Key Points
The oral farnesyltransferase inhibitor, tipifarnib, as a single agent, produces responses in 40% of patients with heavily pretreated PTCL.
Biomarker correlative analysis of pretreatment biopsies suggests increased tipifarnib activity in T-follicular helper phenotype.
Visual Abstract
A phase 2, international, open-label, nonrandomized, single-arm trial was conducted to evaluate the efficacy and safety of tipifarnib, a farnesyltransferase inhibitor, as monotherapy for relapsed/refractory peripheral T-cell lymphoma (PTCL) and to evaluate tumor mutation profile as a biomarker of response. Adults with relapsed/refractory PTCL received tipifarnib 300 mg orally twice daily for 21 days in a 28-day cycle. The primary end point was objective response rate (ORR); secondary end points included ORR, progression-free survival (PFS), duration of response (DOR), and adverse events (AEs) in specific subtypes. Sixty-five patients with PTCL were enrolled: n = 38 angioimmunoblastic T-cell lymphoma (AITL), n = 25 PTCL not otherwise specified, and n = 2 other T-cell lymphomas. The ORR was 39.7% (95% confidence interval [CI], 28.1-52.5) in all patients and 56.3% (95% CI, 39.3-71.8) for AITL. Median PFS was 3.5 months overall (954% CI, 2.1-4.4), and 3.6 months (95% CI, 1.9-8.3) for AITL. Median DOR was 3.7 months (95% CI, 2.0-15.3), and greatest in patients with AITL (7.8 months; 95% CI, 2.0-16.3). The median overall survival was 32.8 months (95% CI, 14.4 to not applicable). Tipifarnib-related hematologic AEs were manageable and included neutropenia (43.1%), thrombocytopenia (36.9%), and anemia (30.8%); other tipifarnib-related AEs included nausea (29.2%) and diarrhea (27.7%). One treatment-related death occurred. Mutations in RhoA, DNMT3A, and IDH2 were seen in 60%, 33%, and 27%, respectively, in the AITL tipifarnib responder group vs 36%, 9%, and 9% in the nonresponder group. Tipifarnib monotherapy demonstrated encouraging clinical activity in heavily pretreated relapsed/refractory PTCL, especially in AITL, with a manageable safety profile. This trial was registered at www.ClinicalTrials.gov as #NCT02464228.
Introduction
Peripheral T-cell lymphomas (PTCLs) are a rare, heterogenous group of lymphoproliferative disorders expressing mature T-cell immunophenotype that account for ∼10% to 15% of lymphomas worldwide.1,2 PTCLs are phenotypically diverse, with >30 different types characterized in the fifth edition of the World Health Organization classification.3,4 In western countries, PTCL accounts for 5% to 10% of all lymphomas, of which PTCL not otherwise specified (PTCL-NOS) is the most common subtype (26%), followed by nodal T follicular helper (TFH) cell lymphoma (nTFHL) angioimmunoblastic type (19%; formerly known at the time of study enrollment as angioimmunoblastic T-cell lymphoma [AITL]), anaplastic large cell lymphoma (ALCL), anaplastic lymphoma kinase-positive (7%); ALCL, anaplastic lymphoma kinase-negative (6%); and enteropathy-associated T-cell lymphoma (<5%).1,5 PTCLs are often associated with frequent mutations affecting IDH2, TET2, DNMT3A, RhoA, and PLCƔ1.6-12
Patients with relapsed/refractory PTCL have a poor outcome with median progression-free survival (PFS) and overall survival (OS) rates ranging from 3 to 4 months and 5 to 6 months, respectively, and <25% survival at 3 years after relapse.1,13,14 Standard first-line treatment for PTCL includes anthracycline-based chemotherapy or the combination of cyclophosphamide/doxorubicin/prednisone + brentuximab vedotin in patients with CD30+ disease.5,15 Current guidelines suggest that patients who achieve a response after primary therapy be considered for autologous stem cell transplant if eligible.5
There is no standard therapy for relapsed/refractory PTCL.15 Because of minimal supportive data, recommendations for regimens based on subtype are lacking in patients with relapsed/refractory disease, with the exception for ALCL.5 Treatment options include clinical trials, single agents (eg, brentuximab vedotin, romidepsin, belinostat, and pralatrexate), or combination regimens (eg, DHAP [dexamethasone, high-dose cytarabine and cisplatin] or ICE [ifosfamide, carboplatin, and etoposide]).5 Eligible patients who achieve a response may proceed to potentially curative allogeneic stem cell transplant.5
Tipifarnib is a well-characterized potent, oral, selective small-molecule inhibitor of farnesyltransferase that has shown activity in hematologic malignancies, including acute myeloid leukemia, myelodysplastic syndrome, and lymphomas, in several clinical trials.16-18 Farnesyltransferase inhibitors (FTIs) were initially developed to target cancers with RAS mutations and have demonstrated clinical utility in HRAS mutant head and neck squamous cell carcinoma.19 In addition, FTIs have demonstrated interesting clinical activity in non–RAS-driven malignancies, including acute myeloid leukemia and T-cell lymphomas.20-26
A prior phase 2 trial of tipifarnib in patients with relapsed/refractory aggressive lymphoma reported a 31% (11/36) overall response rate in the T-cell and Hodgkin lymphoma group, and a 50% (4/8) overall response rate in patients with PTCL.27 Building on these data, we conducted a multicenter, international, open-label, nonrandomized, single-arm phase 2 study to further evaluate the efficacy and safety of oral tipifarnib in a larger cohort of patients with relapsed/refractory PTCL with an emphasis on investigating molecular biomarkers that may correlate with clinical activity.
