 200 μL) injection.
Statistical analysis
All data were analyzed with GraphPad Prism (version 9.4.0, Research Resource Identifiers: SCR_002798). Data show means and standard deviations. Statistical tests were carried out by 2-sided Student t test, 1-way analysis of variance, and 2-way analysis of variance, and were adjusted with Bonferroni correction. The correlation between the percentage of CAR+ cells and the concentration of IL-15 was calculated with Pearson correlation analysis. Kaplan-Meier plots were used to display survival data with statistical testing by log-rank tests. NS indicates no significance, ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, and ∗∗∗∗P < .0001.
Results
Generation of CD70-specific CAR NK cells expressing IL-15
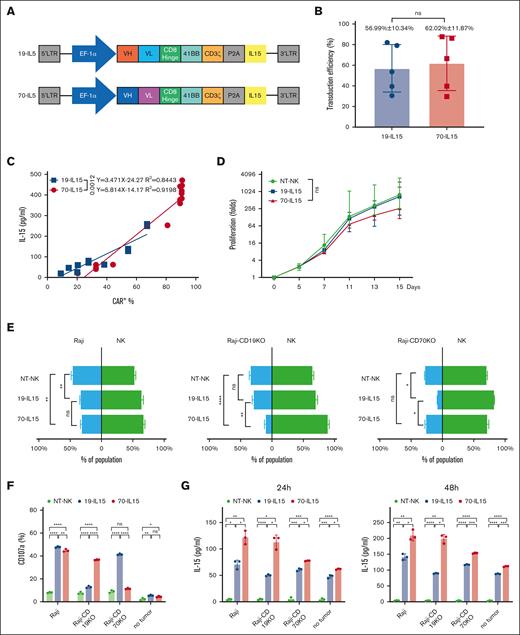
First, we generated a CD70-targeting CAR (CD70-CAR) construct by cloning the anti-CD70 antibody scFv (41D12) in-frame into a second-generation CAR backbone.26 Because NK cell activity is enhanced by IL-15,31 CD70-CAR construct also contained complementary DNA for the expression of human IL-15 (CD70-CAR-IL-15). We also established an IL-15–producing CD19-targeting CAR (CD19-CAR-IL-15) by cloning the anti-CD19 scFv (FMC63) into the same construct (Figure 1A). Two kinds of CAR NK cells were prepared using human CBMC-derived NK cells that were genetically modified by BaEV pseudotyped lentivirus carrying CD19-CAR-IL-15 or CD70-CAR-IL-15 (supplemental Figure 2A). Transgene integration by BaEV pseudotyped lentivirus resulted in similar transduction rates between CD19-CAR-IL-15 and CD70-CAR-IL-15 vector (Figure 1B). The level of IL-15 secreted by CAR NK cells was positively correlated with the number of CAR+ cells (Figure 1C), and no impairment of cellular proliferation was observed during ex vivo expansion supported by the feeder cells (Figure 1D).
