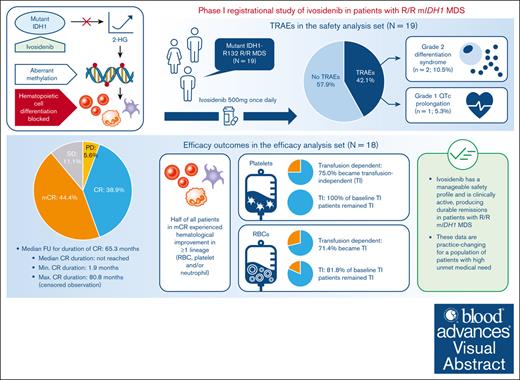
Key Points
Ivosidenib resulted in a CR rate of 38.9% and an OR rate of 83.3% in mIDH1 R/R MDS; median duration of response was not reached.
Median OS in this R/R MDS cohort was ∼36 months; ∼75% of RBC- and platelet-transfusion–dependent patients became transfusion independent.
Visual Abstract
Ivosidenib is a first-in-class mutant isocitrate dehydrogenase 1 (mIDH1) inhibitor with efficacy and tolerability in patients with advanced mIDH1 hematologic malignancies, leading to approval in frontline and relapsed/refractory (R/R) mIDH1 acute myeloid leukemia. We report final data from a phase 1 single-arm substudy of once-daily ivosidenib in patients with R/R mIDH1 myelodysplastic syndrome (MDS) after failure of standard-of-care therapies. Primary objectives were to determine safety, tolerability, and clinical activity. The primary efficacy end point was the complete remission (CR) + partial remission (PR) rate. Nineteen patients were enrolled; 18 were included in the efficacy analysis. Treatment-related adverse events occurred in 8 (42.1%) patients, including a grade 1 QT interval prolongation in 1 (5.3%) patient and grade 2 differentiation syndrome in 2 (10.5%) patients. Rates of CR + PR and objective response (CR + PR + marrow CR) were 38.9% (95% confidence interval [CI], 17.3-64.3) and 83.3% (95% CI, 58.6-96.4), respectively. Kaplan-Meier estimates showed a 68.6% probability of patients in CR achieving a remission duration of ≥5 years, and a median overall survival of 35.7 months. Of note, 71.4% and 75.0% baseline red blood cell (RBC)- and platelet-transfusion-dependent patients, respectively, became transfusion independent (TI; no transfusion for ≥56 days); 81.8% and 100% of baseline RBC and platelet TI patients, respectively, remained TI. One (5.3%) patient proceeded to a hematopoietic stem cell transplant. In conclusion, ivosidenib is clinically active, with durable remissions and a manageable safety profile observed in these patients. This trial was registered at www.ClinicalTrials.gov as #NCT02074839.
Introduction
Myelodysplastic syndromes (MDSs) are a heterogenous group of clonal stem cell disorders characterized by ineffective hematopoiesis, resulting in peripheral blood cytopenias and an increased risk of transformation to acute myeloid leukemia (AML).1,2 Standard-of-care therapy for MDS is determined by disease risk and patient prognosis, according to the revised International Prognostic Scoring System (IPSS-R) and, more recently, the inclusion of somatic mutational profiles (molecular IPSS [IPSS-M]).3,4 Patients with low-risk MDS are managed with supportive care and agents that improve cytopenias, whereas patients with high-risk MDS are generally treated with hypomethylating agent (HMA)-based therapies.2 Although an overall response rate of ∼50% is observed with first-line HMA treatment, complete remissions (CRs) are uncommon, therapeutic benefits are transient, and progression is near universal within a period of months to years.5,6 Therapeutic options for HMA-resistant or -refractory MDS are scarce, with no approved standard-of-care second-line treatments currently available, and with poor overall survival (OS) outcomes demonstrating an unmet need for these patients.6-8
Somatic mutations in the isocitrate dehydrogenase 1 (IDH1) gene have been reported in ∼3% of patients with MDS.9IDH1 mutations are often associated with high-risk MDS, neutropenia, and elevated bone marrow blast counts, and patients with mutant IDH1 (mIDH1) MDS typically have a worse prognosis, including a higher risk of progression to AML, compared with patients with wild-type IDH1 MDS.10-12
IDHs are homodimeric enzymes involved in numerous cellular processes, including DNA modification and adaptation to hypoxia, and catalysis of the oxidative decarboxylation of isocitrate to α-ketoglutarate (α-KG).9IDH1 mutations most often arise at a single amino acid residue, arginine 132, within the active site of IDH1.13,14 The IDH1 mutation reduces the ability to convert isocitrate to α-KG, and instead mIDH1 gains a novel capacity to catalyze the nicotinamide adenine dinucleotide phosphate-dependent reduction of α-KG to the R enantiomer of 2-hydroxyglutarate (2-HG), an oncometabolite.9,15 2-HG competitively inhibits various key epigenetic regulators, including histone lysine demethylases and members of the ten-eleven-translocation family of 5-methylcytosine hydroxylases.9 Inhibition of these enzymes leads to a hypermethylation signature that alters gene expression, thereby preventing differentiation of hematopoietic stem cells into mature blood cells and contributing to oncogenesis.9,16 Direct inhibition of mIDH1 suppresses production of 2-HG, thereby enabling blast cell differentiation, potentially reducing the rate of oncogenic transformation.17,18
Ivosidenib (AG-120), a first-in-class mIDH1 inhibitor, has been approved by the US Food and Drug Administration as monotherapy or in combination with azacitidine in adult patients with newly diagnosed mIDH1 AML aged >75 years or who have comorbidities that preclude use of intensive induction. Ivosidenib monotherapy has also been approved for adult patients with mIDH1 relapsed/refractory (R/R) AML and recently for patients with R/R MDS.19-22
In the first-in-human study of ivosidenib for patients with mIDH1 advanced hematologic malignancies (ClinicalTrials.gov identifier: NCT02074839), 12 patients with R/R MDS received ivosidenib 500 mg once daily.23 Based on encouraging safety and efficacy findings, including an overall response rate of 75%, the study was amended to enroll additional patients with R/R MDS.24,25 We herein report the final analysis of data for patients with R/R mIDH1 MDS.
Methods
Study design
We conducted a phase 1, open-label, single-arm, first-in-human multinational substudy to assess ivosidenib for patients with mIDH1 R/R MDS. This substudy was performed in compliance with the International Council for Harmonization Good Clinical Practice Guideline, in accordance with the Declaration of Helsinki, and was approved by the relevant institutional review boards. All patients provided written informed consent before screening and enrollment.
Patient inclusion/exclusion criteria
Key inclusion criteria were an age of ≥18 years; documented mIDH1-R132 MDS; and R/R MDS defined as MDS that has relapsed (according to modified International Working Group [mIWG] 2006 criteria) after, or is refractory to, ≥1 of the following: high-intensity chemotherapy or intensive combination chemotherapy with investigational agents, novel combinations of standard treatments, hematopoietic stem cell transplant (HSCT), and/or HMA-based therapy. MDS refractory to HMA treatment per mIWG 2006 criteria was defined as the absence of CR, marrow CR (mCR), partial remission (PR), or hematologic improvement (HI) after a minimum of 4 cycles or if patients had disease progression before 4 cycles of HMA treatment.26 Key exclusion criteria were prior treatment with an mIDH1 inhibitor; HSCT within 60 days of study start; documented AML (≥20% bone marrow or peripheral blood blasts); and treatment with systemic anticancer therapy, or radiotherapy, or an investigational agent within 14 days before first dose of study drug.
