Genetic and pharmacological inhibition of BTK increases dasatinib sensitivity in vitro and in vivo including in E2A-PBX1+/pre-BCR+ ALL cells.
The combination therapy of dasatinib with ibrutinib reduces significantly CNS-infiltrating E2A-PBX1+/preBCR+ ALL after in vivo treatments.
Visual Abstract
The t(1;19) translocation, encoding the oncogenic fusion protein E2A (TCF3)-PBX1, is involved in acute lymphoblastic leukemia (ALL) and associated with a pre–B-cell receptor (preBCR+) phenotype. Relapse in patients with E2A-PBX1+ ALL frequently occurs in the central nervous system (CNS). Therefore, there is a medical need for the identification of CNS active regimens for the treatment of E2A-PBX1+/preBCR+ ALL. Using unbiased short hairpin RNA (shRNA) library screening approaches, we identified Bruton tyrosine kinase (BTK) as a key gene involved in both proliferation and dasatinib sensitivity of E2A-PBX1+/preBCR+ ALL. Depletion of BTK by shRNAs resulted in decreased proliferation of dasatinib-treated E2A-PBX1+/preBCR+ cells compared with control-transduced cells. Moreover, the combination of dasatinib with BTK inhibitors (BTKi; ibrutinib, acalabrutinib, or zanubrutinib) significantly decreased E2A-PBX1+/preBCR+ human and murine cell proliferation, reduced phospholipase C gamma 2 (PLCG2) and BTK phosphorylation and total protein levels and increased disease-free survival of mice in secondary transplantation assays, particularly reducing CNS-leukemic infiltration. Hence, dasatinib with ibrutinib reduced pPLCG2 and pBTK in primary ALL patient samples, including E2A-PBX1+ ALLs. In summary, genetic depletion and pharmacological inhibition of BTK increase dasatinib effects in human and mouse with E2A-PBX1+/preBCR+ ALL across most of performed assays, with the combination of dasatinib and BTKi proving effective in reducing CNS infiltration of E2A-PBX1+/preBCR+ ALL cells in vivo.
Introduction
Acute lymphoblastic leukemia (ALL) is the most common cancer in children and is associated with poor prognosis in adults.1 Even though the prognosis and treatment of ALL have significantly improved through intensified and stratified chemotherapy regimens,2 central nervous system (CNS) relapse remains a main therapeutic obstacle to treat ALL. CNS involvement is observed in about 3% to 5% of patients at initial diagnosis and 30% to 40% of patients at relapse, including after allogeneic hematopoietic cell transplantation.3-5 Therefore, a major clinical challenge in ALL is to identify and establish effective therapies in the context of CNS infiltration by ALL cells.
ALL constitutes a family of genetically, morphologically, immunophenotypically, and clinically heterogeneous lymphoid neoplasms derived from B- and T-lymphoid progenitors.6 Among the B-ALL, a distinct immunophenotype characterized by expression and tonic functional activity of the pre–B-cell receptor (preBCR) defines a novel ALL subtype (preBCR+ ALL), accounting for 15% of patients with ALL.7 Half of preBCR+ ALL cases harbor the chromosomal translocation t(1:19) (q23;p13), coding for the chimeric transcription factor E2A (TCF3)-PBX1 and affecting from 5% to 7% of pediatric and adult patients with ALL.8 This genomic rearrangement, which fuses the TCF3 with the homeobox gene PBX1,9 serves as the initiating driver mutation in a phenotypically and genetically distinct subtype of ALL, which has also been associated with a higher incidence of CNS infiltration.10,11 In E2A-PBX1+ ALL, pathways downstream the preBCR are directly involved in CNS-infiltrating ALL cells and the in vivo CNS infiltration of E2A-PBX1+/preBCR+ cells was observed in different mouse models.12-15
In the last decades, several small-molecule inhibitors have shown in vitro and in vivo antitumor activity and their approval for clinical use as novel cancer treatments has drastically increased.16 Dasatinib is an oral tyrosine kinase inhibitor (TKI), approved by the US Food and Drug Administration as effective therapy in the treatment of chronic myeloid leukemia and Philadelphia chromosome-positive ALL.17,18 Recent studies have suggested dasatinib as a promising targeted therapy for other ALL subtypes, including preBCR+ ALL.8,12,19-21 Moreover, dasatinib has been described to cross the blood-brain barrier.22
In this study, we aimed to identify novel targets to increase dasatinib efficacy, particularly with CNS activity, using genetic and pharmacological approaches. We identified and validated Bruton tyrosine kinase (BTK) as therapeutic target in murine models, human cell lines and human primary E2A-PBX1+/preBCR+ ALL, established combination therapies in vitro and in vivo of BTK inhibitors (BTKi) with dasatinib and demonstrated higher efficacy of the combination therapy in CNS-infiltrating E2A-PBX1+/preBCR+ ALL cells in in vivo transplantation models.
Materials and methods
shRNA knockdown, lentiviral transfection, and competition growth assays
Individual BTK-2 and BTK-3 short hairpin RNA (shRNA) sequences (supplemental Table 1) were cloned into the BstXI site of the p309 lentiviral vector, for stable transduction in ALL cell lines of human.23,24 The lentivirus generation was performed by cotransfection of shRNA constructs with pCMV-dR8.2 (packaging) and pCMV-VSVG (envelope) plasmids into HEK293T cells. Afterwards, human leukemia cells were transduced with viral supernatant collected 48 hours after transfection of HEK293T cells, using spinoculation (2500 rpm, 37°C for 3 hours) in the presence of 4 μg/mL polybrene. Cells were cultured for 2 days before selection with 1 μg/mL puromycin (Invitrogen). After 4 days of selection, cells with control shRNAs (shLuc-mCherry) and shRNA knockdown for BTK (shBTK-2-mCherry and shBTK-3-mCherry) were mixed 1:1 with cells containing a control vector (shLuc-GFP), cultured for 24 days and monitored by flow cytometry for mCherry+ and green fluorescence protein (GFP)+ cells every 3 days. After puromycin selection, the knockdowns of the single shRNAs were confirmed by immunoblotting and quantitative reverse transcription polymerase chain reaction (qRT-PCR).
Western blotting
Proteins were isolated using a modified radio-immunoprecipitation assay lysis buffer (50 mM Tris HCl, 1% NP-40, 1% natrium-deoxycholate, 150 mM NaCl, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, and 1 mM Na3VO4), and 1× protease inhibitor cocktail (Roche).12 Proteins were immunodetected with the following antibodies: rabbit anti-BTK (D3H5) (catalog no. 8547; Cell Signaling Technology), rabbit antiphospho-BTK (D9T6H) (catalog no. 87141; Cell Signaling Technology), rabbit antiphospholipase C gamma 2 (PLCG2) (catalog no. 3872; Cell Signaling Technology), rabbit anti-phospho-PLCG2 (D9T6H) (catalog no. 87141; Cell Signaling Technology), rabbit anti-GAPDH (14C10) (catalog no. 2118; Cell Signaling Technology) antibodies.
qRT-PCR
The RNeasy Mini Kit (QIAGEN) was used to isolate the RNA, whereas the complementary DNA was synthesized using the SuperScript III Reverse Transcriptase Kit (Life Technologies) according to the manufacturer’s recommendations. Relative expression of BTK (Hs04999593) gene and of ACTB (Hs01060665_g1) housekeeping gene were quantified using an LightCycler 480 II Thermocycler (Roche) with TaqMan gene expression assays from Thermo Fisher and Light Cycler 480 Probes Master (Roche) at an annealing temperature of 60°C.
Human cell lines
Human E2A-PBX1+/preBCR+ (RCH-ACV and 697) cell lines and E2A-PBX1− (REH and SEM) cell lines were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum, 100 U/mL penicillin-streptomycin. B-ALL cell lines were authenticated in 2020 in German Collection of Microorganisms and Cell Cultures (DSMZ), after being obtained from DSMZ (Braunschweig, Germany) in 2013. Cell lines were negatively tested for mycoplasma contamination by PCR. Human cell lines treated with vehicle (dimethyl sulfoxide [DMSO]), dasatinib (BMS-354825), ibrutinib (PCI-32765), acalabrutinib (ACP-196), and zanubrutinib (BGB-3111) from Selleck Chemicals (Houston, TX), as well as with the combination of dasatinib with BTKis, at the indicated concentrations. Titration curves were performed using increasing drug concentrations and cells were counted by trypan blue assay after 3 days.