IL-15–expressing CAR NK cells exhibit robust antigen-specific cytotoxicity and IL-15 secretion against Raji cells. (A) Schematic representation of the lentiviral vectors encoding anti-CD70 CAR (70-IL-15) and anti-CD19 CAR (19-IL-15). (B) Percentage transduced NK cells expressing anti-CD70 CAR or anti-CD19 CAR 2 days after transduction (n = 5). Data show mean ± standard deviation (SD), representative of 3 independent experiments. (C) Correlation analysis between IL-15 production and percentage of CAR+ NK cells, 48 hours after anti-CD70 CAR or anti-CD19 CAR lentiviral transduction. Simple line regression was used to fit the correlation between IL-15 concentration and the percentage of CAR+ cells. (D) Proliferation of anti-CD70 CAR, anti-CD19 CAR NK cells, or non-NT-NK cells after stimulation with irradiated K562-mbIL21-mb41BBL feeder cells for 15 days. Data were representative of mean ± SD; n = 5. (E) Flow cytometry–based cytotoxicity assay of indicated CAR NK cells against Raji, CD19 KO Raji, or CD70 KO Raji. Bars in green indicate the percentage of NT-NK or CAR NK cells, and bars in blue indicate target tumor cells in the cocultured system. Data were representative of mean ± SD of 3 independent experiments; ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, and ∗∗∗∗P < .0001. Two-way analysis of variance (ANOVA) and Tukey tests were used for P values. (F) The indicated Raji lymphoma cells were cocultured with the indicated CAR-transduced NK cells at a ratio of 1:1 for 5 hours, respectively, and then the percentage of CD107a+ cells in each group were detected by flow cytometry. ∗∗P < .01, ∗∗∗P < .001, and ∗∗∗∗P < .0001. P values were determined by 1-way ANOVA test. (G) Enzyme-linked immunosorbent assay detection of IL-15 in the supernatants after the indicated Raji cells were cocultured with the indicated CAR-transduced NK cells at a ratio of 1:2 for 24 hours or 48 hours. Data show mean ± SD, and P values were calculated by 1-way ANOVA and Tukey test; ∗∗P < .01, ∗∗∗P < .001, and ∗∗∗∗P < .0001. ns, no significance.
IL-15–expressing CAR NK cells exhibit robust antigen-specific cytotoxicity and IL-15 secretion against Raji cells. (A) Schematic representation of the lentiviral vectors encoding anti-CD70 CAR (70-IL-15) and anti-CD19 CAR (19-IL-15). (B) Percentage transduced NK cells expressing anti-CD70 CAR or anti-CD19 CAR 2 days after transduction (n = 5). Data show mean ± standard deviation (SD), representative of 3 independent experiments. (C) Correlation analysis between IL-15 production and percentage of CAR+ NK cells, 48 hours after anti-CD70 CAR or anti-CD19 CAR lentiviral transduction. Simple line regression was used to fit the correlation between IL-15 concentration and the percentage of CAR+ cells. (D) Proliferation of anti-CD70 CAR, anti-CD19 CAR NK cells, or non-NT-NK cells after stimulation with irradiated K562-mbIL21-mb41BBL feeder cells for 15 days. Data were representative of mean ± SD; n = 5. (E) Flow cytometry–based cytotoxicity assay of indicated CAR NK cells against Raji, CD19 KO Raji, or CD70 KO Raji. Bars in green indicate the percentage of NT-NK or CAR NK cells, and bars in blue indicate target tumor cells in the cocultured system. Data were representative of mean ± SD of 3 independent experiments; ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, and ∗∗∗∗P < .0001. Two-way analysis of variance (ANOVA) and Tukey tests were used for P values. (F) The indicated Raji lymphoma cells were cocultured with the indicated CAR-transduced NK cells at a ratio of 1:1 for 5 hours, respectively, and then the percentage of CD107a+ cells in each group were detected by flow cytometry. ∗∗P < .01, ∗∗∗P < .001, and ∗∗∗∗P < .0001. P values were determined by 1-way ANOVA test. (G) Enzyme-linked immunosorbent assay detection of IL-15 in the supernatants after the indicated Raji cells were cocultured with the indicated CAR-transduced NK cells at a ratio of 1:2 for 24 hours or 48 hours. Data show mean ± SD, and P values were calculated by 1-way ANOVA and Tukey test; ∗∗P < .01, ∗∗∗P < .001, and ∗∗∗∗P < .0001. ns, no significance.