Patients and methods
Eligible patients were aged ≥18 years with relapsed/refractory PTCL (per 2016 World Health Organization Classification of Lymphoid Neoplasms) and measurable disease according to the Lugano classification.28 Subsets of PTCL included in the study were PTCL, PTCL-NOS, and AITL (nTFHL-angioimmunoblastic-type); nTFHL-angioimmunoblastic-type will be referred to as AITL throughout the paper. Patients had an Eastern Cooperative Oncology Group performance status score of 0 to 2 and had received ≥1 prior conventional systemic cytotoxic therapy and/or localized radiation within ≥2 weeks before enrollment. Laboratory requirements were absolute neutrophil count of ≥1000/μL; platelet count of ≥50 000/μL; hemoglobin of ≥8.0 g/dL; serum creatinine ≤1.5 times the upper limit of normal; and aspartate aminotransferase (serum glutamic-oxaloacetic transaminase) and alanine aminotransferase (serum glutamic-pyruvic transaminase) of ≤3 times the upper limit of normal. Patients were excluded if they had prior treatment with an FTI. Full eligibility criteria are provided in supplemental Table 1. The study was conducted at 14 centers in the United States, Spain, and the Republic of Korea, in accordance with the International Conference on Harmonization Good Clinical Practice guidelines and the Declaration of Helsinki. (ClinicalTrials.gov identifier: NCT02464228). The protocol was approved by the independent ethics committee or institutional review boards before the start of the trial. Patients provided their written consent to participate in the study. The trial was designed by the study sponsor Kura Oncology, Inc in collaboration with the investigators. Data were collected by the clinical investigators and their research staff and were analyzed by the trial sponsor. All the authors vouch for the completeness and accuracy of the data and adherence of the trial to the protocol.
The multistage study design assumed enrollment of patients in 3 consecutive groups leading to the overall assessment of the clinical activity of tipifarnib in AITL and PTCL-NOS disease subtypes. The starting dose and schedule, which were updated throughout the study (see “Amendments” in supplemental Table 2), were derived from studies evaluating 2 main dosing strategies: 3 weeks on/1 week off dosing (twice daily on days 1-21 in 28-day treatment cycles), and alternative week dosing (twice daily on days 1-7 and days 15-21 in 28-day treatment cycles).20,27,29-31 Investigators were permitted to deescalate by 100 mg per dose based on tolerability to control treatment-related and/or treatment-emergent toxicities. Growth factors were permitted at investigator discretion. Disease evaluation was determined at baseline and on day 22 ± 5 days of cycles 2, 4, 6, etc, and approximately every 12 weeks thereafter until disease progression by either positron emission tomography–computed tomography or contrast-enhanced spiral computed tomography scan or magnetic resonance imaging. Efficacy was assessed according to the Lugano classification.28
Statistical analysis
The primary objective was to determine the antitumor activity of tipifarnib in patients with relapsed/refractory PTCL in terms of investigator-assessed objective response rate (ORR; partial response [PR] + complete response [CR]). The ORR was estimated in the efficacy evaluable analysis set (patients who received at least 1 dose of tipifarnib and had at least 1 disease response assessment after dosing) and was used to analyze study sample subgroups. Secondary objectives included the overall PFS and duration of response (DOR) in PTCL, the safety and tolerability of tipifarnib, and the ORR, PFS, and DOR according to disease subtypes. Exploratory studies included analysis of tumor tissues for molecular biomarkers that correlate with clinical activity of tipifarnib. The study assumed that all patients, despite sample heterogeneity in terms of subtypes, had the same true ORR. The null hypothesis (ORR = 10%) was tested at α = .05 significance level using a 2-sided binomial test for the first 18 patients, with an interested response rate of 30%. The study accrued a total sample size of 65 safety-evaluable patients (all enrolled patients who received at least 1 dose of tipifarnib). This sample size was sufficient for both safety and efficacy end points (supplemental Statistics Information).
Correlative biomarker studies
Formalin-fixed paraffin embedded tumor tissue from prior archival specimens were collected in accordance with the study informed consent. Genomic DNA underwent whole-exome sequencing using hybrid capture probes (Agilent SureSelect All Exon version 5, 50 Mb) according to the manufacturer’s protocol. After removal of low-quality reads (fastp version 0.19.4), sequencing data were aligned to the human genome (hg19; bwa-mem aligner version 0.7.10). Global variants were called using TNscope (version sentieon-genomics-201911) and raw variants were annotated (eg, somatic vs germ line) using Golden Helix’s VarSeq program. Ensembl Variant Effect Predictor version 105 was used to annotate each variant and each variant was classified based on single nucleotide polymorphism database, COSMIC, and ClinVar data. Somatic variants that were classified as “pathogenic” or “likely pathogenic” were used to create oncoplots for all participants with available whole-exome sequencing data in the AITL and PTCL-NOS cohorts.