IPSS-R calculation
IPSS-R scores were calculated at diagnosis and at screening. Please see the supplemental Materials for further details regarding IPSS-R calculations.3
Study schedule and treatment
Patients underwent a screening period of 28 days before day 1 and then received ivosidenib orally at the recommended phase 2 dose of 500 mg once daily from days 1 through 28 in 28-day cycles until disease progression, development of unacceptable toxicity, HSCT, or another prespecified end-of-treatment criterion. There was a 28-day safety follow-up period after treatment followed by a survival follow-up period.
Objectives and end points
The primary objectives were to determine the safety, tolerability, and clinical activity of ivosidenib in patients with mIDH1 R/R MDS. Adverse events (AEs) of special interest were differentiation syndrome, leukocytosis, and corrected QT interval (QTc) prolongation. The primary efficacy end point was the CR + PR rate. Key secondary efficacy end points were duration of CR + PR, acquisition/maintenance of transfusion independence, time to transfusion independence, and duration of transfusion independence. Transfusion independence was defined as no transfusion (red blood cells [RBCs] or platelets) for at least 56 days. Other efficacy end points included mCR, objective response rate (CR + PR + mCR), HI, and OS. Responses and HI were assessed according to mIWG 2006 criteria, the details of which are provided in the supplemental Materials. Patients needed to meet certain pretreatment criteria to be evaluated for HI (Table 1).26 For those patients alive at data cutoff, OS was reported as a censored observation.
Efficacy outcomes in the efficacy analysis set, according to IWG 2006 response criteria
| Efficacy outcomes . | MDS substudy efficacy analysis set (N = 18) . | 95% CI . |
|---|---|---|
| Primary end point, n (%) | ||
| CR + PR | 7 (38.9) | (17.3-64.2) |
| Secondary end points | ||
| Time to CR + PR, median mo (min, max) | 1.87 (1.0, 5.6) | (58.6-96.4) |
| Duration of CR + PR,∗ median mo (min, max) | NR (1.9, NR) | |
| Probability of patients maintaining CR + PR,∗ n (%) | ||
| At 3 mo | 85.7 | |
| At 24 mo | 68.6 | |
| At 60 mo | 68.6 | |
| Best overall response | ||
| ORR† | 15 (83.3) | (58.6-96.4) |
| CR | 7 (38.9) | (17.3-64.3) |
| PR | 0 | (0.0-18.5) |
| mCR | 8 (44.4) | (21.5-96.2) |
| SD | 2 (11.1) | (1.4-34.7) |
| PDis | 1 (5.6) | (0.1-27.3) |
| Time to OR,† median months (min, max) | 0.99 (0.9, 4.6) | |
| HI in non-CR/PR patients, n (%) | ||
| Erythrocyte lineage | 2 (18.2)‡ | |
| Platelet lineage | 2 (25.0)§ | |
| Neutrophil lineage | 4 (57.1)‖ | |
| Any HI lineage | 4 (36.4)¶ | |
| OS,∗ median months, [95% CI] (min, max) | 35.7 [13.1, NE] (3.7#, 88.7#) | |
| OS rate,∗(%) | ||
| 1 y | 86.9 | |
| 3 y | 46.3 | |
| 5 y | 46.3 | |
| 7 y | 46.3 |
| Efficacy outcomes . | MDS substudy efficacy analysis set (N = 18) . | 95% CI . |
|---|---|---|
| Primary end point, n (%) | ||
| CR + PR | 7 (38.9) | (17.3-64.2) |
| Secondary end points | ||
| Time to CR + PR, median mo (min, max) | 1.87 (1.0, 5.6) | (58.6-96.4) |
| Duration of CR + PR,∗ median mo (min, max) | NR (1.9, NR) | |
| Probability of patients maintaining CR + PR,∗ n (%) | ||
| At 3 mo | 85.7 | |
| At 24 mo | 68.6 | |
| At 60 mo | 68.6 | |
| Best overall response | ||
| ORR† | 15 (83.3) | (58.6-96.4) |
| CR | 7 (38.9) | (17.3-64.3) |
| PR | 0 | (0.0-18.5) |
| mCR | 8 (44.4) | (21.5-96.2) |
| SD | 2 (11.1) | (1.4-34.7) |
| PDis | 1 (5.6) | (0.1-27.3) |
| Time to OR,† median months (min, max) | 0.99 (0.9, 4.6) | |
| HI in non-CR/PR patients, n (%) | ||
| Erythrocyte lineage | 2 (18.2)‡ | |
| Platelet lineage | 2 (25.0)§ | |
| Neutrophil lineage | 4 (57.1)‖ | |
| Any HI lineage | 4 (36.4)¶ | |
| OS,∗ median months, [95% CI] (min, max) | 35.7 [13.1, NE] (3.7#, 88.7#) | |
| OS rate,∗(%) | ||
| 1 y | 86.9 | |
| 3 y | 46.3 | |
| 5 y | 46.3 | |
| 7 y | 46.3 |
NR, not estimable; OR, objective response; ORR, objective response rate; PDis, progressive disease; SD, stable disease.
Kaplan-Meier estimate of duration of CR + PR.
OR comprised CR, PR, or mCR.
% based on number of patients with mCR and pretreatment hemoglobin <11 g/dL (N1 = 11).
% based on number of patients with mCR and pretreatment platelet count <100 × 109/L (N2 = 8).
% based on patients with mCR and pretreatment absolute neutrophil count <1.0 × 109/L (N3 = 7).
% based on patients with mCR and who satisfy the pretreatment criteria for HI-erythrocyte, HI-platelet, or HI-neutrophil (N4 = 11).
Censored observation.
Furthermore, translational, pharmacokinetic (PK), and pharmacodynamic (PD) analyses were performed. Translational analyses included baseline next-generation sequencing and longitudinal analysis of mIDH1 variant allele frequency (VAF). Next-generation sequencing results were used to retrospectively derive the IPSS-M score.
Statistical analysis
Investigator-assessed response rates were evaluated as a binomial proportion and presented with associated exact binomial 95% confidence interval (CI). A CR + PR rate with an exact binomial 95% CI with a lower bound that excluded 10% was considered clinically meaningful. Approximately 23 eligible patients (dose escalation/expansion population + substudy population) were planned based on testing a null CR + PR rate of 10% compared with a target CR + PR rate of 33%, with 80% power and a 2-sided α of 0.05. Based on efficacy results from the data analysis reported here, it was agreed that enrollment of 18 patients adequately supported the planned efficacy evaluation. Summaries were produced for patient disposition, demographic and baseline disease characteristics, efficacy, safety, PK, and PD, as appropriate.