CD70 CAR NK cells demonstrate potent in vitro activity against CD19− BCL
To evaluate the target-specific cytotoxicity of CD70-CAR or CD19-CAR NK cells, we cocultured them with target cells Raji, CD70− Raji (Raji-CD70KO) or CD19− Raji (Raji-CD19KO), respectively. CD70-CAR NK cells eliminated Raji and Raji-CD19KO cells significantly better than NT-NK cells, however, did not significantly kill Raji-CD70KO cells; whereas CD19-CAR NK elicited strong cytotoxicity in Raji and Raji-CD70KO cells but not in Raji-CD19KO cells (Figure 1E). NK cell degranulation was evaluated by flow cytometry detecting cell surface CD107a. Both CD19-CAR NK and CD70-CAR NK cells demonstrated markedly increased expression of CD107a when they were cocultured with Raji cells. In contrast, these 2 kinds of CAR NK cells showed minimal expression of CD107a without stimulation by tumor cells. In Raji-CD70KO cells or Raji-CD19KO cells, antigen-specific CD107a degranulation was confirmed when treated with CD19-CAR NK or CD70-CAR NK cells, respectively (Figure 1F). Similar results were obtained in Jeko-1 cells and its variants (supplemental Figure 2B-C). Taken together, our data indicate that CD70-CAR NK cells had potent cytolytic activity against CD19− BCL cells via CD70 antigen–specific effector function.
Next, we explored the relationship between IL-15 secretion and antigen stimulation. Raji cells and its variants were cocultured with CD19-CAR NK, CD70-CAR NK, or NT-NK cells in presence of IL-2 for 24 and 48 hours, respectively. Regardless of antigen stimulation, IL-15 secreted by NT-NK cells was minimal. CD19-CAR NK and CD70-CAR NK cells demonstrated a significantly increased IL-15 secretion in a time-dependent and antigen-dependent manner. CD19-CAR NK and CD70-CAR NK cells also produce IL-15 in the absence of antigen stimulation, which were associated with IL-2–mediated CAR NK cell proliferation. However, we also observed that IL-15 level was significantly higher in supernatants in samples with CD70-CAR NK rather than CD19-CAR NK cells (Figure 1G). We reasoned that CD70 expressed on activated CAR NK cells during in vitro cultures results in an enhanced proliferation of CAR NK cells and increased secretion of IL-15 upon antigen stimulation. This hypothesis was experimentally verified when we compared expression of CD70 and proliferation of 2 kinds of CAR-NK and NT-NK cells (supplemental Figure 3).
CD70-CAR-NK cells exert greater antitumor activity in mice models bearing CD19− BCL
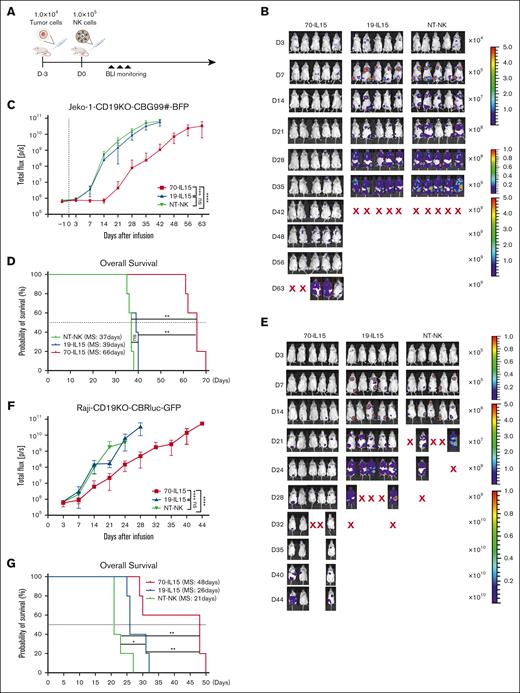
Next, we investigated whether CD70-CAR NK cells are effective in controlling growth of CD19− BCL cells in vivo. Jeko-1-CD19KO-CBG99#-BFP cells (1 × 104) were used to establish a mouse model. After 3 days, lymphoma-bearing mice were treated with 1 × 105 NT-NK, CD19-CAR NK, or CD70-CAR NK cells, respectively (Figure 2A). Mice that received NT-NK or CD19-CAR NK cells showed rapid disease progression and early mortality (Figure 2B). On the contrary, CD70-CAR NK injection substantially inhibited tumor growth and translated into a notable improvement in tumor-free survival (Figure 2B-D). We also established another lymphoma model by injection of Raji-CD19KO-CBRluc-GFP, in which we tested the cytotoxicity of CD70-CAR NK cells against CD19− Raji cells by injecting these mice with 1 × 105 CAR NK or NT-NK cells. Bioluminescence imaging revealed that CD19-CAR NK and NT-NK cells did not achieve tumor clearance, whereas CD70-CAR NK cells exerted greater antitumor activity and showed significantly longer median survival (Figure 2E-G).
