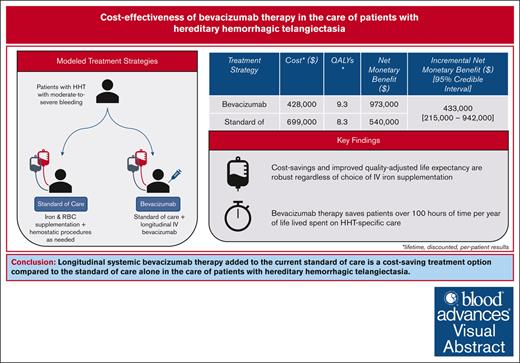
Longitudinal systemic bevacizumab is a cost-saving therapeutic option in the care of patients with HHT with moderate-to-severe bleeding.
Cost savings are mediated by decreased need for iron and red blood cell supplementation, hemostatic procedures, and hospitalizations.
Visual Abstract
No US Food and Drug Administration- or European Medicines Agency-approved therapies exist for bleeding due to hereditary hemorrhagic telangiectasia (HHT), the second-most common inherited bleeding disorder worldwide. The current standard of care (SOC) includes iron and red cell supplementation, alongside the necessary hemostatic procedures, none of which target underlying disease pathogenesis. Recent evidence has demonstrated that bleeding pathophysiology is amenable to systemic antiangiogenic therapy with the anti-vascular endothelial growth factor bevacizumab. Despite its high cost, the addition of longitudinal bevacizumab to the current SOC may reduce overall health care resource use and improve patient quality of life. We conducted, to our knowledge, the first cost-effectiveness analysis of IV bevacizumab in patients with HHT with the moderate-to-severe phenotype, comparing bevacizumab added to SOC vs SOC alone. The primary outcome was the incremental net monetary benefit (iNMB) reported over a lifetime time horizon and across accepted willingness-to-pay thresholds, in US dollar per quality-adjusted life year (QALY). Bevacizumab therapy accrued 9.3 QALYs while generating $428 000 in costs, compared with 8.3 QALYs and $699 000 in costs accrued in the SOC strategy. The iNMB of bevacizumab therapy vs the SOC was $433 000. No parameter variation and no scenario analysis, including choice of iron supplementation product, changed the outcome of bevacizumab being a cost-saving strategy. Bevacizumab therapy also saved patients an average of 133 hours spent receiving HHT-specific care per year of life. In probabilistic sensitivity analysis, bevacizumab was favored in 100% of all 10 000 Monte Carlo iterations across base-case and all scenario analyses. Bevacizumab should be considered for more favorable formulary placement in the care of patients with moderate-to-severe HHT.
Introduction
Hereditary hemorrhagic telangiectasia (HHT) is the second-most common inherited bleeding disorder worldwide, afflicting 1 in 5000 or 1.4 million persons, with twice the prevalence of hemophilia A and 12 times that of hemophilia B.1 Vascular lesions that develop with disease progression result in recurrent mucosal bleeding, leading to iron deficiency anemia in most patients.2-4 As such, HHT is associated with substantial morbidity and mortality at every year lived, with significant decreases in both quality of life and life expectancy.5-8 Unlike the inherited bleeding disorders hemophilia and von Willebrand disease, there are no US Food and Drug Administration (FDA)- or EMA-approved therapies for HHT-associated bleeding.9 The current standard of care (SOC) consists of red blood cell (RBC) transfusion, IV iron supplementation, and local hemostatic procedures (ie, nasal and gastrointestinal).1,4 Such interventions may help manage the symptoms of HHT but do not target its underlying pathogenesis, burdening patients with the need for lifelong, recurrent exposure to the risks of repeat iron and RBC support, as well as surgical procedures, all alongside an increasing age-dependent hazard of bleeding and bleeding-associated complications.10
Bleeding pathophysiology in HHT is amenable to vascular endothelial growth factor inhibition, and the monoclonal anti-vascular endothelial growth factor antibody bevacizumab has shown great promise as a disease-modifying therapeutic.4 The multisite, international, observational International HHT Intravenous Bevacizumab Investigative Team Study of Bleeding (InHIBIT-Bleed) was the largest clinical study evaluating a therapeutic intervention in HHT (n = 238) to date and used a retrospective pre- vs a prospective post-bevacizumab intrapatient comparison study design.9 InHIBIT-Bleed demonstrated that longitudinal IV bevacizumab significantly improved clinical symptoms, raised hemoglobin levels, and nearly abrogated the need for IV iron infusions and RBC transfusions for patients with HHT. Although bevacizumab is an expensive biologic agent with a financial barrier to access in the United States, we hypothesized that reduction in health care resource utilization and improvement in transfusion dependence would improve the quality of life for patients with HHT at costs commensurate with added clinical value (ie, that bevacizumab is a cost-effective intervention).11 Accordingly, we conducted, to our knowledge, the first cost-effectiveness analysis of IV bevacizumab therapy in HHT.
Methods
Model Structure
We built a Markov cohort simulation model of adult patients with a diagnosis of HHT to examine the cost-effectiveness of treatment with (1) bevacizumab added to the current SOC (hereon, referred to as “bevacizumab” for simplicity) vs (2) the current SOC alone. Rates of RBC transfusion, iron infusion, and health care resource utilization in both treatment arms was informed by InHIBIT-Bleed study intrapatient comparison data for pre- vs post-bevacizumab that included descriptive statistics for (1) RBC transfusions, (2) IV iron infusions, (3) hemostatic procedures (number and type), (4) hospitalizations (number and length), and (5) emergency department visits.9 During each model cycle, patients may experience RBC- and iron-related adverse events including delayed hemolytic transfusion reactions, transfusion-associated circulatory overload, transfusion-related acute lung injury, posttransfusion purpura, or iron infusion-related anaphylaxis, which may lead to death. Transition probabilities for these adverse events were sourced from hemovigilance reports including the 2015 National Blood Collection and Utilization Survey, 2008-2018 World Health Organization Vigibase data, 2014-2019 FDA Adverse Event Reporting System, Medicare data (2010-2013), and the 2021 Serious Hazards of Transfusion report.12-16 These adverse events were also nullified in a scenario analysis to show the effect of the complete absence of RBC and iron-related adverse events on whether the model result changed.