Categorical data were summarized by frequency distributions (number and percentages of patients). Continuous data were summarized by descriptive statistics (mean, standard deviation, median, minimum, and maximum). Time-to-event end points were estimated using the Kaplan-Meier method. Point estimates and 95% CIs were provided as appropriate; and estimates of the median and other quantiles, as well as individual time points (such as 3-, 6-, and 12-month rates), were generated.
Supplemental materials
Please see “Methods” of the supplemental Materials for additional inclusion/exclusion criteria; further details on the study schedule; the safety, efficacy, translational (including baseline IPSS-M calculations), and PK/PD analyses; as well as descriptions of the analysis sets evaluated in this substudy; a comprehensive list of end points (supplemental Table 1); and a schedule of study assessments (supplemental Table 2).
This substudy was performed in compliance with the International Council for Harmonization Good Clinical Practice Guideline, in accordance with the Declaration of Helsinki and was approved by the relevant institutional review boards. All patients provided written informed consent before screening and enrollment.
Results
Patient demographics and baseline characteristics
As of 26 September 2022, 19 patients with R/R MDS had been enrolled and were included in the full analysis set. One patient had MDS that was refractory to an investigational agent and had not received prior intensive chemotherapy or HMAs. Therefore, the patient did not meet an inclusion criterion for the MDS substudy and was excluded from the efficacy analysis but was included in the safety analysis.
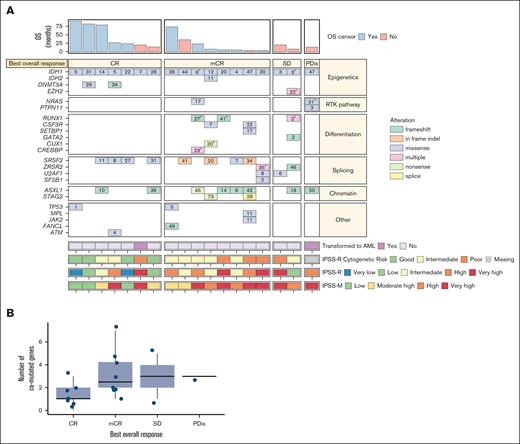
Most patients were male (78.9%), the median age was 73.0 years (range, 52-82 years) and a high proportion (78.9%) had received prior HMAs (Table 2; Figure 1). Of 18 patients with an available IPSS-R score at screening, 77.8% had an IPSS-R score of >3 (intermediate risk or higher); 42.1% of patients had an IPSS-M score that was in the highest risk category (“very high”). IPSS-M scoring tended to upstage patients’ risk category vs their IPSS-R score at screening (Figure 2A). This aligns with restratification data reported after development of the IPSS-M model, whereby 74% of patients were upstaged and 26% were downstaged from their original IPSS-R risk category.4Table 2 provides further demographic and disease characteristics data.
Baseline patient demographics and disease characteristics for the full analysis set
| Characteristic . | (N = 19) . |
|---|---|
| Sex, n (%) | |
| Female | 4 (21.1) |
| Male | 15 (78.9) |
| Age, median (min, max), y | 73.0 (52, 82) |
| Race | |
| White | 15 (78.9) |
| Black or African American | 1 (5.3) |
| Not reported | 3 (15.8) |
| Ethnicity | |
| Hispanic or Latino | 1 (5.3) |
| Not Hispanic or Latino | 12 (63.2) |
| Not reported | 6 (31.6) |
| ECOG PS | |
| 0 | 5 (26.3) |
| 1 | 11 (57.9) |
| 2 | 3 (15.8) |
| Prior therapy, n (%) | |
| Intensive chemotherapy | 3 (15.8) |
| One line of HMA-based therapy | 14 (73.7) |
| Two lines of HMA-based therapy | 1 (5.3) |
| Investigational | 3 (15.8) |
| Other∗ | 1 (5.3) |
| IPSS-R score at initial diagnosis, n (%)† | |
| ≤1.5 (very low) | 0 |
| >1.5-3 (low) | 2 (10.5) |
| >3-4.5 (intermediate) | 6 (31.6) |
| >4.5-6 (high) | 2 (10.5) |
| >6 (very high) | 2 (10.5) |
| Unknown | 7 (36.8) |
| IPSS-R score at screening, n (%) | |
| ≤1.5 (very low) | 0 |
| >1.5-3 (low)‡ | 4 (21.1) |
| >3-4.5 (intermediate)§ | 8 (42.1) |
| >4.5-6 (high)‖ | 3 (15.8) |
| >6 (very high)¶ | 3 (15.8) |
| Unknown# | 1 (5.3) |
| IPSS-M score at screening, n (%)∗∗ | |
| Low | 3 (15.8) |
| Moderate low | 1 (5.3) |
| Moderate high | 4 (21.1) |
| High | 3 (15.8) |
| Very high | 8 (42.1) |
| WHO classification at screening, n (%) | |
| MDS with excess blasts (MDS-EB1)†† | 4 (21.1) |
| MDS with excess blasts (MDS-EB2)‡‡ | 1 (5.3) |
| MDS, with MLD (MDS-MLD) | 4 (21.1) |
| MDS, unclassifiable | 10 (52.6) |
| Cytogenetic result, n (%) | |
| Normal | 9 (47.4) |
| Abnormal | 9 (47.4) |
| Missing | 1 (5.3) |
| Characteristic . | (N = 19) . |
|---|---|
| Sex, n (%) | |
| Female | 4 (21.1) |
| Male | 15 (78.9) |
| Age, median (min, max), y | 73.0 (52, 82) |
| Race | |
| White | 15 (78.9) |
| Black or African American | 1 (5.3) |
| Not reported | 3 (15.8) |
| Ethnicity | |
| Hispanic or Latino | 1 (5.3) |
| Not Hispanic or Latino | 12 (63.2) |
| Not reported | 6 (31.6) |
| ECOG PS | |
| 0 | 5 (26.3) |
| 1 | 11 (57.9) |
| 2 | 3 (15.8) |
| Prior therapy, n (%) | |
| Intensive chemotherapy | 3 (15.8) |
| One line of HMA-based therapy | 14 (73.7) |
| Two lines of HMA-based therapy | 1 (5.3) |
| Investigational | 3 (15.8) |
| Other∗ | 1 (5.3) |
| IPSS-R score at initial diagnosis, n (%)† | |
| ≤1.5 (very low) | 0 |
| >1.5-3 (low) | 2 (10.5) |
| >3-4.5 (intermediate) | 6 (31.6) |
| >4.5-6 (high) | 2 (10.5) |
| >6 (very high) | 2 (10.5) |
| Unknown | 7 (36.8) |
| IPSS-R score at screening, n (%) | |
| ≤1.5 (very low) | 0 |
| >1.5-3 (low)‡ | 4 (21.1) |
| >3-4.5 (intermediate)§ | 8 (42.1) |
| >4.5-6 (high)‖ | 3 (15.8) |
| >6 (very high)¶ | 3 (15.8) |
| Unknown# | 1 (5.3) |
| IPSS-M score at screening, n (%)∗∗ | |
| Low | 3 (15.8) |
| Moderate low | 1 (5.3) |
| Moderate high | 4 (21.1) |
| High | 3 (15.8) |
| Very high | 8 (42.1) |
| WHO classification at screening, n (%) | |
| MDS with excess blasts (MDS-EB1)†† | 4 (21.1) |
| MDS with excess blasts (MDS-EB2)‡‡ | 1 (5.3) |
| MDS, with MLD (MDS-MLD) | 4 (21.1) |
| MDS, unclassifiable | 10 (52.6) |
| Cytogenetic result, n (%) | |
| Normal | 9 (47.4) |
| Abnormal | 9 (47.4) |
| Missing | 1 (5.3) |
EB, excess blasts; ECOG PS, Eastern Cooperative Oncology Group performance status; max, maximum; min, minimum; MLD, multilineage dysplasia; n, number of patients; N, total number of patients; SD, standard deviation; WHO, World Health Organization.