In vivo antitumor activity of IL-15–producing CAR NK cells. (A) Experimental scheme of the in vivo evaluation of a single dose of IL-15–producing CAR NK cells in a Jeko-1-CD19KO-CBG-BFP xenograft model. (B) Bioluminescent imaging analysis in Jeko-1-CD19KO-CBG-BFP tumor–bearing mice after the infusion of indicated CAR NK cells (n = 5). (C) The average radiance measured over the course of 63 days as an indication for Jeko-1-CD19KO-CBG-BFP tumor growth in vivo. (D) Kaplan-Meier plots showing the survival of tumor-bearing mice treated with the indicated CAR NK cells. MS, median survival. ∗∗P < .01, ∗∗∗P < .001, and ∗∗∗∗P < .0001. P values were determined by 2-way ANOVA and log-rank test. (E) Bioluminescent imaging analysis in Raji-CD19KO-CBRluc-BFP tumor–bearing mice after the infusion of indicated CAR NK cells (n = 5). (F) The average radiance measured over the course of 44 days as an indication for Raji-CD19KO-CBRluc-BFP tumor growth in vivo. (G) Kaplan-Meier plots showing survival of tumor bearing mice treated with the indicated CAR NK cells. ∗P < .05, ∗∗P < .01, and ∗∗∗∗P < .0001. P values were from 2-way ANOVA and log-rank test.
In vivo antitumor activity of IL-15–producing CAR NK cells. (A) Experimental scheme of the in vivo evaluation of a single dose of IL-15–producing CAR NK cells in a Jeko-1-CD19KO-CBG-BFP xenograft model. (B) Bioluminescent imaging analysis in Jeko-1-CD19KO-CBG-BFP tumor–bearing mice after the infusion of indicated CAR NK cells (n = 5). (C) The average radiance measured over the course of 63 days as an indication for Jeko-1-CD19KO-CBG-BFP tumor growth in vivo. (D) Kaplan-Meier plots showing the survival of tumor-bearing mice treated with the indicated CAR NK cells. MS, median survival. ∗∗P < .01, ∗∗∗P < .001, and ∗∗∗∗P < .0001. P values were determined by 2-way ANOVA and log-rank test. (E) Bioluminescent imaging analysis in Raji-CD19KO-CBRluc-BFP tumor–bearing mice after the infusion of indicated CAR NK cells (n = 5). (F) The average radiance measured over the course of 44 days as an indication for Raji-CD19KO-CBRluc-BFP tumor growth in vivo. (G) Kaplan-Meier plots showing survival of tumor bearing mice treated with the indicated CAR NK cells. ∗P < .05, ∗∗P < .01, and ∗∗∗∗P < .0001. P values were from 2-way ANOVA and log-rank test.
To investigate the relevance of CD70 as a diffuse LBCL (DLBCL) marker, we analyzed differential expression of CD70 between multiple cancer types and normal tissues and found that transcripts of CD70 were upregulated in DLBCL but minimally expressed in normal tissues. By immunohistochemical staining we also observed CD70 protein to be broadly expressed in DLBCL. Next, we examined the frequency of CD19+CD70+ tumor cells in biopsy samples obtained from 2 patients with DLBCL and demonstrated higher frequencies of CD19+CD70+ cells (supplemental Figure 4).