In addition, patients may also discontinue bevacizumab due to adverse events, after which they return to the SOC treatment paradigm, no longer receiving bevacizumab and so experiencing a return of bleeding events to the status quo (ie, SOC). All transition probabilities were directly informed by InHIBIT study data. Treatment-specific patient time and lost wages (up to age 65 years, representing the assumed age of retirement in the United States) were also accounted for across IV infusions, emergency department visits, hospitalizations, and hemostatic procedures.
We assumed a starting age of 63 years to reflect the mean age of patients enrolled in InHIBIT-Bleed and further explored the effect of earlier bevacizumab initiation in sensitivity analysis. Transition-state cycles were 6 months in duration, in line with the lengths of the pre- and post-bevacizumab portions of the InHIBIT study, with a lifetime time horizon and a 62:38 male:female sex mix, reflecting the study results. The primary outcome was either the incremental cost-effectiveness ratio or the incremental net monetary benefit (iNMB) if the intervention was found to be cost saving (ie, intervention saves costs while yielding increased quality-adjusted life expectancy). The secondary outcome was the aggregated patient-time spent receiving HHT-specific care. We performed this analyses from both the US health care system and societal perspectives (ie, incorporating wages lost due to time spent on HHT-specific care as part of overall costs),17 doing so across a range of conventionally accepted willingness-to-pay (WTP) thresholds in the United States ($50 000-$150 000 per quality-adjusted life year [QALY]) and discounting cost and effectiveness by 3% annually, as recommended in the US context.17,18 We constructed our model using TreeAge Pro Healthcare 2023 (TreeAge Software, Williamstown, MA). Consolidated Health Economic Evaluation Reporting Standards guidelines were implemented when applicable.
Model assumptions
Age- and disease-specific annual background mortality probabilities for patients living with HHT were informed by 2022 US Life Tables and a population-based study comparing mortality risk in an HHT cohort with that of a matched cohort of individuals without HHT.5,19 Base-case estimates and ranges for all input parameters used in the model are reported in Table 1. Ferric carboxymaltose was chosen as the base-case iron supplement given its market dominance among IV iron products, estimated at >47% US market share.20-23 We explored this assumption extensively both in the sensitivity analyses and also scenario analyses that used use of ferumoxytol and iron dextran in place of ferric carboxymaltose, as described below in “Sensitivity and scenario analyses.” To account for aggregated patient-time spent received HHT-related care, we assumed that all infusion types (RBC, iron, and bevacizumab) cost patients half of 1 working day or 4 hours of wages, with hemostatic procedures costing 1 working day (8 hours) and hospitalization costing 5.5 days. In addition, although patients who undergo bevacizumab discontinuation largely do so in the first few months of drug use, we extrapolated bevacizumab discontinuation to continue beyond the first year of its successful use, doing so at 10% of the initial discontinuation rate annually. All of these assumptions were extensively tested in the sensitivity analysis to see if any affect model results (ie, which strategy is the cost-effective strategy).
Base-case input parameters and probability distributions
| Input parameter . | Value . | Probability distribution used in probabilistic sensitivity analysis . | Data source . |
|---|---|---|---|
| Cohort age at start | 63 | Fixed | InHIBIT-Bleed9 |
| Discount rate | 0.03 | Fixed | Sanders et al17 |
| Patient weight, mean, kg | 84.05 | Fixed | CDC24 |
| Mean US hourly wage, USD | 34.10 | Fixed | US Bureau of Labor Statistics25 |
| Hazard ratio of mortality, HHT : non-HHT individuals | 2.0 | Fixed | Donaldson et al5 |
| Median number of RBC units transfused, 6 mo before treatment | 9 | γ (0.9138, 0.06741) | InHIBIT-Bleed9 |
| Median number of RBC units transfused, first 6 mo on Bev | 0 | γ (0.3832, 0.1571) | InHIBIT-Bleed9 |
| Median number of iron units infused, 6 mo before treatment | 8 | γ (1.680, 0.1472) | InHIBIT-Bleed9 |
| Median number of iron units infused, first 6 mo on Bev | 2 | γ (0.6645, 0.2086) | InHIBIT-Bleed9 |
| Mean number of ED visits or admissions, 6 mo before treatment | 0.96 | β-PERT (0.48, 1.44) | InHIBIT-Bleed9 |
| Mean number of ED visits or admissions, first 6 mo on Bev | 0.30752 | β-PERT (0.1538, 0.4613) | InHIBIT-Bleed9 |
| Mean number of endoscopic hemostatic procedures, 6 mo before treatment | 0.60123 | β-PERT (0.3006, 0.9018) | InHIBIT-Bleed9 |
| Mean number of endoscopic hemostatic procedures, first 6 mo on Bev | 0.06641 | β-PERT (0.0332, 0.0996) | InHIBIT-Bleed9 |
| Mean number of ENT hemostatic procedures, 6 mo before treatment | 0.47573 | β-PERT (0.0996, 0.7135) | InHIBIT-Bleed9 |