Murine leukemia cells
Mouse leukemia E2A-PBX1+/preBCR+ (#159) and E2A-PBX1+/preBCR− (#1496) cells (25 000 per well) were cultured in methylcellulose medium (M3234, StemCell Technologies) supplemented with 10 ng/mL interleukin 7 (IL7) (Miltenyi Biotec). Leukemias cells were treated at the indicated concentrations in semisolid methylcellulose medium (M3234, StemCell Technologies) supplemented with 10 ng/mL IL7 (Miltenyi Biotec). Titration curves were performed as described elsewhere.12
Primary ALL samples
Primary ALL samples were obtained from tissue bank of the University of Freiburg Medical School, Department of Hematology/Oncology, and primary E2A-PBX1+ ALLs from the tissue bank of the ALL BFM study group. Primary ALL samples were treated with vehicle (DMSO), dasatinib, and ibrutinib from Selleck Chemicals, as well as with the combination of dasatinib with ibrutinib, at the indicated concentrations. Experiments with patient samples were performed in accordance with the tenets of the Declaration of Helsinki and were approved by the ethics commission from the University of Freiburg (ethical vote, approval number 279/17). All patients were informed by a physician and signed an informed consent form.
Antibodies and flow cytometry analysis
Phospho-flow and flow cytometry analysis were performed using standard conditions as described elsewhere.23 For flow cytometry analysis the following antibodies were purchased: PLCG2– (K86-689.37) and BTK– (A3C6E2) antibodies from BD Biosciences (Franklin Lakes, NJ), 7-AAD-Ab (559925), CD34 (P28906) Abs from BD Pharmigen (San Diego, CA), CD45– (30-F11), CD19– (6D5), B220– (RA3-6B2), CD43– (S11) and CD117– (2B8) Abs from BioLegend (San Diego, CA). The BD LSRFortessa and FACS Aria III (BD Biosciences, Heidelberg, Germany) were used for analyzing and sorting.
Bone marrow transplantation assays
Secondary bone marrow transplantation assays were performed using 1000 murine E2A-PBX1+/preBCR+ leukemia cells per recipient mouse, following sublethal irradiation (6 Gy) of healthy recipient mice. Mice were treated with intraperitoneal injections with: vehicle (30% PEG300, 5% Tween 80, 5% DMSO dissolved in phosphate-buffered saline), dasatinib (5 mg/kg body weight per day [b.w./d]), ibrutinib (20 mg/kg b.w./d), dasatinib in combination with ibrutinib (5 mg + 20 mg/kg b.w./d), acalabrutinib (20 mg/kg b.w./d), dasatinib in combination with acalabrutinib (5 mg + 20 mg/kg b.w./d), zanubrutinib (10 mg/kg b.w./d), or dasatinib in combination with zanubrutinib (5 mg + 10 mg/kg b.w./d) from Selleck Chemicals. Immunohistochemical analysis, cell blood counts, flow cytometry analysis for GFP+ cells and phospho-proteins and cell isolation for RNA sequencing and proteomics were performed after sacrificing animals with clinical signs of disease (lymphadenopathies, shivering, or weakness) to analyze the efficacy of dasatinib and BTKi in an in vivo mouse transplantation model and identify mechanism of targeted therapy resistance. No animals were excluded from the analysis. No randomization method was used to allocate the animals to an experimental group. The investigators were not blinded to the group location during the experiment.
Isolation of leukemia cells from CNS
Single cell suspensions were generated from fresh tissue from euthanized mice. After isolation, both brain and spinal cord were cut in small pieces by scalpel and then were resuspended for 1 hour in the cell dissociation solution Accumax (Sigma-Aldrich, St. Louis, MO) at room temperature, followed by washing twice in phosphate-buffered saline and GFP detection by flow cytometry.
Immunohistochemistry
Tissues were fixed in 10% formalin at 4°C overnight and embedded in paraffin.25 Following dewaxing and antigen retrieval for 10 minutes at 96°C in 0.1 M citrate buffer, incubation with polyclonal goat anti-GFP antibody (1:1000 dilution; ab5450; Abcam, Cambridge, MA), was carried out overnight. Sections were incubated with biotin-conjugated anti-goat secondary antibody (1:200 dilution; BA-9500-1.5; Vector Laboratories, Newark, CA). Immunostainings were quantified using QuPath (v.0.2.0) software that enables processing of digitalized images.26 The images were transformed to DAB and GFP positivity was measured by pixel quantification by a single cutoff threshold of 0.20.
RNA sequencing and bioinformatics analysis
RNA from CNS of vehicle, dasatinib, ibrutinib, and dasatinib + ibrutinib-treated mice was isolated using the RNeasy Plus Micro Kit (Qiagen) according to the manufacturer’s instructions. RNA was used for RNA sequencing analysis following library preparation using the Illumina Stranded Total RNA Prep, ligation with Ribo-Zero Plus kit.
Sequencing was performed in the Max-Planck-Institute für Immunbiology und Epigenetics using NovaSeq 6000. Sequencing data were processed using nf-core/rna-seq pipeline (version 3.8).27 Briefly, read quality was assessed by FastQC and MultiQC. Reads were trimmed with TrimGalore to remove adapter sequences and poor-quality reads. Ribosomal RNA was removed with SortMeRNA as preprocessing filtering tools. Filtered reads were then aligned to the mouse genome (mm10) using STAR and read counts per gene were quantified using Salmon and post-processed with SAMtools and picard MarkDuplicates. In later steps, we used DESeq2 to remove genes with low counts keeping genes with at least 10 counts in a minimal number of samples (n = 4). The differential gene expression analysis was performed with the limma + voom pipeline (v. 3.16).28 The significance threshold was set to adjusted P value < .05.
Experiments with patient samples were approved by the ethics commission from the University of Freiburg (ethical vote, approval number 279/17). All experiments with mice were performed in accordance with relevant guidelines and regulations and were approved by the “Regierungspräsidium Freiburg” (number G-17/148).
Results
Identification of common genes and pathways involved in proliferation and dasatinib sensitivity using genetic screening approaches in E2A-PBX1+ leukemia cells
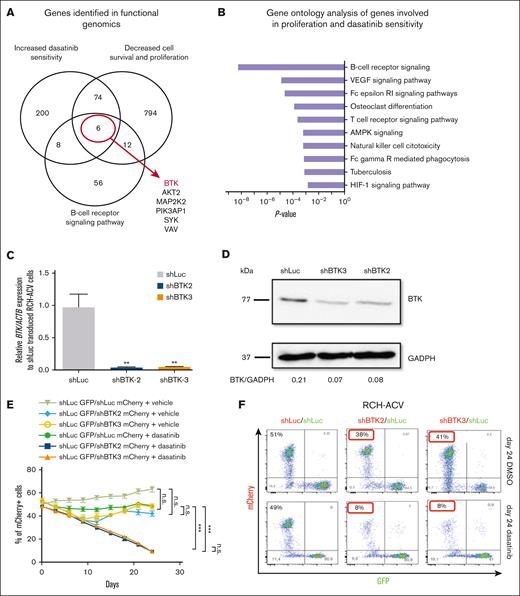
Functional genomic screens based on ultracomplex shRNA libraries were used in human E2A-PBX1+ ALL cells to identify novel genes and pathways involved in cell proliferation, survival,23 and in dasatinib sensitivity and resistance.24 Among the 2 previously performed shRNA screens, we identified 80 common genes from 3158 targeted genes, which significantly decreased cell proliferation and increased dasatinib sensitivity (Figure 1A; supplemental Table 2). From the genes identified in both shRNA screens, we performed gene ontology analysis. Interestingly, the B-cell receptor (BCR) signaling pathway was enriched in both shRNA screens (Figure 1B). Only 6 genes were identified as common genes in both screens belonging to the BCR signaling pathway (BTK, AKT2, MAP2K2, PIK3AP1, SYK, and VAV). In our previous work, we elucidated the role of PI3K/AKT/MTOR signaling pathway23 and SYK29 in E2A-PBX1+/preBCR+ ALL. Knowing the central role of BTK in the pre-BCR signaling pathway and the recent development of high potency BTKi with CNS activity, we focused our studies on this gene (supplemental Table 1; supplemental Figure 1).