Patient received lenalidomide.
Six patients did not have an IPSS-R score at diagnosis due to being treated locally before being referred to the clinical trial site.
Karyotypes in the IPSS-R low-risk group were: 46, XX[20]; 46, XY[20]; 46 XY[20]; and normal male karyotype.
Karyotypes in the IPSS-R intermediate-risk group were: 46, XX [20]; 47,XY,+21[1]/46,XY,DEL(7)(Q22)[1]/46,XY[18]; 43,XY,+1,DER(1;15)(Q10;Q10),-5,-9,-12 (1)/46,XY,DUP(1)[19]; 46, XX[20]; unknown; 46,X,DEL(X)(Q26),T(1;17)(Q12;Q25),T(12;16)(Q15;P11.2)[4]/46,XX,T(3;16)(P21;P13.1)[2]/46,XX[7]//46,XY[2]; and normal male karyotype; 46,XY[4].
Karyotypes in the IPSS-R high-risk group were: 47,XY,+8[3]/46,XY[17] (20 male metaphases [3 abnormal and 17 normal] were analyzed; the previously reported clone remained in the bone marrow, indicating persistent disease); 47,XY,+8[6]/46,XY[14]; and 46, XY[20].
Karyotypes in the IPSS-R very-high-risk group were: 45,XY,−7[17]/46,XY,DER(7;18)(P10;Q10),+11[3] (20 abnormal male metaphases were analyzed; the previously reported abnormal clones remain fully predominant in the bone marrow indicating persistent disease); 46,XY,+1,DER(1;7)(Q10;P10)[3]; and 47,XX,+21[13]/46,XX[7] (abnormal result showing previously unreported abnormality).
The karyotype in the patient whose IPSS-R score was unknown at screening was 46,XY[20].
Derived retrospectively by internal sponsor review.
MDS-EB1: 5% to 9% of bone marrow cells, or 2% to 4% of blood cells, are blasts.
MDS-EB2: 10% to 19% of bone marrow cells, or 5% to 19% of blood cells, are blasts.
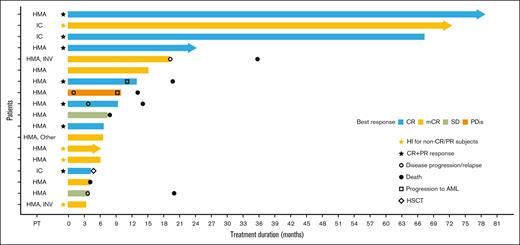
Treatment duration and response to ivosidenib. Swimmer plot of treatment duration and best overall response in the efficacy analysis set (N = 18)a. aOne patient was excluded from the efficacy analysis because of not meeting an inclusion criterion. IC, intensive chemotherapy; INV, investigational agent; PDis, progressive disease; PT, prior therapy; SD, stable disease.
Treatment duration and response to ivosidenib. Swimmer plot of treatment duration and best overall response in the efficacy analysis set (N = 18)a. aOne patient was excluded from the efficacy analysis because of not meeting an inclusion criterion. IC, intensive chemotherapy; INV, investigational agent; PDis, progressive disease; PT, prior therapy; SD, stable disease.
Response rates vs cytogenetic risk categories for the efficacy analysis set. (A-B) Heat map showing IPSS-R, IPSS-M, and IPSS-R cytogenetic risk categories, best overall response, baseline comutations, baseline VAF, and OS in the efficacy analysis set (A; N = 18) and distribution of number of comutated genes with best overall response (B; N = 18). Please note that the numbers within the boxes correspond to the VAF of the alteration. aIf a patient had multiple alterations in a gene, the largest VAF was shown. bThis patient did not have any of the comutations evaluated in the panel but did have a poor-risk karyotype, including monosomy 7. PDis, progressive disease; RTK, receptor tyrosine kinase; SD, stable disease.
Response rates vs cytogenetic risk categories for the efficacy analysis set. (A-B) Heat map showing IPSS-R, IPSS-M, and IPSS-R cytogenetic risk categories, best overall response, baseline comutations, baseline VAF, and OS in the efficacy analysis set (A; N = 18) and distribution of number of comutated genes with best overall response (B; N = 18). Please note that the numbers within the boxes correspond to the VAF of the alteration. aIf a patient had multiple alterations in a gene, the largest VAF was shown. bThis patient did not have any of the comutations evaluated in the panel but did have a poor-risk karyotype, including monosomy 7. PDis, progressive disease; RTK, receptor tyrosine kinase; SD, stable disease.
At the time of data cutoff, 4 (21.1%) patients remained on treatment and 15 (78.9%) had discontinued treatment. Reasons for discontinuation included disease progression (n = 6 patients [31.6%]); non–treatment-related AEs (TRAEs) (n = 2 [10.5%]; sepsis and fatigue); progression of a concomitant medical condition, treating physician’s decision, and withdrawal of consent (n = 1 [5.3%] for each category). One patient (5.3%) proceeded to an HSCT before data cutoff and another patient proceeded to an HSCT after data cutoff. One patient withdrew to take ivosidenib commercially, and another patient did not have recovery of platelet counts.
Of the 19 patients, 7 had died: 2 (10.5%) on study treatment, and 5 (26.3%) after study treatment completion. Median treatment duration was 9.3 months (range, 3.3-78.8 months) and the median follow-up period for OS analysis was 27.1 months (range, 3.7-88.7 months).
Safety
In the safety analysis set (N = 19), 18 patients (94.7%) experienced ≥1 treatment-emergent AEs (TEAEs), and 12 (63.2%) experienced a grade ≥3 TEAE. Eight (42.1%) patients experienced ≥1 TRAE, almost all of which were mild to moderate in severity. Grade 3 TRAEs were reported in 2 (10.5%) patients (fatigue in 1 patient, and hyponatremia in another). Three (15.8%) patients experienced a serious TRAE during the study period (grade 2 rash and skin infection in 1 [5.3%] patient, and grade ≤2 differentiation syndrome in 2 [10.5%] patients). No patients discontinued treatment because of TRAEs.
One patient with grade 2 differentiation syndrome had their treatment held until the AE had resolved, and the other patient experienced 2 events of differentiation syndrome (grade 2, then grade 1) for which treatment was reduced and held until this AE resolved; this patient experienced a third grade 1 differentiation syndrome event (day 122), which required supportive care and was ongoing at data cutoff. This patient came off the study on day 457, received ivosidenib + decitabine for 8 months, and subsequently died 6 months after discontinuing this combination. Neither patient permanently discontinued ivosidenib because of differentiation syndrome.