| Mean number of ENT hemostatic procedures, first 6 mo on Bev | 0.14740 | β-PERT (0.073698002, 0.221094006) | InHIBIT-Bleed9 |
| Transition probabilities | |||
| Probability of major AE leading to Bev discontinuation, first year of treatment | 0.02363 | β-PERT (0.0118, 0.0354) | InHIBIT-Bleed9 |
| Assumed of major AE leading to Bev discontinuation, all subsequent years | 0.00239 | β-PERT (0.001194, 0.003582) | Assumed - 10% of first year probability |
| Probability of delayed hemolytic transfusion reaction | 4.55E-05 | β-PERT (2.27E-05, 6.82E-05) | Goel et al13 |
| Probability of transfusion aasociated circulatory overload | 0.000111 | β-PERT (5.56E-05, 1.67E-04) | Goel et al13 |
| Probability of transfusion-related acute lung injury | 1.67E-05 | β-PERT (8.33E-06, 2.50E-05) | Goel et al13 |
| Probability of PTP | 7.69E-08 | β-PERT (3.85E-08, 1.15E-07) | Goel et al13 |
| Probability of ferric carboxymaltose anaphylaxis | 3.31E-05 | β-PERT (1.66E-05, 4.97E-05) | Durup et al14 |
| Probability of ferumoxytol anaphylaxis | 3.41E-04 | β-PERT (1.71E-04, 5.12E-04) | Wang et al15 |
| Probability of iron dextran anaphylaxis | 7.90E-06 | β-PERT (3.95E-06, 1.19E-05) | Durup et al14 |
| Probablity of death from DHTR | 0.038 | β-PERT (0.019, 0.057) | Davies et al26 |
| Probability of death from TACO | 0.084 | β-PERT (0.042, 0.13) | 2021 SHOT Report12 |
| Probability of death from TRALI | 0.075 | β-PERT (0.0375, 0.1125) | Popovsky27 |
| Probability of death from PTP | 0.046 | β-PERT (0.023, 0.069) | Davies et al26 |
| Probability of death due to Ferric carboxymaltose anaphylaxis | 0.002 | β-PERT (0.001, 0.003) | Trumbo et al16 |
| Probability of death due to ferumoxytol anaphylaxis | 0.13 | β-PERT (0.067, 0.20) | Trumbo et al16 |
| Probability of death due to iron dextran anaphylaxis | 0.045 | β-PERT (0.022, 0.068) | Trumbo et al16 |
| Utility values | |||
| Utility of HHT disease state | Age-dependent | β-PERT | Zarrabeitia et al6 |
| Utility increment of anemia improvement (severe to moderate) | 0.05 | β-PERT (0.045, 0.055) | GBD 201928 |
| Estimated disutility of major AE due to Bev | –0.00261 | β-PERT (–0.00288, –0.00235) | Assumed |
| Disutility of transfusion-related acute lung injury | –0.01538 | β-PERT (–0.01692, –0.0547) | van Eerd et al29 |
| Disutility of delayed hemolytic transfusion reaction | –0.00608 | β-PERT (–0.00668, –0.00547) | Lloyd et al30 |
| Disutility of transfusion-associated circulatory overload | –0.01538 | β-PERT (–0.01692, –0.0547) | van Eerd et al29 |
| Disutility of PTP | –0.00885 | β-PERT (–0.00973, –0.00796) | Di Minno et al31 |
| Disutility of iron anaphylaxis | –0.000740 | β-PERT (–0.000814, –0.000665) | Shaker et al32 |
| Costs (USD) | |||
| Cost of bevacizumab per mg | 7.0752 | Fixed | HCPCS 4436311 |
| Cost per unit of RBC | 135.52 | Fixed | HCPCS P902111 |
| Cost of ferric carboxymaltose per infusion | 826.5 | Fixed | HCPCS J143911 |
| Cost of ferumoxytol per infusion | 283.56 | Fixed | HCPCS Q013811 |
| Cost of iron dextran per infusion | 33.16 | Fixed | HCPCS J175011 |
| Cost of ED visit | 381.61 | γ (16, 0.04192) | HCPCS 9928411 |
| Cost of hospital admission | 37266.09 | γ (16, 0.0004293) | Harder et al33 |
| Cost of endoscopic hemostatic procedure | 1412.25 | γ (16, 0.01133) | HCPCS 4439111 |
| Cost of ENT hemostatic procedure | 8501.26 | γ (16, 0.001882) | Rudmik et al, Healthcare Bluebook34,35 |
| Cost of delayed hemolytic transfusion reaction treatment | 1537 | γ (16, 0.01041) | Janssen et al36 |
| Cost of transfusion-associated circulatory overload treatment | 4830 | γ (16, 0.003313) | Janssen et al36 |
| Cost of transfusion-related acute lung injury | 9959 | γ (16, 0.001607) | Janssen et al36 |
| Cost of PTP treatment | 2536.58 | γ (16, 0.006308) | Davies et al26 |
| Cost of ferric carboxymaltose anaphylaxis treatment | 2124.61 | γ (16, 0.007531) | Trumbo et al16 |
| Cost of ferumoxytol anaphylaxis treatment | 9491.83 | γ (16, 0.001686) | Trumbo et al16 |
| Cost of iron dextran anaphylaxis treatment | 10016.19 | γ (16, 0.001597) | Trumbo et al16 |
| Input parameter . | Value . | Probability distribution used in probabilistic sensitivity analysis . | Data source . |
|---|---|---|---|
| Cohort age at start | 63 | Fixed | InHIBIT-Bleed9 |
| Discount rate | 0.03 | Fixed | Sanders et al17 |
| Patient weight, mean, kg | 84.05 | Fixed | CDC24 |
| Mean US hourly wage, USD | 34.10 | Fixed | US Bureau of Labor Statistics25 |
| Hazard ratio of mortality, HHT : non-HHT individuals | 2.0 | Fixed | Donaldson et al5 |
| Median number of RBC units transfused, 6 mo before treatment | 9 | γ (0.9138, 0.06741) | InHIBIT-Bleed9 |
| Median number of RBC units transfused, first 6 mo on Bev | 0 | γ (0.3832, 0.1571) | InHIBIT-Bleed9 |
| Median number of iron units infused, 6 mo before treatment | 8 | γ (1.680, 0.1472) | InHIBIT-Bleed9 |
| Median number of iron units infused, first 6 mo on Bev | 2 | γ (0.6645, 0.2086) | InHIBIT-Bleed9 |