BTK-depletion decreases the proliferation of human E2A-PBX1+/preBCR+ RCH-ACV cells treated with dasatinib. (A) Using a shRNA library screen approach, the BTK was identified as key gene involved to increase sensitivity to dasatinib in E2A-PBX1+/preBCR+ leukemia cells. (B) Gene ontology analysis of pathways enriched in genes increasing sensitivity to dasatinib. Only the top 10 KEGG pathways are shown. (C) RT-qPCR shows efficient shRNA-mediated knockdown of BTK2 and BTK3. (D) Western blot analysis (representative of 3 independent experiments) shows the protein levels following knockdown by sh-BTK2 and sh-BTK3 constructs in RCH-ACV cells. Messenger RNA (mRNA) and protein were extracted from the same stably transduced expanded sh-Luc control cells and sh-BTK2/sh-BTK3 knockdown cells used for (C) and (D) experiments. GADPH was used as loading control. Densitometry values were calculated using ImageJ software. (E) Graph shows the percentage of mCherry+ cells transduced with shRNAs for luciferase (control) or shRNA constructs of BTK2 or BTK3 and treated with dasatinib 20 nM for 24 days. Data represent the mean ± standard of error mean (SEM) of 3 independent experiments. Statistical analysis performed by nonparametric Mann-Whitney test. n.s., not significant; ∗∗∗P < .001. (F) Dot plot proportion of mCherry+ and GFP+ cells in flow cytometry at day 24 of culture of a representative experiment, in which RCH-ACV cells were transduced with control shRNA (shLuc, luciferase) or shRNA for BTK (shBTK2 and shBTK3) with a mCherry as fluorescence marker. KEGG, Kyoto Encyclopedia of Genes and Genomes.
BTK-depletion decreases the proliferation of human E2A-PBX1+/preBCR+ RCH-ACV cells treated with dasatinib. (A) Using a shRNA library screen approach, the BTK was identified as key gene involved to increase sensitivity to dasatinib in E2A-PBX1+/preBCR+ leukemia cells. (B) Gene ontology analysis of pathways enriched in genes increasing sensitivity to dasatinib. Only the top 10 KEGG pathways are shown. (C) RT-qPCR shows efficient shRNA-mediated knockdown of BTK2 and BTK3. (D) Western blot analysis (representative of 3 independent experiments) shows the protein levels following knockdown by sh-BTK2 and sh-BTK3 constructs in RCH-ACV cells. Messenger RNA (mRNA) and protein were extracted from the same stably transduced expanded sh-Luc control cells and sh-BTK2/sh-BTK3 knockdown cells used for (C) and (D) experiments. GADPH was used as loading control. Densitometry values were calculated using ImageJ software. (E) Graph shows the percentage of mCherry+ cells transduced with shRNAs for luciferase (control) or shRNA constructs of BTK2 or BTK3 and treated with dasatinib 20 nM for 24 days. Data represent the mean ± standard of error mean (SEM) of 3 independent experiments. Statistical analysis performed by nonparametric Mann-Whitney test. n.s., not significant; ∗∗∗P < .001. (F) Dot plot proportion of mCherry+ and GFP+ cells in flow cytometry at day 24 of culture of a representative experiment, in which RCH-ACV cells were transduced with control shRNA (shLuc, luciferase) or shRNA for BTK (shBTK2 and shBTK3) with a mCherry as fluorescence marker. KEGG, Kyoto Encyclopedia of Genes and Genomes.
BTK plays a key role in proliferation, survival, and dasatinib sensitivity in human E2A-PBX1+/preBCR+ ALL cells
Validation and functional characterization of the identified BTK gene was performed using a genetic shRNA–mediated knockdown approach followed by proliferation competition assays. Single shRNAs (sh-BTK2 and sh-BTK3) were used to deplete efficiently BTK in human E2A-PBX1+/preBCR+ RCH-ACV cells using lentiviral vectors (Figure 1C-D; supplemental Table 2). Stably transduced sh-Luc control cells and sh-BTK2/sh-BTK3 knockdown cells were treated in vitro with vehicle (DMSO) or TKI dasatinib (20 nM) for 24 days. Depletion of BTK by both shRNA constructs reduced the proliferation and survival, even in the presence of dasatinib, of transduced RCH-ACV cells compared with sh-Luc control–transduced cells and to vehicle (DMSO) treated cells in competition assays (Figure 1E-F; supplemental Figure 2), thereby validating BTK as a key gene for cell proliferation/survival and dasatinib sensitivity in human E2A-PBX1+/preBCR+ ALL.
In vitro efficacy of pharmacologic targeted therapy with dasatinib in combination with BTKi in human E2A-PBX1+/preBCR+ ALL cells
To establish a combination therapy that increases dasatinib sensitivity especially in CNS in E2A-PBX1+/preBCR+ ALL, a pharmacologic approach was explored to test the effects of dasatinib in combination with the BTKi ibrutinib, which is known to penetrate the CNS very efficiently and has been established for the treatment of relapsed/refractory primary CNS-lymphoma30,31 and CNS-infiltrating mantle cell lymphoma.32 We hypothesized, that second generation BTKis (acalabrutinib and zanubrutinib) would also penetrate the CNS like ibrutinib and thus were tested in combination with dasatinib. Indeed, clinical trials testing the efficacy of acalabrutinib in CNS-lymphoma are ongoing (NCT04548648).
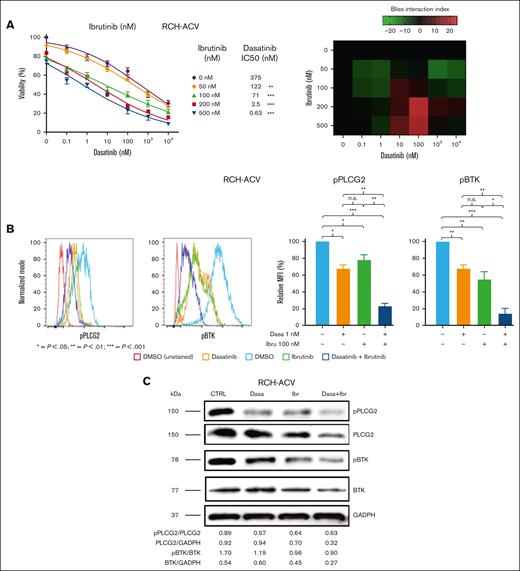
First, we treated human RCH-ACV, 697, REH, and SEM ALL cells with single substances and in combination. Dasatinib efficiently suppressed cell proliferation in E2A-PBX1+/preBCR+ RCH-ACV and 697 cell lines,29 with a dasatinib 50% inhibitory concentration (IC50) of 415 nM (RCH-ACV) and 247 nM (697), as previously shown (supplemental Figure 3A). RCH-ACV and 697 showed similar sensitivity to BTKi, (IC50 = 327 and 328 nM, respectively), as well as for acalabrutinib (IC50 = 129 and 121 nM, respectively) and zanubrutinib (IC50 = 220 and 193 nM, respectively) (supplemental Figure 3B-D). Moreover, we observed a consistent statistically significant difference in sensitivity to single treatments dasatinib and BTKis between human E2A-PBX1+/preBCR+ and E2A-PBX1−/preBCR− cell lines (supplemental Figure 3A-D). Furthermore, combination treatments with dasatinib and BTKis showed interactions at several concentrations as shown by the bliss index33 on proliferation assays in human E2A-PBX1+/preBCR+ RCH-ACV and 697 ALL cell lines (Figure 2A; supplemental Figures 4A-B and 5A-B) but not in human E2A-PBX1−/preBCR− ALL SEM and REH cell lines at least at the selected concentrations (supplemental Figure 6A-B). However, we cannot exclude negative, additive or synergistic effects at lower/higher concentrations in those cells. These data demonstrate that BTKis increase dasatinib sensitivity in human E2A-PBX1+/preBCR+ but not in E2A-PBX1−/preBCR− ALLs, suggesting some selectivity.