QTc increases of a grade ≤2 severity occurred in 2 patients (10.5%); only 1 of these QTc increases was considered related to treatment, and neither event required ivosidenib dose modification. Fewer than half of patients were taking concomitant QTc-prolonging medications, with ondansetron being the most common (36.8% of patients); please see supplemental Materials for further details on other concomitant QTc-prolonging medications.
Two patients had TEAEs, which led to permanent discontinuation of ivosidenib; 1 patient discontinued because of sepsis, and another patient discontinued because of grade 3 fatigue related to underlying MDS. Both AEs were considered to be due to disease progression and not related to treatment; both patients subsequently died. Table 3 provides further details on TEAEs and TRAEs.
Overview of TEAEs
| AE outcomes, n (% patients) . | Safety analysis set (N = 19) . |
|---|---|
| AE summary | |
| Any TEAE | 18 (94.7) |
| Any treatment-related SAE | 3 (15.8) |
| Any grade ≥3 treatment-related TEAE | 2 (10.5) |
| Any TEAE leading to ivosidenib discontinuation∗ | 2 (10.5) |
| Any TEAE leading to ivosidenib interruption | 5 (26.3) |
| Any treatment-related TEAE leading to ivosidenib dose modification† | 4 (21.1) |
| TEAEs by preferred term considered possibly or probably related to treatment‡ | |
| Any-grade | 8 (42.1) |
| Fatigue | 3 (15.8) |
| Diarrhea | 2 (10.5) |
| Differentiation syndrome§ | 2 (10.5) |
| Rash | 2 (10.5) |
| Dyspnea | 2 (10.5) |
| ECG QT prolonged‖ | 1 (5.3) |
| Dyspepsia | 1 (5.3) |
| Decreased appetite | 1 (5.3) |
| Skin infection | 1 (5.3) |
| Anemia | 1 (5.3) |
| Platelet count decrease | 1 (5.3) |
| Blood alkaline phosphatase increased | 1 (5.3) |
| Hyponatremia | 1 (5.3) |
| Grade 3 or higher | |
| Fatigue | 1 (5.3) |
| Hyponatremia | 1 (5.3) |
| AE outcomes, n (% patients) . | Safety analysis set (N = 19) . |
|---|---|
| AE summary | |
| Any TEAE | 18 (94.7) |
| Any treatment-related SAE | 3 (15.8) |
| Any grade ≥3 treatment-related TEAE | 2 (10.5) |
| Any TEAE leading to ivosidenib discontinuation∗ | 2 (10.5) |
| Any TEAE leading to ivosidenib interruption | 5 (26.3) |
| Any treatment-related TEAE leading to ivosidenib dose modification† | 4 (21.1) |
| TEAEs by preferred term considered possibly or probably related to treatment‡ | |
| Any-grade | 8 (42.1) |
| Fatigue | 3 (15.8) |
| Diarrhea | 2 (10.5) |
| Differentiation syndrome§ | 2 (10.5) |
| Rash | 2 (10.5) |
| Dyspnea | 2 (10.5) |
| ECG QT prolonged‖ | 1 (5.3) |
| Dyspepsia | 1 (5.3) |
| Decreased appetite | 1 (5.3) |
| Skin infection | 1 (5.3) |
| Anemia | 1 (5.3) |
| Platelet count decrease | 1 (5.3) |
| Blood alkaline phosphatase increased | 1 (5.3) |
| Hyponatremia | 1 (5.3) |
| Grade 3 or higher | |
| Fatigue | 1 (5.3) |
| Hyponatremia | 1 (5.3) |
ECG QT, electrocardiogram QT interval; n, number of patients; SAE, serious AE.
These AEs were grade 5 sepsis and grade 3 fatigue; neither was considered related to ivosidenib.
Modification refers to dose reduction, interruption, or discontinuation.
Six patients experienced multiple treatment-related TEAEs.
One patient experienced 3 differentiation syndrome events (grade 1, 2 events; and grade 2, 1 event) and the other patient experienced 1 grade 2 differentiation syndrome event.
Grade 1 severity.
Efficacy
Clinical activity
In the efficacy analysis set (n = 18), the rate of CR + PR (primary end point) was 38.9% (n = 7 patients, all of whom experienced a CR; 95% CI, 17.3-64.3; Figure 1). Median time to CR was 1.87 months (range, 1.0-5.6 months; Table 1). Remissions were durable, with a Kaplan-Meier estimate showing a 68.6% probability of CR patients experiencing a remission duration of at least 5 years (supplemental Figure 1). Median duration of CR has not yet been reached, according to Kaplan-Meier analyses, and maximum CR duration was 80.8 months (censored observation). No impact on median CR duration was shown when censoring for transplant. Fifteen patients (83.3%; 95% CI, 58.6-96.4) experienced an objective response. Of 8 patients (44.4%) experiencing mCR, 4 (50.0%) experienced HI in ≥1 lineage (erythrocyte, platelet, and/or neutrophil). Two (25.0%) patients with mCR had an improvement in erythrocyte counts, 2 had an improvement in platelet counts, and 4 (50.0%) had an improvement in neutrophil counts, according to mIWG 2006 response criteria (Table 1).
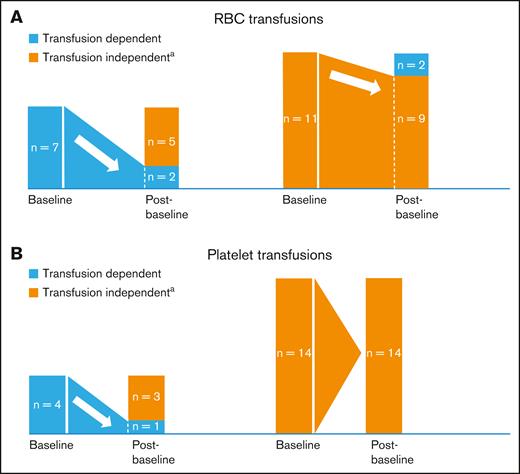
Five of 7 (71.4%) and 3 of 4 (75.0%) baseline RBC-transfusion– and platelet-transfusion–dependent patients, respectively, became transfusion independent (TI; no transfusion during ≥56 days) after baseline; 9 of 11 (81.8%), and all 14 (100%) baseline RBC- and platelet-TI patients, respectively, remained TI after baseline (Figure 3). Median time to any transfusion independence was 2.43 months (range, 0.03-5.36 months); median duration of any transfusion independence was not reached (range, 1.9-78.8 months [censored observation]). Supplemental Materials provide further information on response and transfusion independence outcomes.
Red blood cell and platelet transfusion requirements. (A-B) Proportions of patients with postbaseline RBC (A) or platelet transfusion (B) independence (N = 18). aPostbaseline transfusion independence was defined as no transfusion for at least one 56-day period. n, number of patients.
Red blood cell and platelet transfusion requirements. (A-B) Proportions of patients with postbaseline RBC (A) or platelet transfusion (B) independence (N = 18). aPostbaseline transfusion independence was defined as no transfusion for at least one 56-day period. n, number of patients.