| Mean number of ED visits or admissions, 6 mo before treatment | 0.96 | β-PERT (0.48, 1.44) | InHIBIT-Bleed9 |
| Mean number of ED visits or admissions, first 6 mo on Bev | 0.30752 | β-PERT (0.1538, 0.4613) | InHIBIT-Bleed9 |
| Mean number of endoscopic hemostatic procedures, 6 mo before treatment | 0.60123 | β-PERT (0.3006, 0.9018) | InHIBIT-Bleed9 |
| Mean number of endoscopic hemostatic procedures, first 6 mo on Bev | 0.06641 | β-PERT (0.0332, 0.0996) | InHIBIT-Bleed9 |
| Mean number of ENT hemostatic procedures, 6 mo before treatment | 0.47573 | β-PERT (0.0996, 0.7135) | InHIBIT-Bleed9 |
| Mean number of ENT hemostatic procedures, first 6 mo on Bev | 0.14740 | β-PERT (0.073698002, 0.221094006) | InHIBIT-Bleed9 |
| Transition probabilities | |||
| Probability of major AE leading to Bev discontinuation, first year of treatment | 0.02363 | β-PERT (0.0118, 0.0354) | InHIBIT-Bleed9 |
| Assumed of major AE leading to Bev discontinuation, all subsequent years | 0.00239 | β-PERT (0.001194, 0.003582) | Assumed - 10% of first year probability |
| Probability of delayed hemolytic transfusion reaction | 4.55E-05 | β-PERT (2.27E-05, 6.82E-05) | Goel et al13 |
| Probability of transfusion aasociated circulatory overload | 0.000111 | β-PERT (5.56E-05, 1.67E-04) | Goel et al13 |
| Probability of transfusion-related acute lung injury | 1.67E-05 | β-PERT (8.33E-06, 2.50E-05) | Goel et al13 |
| Probability of PTP | 7.69E-08 | β-PERT (3.85E-08, 1.15E-07) | Goel et al13 |
| Probability of ferric carboxymaltose anaphylaxis | 3.31E-05 | β-PERT (1.66E-05, 4.97E-05) | Durup et al14 |
| Probability of ferumoxytol anaphylaxis | 3.41E-04 | β-PERT (1.71E-04, 5.12E-04) | Wang et al15 |
| Probability of iron dextran anaphylaxis | 7.90E-06 | β-PERT (3.95E-06, 1.19E-05) | Durup et al14 |
| Probablity of death from DHTR | 0.038 | β-PERT (0.019, 0.057) | Davies et al26 |
| Probability of death from TACO | 0.084 | β-PERT (0.042, 0.13) | 2021 SHOT Report12 |
| Probability of death from TRALI | 0.075 | β-PERT (0.0375, 0.1125) | Popovsky27 |
| Probability of death from PTP | 0.046 | β-PERT (0.023, 0.069) | Davies et al26 |
| Probability of death due to Ferric carboxymaltose anaphylaxis | 0.002 | β-PERT (0.001, 0.003) | Trumbo et al16 |
| Probability of death due to ferumoxytol anaphylaxis | 0.13 | β-PERT (0.067, 0.20) | Trumbo et al16 |
| Probability of death due to iron dextran anaphylaxis | 0.045 | β-PERT (0.022, 0.068) | Trumbo et al16 |
| Utility values | |||
| Utility of HHT disease state | Age-dependent | β-PERT | Zarrabeitia et al6 |
| Utility increment of anemia improvement (severe to moderate) | 0.05 | β-PERT (0.045, 0.055) | GBD 201928 |
| Estimated disutility of major AE due to Bev | –0.00261 | β-PERT (–0.00288, –0.00235) | Assumed |
| Disutility of transfusion-related acute lung injury | –0.01538 | β-PERT (–0.01692, –0.0547) | van Eerd et al29 |
| Disutility of delayed hemolytic transfusion reaction | –0.00608 | β-PERT (–0.00668, –0.00547) | Lloyd et al30 |
| Disutility of transfusion-associated circulatory overload | –0.01538 | β-PERT (–0.01692, –0.0547) | van Eerd et al29 |
| Disutility of PTP | –0.00885 | β-PERT (–0.00973, –0.00796) | Di Minno et al31 |
| Disutility of iron anaphylaxis | –0.000740 | β-PERT (–0.000814, –0.000665) | Shaker et al32 |
| Costs (USD) | |||
| Cost of bevacizumab per mg | 7.0752 | Fixed | HCPCS 4436311 |
| Cost per unit of RBC | 135.52 | Fixed | HCPCS P902111 |
| Cost of ferric carboxymaltose per infusion | 826.5 | Fixed | HCPCS J143911 |
| Cost of ferumoxytol per infusion | 283.56 | Fixed | HCPCS Q013811 |
| Cost of iron dextran per infusion | 33.16 | Fixed | HCPCS J175011 |
| Cost of ED visit | 381.61 | γ (16, 0.04192) | HCPCS 9928411 |
| Cost of hospital admission | 37266.09 | γ (16, 0.0004293) | Harder et al33 |
| Cost of endoscopic hemostatic procedure | 1412.25 | γ (16, 0.01133) | HCPCS 4439111 |
| Cost of ENT hemostatic procedure | 8501.26 | γ (16, 0.001882) | Rudmik et al, Healthcare Bluebook34,35 |
| Cost of delayed hemolytic transfusion reaction treatment | 1537 | γ (16, 0.01041) | Janssen et al36 |
| Cost of transfusion-associated circulatory overload treatment | 4830 | γ (16, 0.003313) | Janssen et al36 |
| Cost of transfusion-related acute lung injury | 9959 | γ (16, 0.001607) | Janssen et al36 |
| Cost of PTP treatment | 2536.58 | γ (16, 0.006308) | Davies et al26 |
| Cost of ferric carboxymaltose anaphylaxis treatment | 2124.61 | γ (16, 0.007531) | Trumbo et al16 |
| Cost of ferumoxytol anaphylaxis treatment | 9491.83 | γ (16, 0.001686) | Trumbo et al16 |
| Cost of iron dextran anaphylaxis treatment | 10016.19 | γ (16, 0.001597) | Trumbo et al16 |
AE, adverse event; Bev, bevacizumab; ED, emergency department; DHTR, delayed hemolytic transfusion reactions; ENT, ear-nose-throat; PERT, program evaluation and review technique; PTP, posttransfusion purpura; TACO, transfusion-associated circulatory overload; TRALI, transfusion-related acute lung injury; USD, US dollars.