In vitro effect of combined therapy with dasatinib and BTKi on human E2A-PBX1+/preBCR+ ALL cell proliferation and on PLCG2 and BTK phosphorylation. (A) Left panels, titration curves for E2A-PBX1+/preBCR+ human (RCH-ACV) cells with increasing concentrations of dasatinib and ibrutinib combination treatments. Viable RCH-ACV cells were counted with trypan blue exclusion assay after 3 days. Data represent IC50 calculated using nonlinear regression analysis and curves were compared with the sum-of-squares F test. Dose-response curves of each BTKi concentration were compared with the dose-response curve of the vehicle-treated cells as controls. n.s., not significant; ∗∗P < .001; ∗∗∗P < .0001. Right panel, heat map representation of Bliss interaction index for RCH-ACV cells treated with dasatinib and ibrutinib. Three independent experiments were performed. (B) In vitro effects of the combined therapy with dasatinib and BTKi in human RCH-ACV cells on the phosphorylation status of the key pre-BCR+ pathway proteins PLCG2 and BTK, after 30 minutes of treatment. One representative graph per antibody is shown. Relative fold median fluorescence intensity (MFI) change of cells treated with inhibitors or vehicle are illustrated. Data represent mean of 3 independent experiments + SEM. (C) Western blot analysis (representative of 3 independent experiments) shows the protein levels of BTK, pBTK, PLCG2, and pPLCG2 following dasatinib (1 nM), ibrutinib (100 nM), and dasatinib (1 nM) + ibrutinib (100 nM) treatments compared with control, in human RCH-ACV ALL cells. GADPH was used as loading control. Densitometry values were calculated using ImageJ software.
In vitro effect of combined therapy with dasatinib and BTKi on human E2A-PBX1+/preBCR+ ALL cell proliferation and on PLCG2 and BTK phosphorylation. (A) Left panels, titration curves for E2A-PBX1+/preBCR+ human (RCH-ACV) cells with increasing concentrations of dasatinib and ibrutinib combination treatments. Viable RCH-ACV cells were counted with trypan blue exclusion assay after 3 days. Data represent IC50 calculated using nonlinear regression analysis and curves were compared with the sum-of-squares F test. Dose-response curves of each BTKi concentration were compared with the dose-response curve of the vehicle-treated cells as controls. n.s., not significant; ∗∗P < .001; ∗∗∗P < .0001. Right panel, heat map representation of Bliss interaction index for RCH-ACV cells treated with dasatinib and ibrutinib. Three independent experiments were performed. (B) In vitro effects of the combined therapy with dasatinib and BTKi in human RCH-ACV cells on the phosphorylation status of the key pre-BCR+ pathway proteins PLCG2 and BTK, after 30 minutes of treatment. One representative graph per antibody is shown. Relative fold median fluorescence intensity (MFI) change of cells treated with inhibitors or vehicle are illustrated. Data represent mean of 3 independent experiments + SEM. (C) Western blot analysis (representative of 3 independent experiments) shows the protein levels of BTK, pBTK, PLCG2, and pPLCG2 following dasatinib (1 nM), ibrutinib (100 nM), and dasatinib (1 nM) + ibrutinib (100 nM) treatments compared with control, in human RCH-ACV ALL cells. GADPH was used as loading control. Densitometry values were calculated using ImageJ software.
Phosphorylation of PLCG2 and BTK decreases after combination therapy of dasatinib with BTKi in human E2A-PBX1+/preBCR+ ALL cells
PLCG2 plays a critical role on BCR function and pPLCG2 is hyperactivated and has a pathogenic role in E2A-PBX1+/preBCR+ ALL.29 BTK is a key mediator of the pre-BCR pathway, located upstream of PLCG2, and pBTK levels increase after BCR signaling activation.34 Therefore, we analyzed changes in phosphorylation status of pPLCG2 (Tyr 753) and pBTK (Tyr 223) using phospho-specific flow cytometry (phospho-flow) in ALL human cell lines after treatment with dasatinib in combination with BTKi. Treatments with single compounds reduced significantly pPLCG2 and pBTK levels in human E2A-PBX1+/preBCR+ RCH-ACV and 697 ALL cell lines compared with vehicle-treated cells. Combination treatments of dasatinib with BTKi further reduced pPLCG2 and pBTK levels compared with vehicle and single treatments (Figure 2B; supplemental Figure 7A-C). As expected, human E2A-PBX1−/preBCR− SEM and REH ALL cell lines showed no significant differences in pPLCG2 and pBTK levels after treatment with single substances and dasatinib in combination with BTKi (supplemental Figure 7A-C). Moreover, the phosphorylation status of PLCG2 and BTK was assessed by western blot. pPLCG2 levels decreased after single treatments with dasatinib and BTKi. However, the combination of dasatinib and BTKi did not decrease further pPLCG2 levels. In contrast, pBTK levels were slightly decreased after the combination of dasatinib and BTKi in human RCH-ACV ALL cell lines compared with the vehicle and single treatments. A decrease of total PLCG2 and BTK protein levels was also observed (Figure 2C). In summary, these data indicate that dasatinib and BTKis, alone and in combination, decrease phosphorylation and activation of key proteins PLCG2 and BTK involved in the pre-BCR signaling pathway.
BTK inhibition enhances in vitro efficacy of dasatinib in murine E2A-PBX1+/preBCR+ but not in E2A-PBX1+/preBCR− ALL cells
To confirm that BTK inhibition enhances the effect of TKI dasatinib in E2A-PBX1+/preBCR+ ALL cells, we followed a cross-species comparative approach using mouse ALL cell lines derived from our previously described conditioning E2A-PBX1 knock-in mouse model.29 As previously shown, murine E2A-PBX1+/preBCR+ #159 ALL cells were more sensitive to dasatinib and BTKi than murine E2A-PBX1+/preBCR− #1496 ALL cells (supplemental Figure 8A-D). The IC50 of dasatinib was remarkably reduced in combination with BTKi at different concentrations in murine E2A-PBX1+/preBCR+ #159 cells but not in E2A-PBX1+/preBCR− #1496 ALL cells (supplemental Figure 9A-B). Similarly, to the human E2A-PBX1−/preBCR− ALL cell lines, we cannot exclude additive, synergistic or negative effects at higher or lower concentrations of the BTKis in murine E2A-PBX1+/preBCR− #1496 ALL cells.
We also analyzed the phosphorylation status of pPLCG2 and pBTK in murine ALL cell lines after the treatment with BTKis as monotherapies and in combination with dasatinib by phospho-flow. Combination therapies of BTKi with dasatinib reduced the pPLCG2 and pBTK in murine E2A-PBX1+/preBCR+ #159 but not E2A-PBX1+/preBCR− #1496 ALL cell lines compared with treatment with single substances and with vehicle-treated cells (supplemental Figures 10A-B and 11). Western blot analysis of murine E2A-PBX1+/preBCR+ #159 ALL cells confirmed the decrease of pPLCG2 and pBTK and, similar to human E2A-PBX1+/preBCR+ ALL cells, a decrease of total PLCG2 and BTK proteins after the treatment with single compounds and combination therapies was noted (supplemental Figure 10B).
These data indicate that BTKis increase dasatinib effects on colony forming assays and reduction of PLCG2 and BTK phosphorylation in murine E2A-PBX1+/preBCR+ but not in murine E2A-PBX1+/preBCR− ALLs, suggesting some selectivity for preBCR signaling inhibition.