Kaplan-Meier analyses showed a median OS duration estimate of 35.7 months (range, 3.7-88.7 months; 95% CI, 13.1-not reached) and the probabilities of patients being alive at 1, 3, and 5 years were 86.9%, 46.3%, and 46.3%, respectively (Table 1; supplemental Figure 2). Patients in “high” or “very high” IPSS-M risk categories tended to have shorter OS than patients in “moderately high” or “low” IPSS-M risk categories (Figure 2A).
Progression to AML
Two (11.1%) patients progressed to AML, both of whom still had a detectable IDH1 mutation at their last bone marrow assessment. At baseline, 1 patient’s MDS had +8 on cytogenetic evaluations and ASXL1, NRAS, and PTPN11 comutations; with 4% bone marrow blasts; and a high IPSS-R score at diagnosis. This patient had a best response of progressive disease. The other patient’s MDS had a poor-risk/monosomy 7 karyotype, 19% bone marrow blasts, and a very-high-risk IPSS-R score at diagnosis. This patient initially had an overall duration of remission of 8.3 months, including a CR duration of 5.6 months, but then subsequently developed AML.
Correlative analyses
IDH1 R132 frequency
All 19 patients had at least 1 IDH1-R132 mutation detected in the bone marrow and/or peripheral blood; 2 patients had >1 variant detected. Please see supplemental Materials for further details on these 2 patients. R132C was the most common IDH1 variant (Figure 4).
IDH1 mutation type, based on central testing (n [% patients]; N = 19).
Baseline mIDH1 VAF and clinical response
Median baseline mIDH1 VAF was 19.7%; patients in CR (n = 7) had a numerically lower median mIDH1 VAF at baseline than those patients in mCR (n = 8; 14% vs 25%). Median mIDH1 VAF was 3% in the group of 3 nonresponders, which included 2 patients with stable disease and a very low mIDH1 VAF, and 1 patient with progressive disease and a high mIDH1 VAF (Figure 5A).
Baseline and longitudinal mIDH1 VAF and clinical response. Association of NGS-assessed baseline mIDH1 VAF with response in the efficacy analysis set (A; N = 18) and longitudinal mIDH1 VAF as measured in BMMCs or PBMCs, stratified by BOR (B; N = 18). For panel A, please note: PB data are only plotted for patients who did not have BM samples available. Of 18 patients, 4 had NGS results available for BM only, 3 had NGS results available for PB only, and the remaining 11 had results available for both sample types. For panel B, please note: baseline mIDH1 VAF is plotted in gray, and the minimum posttreatment mIDH1 VAF is plotted in orange (indicating persistent mIDH1) or cyan (for mIDH1 clearance, defined as having a measured VAF below the validated LOD of the assay, which was 0.02%). Data are stratified by BOR and sample type. Lines connect pretreatment and posttreatment data for patients with data available. BM, bone marrow; BMMCs, bone marrow mononuclear cells; BOR, best overall response; LOD, limit of detection; NGS, next-generation sequencing; PB, peripheral blood; PBMCs, peripheral blood mononuclear cells; PDis, progressive disease; SD, stable disease; Tx, treatment.
Baseline and longitudinal mIDH1 VAF and clinical response. Association of NGS-assessed baseline mIDH1 VAF with response in the efficacy analysis set (A; N = 18) and longitudinal mIDH1 VAF as measured in BMMCs or PBMCs, stratified by BOR (B; N = 18). For panel A, please note: PB data are only plotted for patients who did not have BM samples available. Of 18 patients, 4 had NGS results available for BM only, 3 had NGS results available for PB only, and the remaining 11 had results available for both sample types. For panel B, please note: baseline mIDH1 VAF is plotted in gray, and the minimum posttreatment mIDH1 VAF is plotted in orange (indicating persistent mIDH1) or cyan (for mIDH1 clearance, defined as having a measured VAF below the validated LOD of the assay, which was 0.02%). Data are stratified by BOR and sample type. Lines connect pretreatment and posttreatment data for patients with data available. BM, bone marrow; BMMCs, bone marrow mononuclear cells; BOR, best overall response; LOD, limit of detection; NGS, next-generation sequencing; PB, peripheral blood; PBMCs, peripheral blood mononuclear cells; PDis, progressive disease; SD, stable disease; Tx, treatment.
Longitudinal mIDH1 VAF and clinical response
No clear trends were observed with longitudinal VAF and clinical response. Although clearance of IDH1 mutations was uncommon, responders with an available baseline sample tended to experience reduction in mIDH1 VAF. However, mIDH1 VAF clearance in the peripheral blood was observed in 1 patient with stable disease, suggesting that reduction of mIDH1 VAF is not always indicative of clinical response (Figure 5B).
IDH1 MUTATION CLEARANCE IN THE BONE MARROW IN RESPONDERS
Eighteen patients had longitudinal assessment of IDH1 mutation burden in the bone marrow while on treatment. Fifteen (83.3%) of those patients were responders, with 7 patients achieving a CR (46.7% of responders) and 8 patients achieving an mCR (53.3% of responders). Of this group of responders, IDH1 mutation clearance was observed in 1 of 7 (14.3%) CRs, and 1 of 8 (12.5%) mCRs.
IDH1 MUTATION CLEARANCE IN THE PERIPHERAL BLOOD IN RESPONDERS
Seventeen patients had on-treatment peripheral blood assessments, 14 (82.4%) of whom were responders. In this group of responders, IDH1 mutation clearance was observed for 2 of 7 (28.6%) patients with CR, and 2 of 7 patients with mCR.
Comutated genes
The median number of comutated genes was 2 (range, 0-7). Patients with a CR had fewer comutated genes than patients with no response (median of 1 vs 3; Figure 2B). The most common comutated genes were SRSF2 and ASXL1, both occurring in 8 patients (42.1%), as well as RUNX1 (n = 3 patients [15.8%]; supplemental Figure 3). All 8 patients with SRSF2 comutations, and 6 of 8 patients with ASXL1 comutations experienced CR or mCR. Two of 3 patients with RUNX1 comutations experienced mCRs; both patients were alive at data cutoff, with 1 patient having experienced an OS duration of ∼2 years.
TP53 was mutated at baseline in 2 patients, 1 of whom experienced CR (baseline VAF of 1%; remission duration: 65.3 months) and another of whom experienced mCR (baseline VAF of 5%; remission duration: 70.9 months; Figure 2A); both remissions were ongoing at the time of data cutoff. Two patients had comutations in receptor tyrosine kinase pathway genes: 1 patient with an NRAS comutation who experienced mCR, and another with both NRAS and PTPN11 comutations who experienced progressive disease.
PK/PD analyses
Ivosidenib (500 mg) reached steady-state exposure within 14 days of continuous daily dosing. Ivosidenib rapidly reduced both plasma and bone marrow levels of 2-HG, preceding changes in mIDH1 VAF, and demonstrating on-target effects, with plasma 2-HG levels at steady state (day 1 of cycle 2) resembling those of volunteers (72.6 ± 21.8 ng/mL; data not published). At steady state, more than 90% reduction of 2-HG in plasma and bone marrow was seen across the observed range of plasma ivosidenib AUC0-24 values, demonstrating sustained duration of inhibition. Of the 14 evaluable patients on day 1 of cycle 2, 12 (85.7%) showed inhibition of 2-HG plasma levels of at least 94%. Supplemental Materials provides further PK/PD data, including supplemental Table 3.