Costs
All costs were adjusted to 2023 US dollars using the medical component of the consumer price index.37 The costs of bevacizumab, RBCs, iron supplementation, emergency department visits, and endoscopic hemostatic procedures were obtained from the 2023 Centers for Medicare & Medicaid Services Hospital Outpatient Prospective Payment System.11 We assumed a mean patient body weight of 84.05 kg, as per the January 2021 US Vital and Health Statistics report.24 HHT-specific hospitalization costs were sourced from a retrospective cross-sectional analysis of 2000 to 2014 Nationwide Impatient Sample discharge data.33 The cost of intranasal hemostatic procedures (including embolization, passage ablation, and arterial ligation) were sourced from Healthcare Bluebook and previous economic evaluations of epistaxis treatment in the United States.34,35,38
QALYs
Health outcomes estimated by our model were expressed in QALYs, a measure that accounts for both health-related quality of life and length of life. QALYs were informed by age-specific EuroQol-5D index values derived from 1 of the only large (n = 187) quality of life studies in patients with HHT worldwide.6 The EuroQol-5D is a preference-based health-related quality-of-life tool used to measure patient-reported health across the dimensions of mobility, self-care, usual activities, pain or discomfort, and anxiety or depression.39,40 Its use has been validated in variety of clinical settings, including in multiple hematologic malignancies and bleeding disorders such as hemophilia.41,42 To capture the utility increment for patients in the bevacizumab treatment arm we used anemia severity–specific (ie, patient population improved from severe to moderate anemia) utilities derived from the 2019 Global Burden of Disease Study.28 The Global Burden of Disease Study estimates the incidence, prevalence, mortality, years of life lost, and disability-adjusted life years due to >360 diseases and injuries across 204 countries and territories. Principle input data include censuses, civil registration and vital statistics, disease registries, and household surveys.43,44 Please see the supplemental Appendix for further detail.
Sensitivity and scenario analyses
We quantified the sampling uncertainty within the parameters of our Markov model by performing 1-way (deterministic) and probabilistic sensitivity analyses (PSAs). In deterministic sensitivity analyses, utilities and nonutility parameters were varied by ±10% and ±50%, respectively. In the PSA, data-driven probability distributions for input parameters with probabilistic uncertainty were assigned (Table 1). We used β-PERT distributions for utilities and transition probabilities and γ distributions for costs, while simultaneously ensuring random draws from each distribution via 10 000 second order Monte Carlo simulations. γ distributions were used to best model the RBC and iron supplementation count requirements reported for pre- (retrospective) and post-bevacizumab (prospective) treatment in InHIBIT-Bleed patients. We concluded by examining scenario analyses using iron dextran and ferumoxytol for IV iron supplementation as alternative formulations. This purposefully covers a range of choices that include the least costly per infusion product (ie, dextran) and the most (ie, ferric carboxymaltose from base case). The costs of all IV iron products were sourced from the Centers for Medicare & Medicaid Services Hospital Outpatient Prospective Payment System, whereas the rates of anaphylaxis (and subsequent mortality) were informed by the World Health Organization VigiBase and data from the FDA Adverse Event Reporting System, which as noted before (see “Model Structure”) were separately nullified in an additional scenario analysis.14,16
Results
Base-case cost-effectiveness analysis
The estimated total cost, QALYs, and NMBs associated with each treatment strategy at a lifetime time horizon are reported in Table 2. In the base case, bevacizumab vs SOC alone costs $428 000 and $699 000 while accruing 9.3 and 8.3 QALYs, respectively. The iNMB with bevacizumab was $433 000 (95% credible interval, 215 000-942 000) from the US health system perspective and $444 000 (95% credible interval, 217 000-973 000) from the societal perspective, at a WTP of $150 000 per QALY. Bevacizumab is also predicted to save patients 2034 hours over a lifetime or 133 hours per year lived compared with the SOC alone.
Base-case results and NMBs for bevacizumab plus SOC vs SOC alone, from a US health system and societal perspective
| Strategy . | Cost . | Incremental cost . | Effectiveness (QALY) . | Incremental effectiveness (QALY) . | NMB . | iNMB (95% credible interval) . |
|---|---|---|---|---|---|---|
| Base-case model (health system perspective) | ||||||
| Bevacizumab | $428 000 | — | 9.3 | 1.0 | $973 000 | $433 000 (215 000-942 000) |
| SOC | $699 000 | $271 000 | 8.3 | — | $540 000 | |
| Base-case model (societal perspective) | ||||||
| Bevacizumab | $435 000 | — | 9.3 | 1.0 | $967 000 | $444 000 (217 000-973 000) |
| SOC | $717 000 | $281 000 | 8.3 | — | $523 000 | |
| Strategy . | Cost . | Incremental cost . | Effectiveness (QALY) . | Incremental effectiveness (QALY) . | NMB . | iNMB (95% credible interval) . |
|---|---|---|---|---|---|---|
| Base-case model (health system perspective) | ||||||
| Bevacizumab | $428 000 | — | 9.3 | 1.0 | $973 000 | $433 000 (215 000-942 000) |
| SOC | $699 000 | $271 000 | 8.3 | — | $540 000 | |
| Base-case model (societal perspective) | ||||||
| Bevacizumab | $435 000 | — | 9.3 | 1.0 | $967 000 | $444 000 (217 000-973 000) |
| SOC | $717 000 | $281 000 | 8.3 | — | $523 000 | |
Bevacizumab is a cost-saving treatment strategy that accrues NMB.