Repetitive administration of low-dose CD70-CAR NK cells produces a potent and durable antilymphoma response
Limited proliferation and in vivo persistence of NK cells after adoptive transfer can potentially hinder antitumor efficacy.19,20 We thus investigated whether administration of high-dose or multiple infusions instead of single infusion of CAR NK cells could improve the efficacy of CAR-NK cell therapy. After establishing a mixed tumor model by injecting NSG mice with CD19KO Raji and wild-type Raji cells at a ratio of 7:3, we treated the mice using high-dose CAR NK cells that were infused at an effector-to-target ratio of 20:1. Unfortunately, the mice died within 1 to 2 weeks after CAR NK cell infusion (data not shown), which might be because of potential systemic toxic effect of excessive IL-15, as reported by Christodoulou et al.32 To overcome this, mice were administrated repeatedly with CAR NK cells at an effector-to-target ratio of 10:1 on day 3 and day 8 after injection of Raji cells (Figure 3A). Remarkably, the mice administrated with CD70-CAR NK cells showed a striking efficacy in the control of tumor burden with efficient tumor eradication in 4 of 5 mice (Figure 3C-E), therefore resulting in long-term survival (Figure 3B). In contrast, CD19-CAR NK cells failed to eradicate tumors in this mixed tumor model although they mediated a slightly greater antitumor activity than NT-NK cells against Raji cells but not Raji-CD19KO (Figure 3D-E). We euthanized the mice after 113 days and found no tumor cells in the peripheral blood, bone marrow, liver, spleen, or lung of these mice; however, CAR NK cells were still detectable in the spleen, suggesting the long-term persistence of CAR NK cells in vivo (supplemental Figure 5).
Two doses of CD70-CAR-IL-15 NK treatment eliminates Raji lymphoma cells in xenograft model. (A) Scheme of the in vivo evaluation of 2 doses of CD70-CAR-IL-15 NK infusion. (B) Kaplan-Meier plots showing the survival of tumor-bearing mice injected with the indicated CAR NK cells over 113 days. ∗∗P < .01. P values were determined by log-rank test. (C) Bioluminescent imaging without filters captures all luminescent signals in vivo (top), including Raji-CBG99#-BFP and Raji-CD19KO-CBRluc-GFP, and total flux analysis quantifies total tumor burden (bottom); n = 5 in the CAR NK group, and n = 3 in the NT-NK group. (D) Bioluminescent imaging with filter that selectively captures Raji-CBG99#-BFP luminescent signals in vivo (top) and the quantification of luminescence over time (bottom); n = 5 in the CAR NK group; and n = 3 in the NT-NK group. (E) Bioluminescent imaging with filter that selectively captures Raji-CD19KO-CBRluc-GFP luminescent signals in vivo (top) and the quantification of luminance over time (bottom); n = 5 in the CAR NK group; and n = 3 in the NT-NK group. ∗∗∗P < .001 and ∗∗∗∗P < .0001. P values were determined by 2-way ANOVA test, and Tukey test was engaged for post hoc analysis.
Two doses of CD70-CAR-IL-15 NK treatment eliminates Raji lymphoma cells in xenograft model. (A) Scheme of the in vivo evaluation of 2 doses of CD70-CAR-IL-15 NK infusion. (B) Kaplan-Meier plots showing the survival of tumor-bearing mice injected with the indicated CAR NK cells over 113 days. ∗∗P < .01. P values were determined by log-rank test. (C) Bioluminescent imaging without filters captures all luminescent signals in vivo (top), including Raji-CBG99#-BFP and Raji-CD19KO-CBRluc-GFP, and total flux analysis quantifies total tumor burden (bottom); n = 5 in the CAR NK group, and n = 3 in the NT-NK group. (D) Bioluminescent imaging with filter that selectively captures Raji-CBG99#-BFP luminescent signals in vivo (top) and the quantification of luminescence over time (bottom); n = 5 in the CAR NK group; and n = 3 in the NT-NK group. (E) Bioluminescent imaging with filter that selectively captures Raji-CD19KO-CBRluc-GFP luminescent signals in vivo (top) and the quantification of luminance over time (bottom); n = 5 in the CAR NK group; and n = 3 in the NT-NK group. ∗∗∗P < .001 and ∗∗∗∗P < .0001. P values were determined by 2-way ANOVA test, and Tukey test was engaged for post hoc analysis.