Discussion
This is currently the largest prospective study performed specifically in patients with mIDH1 R/R MDS, with final results demonstrating an acceptable safety profile and clinically meaningful activity of ivosidenib in a molecularly defined mIDH1 R/R MDS population with a poor prognosis. No new safety signals or trends were observed, demonstrating the long-term tolerability of ivosidenib monotherapy, further confirmed by the median treatment duration of 9.3 months.23,27,28 Most TEAEs were not treatment-related, and those that were related were typically of a lower grade and could be managed with standard-of-care interventions.
Two patients (10.5%) experienced differentiation syndrome events of a grade ≤2 severity, both of whom were managed with standard therapeutic approaches and remained on ivosidenib treatment. No patients experienced grade ≥3 differentiation syndrome. In comparison, albeit in larger patient cohorts, rates of grade ≥3 differentiation syndrome occurred in 9.0% and 5.0% of patients receiving ivosidenib monotherapy for newly diagnosed or R/R mIDH1 AML, respectively, and 7.0% of patients with mIDH2 R/R AML who were treated with mIDH2 inhibitor enasidenib.23,27,29 A lower incidence of treatment-related QTc prolongation was observed (grade 1 event in 1 patient [5.3%]; no grade ≥3 QTc increases), likely because fewer prophylactic concomitant QTc-prolonging agents were administered in this study compared with AML cohorts receiving ivosidenib.23 Only 2 grade 3 TRAEs (fatigue and hyponatremia) were reported; neither led to treatment discontinuation. Overall, these data support an improved safety profile compared with that observed in patients with AML, which may be secondary to the lower disease burden at baseline, and which may also support earlier use of ivosidenib within the treatment paradigm.
Ivosidenib demonstrated a favorable CR + PR rate (38.9% of patients; 95% CI, 17.3-64.3; primary end point), exceeding the prespecified definition of clinically relevant activity in this high-risk population. All 7 responders who achieved a CR by IWG 2006 response criteria had durable responses, with Kaplan-Meier estimates showing a 68.6% probability of achieving a CR duration of at least 5 years. The median duration of CR was not reached (95% CI, 1.9-not reached) during a median follow-up period of 65.3 months for these patients, among whom 2 patients transitioned to HSCT (1 before, and 1 after, data cutoff).
Half of patients in mCR experienced HI in at least 1 lineage (erythrocyte, platelet, and/or neutrophil), helping to confirm that ongoing ivosidenib therapy provides clinical benefit, as demonstrated in prior studies of ivosidenib in other settings.23,27 Importantly, ∼75% of RBC- or platelet-transfusion–dependent patients at baseline achieved transfusion independence, and almost all patients with RBC or platelet transfusion independence at baseline maintained their transfusion independence status (81.8% and 100.0%, respectively). These outcomes are of particular relevance for patients who experience the detrimental clinical, economic, and quality-of-life effects from frequent transfusion requirements, a defining feature of advanced MDS.30-33
OS outcomes in patients with HMA-refractory MDS are generally poor, with 1 study reporting a median OS duration of 5.6 months and 1- and 2-year survival estimates of 28.9% and 15.3%, respectively, in patients with high-risk R/R MDS.7 In our substudy, median OS duration was 35.7 months and the probabilities of patients being alive for at least 1 year and 5 years were 86.9% and 46.3%, respectively, according to Kaplan-Meier analysis. Although it should be noted that our study sample size was small, and not solely comprised of patients with high-risk disease, although they did form a substantial proportion of the patient population.
IDH1 mutations are often associated with a higher risk of leukemic transformation in patients with MDS or myeloproliferative neoplasms and ivosidenib may prolong the time to transformation to AML.34,35 In this study, 2 (11.1%) patients progressed to AML during a median follow-up period of 27.1 months, which appears favorable compared with a retrospective review in which almost half of patients (48%) with R/R MDS after HMA therapy experienced transformation to AML during a median follow-up period of 19.5 months. However, the patients included in the retrospective review who developed AML tended to be younger (aged <65 years) with higher-risk MDS and often had TP53 mutations, and thus differ somewhat from our overall study population.36
Overall, comutations were heterogenous and typical for MDS, suggesting generalizability of these results to a larger MDS population. No clear correlation between IDH1 mutation clearance and clinical response or other efficacy outcomes was reported in our study, which differs from what was observed in a cohort with R/R AML who received ivosidenib.23 However, this difference should be interpreted cautiously because of the small sample size. Although IDH1 mutation clearance was uncommon in this study, most responding patients demonstrated reduction of mIDH1 VAF while on treatment.
PK and PD parameters in patients with R/R MDS were comparable with those in patients with R/R AML treated with ivosidenib 500 mg once daily.23 After multiple ivosidenib doses, steady-state exposure was achieved within 14 days, with only minor accumulation and plasma 2-HG decreased to similar levels observed in healthy participants, with >90% reduction of 2-HG in plasma and the bone marrow in the majority of patients, regardless of response, demonstrating on-target effects.37,38
Study limitations were the small sample size, which may have led to our study being underpowered to detect infrequent events such as QT prolongation, as well as the single-arm study design; however, both limitations are expected because of the relative rarity of the IDH1 mutation in patients with MDS.
In conclusion, ivosidenib induced durable remissions in patients with mIDH1 R/R MDS, including a substantial proportion of CRs, accompanied by low rates of serious or severe TEAEs. Ivosidenib therefore represents a well-tolerated and efficacious oral therapy for patients with this aggressive life-threatening disease who currently have no approved disease-modifying therapeutic options and may potentially change the future treatment landscape for this poor-prognosis population. Data from this study will form the basis of an upcoming priority review by the US Food and Drug Administration, and the efficacy and safety of ivosidenib are being further studied in patients with R/R mIDH1 MDS in the ongoing phase 2 IDIOME study (NCT03503409).39
Acknowledgments
Medical writing assistance was provided by Melody Watson on behalf of Bioscript Group, Macclesfield, UK, and funded by Servier, LLC.
This study was funded by Agios Pharmaceuticals, Inc. Servier Pharmaceuticals, LLC, has completed the acquisition of Agios’ oncology business.
C.D.D. is a V Foundation Lloyd Family Scholar and Scholar in Clinical Research of The Leukemia & Lymphoma Society.