Sensitivity analyses
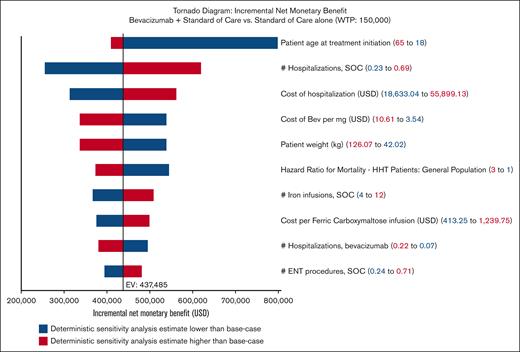
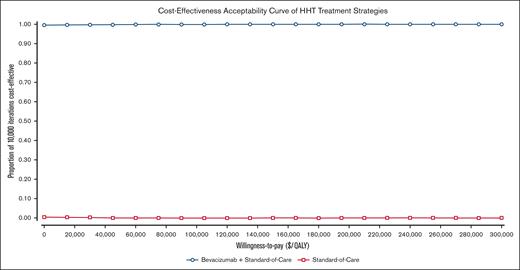
One-way sensitivity analyses identified no parameter changed that caused the iNMB to fall below $0, always favoring bevacizumab (Figure 1). The top 5 parameters to which the model was most sensitive to were, in order: patient age at treatment initiation, the number of hospitalizations experienced under the current SOC, the cost of hospitalization, the cost of bevacizumab, and patient weight. No change in any of these parameters changed the model outcome to favor SOC (Figure 1). Of note, varying the rate of bevacizumab discontinuation did not change the model outcome; in fact, it did not affect the iNMB by >10% (Figure 1). In PSAs, bevacizumab was favored over SOC alone in 100% of the 10 000 Monte Carlo iterations: cost saving in 99.4% and, at worst, cost-effective in the remaining 0.6% of iterations (Figure 2).
One-way sensitivity analysis tornado diagram demonstrating NMBs produced with bevacizumab compared with SOC alone. Each row illustrates analysis results when 1 parameter is varied across its range. Parameters that produced >10% change in iNMB when varied were included. Ranges used in analyses are detailed in Table 1. Blue denotes iNMB changes associated with lower values, whereas red denotes iNMB changes associated with higher values. EV, expected value; USD, US dollars.
One-way sensitivity analysis tornado diagram demonstrating NMBs produced with bevacizumab compared with SOC alone. Each row illustrates analysis results when 1 parameter is varied across its range. Parameters that produced >10% change in iNMB when varied were included. Ranges used in analyses are detailed in Table 1. Blue denotes iNMB changes associated with lower values, whereas red denotes iNMB changes associated with higher values. EV, expected value; USD, US dollars.
Cost-effectiveness acceptability curve of probabilistic sensitivity analysis for bevacizumab added to the SOC vs SOC alone, across a range of WTP thresholds. Ranges used in analysis are detailed in Table 1.
Cost-effectiveness acceptability curve of probabilistic sensitivity analysis for bevacizumab added to the SOC vs SOC alone, across a range of WTP thresholds. Ranges used in analysis are detailed in Table 1.
Scenario analyses
All our scenario analyses for different iron products (ie, rather than ferric carboxymaltose) show that bevacizumab continues to be the cost-effective strategy (Table 3). Specifically, with ferumoxytol as the choice of iron supplementation, bevacizumab costs $407 000 and accrues 9.3 QALYs, compared with $598 000 in costs and 8.3 QALYs in the SOC alone. The iNMB with bevacizumab was $354 000 (95% credible interval [CI], 181 000-652 000), and bevacizumab was preferred in 100% of the 10 000 Monte Carlo iterations in PSA. When iron dextran is used as the choice of iron supplementation, bevacizumab costs $398 000 and generates 9.3 QALYs, compared with $552 000 in costs and 8.3 QALYs accrued in the SOC alone. The iNMB with bevacizumab was $316 000 (95% CI, 147 000 - 567 000), and bevacizumab was preferred in 100% of the 10 000 Monte Carlo iterations in PSA.
Scenario analyses of base-case results and NMBs using alternative IV iron supplementation, ferumoxytol and iron dextran
| Strategy . | Cost . | Incremental cost . | Effectiveness (QALY) . | Incremental effectiveness (QALY) . | NMB . | iNMB (95% credible interval) . |
|---|---|---|---|---|---|---|
| Health system perspective (ferumoxytol) | ||||||
| Bevacizumab | $407 000 | — | 9.3 | 1.0 | $994 000 | $354 000 (181 000-652 000) |
| SOC | $598 000 | 191 000 | 8.3 | — | $640 000 | |
| Societal perspective (ferumoxytol) | ||||||
| Bevacizumab | $414 000 | — | 9.3 | 1.0 | $987 000 | $364 000 (182 000-680 000) |
| SOC | $615 000 | $201 000 | 8.3 | — | $623 000 | |
| Health system perspective (iron dextran) | ||||||
| Bevacizumab | $398 000 | — | 9.3 | 1.0 | $1 003 000 | $316 000 (147 000-567 000) |
| SOC | $552 000 | $154 000 | 8.3 | — | $687 000 | |
| Societal perspective (iron dextran) | ||||||
| Bevacizumab | $405 000 | — | 9.3 | 1.0 | $996 000 | $326 000 (151 000-592 000) |
| SOC | $569 000 | $164 000 | 8.3 | — | $670 000 | |
| Strategy . | Cost . | Incremental cost . | Effectiveness (QALY) . | Incremental effectiveness (QALY) . | NMB . | iNMB (95% credible interval) . |
|---|---|---|---|---|---|---|
| Health system perspective (ferumoxytol) | ||||||
| Bevacizumab | $407 000 | — | 9.3 | 1.0 | $994 000 | $354 000 (181 000-652 000) |
| SOC | $598 000 | 191 000 | 8.3 | — | $640 000 | |
| Societal perspective (ferumoxytol) | ||||||
| Bevacizumab | $414 000 | — | 9.3 | 1.0 | $987 000 | $364 000 (182 000-680 000) |
| SOC | $615 000 | $201 000 | 8.3 | — | $623 000 | |
| Health system perspective (iron dextran) | ||||||
| Bevacizumab | $398 000 | — | 9.3 | 1.0 | $1 003 000 | $316 000 (147 000-567 000) |
| SOC | $552 000 | $154 000 | 8.3 | — | $687 000 | |
| Societal perspective (iron dextran) | ||||||
| Bevacizumab | $405 000 | — | 9.3 | 1.0 | $996 000 | $326 000 (151 000-592 000) |
| SOC | $569 000 | $164 000 | 8.3 | — | $670 000 | |
Bevacizumab remains a cost-saving treatment with added NMB regardless of choice of iron supplementation.