Increased IL-15 by repetitive administration of CAR-NK cells enhances proliferation and persistence in vivo
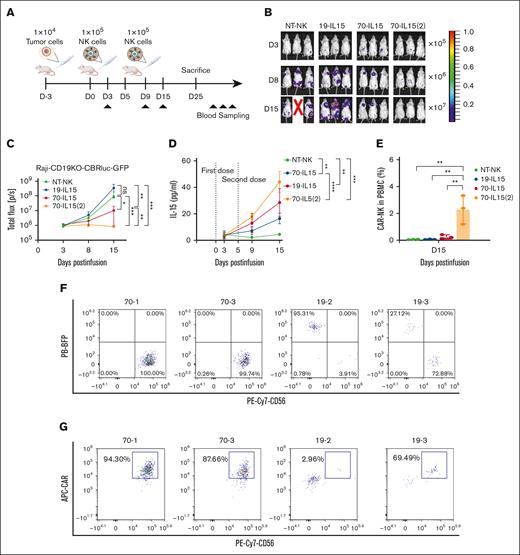
IL-15 is well known to induce STAT5 phosphorylation, which is necessary for NK cell survival, proliferation, and effector function.33 Given that repetitive infusion of CD70-CAR NK cells caused their persistence in vivo, we studied the potential mechanism by which repetitive infusion elicits durable remission. Mice bearing Raji-CD19KO cells received CD19-CAR NK, single dose or 2 infusions of CD70-CAR NK cells (Figure 4A). Repetitive administration of CD70-CAR NK cells was more effective in reducing tumor burden compared with single infusion of CAR-NK cells (Figure 4B-C). To elucidate the mechanisms underlying the significantly greater in vivo antilymphoma capacity associated with repetitive administration, we collected blood samples from the mice on days 3, 9, and 15 after the first infusion of CAR-NK cells and measured IL-15 levels in the plasma. Blood plasma IL-15 concentration continued to rise in the mice infused with either 1 dose or 2 doses of CD70-CAR NK cells and the highest concentration of IL-15 was observed in the mice that received 2 infusions of CD70-CAR NK cells at day 15 (Figure 4D). In the meantime, the mice treated with 2 doses of CD70-CAR NK showed a significantly higher number of CAR-NK cells in the peripheral blood compared with all the other groups (Figure 4E). Next, we evaluated the in vivo persistence of CAR-NK cells and tumor cells by flow cytometry analysis at the end experiments. At day 25, 2 mice infused with CD19-CAR NK and 2 mice that received repetitive doses of CD70-CAR NK cells were still alive. The number of human CD45+ cells in the bone marrow, spleen, lung, and liver was assessed. Flow cytometry analysis revealed that the number of CD45+ cells were higher in CD70-CAR NK–treated mice than in those treated with CD19-CAR NK cells (supplemental Figure 6). Among them, the frequency of CAR NK cells was higher in the bone marrow of mice infused with CD70-CAR NK whereas the frequency of tumor cells was less than that of the mice that received CD19-CAR NK cells (Figure 4F). Taken together, these results indicated that increased levels of IL-15 resulting from repetitive administration endowed the CAR NK cells with greater cellular proliferation, long-term persistence, and enhanced efficacy in eliminating lymphoma xenografts in vivo.