Authorship
Contribution: C.D.D. designed and performed research, collected, analyzed, and interpreted data, contributed to the writing of the manuscript, and critically reviewed the manuscript; G.J.R. performed research, collected, analyzed, and interpreted data, and critically reviewed the manuscript; J.M.W. performed research, collected, analyzed, and interpreted data, contributed to the writing of the manuscript, and critically reviewed the manuscript; Y.F.M. performed research, collected, analyzed, and interpreted data, contributed to the writing of the manuscript, and critically reviewed the manuscript; G.T.P performed research, collected data, and critically reviewed the manuscript; P.B. performed research, collected, analyzed, and interpreted data, contributed to the writing of the manuscript, and critically reviewed the manuscript; S.d.B. performed research, collected data, and critically reviewed the manuscript; A.S. performed research, collected data, and critically reviewed the manuscript; J.M.F. designed research, collected data, and critically reviewed the manuscript; M.L.A. performed research, collected data, and critically reviewed the manuscript; D.A.S. performed research, collected data, contributed to the writing of the manuscript, and critically reviewed the manuscript; M.H. designed and performed research, analyzed and interpreted data, contributed to the writing of the manuscript, and critically reviewed the manuscript; D.M.M. designed and performed research, analyzed and interpreted data, contributed to the writing of the manuscript and critically reviewed the manuscript; X.B. designed and performed research, analyzed and interpreted data, performed statistical analyses, contributed to the writing of the manuscript and critically reviewed the manuscript; P.A.P. designed and performed research, analyzed and interpreted data, contributed to the writing of the manuscript and critically reviewed the manuscript; S.M.K. designed and performed research, analyzed and interpreted data, contributed to the writing of the manuscript, and critically reviewed the manuscript; G.G.-M. performed research, collected data, and critically reviewed the manuscript; and A.T.F. designed and performed research, collected, analyzed, and interpreted data, contributed to the writing of the manuscript, and critically reviewed the manuscript.
Conflict-of-interest disclosure: C.D.D. received research funding from AbbVie, Astex, ImmuneOnc, Bristol Myers Squibb, Cleave, Foghorn, Loxo, Rigel, Servier; consulting fees from Amgen, AbbVie, Astellas, Bristol Myers Squibb, GenMab, GlaxoSmithKline, Gilead, Jazz, Schrodinger, Servier, Stemline; honoraria for educational events from AbbVie, Astellas, Bristol Myers Squibb, Jazz, and Servier; meeting support from Servier; and has participated on a GenMab data safety board. G.J.R. has received consulting fees from Janssen, Amgen, Celgene, Novartis, Pfizer, AbbVie, Argenx, Jazz Pharmaceuticals, Roche, Daiichi Sankyo, Takeda, GlaxoSmithKline, Bristol Myers Squibb, Blueprint Medicines, bluebird bio, Jasper Pharmaceuticals, Syndax, Molecular Partners, Ellipses Pharma, AstraZeneca, Caribou and Rigel, and research funding from Janssen. J.M.W. has received consulting fees from Rigel, Servier; participated on safety monitoring or advisory boards for Rigel, Servier, Bristol Myers Squibb, Daiichi Sankyo, Aptose, Reven Pharma, and Rafael Pharma; and has received funding from Takeda and Immune Systems Key, Ltd. Y.F.M. has received consulting fees from GERON Pharmaceuticals, Kura Oncology, Blueprint Medicines, OncLive, MD Education, Sierra Oncology, Stemline Therapeutics, MorphoSys, Taiho Oncology, Rigel Pharmaceuticals, and Novartis, and support for attending meetings/travel from Blueprint Medicines, MD Education, and MorphoSys. P.B. has received consulting fees from MBS Pharma and ONO Pharmaceuticals; honoraria from Rigel Pharma and Bristol Myers Squibb; support for meetings/travel from KITE Pharma and Rigel Pharma; and has participated on data safety monitoring/advisory boards for Protagonist Therapeutics and KITE Pharma. S.d.B. has received support from Bristol Myers Squibb; research funding from Auron and Forma; consulting fees from Bristol Myers Squibb, GlaxoSmithKline, Remix, Servier, and Syndax; honoraria for speakers’ bureaus from AbbVie, Astellas, Bristol Myers Squibb, Jazz Pharmaceuticals, and Servier; honoraria from Loxo; and travel expenses from AbbVie and Servier. A.S. participated on speaker bureaus for Amgen and advisory boards for Sanofi and Daiichi-Sankyo. J.M.F. received institutional grants from Actinium, Astellas, Roivant, Celgene, Novartis, Takeda, Sellas, Kura, Pfizer, Servier, and Chordia; consulting fees from CTI Biopharma, Lava, Remix, Bristol Myers Squibb, and MJH LifeSciences; honoraria from Aptitude Health, AmerisourceBergen/IntrinsiQ Specialty Solutions, and MJH LifeSciences; is a member of the NCI Leukemia steering committee; and has stock options in Aurinia. D.A.S. has received consulting fees from AbbVie, Affimed, Gilead, Incyte, Intellisphere, Molecular Partners, PGEN Therapeutics, Takeda, and Zentalis; has participated on advisory boards for AvenCell, bluebird bio, Bristol Myers Squibb, Intellia, Jasper Therapeutics, KITE Pharma, Magenta Therapeutics, Nkarta, Novartis, Shattuck Labs, Servier, Syndax, and Syros; and reports payments from Aprea and Jazz were received by the Moffitt Cancer Center. D.M.M., M.H., X.B., P.A.P., and S.M.K. are employees of Servier, LLC. A.T.F. has received personal fees from Orum, Takeda, Servier, Amgen, Autolus, Rigel, Pfizer, Daiichi Sankyo, Forma, PureTech, EnClear, Genentech, Ipsen, AbbVie, Mablytics, Immunogen, Astellas, Bristol Myers Squibb/Celgene, Novartis, Agios, MorphoSys, Kite, Foghorn, Blueprint, Kura, and Trillium; and grants from AbbVie, Bristol Myers Squibb/Celgene, and Agios/Servier, outside the submitted work. The remaining authors declare no competing financial interests.
Correspondence: Courtney D. DiNardo, Division of Cancer Medicine, Department of Leukemia, University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Unit 428, Houston, TX 77030; email: cdinardo@mdanderson.org.
References
Author notes
Presented in poster form at the 28th annual meeting of the European Hematology Association, Frankfurt, Germany, 8 to 11 June 2023.
All authors had access to the primary clinical trial data. Deidentified individual participant data that underlie the reported results, and the study protocol, will be made available 3 months after publication for a period of 5 years after the publication date, available upon reasonable request from a qualified medical or scientific professional for the specific purpose laid out in that request. The data for this request will be available after a data access agreement has been signed. Please send your data-sharing request via https://clinicaltrials.servier.com/data-request-portal/.
The full-text version of this article contains a data supplement.

![IDH1 mutation type, based on central testing (n [% patients]; N = 19).](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/8/15/10.1182_bloodadvances.2023012302/1/m_blooda_adv-2023-012302-gr4.jpeg?Expires=1769090407&Signature=krAd5nLJWkkUZRevMsiIUNDX~88IAsoQBN-ovwJ1l~JOCwjXhHe4Dg9hZt5-URbintH-JaodVt7C8BOSLpW5P2d6vi7OjadrLKxzEdB07mk0OYvQrAQVGxq9cpsqRm0-THCrWpP4ogDNxo3cDgSCWtfP42Y8ESSqNGiCRnZwmYi8tLzaMEVisO3ev15-h-baUv6x9uUn7ZRZrQ09jdkJ9g32lwEAHtitL5qpA6AxAVkTafcfgvU2gQyIWenu~SD9xyZSWLEunOgLynq3oAZnuRp7AO2byZJFl0RON1xf0iVS37HEPEg4pj7tBLfy-ap-LhHseGgrcWwZWWDq33Rm5Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