In a third scenario analysis when RBC- and iron-related adverse events were nullified, bevacizumab remains the cost-effective strategy, accruing 9.3 QALYs at $428 000 in costs compared with 8.3 QALYs and $700 000 in the SOC alone (Table 4). The iNMB with bevacizumab was $432 000 (95% CI, 217 000-941 000) and was preferred over the SOC alone in 100% of the 10 000 Monte Carlo iterations in PSA.
Scenario analysis of base-case results and NMBs assuming no adverse events from iron or RBC supplementation
| Strategy . | Cost . | Incremental cost . | Effectiveness (QALY) . | Incremental effectiveness (QALY) . | NMB . | iNMB (95% credible interval) . |
|---|---|---|---|---|---|---|
| Base-case model (health system perspective) | ||||||
| Bevacizumab | $428 000 | — | 9.3 | 1.0 | $973 000 | $432 000 (215 000-941 000) |
| SOC | $700 000 | $272 000 | 8.3 | — | $541 000 | |
| Base-case model (societal perspective) | ||||||
| Bevacizumab | $435 000 | — | 9.3 | 1.0 | $967 000 | $443 000 (218 000-973 000) |
| SOC | $717 000 | $282 000 | 8.3 | — | $524 000 | |
| Strategy . | Cost . | Incremental cost . | Effectiveness (QALY) . | Incremental effectiveness (QALY) . | NMB . | iNMB (95% credible interval) . |
|---|---|---|---|---|---|---|
| Base-case model (health system perspective) | ||||||
| Bevacizumab | $428 000 | — | 9.3 | 1.0 | $973 000 | $432 000 (215 000-941 000) |
| SOC | $700 000 | $272 000 | 8.3 | — | $541 000 | |
| Base-case model (societal perspective) | ||||||
| Bevacizumab | $435 000 | — | 9.3 | 1.0 | $967 000 | $443 000 (218 000-973 000) |
| SOC | $717 000 | $282 000 | 8.3 | — | $524 000 | |
Bevacizumab remains a cost-saving treatment even when the rates of adverse events from iron and RBC supplementation are reduced to 0.
Discussion
To our knowledge, this is the first cost-effectiveness analysis of any therapeutic in HHT, a multisystem disease with significant population health impact and documented prior inequities.45,46 Our findings suggest that, regardless of the WTP threshold, bevacizumab appears to be a cost-saving intervention that also improves the quality-adjusted life expectancy of patients with HHT.47,48 This overwhelming benefit is mediated by a reduction in the need for all hemostatic procedures, hospitalizations, emergency department visits, RBC transfusions, and iron infusions in a vulnerable patient population. Beyond cost-effectiveness, bevacizumab also saves patients >100 hours per year lived spent on receiving HHT-related care. Moreover, these effects are consistent regardless of the choice of IV iron supplementation.
These results are impactful for several reasons and extend the literature in several ways. First, our model adds to the growing body of clinical evidence, including the InHIBIT-Bleed study and the Second International Guidelines for the Diagnosis and Management of HHT, supporting the use of IV bevacizumab in patients with HHT with a defined phenotype of moderate-to-severe bleeding.9,49 The clinical improvement seen with bevacizumab treatment stands in stark contrast to other systemic agents that were less efficacious when evaluated for HHT-associated bleeding, such as tranexamic acid and oral estrogen.50-52 Nasal pharmacotherapy with timolol, tranexamic acid, and even nasal bevacizumab have also shown no improvement in epistaxis severity compared with placebo.53-55 Estrogen nasal pharmacotherapy has garnered mixed results with no conclusive evidence of benefit.55,56 As such, IV bevacizumab should be strongly considered as a best-choice agent for the treatment of moderate-to-severe HHT-associated bleeding.
Second, although patients seen at an HHT Center of Excellence will be treated according to the international guidelines, only 30 such centers currently exist in North America, and the majority of community-based physicians remain less familiar with bevacizumab use in HHT or HHT itself.4 This lack of awareness, combined with underrecognition and delayed diagnosis, a substantial problem in HHT care, means that many patients currently experience worse outcomes and quality of life than is necessary, at an increased cost to all payers.57,58 Our work highlights the importance of educating both physicians and patients about the benefit of bevacizumab therapy in HHT, as well as providing a quantitative, value-based analysis for the inclusion of an HHT-specific bevacizumab indication for those making coverage decisions. Finally, our analysis further supports the value of decision science and cost-effectiveness studies in evaluating therapeutic options in multisystemic, rare disease spaces such as HHT, especially because patients would benefit before large randomized controlled trials can accrue and reach completion.