In vivo efficacy of combination dasatinib and BTKi in E2A-PBX1+/preBCR+ leukemias
To further validate and confirm the efficacy of the combination of dasatinib and BTKi, we analyzed in vivo treatments after transplantation of murine E2A-PBX1+/preBCR+ ALL cells in sublethal irradiated healthy mouse recipients (Figure 3A). As expected, in vivo treatment with dasatinib in combination with BTKi, prolonged disease-free survival compared with vehicle- and single-treated mice (Figure 3B). The combination of dasatinib with BTKi–reduced leukemia cell infiltration of several organs such as liver, spleen, and lymph nodes, as seen by hematoxylin and eosin stainings and immunostainings by anti-GFP antibodies (Figure 3C; supplemental Figure 12A-B). Mice treated with dasatinib combined with BTKi developed less lymphadenopathy, hepatomegaly, and splenomegaly (supplemental Figure 13A). Bone marrow insufficiency (anemia and thrombocytopenia), and leukocytosis were less pronounced in mice treated with the combination of dasatinib and BTKi (supplemental Figure 13B). We showed presence of murine E2A-PBX1+/preBCR+ leukemia cells in several organs by immunohistological stainings for GFP and we additionally quantified GFP+ leukemia cells by flow cytometry. We confirmed that mice treated with the combination of dasatinib and BTKi showed less infiltration of GFP+ leukemia cells in analyzed organs and tissues (Figure 3C; supplemental Figures 14 and 15).
In vivo sensitivity of E2A-PBX1+/preBCR+ leukemia cells to the combination of dasatinib and BTKi. (A) Schematic representation of secondary transplantations of E2A-PBX1+/preBCR+ leukemia cells in healthy recipients and in vivo treatment with vehicle, dasatinib, BTKis, and combination of dasatinib with BTKis. (B) E2A-PBX1+ leukemia cells from preBCR+ leukemia were transplanted into sublethally irradiated recipient healthy C57/BL6 mice. Mice were treated for 20 days with vehicle (n = 10), dasatinib (n = 10), ibrutinib (n = 10), dasatinib + ibrutinib (n = 10), acalabrutinib (n = 5), dasatinib + acalabrutinib (n = 5), zanubrutinib (n = 5), and dasatinib + zanubrutinib (n = 5). In vivo dasatinib + BTKi treatments of pre-BCR+ leukemia in a secondary transplantation assay led to significantly prolonged disease-free survival compared with vehicle-treated mice. Statistical analysis was performed by log-rank test. (C) Immunohistochemical staining of bone marrow (BM) sections with anti-GFP antibody showing a decrease in GFP+ E2A-PBX1+/pre-BCR+ leukemic cells infiltration in double-treated mice compared with single- and vehicle-treated mice. Samples were collected from euthanized animals with signs of disease from vehicle- (mean = 18 days), dasatinib- (mean = 24 days), ibrutinib- (mean = 30 days), and dasatinib + ibrutinib– (mean = 37 days) treated animals. Objective lens: 20×; scale bar: 200 μm. GFP positivity was measured using the QuPath software after transformation of images to DAB and staining intensity quantified by a single cutoff threshold of 0.20. (D) The phosphorylation status of p-PLCG2 (Tyr 753) and p-BTK (Tyr 223) of BM murine cells by phospho-specific flow cytometry. The phosphorylation of both PLCG2 and BTK decrease significantly in the BM isolated from dasatinib + ibrutinib–treated mice. Fold MFI change of dasatinib-, ibrutinib-, and dasatinib + ibrutinib–treated mice compared with vehicle. Data represent mean of 3 independent experiments + SEM. n.s., not significant; ∗P < .01; ∗∗P < .001; ∗∗∗P < .0001.
In vivo sensitivity of E2A-PBX1+/preBCR+ leukemia cells to the combination of dasatinib and BTKi. (A) Schematic representation of secondary transplantations of E2A-PBX1+/preBCR+ leukemia cells in healthy recipients and in vivo treatment with vehicle, dasatinib, BTKis, and combination of dasatinib with BTKis. (B) E2A-PBX1+ leukemia cells from preBCR+ leukemia were transplanted into sublethally irradiated recipient healthy C57/BL6 mice. Mice were treated for 20 days with vehicle (n = 10), dasatinib (n = 10), ibrutinib (n = 10), dasatinib + ibrutinib (n = 10), acalabrutinib (n = 5), dasatinib + acalabrutinib (n = 5), zanubrutinib (n = 5), and dasatinib + zanubrutinib (n = 5). In vivo dasatinib + BTKi treatments of pre-BCR+ leukemia in a secondary transplantation assay led to significantly prolonged disease-free survival compared with vehicle-treated mice. Statistical analysis was performed by log-rank test. (C) Immunohistochemical staining of bone marrow (BM) sections with anti-GFP antibody showing a decrease in GFP+ E2A-PBX1+/pre-BCR+ leukemic cells infiltration in double-treated mice compared with single- and vehicle-treated mice. Samples were collected from euthanized animals with signs of disease from vehicle- (mean = 18 days), dasatinib- (mean = 24 days), ibrutinib- (mean = 30 days), and dasatinib + ibrutinib– (mean = 37 days) treated animals. Objective lens: 20×; scale bar: 200 μm. GFP positivity was measured using the QuPath software after transformation of images to DAB and staining intensity quantified by a single cutoff threshold of 0.20. (D) The phosphorylation status of p-PLCG2 (Tyr 753) and p-BTK (Tyr 223) of BM murine cells by phospho-specific flow cytometry. The phosphorylation of both PLCG2 and BTK decrease significantly in the BM isolated from dasatinib + ibrutinib–treated mice. Fold MFI change of dasatinib-, ibrutinib-, and dasatinib + ibrutinib–treated mice compared with vehicle. Data represent mean of 3 independent experiments + SEM. n.s., not significant; ∗P < .01; ∗∗P < .001; ∗∗∗P < .0001.
Immunophenotypic analysis of GFP+ leukemia cells reveal significant differences on CD117 expression between E2A-PBX1+/preBCR+ #159 leukemia cells growth in vitro (CD117−) and #159 leukemia cells derived from secondary transplantation experiments and in vivo treatments (CD117+). We speculate that the change in CD117 expression between in vitro and in vivo cell growth might be explained by interactions of leukemic cells with microenvironment (that is presence of stem cell factor in vivo and not in in vitro culture). No difference was found in the immunophenotype from leukemia cells derived from mice treated with dasatinib, ibrutinib, or dasatinib + ibrutinib compared with vehicle-treated mice (supplemental Figure 16).
Hence, we further validated the in vivo effect of dasatinib in combination with ibrutinib on total and phosphorylated PLCG2 and BTK proteins in bone marrow and spleen-infiltrating E2A-PBX1+/preBCR+-GFP+ leukemic cells by phospho-flow. The infiltrating bone marrow and spleen GFP+ cells showed a significant decrease in both pPLCG2 (Tyr 753) and pBTK (Tyr 223) after the combination treatment compared with vehicle and single treatments after in vivo treatment with BTKis and dasatinib (Figure 3D; supplemental Figure 17A-B). A significant decrease in total and phosphorylated BTK and PLCG2 proteins was detected in dasatinib + ibrutinib- compared withvehicle-treated murine E2A-PBX1+/preBCR+ ALL isolated from spleens from treated mice, similar to our in vitro experiments (supplemental Figure 17A-B). These results confirmed the effect of BTKi and dasatinib on pre-BCR signaling pathway in vivo at the protein level
In vivo efficacy of combination treatment of dasatinib and BTKi on CNS-infiltrating murine E2A-PBX1+/preBCR+ ALL cells
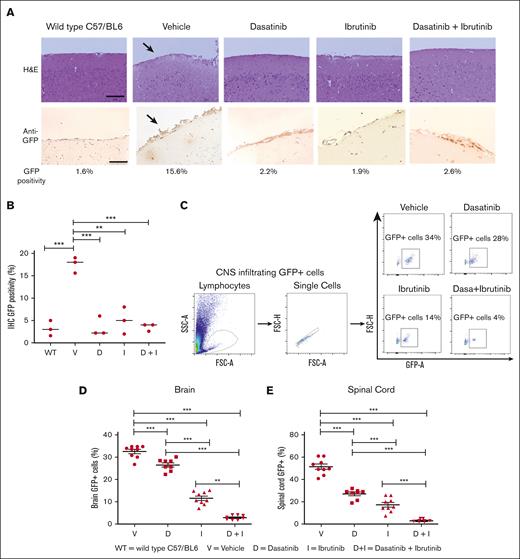
As described previously, both dasatinib and BTKi can penetrate the blood-brain barrier, showing promising results in managing CNS-infiltrating leukemia and lymphomas.22,30-32,35 We hypothesized that the combination of dasatinib with BTKi may be more effective in reducing CNS-infiltrating ALL cells. Therefore, we immune-stained brain sections with anti-GFP antibody measuring GFP positivity by staining intensity in the immunohistochemistry. A significant reduction of GFP intensity from 15% in CNS samples of vehicle-treated to 3% to 5% in dasatinib-, ibrutinib-, and dasatinib-ibrutinib-treated animals was observed (Figure 4A-B). Hence, we quantified the percentage of CNS-infiltrating GFP+ leukemic cells by flow cytometry. Interestingly, GFP+ infiltrating leukemic cells were reduced to 4% in CNS in mice treated with dasatinib and ibrutinib, compared with the vehicle (mean 50%) or single treatments (about 20%), demonstrating superior efficacy of combination treatment for CNS-infiltrating leukemia cells (Figure 4C-E).