Increased plasma IL-15 level and CAR-expressing NK cells by repetitive administration of CAR NK cells. (A) Experimental scheme for quantification of IL-15 production and CAR NK cell persistence in vivo. (B) Bioluminescent imaging of tumor-bearing mice injected with the indicated CAR NK cells; n = 3. 70-IL-15(2) indicates mice treated with 2 dosages of CD70-CAR-IL-15 NK cells. (C) The quantification of tumor luminescence measured at the indicated days after tumor implantation and NK cell injection. ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001; n = 3. P values were determined by 2-way ANOVA. (D) Serial measurement of IL-15 levels in mouse serum on the indicated days; n = 3. (E) The percentage of CAR-expressing NK cells in the peripheral blood of mice on day 15 after injection, analyzed by flow cytometry. ∗∗P < .01, ∗∗∗P < .001, and ∗∗∗∗P < .0001. P values were determined by 2-way ANOVA; n = 3. (F) Representative flow cytometry plots showing the percentage of tumor (BFP+) and NK (CD56+) cells in the bone marrow at day 25 after the first CAR NK cell injection (top). Representative flow cytometry plots showing the percentage of bone marrow CAR+ cells gated in CD56+ NK cells at day 25 after the first CAR NK-cell injection (bottom).
Increased plasma IL-15 level and CAR-expressing NK cells by repetitive administration of CAR NK cells. (A) Experimental scheme for quantification of IL-15 production and CAR NK cell persistence in vivo. (B) Bioluminescent imaging of tumor-bearing mice injected with the indicated CAR NK cells; n = 3. 70-IL-15(2) indicates mice treated with 2 dosages of CD70-CAR-IL-15 NK cells. (C) The quantification of tumor luminescence measured at the indicated days after tumor implantation and NK cell injection. ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001; n = 3. P values were determined by 2-way ANOVA. (D) Serial measurement of IL-15 levels in mouse serum on the indicated days; n = 3. (E) The percentage of CAR-expressing NK cells in the peripheral blood of mice on day 15 after injection, analyzed by flow cytometry. ∗∗P < .01, ∗∗∗P < .001, and ∗∗∗∗P < .0001. P values were determined by 2-way ANOVA; n = 3. (F) Representative flow cytometry plots showing the percentage of tumor (BFP+) and NK (CD56+) cells in the bone marrow at day 25 after the first CAR NK cell injection (top). Representative flow cytometry plots showing the percentage of bone marrow CAR+ cells gated in CD56+ NK cells at day 25 after the first CAR NK-cell injection (bottom).
Discussion
CAR-based cancer immunotherapy for the treatment of BCL has previously focused on the generation of CAR T cells targeting CD19.5 Although this has been remarkably successful, ∼50% of patients were resistant to CAR T-cell therapy or suffered relapse within 1 year after receiving this treatment.6 Among them, approximately one-third of patients with LBCL relapsed, with CD19 loss mediated by mutations and splice variants of the CD19 gene as well as switching of tumor lineage,6 suggesting that there is a need to validate novel immunotherapies directed to alternative antigen. Dual- and triple-specific targeting of B-cell antigen such as CD19, CD20, and CD22 have surfaced as a promising strategy to reduce the risk of relapse by antigen-negative resistance.34,35 In addition, our previous studies demonstrated that targeting CD70 with CAR T cells is effective in eliminating CD19− BCL.26 CD70 is widely expressed on lymphoid cancers including Hodgkin lymphoma, BCL, T-cell lymphoma, multiple myeloma, and acute myeloid leukemia but nearly absent on normal cells and tissues.22,36-38 However, CD70 is also transiently expressed on activated B and T cells, NK cells, and mature dendritic cells.20 Thus, targeting CD70 with CAR-NK cells might raise safety concerns because of on-target off-tumor toxicities. In this study, we generated an anti-CD70 CAR molecule bearing costimulatory 4-1BB and CD3ζ. NK cells from cord blood underwent BaEV pseudotyped lentivirus transduction with the CD70-CAR, demonstrating high transduction rates and suggesting clinical translation of BaEV lentivirus–based CAR NK therapy for the treatment of tumors, which are consistent with a previous report.39 CD70-CAR NK cells efficiently eliminated not only wild-type Raji and Jeko-1 cells but also Raji-CD19KO and Jeko-1-CD19KO cells, which are CD19− but CD70+ cells. Furthermore, treatment of CD70-CAR NK cells displays a high efficacy to inhibit CD19KO B-cell tumors in xenograft mouse models. These data suggest that anti-CD70-CAR NK cells are a new potential treatment for CD70+ lymphoma.