Because of its wide variety of symptoms ranging from epistaxis and gastrointestinal bleeding to less specific signs such as dyspnea from lung arteriovenous malformations or headaches, local and community physicians, including primary care doctors, cardiologists, pulmonologists, gastroenterologists, otolaryngologists, and neurologists, should all be prepared to serve as primary points of contact and care for patients with HHT.4,59 As such, our results have broad implications for providers across the country, regardless of specialty. However, many are unfamiliar with the important, multisystem manifestations of the disease, which may lead to delayed screening, diagnosis, and initiation of guideline-directed therapies.59,60 Therefore, bridging this gap in knowledge while continuing to advocate for multispecialty, interdisciplinary collaboration in the treatment of patients with HHT remains of utmost importance in improving the overall quality of care.
Our model has several strengths. First, our model parameters are directly informed by the real-world clinical outcomes of the largest (n = 238) reported cohort of patients, serving as their own controls with a retrospective pretreatment and prospective posttreatment study design in the multicenter, international InHIBIT-Bleed study. The structure of our model also accounts for a wide variety of possible adverse events, including to RBCs, iron, and bevacizumab, whose probabilities are scaled on an exposure-dependent basis so as to best approximate the risks that real patients face with repeated infusions (Supplemental Appendix for more details). Additionally, we performed scenario analyses to examine the impact of varying iron supplementation products on our model results, purposefully selecting from commonly used agents across low (iron dextran), medium (ferumoxytol), and high (ferric caroxymaltose) price points on a per-infusion basis. We found that regardless of iron product, bevacizumab continues to produce cost savings and increase QALYs when added to the current SOC. A separate scenario analysis adjusts our model to further favor the null hypothesis by eliminating any iron- or RBC-associated adverse events (which the patients in the SOC have greater exposure to), finding that bevacizumab remains a cost-saving intervention even in this case. Finally, another strength of our model is its incorporation of a societal cost perspective, through the quantification of wages and hours lost by patients and caregivers to HHT-related care.
We also recognize several limitations in our model. Although our model is based on prospectively collected health resource utilization data across multiple international centers, the health resource utilization data from these same patients (ie, intrapatient comparison) before initiating bevacizumab was retrospective. Although it is subject to the typical limitations of retrospective data collection, the data were directly pulled by investigators from physician records over the preceding year. Second, these results only apply to patients with HHT with a strictly defined moderate-to-severe phenotype (supplemental Appendix for clinical definition). We also acknowledge that, on a population level, the severity of bleeding symptoms in HHT generally increase with age, and older patients bear a significantly greater burden of disease and subsequent degree of health care resource utilization than their younger counterparts.10,61 As such, the results of our 1-way sensitivity analysis on age must be carefully interpreted in this context; although severe symptoms (epistaxis and gastrointestinal) can and does occur in patients as young as 18 years, the majority of those experiencing moderate-to-severe bleeding are of an older age, on which the base-case and scenario analyses are all focused. Furthermore, alternative oral antiangiogenic agents that are currently underinvestigated for the treatment of HHT-associated bleeding, including pomalidomide, are outside the scope of this analysis.62,63
Third, our model does not explore all the possible choices of IV iron supplementation available to treat HHT-associated bleeding under the current SOC, although it specifically targets the entire range by examining the lowest and highest cost products with consistent results across all tested formulations. Fourth, all economic models are specific to their specific country context, and this model is specific to the US context. Analyses of bevacizumab for HHT outside of the US context may not reach the same conclusion given the different country-specific costs. Fifth, because there are no on-treatment–specific utility data for patients with HHT undergoing bevacizumab treatment, we estimated an incremental utility increase for patients on bevacizumab based on their degree of anemia improvement rather than direct patient reporting of quality of life, although we addressed this limitation in extensive sensitivity analyses. Future, longitudinal quality-of-life studies of systemic bevacizumab treatment in the patients with HHT population will reflect an even more accurate depiction of its utility and impact on individuals living with HHT, although the investment into these would be first best adjudicated with value-of-information analysis, as was recently done in the evaluation of potential future studies for study prioritization in the peripartum thromboprophylaxis space.64
In summary, and to the best of our knowledge, we performed the first cost-effectiveness analysis of systemic bevacizumab therapy in HHT. We found that, regardless of WTP, the addition of longitudinal, IV bevacizumab to the current SOC improves the quality-adjusted life expectancy of patients with HHT and appears to be a cost-saving intervention, compared with the current SOC alone. Separately, bevacizumab also saves patients at least 133 hours in HHT-specific care per year lived in the form of reduced RBC transfusions, IV iron infusions, emergency department visits, hospitalizations, and hemostatic procedures. In the US context, these results strongly suggest that bevacizumab tier placement should be favorably prioritized across all commercial and public funders in the care of individuals living with HHT.
Acknowledgments
This work was supported by funding from the National Heart, Lung, and Blood Institute (T35HL007649 [D.W.] and 1K23HL159313 [H.A.-S.]), as well as the Frederick A. DeLuca Foundation and the Yale Bunker Endowment (G.G.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health.
Authorship
Contribution: D.W., S.I., H.A.-S., and G.G. conceived the study; and all authors wrote and edited the manuscript.
Conflict-of-interest disclosure: H.M.K., in the past 3 years, has received expenses and/or personal fees from UnitedHealth, Element Science, Eyedentifeye, and F-Prime; is a cofounder of Refactor Health and HugoHealth; and is associated with contracts, through Yale New Haven Hospital, from the Centers for Medicare & Medicaid Services and, through Yale University, from the Food and Drug Administration, Johnson & Johnson, Google, and Pfizer. H.A.-S. reports research funding to institution from Agios, Sobi, Novartis, Vaderis, and Amgen, and consultancy fees from Agios, Sobi, Moderna, Novartis, argenx, Forma, and Pharmacosmos. The remaining authors declare no competing financial interests.
Correspondence: George Goshua, Yale University School of Medicine, 333 Cedar St, New Haven, CT 06520; email: george.goshua@yale.edu.
References
Author notes
H.A.-S. and G.G. are joint senior authors.
Data are available on request from the corresponding author, George Goshua (george.goshua@yale.edu).
The full-text version of this article contains a data supplement.