Decrease of CNS infiltration of leukemia cells by combination therapy with dasatinib and BTKi. (A) Upper panels, images of mouse brain rostral leptomeninges slices from WT C57/BL6 mice, vehicle-, dasatinib-, ibrutinib-, and dasatinib + ibrutinib–treated mice tissues, stained using the hematoxylin and eosin (H&E) method. Vehicle-treated mice show an increased level of leukemic infiltration (arrow) compared with the dasatinib-, ibrutinib-, and dasatinib + ibrutinib–treated mice. Samples were collected from sacrificed animals with signs of disease from vehicle- (mean = 18 days), dasatinib (mean = 24 days), ibrutinib (mean = 30 days), and dasatinib + ibrutinib (mean = 37 days) treated animals. Objective lens: 40×; scale bar: 50 μm. Lower panels, immunohistochemical staining of CNS sections with anti-GFP antibody showing less GFP+ E2A-PBX1+/preBCR+ leukemic cells infiltration in double- and single-treated mice compared with vehicle-treated mice. Arrows pointing at leukemic CNS infiltration. Unstained CNS sections are showed. Objective lens: 20×; scale bar: 200 μm. (B) GFP positivity from 3 representative mice of each group was estimated after transformation of images to DAB and GFP staining intensity quantified by a single cutoff threshold of 0.20. Statistical analysis was performed by 1-way analysis of variance (ANOVA), Dunnett multiple comparison test. (C) Single-cell suspensions from fresh tissue from both brain and spinal cord after cutting into small pieces by scalpel and resuspended in a cell dissociation solution were used for quantification of GFP+ cells in flow cytometry. Panels show the gating strategy for phospho-flow cytometry staining for GFP+ murine E2A-PBX1+/pre-BCR+ leukemia cells from vehicle-, dasatinib-, ibrutinib-, and dasatinib + ibrutinib–treated mice. Gating was performed on lymphocytes, single cells, and GFP+ cells. The frequency of GFP+ murine E2A-PBX1+/preBCR+ leukemia cells decrease in the combination treatment. (D-E) Frequency of GFP+ murine leukemia infiltrating (D) the brain and (E) the spinal cord of C57BL/6 WT, mice. Each symbol represents an individual mouse. Statistical analysis was performed by 1-way ANOVA, Dunnett multiple comparison test. Scatter dot plots represent mean ± SEM. ∗∗P < .01; ∗∗∗P < .0001. IHC, immunohistochemistry; WT, wild type.
Decrease of CNS infiltration of leukemia cells by combination therapy with dasatinib and BTKi. (A) Upper panels, images of mouse brain rostral leptomeninges slices from WT C57/BL6 mice, vehicle-, dasatinib-, ibrutinib-, and dasatinib + ibrutinib–treated mice tissues, stained using the hematoxylin and eosin (H&E) method. Vehicle-treated mice show an increased level of leukemic infiltration (arrow) compared with the dasatinib-, ibrutinib-, and dasatinib + ibrutinib–treated mice. Samples were collected from sacrificed animals with signs of disease from vehicle- (mean = 18 days), dasatinib (mean = 24 days), ibrutinib (mean = 30 days), and dasatinib + ibrutinib (mean = 37 days) treated animals. Objective lens: 40×; scale bar: 50 μm. Lower panels, immunohistochemical staining of CNS sections with anti-GFP antibody showing less GFP+ E2A-PBX1+/preBCR+ leukemic cells infiltration in double- and single-treated mice compared with vehicle-treated mice. Arrows pointing at leukemic CNS infiltration. Unstained CNS sections are showed. Objective lens: 20×; scale bar: 200 μm. (B) GFP positivity from 3 representative mice of each group was estimated after transformation of images to DAB and GFP staining intensity quantified by a single cutoff threshold of 0.20. Statistical analysis was performed by 1-way analysis of variance (ANOVA), Dunnett multiple comparison test. (C) Single-cell suspensions from fresh tissue from both brain and spinal cord after cutting into small pieces by scalpel and resuspended in a cell dissociation solution were used for quantification of GFP+ cells in flow cytometry. Panels show the gating strategy for phospho-flow cytometry staining for GFP+ murine E2A-PBX1+/pre-BCR+ leukemia cells from vehicle-, dasatinib-, ibrutinib-, and dasatinib + ibrutinib–treated mice. Gating was performed on lymphocytes, single cells, and GFP+ cells. The frequency of GFP+ murine E2A-PBX1+/preBCR+ leukemia cells decrease in the combination treatment. (D-E) Frequency of GFP+ murine leukemia infiltrating (D) the brain and (E) the spinal cord of C57BL/6 WT, mice. Each symbol represents an individual mouse. Statistical analysis was performed by 1-way ANOVA, Dunnett multiple comparison test. Scatter dot plots represent mean ± SEM. ∗∗P < .01; ∗∗∗P < .0001. IHC, immunohistochemistry; WT, wild type.
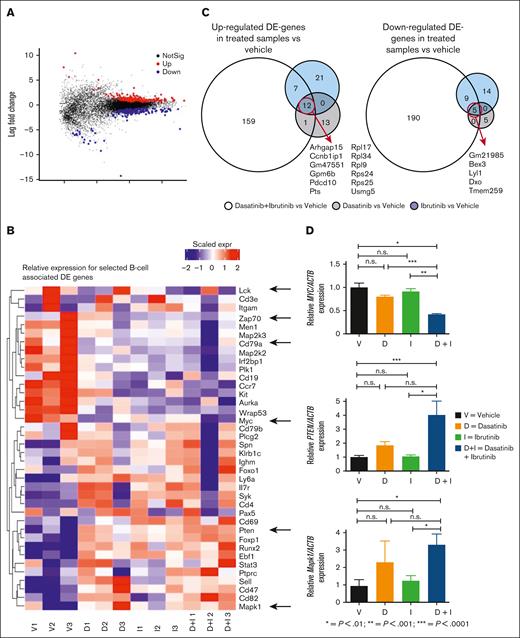
Transcriptomic analysis revealed crucial pathways for resistance in CNS-infiltrating E2A-PBX1+/preBCR+ ALL cells
To elucidate mechanism of resistance to BTKi and dasatinib in CNS-infiltrating leukemia cells and to investigate the CNS homing of E2A-PBX1/preBCR+ ALL cells, we performed global transcriptome analysis by RNA sequencing. Bioinformatics analysis identified 349 genes differentially regulated (159 upregulated and 190 downregulated; P < .05) in the CNS-infiltrating leukemia cells after treatment with the combination of dasatinib with ibrutinib compared with vehicle-treated mice (Figure 5A-C; supplemental Figure 18; supplemental Tables 3-6). Notably, the phosphatase and tensin homolog deleted on chromosome 10 (Pten) (log fold change +0.86; P = .044), the Mitogen-Activated Protein Kinase 1 (Mapk1/Erk2), (log fold change +0.43; P = .043), and the proto-oncogene Myc, which play an important role in ALL, (log fold change −0.9; P = .057) were subsequently validated by qPCR (Figure 5D). Other genes previously involved in the pathophysiology and CNS infiltration in E2A-PBX1+/preBCR+ as Lck, Zap70, and Cd79a,13,15,29 showed a trend for decreased expression under the treatment with BTKi alone or in combination with dasatinib (Figure 5B).