Despite the immense potential of CAR-NK therapies, there are still fundamental issues that need to be addressed to expand their clinical application. The major limitations of NK-cell therapy include relatively limited lifespan and the lack of in vivo persistence in absence of IL-2 or IL-15.19,20 The activation of NK cells results in poor persistence in the circulation that is no longer than ∼2 to 3 weeks.33,40 In addition, allogeneic NK cells will be subject to recognition and rejection by the host T cells, thereby limiting their lifespan in vivo and resulting in reduced clinical activity.41,42 Recently, many strategies have been used to prevent rejection of the allogeneic NK by the host immune cells. For example, knocking out expression of HLA class I molecules on NK cells via gene editing has been shown to prevent immune recognition by mismatch T cells.42 In contrast, high dose and repeated infusion of CAR NK cells might be necessary to enhance the antitumor immune response. Our study showed that 2 doses of CD70-CAR NK cells successfully eradicated CD19− and CD19+ mixed B-cell tumors, and induced a long-term survival, consistent with results from 2 recent studies that used a second-generation CD33-CAR bearing 4-1BB, or CD123-CAR with 2B4 costimulatory domain construct, respectively.43 Importantly, we observed that CD70-CAR NK cells were still detectable in the spleen of all 4 surviving mice for up to 113 days, indicating that the repetitive administration of CAR NK cells enhances proliferation and persistence in vivo.
IL-15, a pleiotropic cytokine, plays an important role in NK cell activation, proliferation, and persistence, but possess less immunosuppression and toxicity.18,32,44-46 Arming CD19-CAR NK cells with secretory IL-15 could overcome loss of metabolic fitness in NK cells, thereby enhancing NK cell potency and persistence.47 In this study, the mice that received 2 doses of CD70-CAR NK showed eradication of tumors, accompanied by increased concentration of plasma IL-15 and enhanced CAR NK proliferation and persistence. These results collectively suggest that repetitive administration–based CAR NK-cell therapy has clinical advantage compared with single dose of CAR-NK cells for the treatment of BCL.
There are some limitations of this study. We only used xenograft models with lymphoma cell lines. Toward the goal of revealing the promising feature of CD70-CAR NK cells for clinical application and evaluating alloimmune pressure, further studies using patient-derived xenograft and humanized mouse model are required.
This work reports a rational approach for the engineering of IL-15–secreting CD70-specific CAR NK cells that can effectively target CD19− BCL. Our results underscore the critical role of repetitive administration of CAR NK cells in elevating plasma levels of IL-15, which endow CAR NK cells’ functional superiority over single dose of CAR NK cells and may provide a significant advantage for clinical translation.
Acknowledgments
This work was supported by funds from the National Natural Science Foundation of China (numbers 81830006 and 82170219) and the Natural Science Foundation of Zhejiang Province (numbers LQ23H080003 and LY23H080004).
Authorship
Contribution: W.Q. and W.D. designed the study; S.G., W.L., X.J., H.L., and J.Q.W. performed preclinical studies; W.Q., W.D., and S.G. analyzed and interpreted data, and drafted the manuscript; and all authors read and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Wenbin Qian, Department of Hematology, The Second Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou 310009, Zhejiang, China; email: qianwb@zju.edu.cn; and Wenhai Deng, Oujiang Laboratory (Zhejiang Lab for Regenerative Medicine, Vision, and Brain Health), School of Laboratory Medicine and Life Sciences, Wenzhou Medical University, Wenzhou 325000, Zhejiang, China; email: dwh@wmu.edu.cn.
References
Author notes
S.G. and W.L. contributed equally to this study.
Data are available on request from the corresponding author, Wenbin Qian (qianwb@zju.edu.cn).
The full-text version of this article contains a data supplement.