RNAseq of E2A-PBX1+/preBCR+ leukemia CNS-infiltrating cells compared across treatments. Bulk RNA sequencing of FACS-sorted CNS-infiltration E2A-PBX1-GFP+ leukemic cells from vehicle- (n = 3), dasatinib- (n = 3), ibrutinib- (n = 3), and dasatinib + ibrutinib–treated mice (n = 3) was performed. (A) MA plot displaying 349 most significant genes (P < .05) between all the treatments. (B) Heat map showing BCR–associated differential expressed (DE) genes. Myc (P = .06) is downregulated in the double-treated mice compared with vehicle-treated mice. A trend for decreased expression of BCR-associated genes Lck, Zap70, and Cd79a showed a trend for decreased expression under the treatment with BTKi alone or in combination with dasatinib. In contrast, Pten (P < .05) and Mapk1/ERK2 (P < .05) are upregulated in the double-treated mice compared with vehicle-treated mice. (C) Venn diagrams showing the most significant in-common up- and downregulated genes between the treatments. (D) Validation of Pten, Myc, and Mapk1 genes by RT-qPCR. Actb was used as a housekeeping gene. The experiment was performed in triplicate. Bars represent the mean and error bars, the SEM, of 3 independent experiments.
RNAseq of E2A-PBX1+/preBCR+ leukemia CNS-infiltrating cells compared across treatments. Bulk RNA sequencing of FACS-sorted CNS-infiltration E2A-PBX1-GFP+ leukemic cells from vehicle- (n = 3), dasatinib- (n = 3), ibrutinib- (n = 3), and dasatinib + ibrutinib–treated mice (n = 3) was performed. (A) MA plot displaying 349 most significant genes (P < .05) between all the treatments. (B) Heat map showing BCR–associated differential expressed (DE) genes. Myc (P = .06) is downregulated in the double-treated mice compared with vehicle-treated mice. A trend for decreased expression of BCR-associated genes Lck, Zap70, and Cd79a showed a trend for decreased expression under the treatment with BTKi alone or in combination with dasatinib. In contrast, Pten (P < .05) and Mapk1/ERK2 (P < .05) are upregulated in the double-treated mice compared with vehicle-treated mice. (C) Venn diagrams showing the most significant in-common up- and downregulated genes between the treatments. (D) Validation of Pten, Myc, and Mapk1 genes by RT-qPCR. Actb was used as a housekeeping gene. The experiment was performed in triplicate. Bars represent the mean and error bars, the SEM, of 3 independent experiments.
In vitro efficacy of pharmacologic targeted therapy with dasatinib in combination with BTKi in primary ALL samples
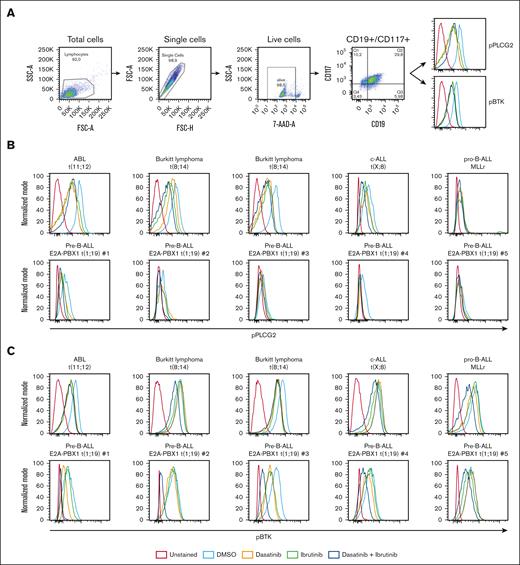
To investigate the effect of the combination of dasatinib and BTKi in primary ALL samples, we performed phospho-flow assessing pPLCG2 (Tyr 753) and pBTK (Tyr 223; Figure 6A). In gated CD19+ CD117+ blasts, the basal levels of pPLCG2 were relatively increased in t(11;12) and in 2 samples from patients with Burkitt lymphoma/leukemia compared with patients with c-ALL and pro–B-ALL. After treatment with dasatinib and ibrutinib, a significant decrease of pBTK and pPLCG2 levels in all samples was observed compared with vehicle-treated samples. In 5 primary pre–B-ALL E2A-PBX1+ t(1;19) samples, we observed a slight decrease of pPLCG2 and a more significant decrease of pBTK after the combination treatment with dasatinib and ibrutinib (Figure 6B-C; supplemental Figure 19).
In vitro effect of combined therapy with dasatinib and BTKi on primary ALL samples. (A) Flow cytometric analysis of primary samples aiming to detect the phosphorylation status of PLCG2 and BTK after 30 minutes of treatment with vehicle (DMSO), dasatinib, ibrutinib, and dasatinib + ibrutinib. Gating strategy on lymphocytes, single cells, 7AAD, CD19+, and CD117+. One representative histogram per antibody is showed. (B-C) Histogram represents the phosphorylation of (B) PLCG2 and (C) BTK in primary ALL samples with different karyotypes, after treatment with vehicle, ibrutinib, dasatinib, and dasatinib + ibrutinib treatment.
In vitro effect of combined therapy with dasatinib and BTKi on primary ALL samples. (A) Flow cytometric analysis of primary samples aiming to detect the phosphorylation status of PLCG2 and BTK after 30 minutes of treatment with vehicle (DMSO), dasatinib, ibrutinib, and dasatinib + ibrutinib. Gating strategy on lymphocytes, single cells, 7AAD, CD19+, and CD117+. One representative histogram per antibody is showed. (B-C) Histogram represents the phosphorylation of (B) PLCG2 and (C) BTK in primary ALL samples with different karyotypes, after treatment with vehicle, ibrutinib, dasatinib, and dasatinib + ibrutinib treatment.
Discussion
The cytoplasmic protein tyrosine kinase BTK is an essential component of the pre-BCR signaling pathway, on which lymphoid cells are dependent for development, proliferation and survival.34 Several BTKi have shown antileukemic activity interfering with the mechanisms of malignant B-cell pathophysiology.36 Treatments with BTKi or dasatinib have been shown to be effective in many different B-cell malignancies.17,18,37-39
Despite encouraging results and breakthrough treatments for ALL, CNS relapse of leukemia remains an unmet clinical need.10,11 In this study, we investigated the role of BTK as key protein for proliferation and survival, as well as BTK inhibition as a strategy to increase dasatinib sensitivity in E2A-PBX1+/preBCR+ ALL, particularly in CNS-infiltrating leukemia cells.
Although dasatinib has promising preclinical efficacy in the treatment of preBCR+ ALL, including E2A-PBX1+ leukemias,8,12,19-21 its efficacy is limited by the development of resistance in vitro and in vivo.24,29 Using a shRNA library screening approach, targeting thousands of genes in human E2A-PBX1+ leukemia cells,23,24 the BTK gene was identified by bioinformatic analysis as a key gene involved in dasatinib sensitivity and on which B-ALL cells are dependent for proliferation. We validated and confirmed the role of BTK as a regulator of proliferation and increasing dasatinib sensitivity of E2A-PBX1+/preBCR+ RCH-ACV cells by competition assays after single shRNA knockdown assays.
Aiming to improve the efficacy of the treatment of CNS-infiltrating E2A-PBX1+/preBCR+ ALL cells, we took advantage of the pharmacological properties of dasatinib and BTKis (ibrutinib, acalabrutinib, and zanubrutinib) to cross the blood-brain barrier and establish combination therapies, which are very effective for the treatment of systemic and CNS-infiltrating disease.
Because of the oncogenic role of BTK in B-cell malignancies, several BTKi have been developed over time are currently approved for patient treatment.37-39 In this study, we particularly focused our attention on the BTKi ibrutinib, acalabrutinib, and zanubrutinib. Ibrutinib is a selective and irreversible inhibitor of BTK, and a promising therapeutic option for preBCR+ or TCF3-rearranged ALL.21 Acalabrutinib and zanubrutinib are potent BTKi, with improved selectivity and lower off-target effects in comparison with ibrutinib.40,41
As expected, the in vitro combination of dasatinib with BTKi displayed at least an additive antiproliferative effect on E2A-PBX1+/preBCR+ human RCH-ACV and 697 cells and murine #159 cells. In addition, the combination therapies led to an in vitro decrease in PLCG2 and BTK expression levels and affected the phosphorylation status of PLCG2 and BTK, as reduced in human and murine E2A-PBX1+/preBCR+ cell lines and spleen-infiltrating ALL cells. Our findings imply that the pharmacological combinations of dasatinib and BTKi cause a high impact on B-cell growth and confirmed BTK as a key regulator of dasatinib sensitivity in vitro.
In addition, using a secondary transplanted E2A-PBX1+ ALL mouse model as a preclinical platform, we validated the therapeutic in vivo efficacy of dasatinib in combination with BTKis. We observed a statistically significant prolonged disease-free survival of mice treated with dasatinib combined with BTKi, implying that the combination therapies might have clinical efficacy in the treatment of a subset of pre–B-ALL. Of note, changes in levels and distribution of E2A-PBX1+/preBCR+ GFP+ infiltrating leukemia cells were visualized in several organs, including the CNS by immunohistochemistry and flow cytometry, in which the combined treatment therapy drastically decreased the percentage of tumor-infiltrating cells. Of note, treatment with BTKi and dasatinib was limited on time (20 days) and we used a single dose proven to be effective. Mice receiving the combined treatment start getting sick and showing CNS-infiltrating ALL cells beyond this period of time. We suggest that continuous treatment with the combination therapy till disease progress or relapse and assessment of maximal tolerated dose in single and combination treatments might improve the clinical efficacy in future clinical trials.
Because of the short time between treatment stop and leukemia development in dasatinib and BTKi-treated mice, we hypothesize that dasatinib and BTKi treatments were able to inhibit efficiently leukemia cell proliferation as long as the treatments were given. Nevertheless, resistant and/or very aggressive leukemic subclones might have been already present under the treatment and might have expanded very quickly after treatment withdrawal. However, we did not confirm dasatinib- and BTKi-resistance in in vitro experiments in leukemia cells from sacrificed mice. These data suggest a continuous treatment with dasatinib and BTKi as effective schedule for possible future clinical trials in human ALL.
Our results show that the combination therapy of dasatinib and BTKis has a drastic impact on CNS-infiltrating E2A-PBX1+/preBCR+ leukemia cells in our mouse model. These data are consistent with the previously discussed in vitro efficacy of the combined therapies on overcoming the dasatinib resistance in E2A-PBX1+/preBCR+ ALL,29 highlighting the impact on CNS-infiltrating leukemic cells.
Moreover, the data from the RNA sequencing analysis showed that the tumor suppressor Pten, which plays a key role in development and maintenance of CNS42 and chemotherapy resistance in T-ALL,43 is upregulated in the CNS of vehicle-treated mice compared with mice treated with dasatinib + ibrutinib. The upregulation of Mapk1/Erk2, involved in CNS-ALL cells survival, in the double-treated mice compared with vehicle-treated mice may indicate therapy resistant mechanism, in which the inhibition of pre-BCR signaling pathway by dasatinib and ibrutinib leads to alternative activation of MAPK signaling pathway.44 The proto-oncogene Myc is instead downregulated in CNS after the double treatment compared with vehicle treatment, suggesting effective inhibition of this oncogenic signaling pathway in CNS-infiltrating cells. We speculate, that E2A-PBX1+/preBCR+ ALL cells resistant to combined treatment with BTKi + dasatinib arising in CNS are dependent on alternative pathways as MAPK signaling pathways, which might be targeted by small-molecule inhibitors as MEK inhibitors.
One of our goals in this study was not only to analyze global transcriptional changes at the messenger RNA level but also at the protein level. The results of our in vitro/in vivo analysis assessed by western blots and phospho-specific flow cytometry indicated a decrease not only on phosphorylated but also on total proteins involved in the pre-BCR signaling pathway as BTK and PLCG2 under treatment with BTKi and dasatinib.
Furthermore, the combination of dasatinib and BTKi also shows activity on reducing pBTK and pPLCG2 in primary ALL with different karyotypes including E2A-PBX1+/preBCR+ ALL, suggesting therapeutic activity of this combination therapy beyond E2A-PBX1+/preBCR+ ALL. In summary, our findings demonstrate promising preclinical in vitro and in vivo efficacy of dasatinib and BTKi, especially in CNS-infiltrating ALL cells, using mouse, human cell lines, and human primary E2A-PBX1+/preBCR+ ALL.
Acknowledgments
The authors acknowledge the Lighthouse Core Facility for their assistance with flow cytomety analysis and quantitative polymerase chain reaction assays, and all members of the Duque laboratory for assistance and helpful comments. The authors also acknowledge Bioinformatics Center, University of Eastern Finland, Kuopio, Finland for computational resources. The authors thank C. Duque-Afonso for graphic design.
This work was supported in part by grants from the German Research Foundation (DFG, DU 1287/5-1) (J.D.-A.), the National Institutes of Health, National Cancer Institute (NIH; CA214888/CA/NCI) (M.L.C.), and the William Lawrence and Blanche Hughes Foundation (M.L.C.). J.D.-A. and M.L.C. received support from the Lucile Packard Foundation for Children's Health, the Child Health Research Institute, and the Stanford NIH-National Center for Advancing Translational Sciences-Clinical and Translational Science Awards (grant #UL1 TR001085). J.D.-A. also received support from the Berta Ottenstein-Program for Advanced Clinician Scientists, Faculty of Medicine, University of Freiburg. J.D.-A. and M.H. received funding from ERA Per Med. JTC 2018 “GEPARD” project.
Authorship
Contributions: G.G. planned and performed the experiments; J.D.-A. designed the experiments; G.G. and T.P. performed mouse experiments and performed the phospho-flow on primary samples; G.G. and A.C. performed immunohistochemical stainings; M.P., D.W.M., and R.S. prepared the paraffin-embedded mice samples blocks; M. Lübbert and T.M. provided reagents; G.G. and T.M. prepared the RNA samples for sequencing; M.H., M. Lahnalampi, and M.V. analyzed the RNA sequencing data; G.G. and M.A.-M. performed the phospho-specific flow cytometry (phospho-flow); M.C.B., K.H., and D.W.M. designed, performed, and analyzed the data from short hairpin RNA library screen assay; L.L., G.C., M. Schrappe, M. Stanulla, and J.D. provided patient samples; M.L.C. provided mouse E2A-PBX1+ leukemia cells; G.G. and J.D.-A. wrote the manuscript, prepared the figures, and analyzed the data of primary ALL; and all authors have access to clinical data, revised the final version of the manuscript, and read and agreed to the published version of the manuscript.
Conflict-of-interest disclosure: J.D.-A. received speakers honoraria from Riemser, Lilly, Ipsen, Roche, Amgen, and AstraZeneca, and travel support from AstraZeneca, Ipsen, Gilead, and Sobi. M.L. received research support from Janssen-Cilag. The remaining authors declare no competing financial interests.
Correspondence: Jesús Duque-Afonso, Department of Hematology/Oncology/Stem Cell Transplantation, Faculty of Medicine University of Freiburg Medical Center, Hugstetterstr. 55, 79106 Freiburg, Germany; email: jesus.duque.afonso@uniklinik-freiburg.de.
References
Author notes
The bulk RNA sequencing data have been deposited in the Gene Expression Omnibus database (accession number GSE221238, token clkhwucmttejpaf at http://ncbi.nlm.nih.gov/geo).
The data generated during and/or analyzed during this study are available upon reasonable request from the corresponding author, Jesús Duque-Afonso (jesus.duque.afonso@uniklinik-freiburg.de.
The full-text version of this article contains a data supplement.