Results
Demographics for all patients enrolled in the study are listed in Table 1. In total, 65 patients with PTCL were enrolled (first subject enrolled on 25 February 2016, last subject completed on 31 March 2021), of whom 38 (58.5%) were diagnosed with AITL, 25 (38.5%) with PTCL-NOS, and 2 (3.1%) with other T-cell lymphomas (referred to hereafter as “other”). The median age of patients was 66.3 years (range, 31.3-87.7) and the majority of patients were White (70.8%) and male (63.1%); 90% of patients had stage III/IV disease. Median prior anticancer therapies was 3 (range, 1-8). Forty percent (40%) of patients received single-agent therapy immediately before tipifarnib, whereas 43% of patients received aggressive multiagent regimens (R-CHOP [rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone]–like or ICE) before tipifarnib. Overall, 44.6% of patients were refractory to immediate prior therapies, and 38.5% had progressed after autologous stem cell transplant.
Baseline patient demographics and clinical characteristics in the safety evaluable set
| . | AITL (n = 38) . | PTCL-NOS∗ (n = 25) . | Total† (N = 65) . |
|---|---|---|---|
| Median age, y (min, max) | 66.27 (41.3, 87.4) | 66.88 (31.3, 87.7) | 66.34 (31.3, 87.7) |
| Sex, n (%) | |||
| Male | 22 (57.9) | 18 (72.0) | 41 (63.1) |
| Female | 16 (42.1) | 7 (28.0) | 24 (36.9) |
| Ethnicity, n (%) | |||
| Hispanic or Latino | 2 (5.3) | 2 (8.0) | 4 (6.2) |
| Not Hispanic or Latino | 36 (94.7) | 23 (92.0) | 61 (93.8) |
| Race, n (%) | |||
| White | 23 (60.5) | 22 (88.0) | 46 (70.8) |
| Black or African American | 1 (2.6) | 1 (4.0) | 3 (4.6) |
| Asian | 12 (31.6) | 0 | 12 (18.5) |
| Other | 2 (5.3) | 2 (8.0) | 4 (6.2) |
| ECOG performance status score, n (%) | |||
| 0 | 18 (47.4) | 8 (32.0) | 26 (40.0) |
| 1 | 13 (34.2) | 16 (64.0) | 31 (47.7) |
| 2 | 7 (18.4) | 1 (4.0) | 8 (12.3) |
| Stage at study entry, n (%) | |||
| I | 1 (1.5) | 0 | 1 (1.5) |
| II | 2 (3.1) | 2 (3.1) | 5 (7.7) |
| III | 11 (16.9) | 6 (9.2) | 17 (26.1) |
| IV | 24 (36.9) | 17 (26.2) | 42 (64.6) |
| Disease status at study entry, n (%)† | |||
| Relapse | 21 (55.3) | 13 (52.0) | 35 (53.8) |
| Refractory | 16 (42.1) | 12 (48.0) | 29 (44.6) |
| Unknown | 1 (2.6) | 0 | 1 (1.5) |
| No. of prior treatments, median (min, max) | 3.0 (1, 7) | 3.0 (1, 8) | 3.0 (1, 8) |
| Prior (any) cancer therapies, n‡(%) | |||
| CHOP + CHOP-like | 22 (57.9) | 15 (60.0) | 37 (56.9) |
| CHOEP + CHOEP-like | 18 (47.4) | 8 (32.0) | 27 (41.5) |
| CHOP-R | 3 (7.9) | 2 (8.0) | 5 (7.7) |
| ICE + ICE-like | 11 (28.9) | 6 (24.0) | 17 (26.2) |
| Single agent‡ | 13 (34.2) | 13 (52.0) | 28 (43.1) |
| Belinostat | 1 (2.6) | 3 (12.0) | 4 (6.2) |
| Brentuximab vedotin | 5 (13.2) | 5 (20.0) | 11 (16.9) |
| Nivolumab | 4 (10.5) | 1 (4.0) | 5 (7.7) |
| Pralatrexate | 1 (2.6) | 4 (16.0) | 5 (7.7) |
| Romidepsin | 8 (21.1) | 7 (28.0) | 17 (26.2) |
| Immediate prior therapy to study drug, n‡(%) | |||
| CHOP-R | 1 (2.6) | 0 (0.0) | 1 (1.5) |
| CHOP + CHOP-like | 7 (18.4) | 4 (16.0) | 11 (16.9) |
| CHOEP + CHOEP-like | 5 (13.2) | 2 (8.0) | 7 (10.8) |
| ICE + ICE-like | 3 (7.9) | 2 (8.0) | 5 (7.7) |
| Single agent | 13 (34.2) | 11 (44.0) | 26 (40.0) |
| Brentuximab vedotin | 4 (10.5) | 4 (16.0) | 8 (12.3) |
| Romidepsin | 5 (13.2) | 1 (4.0) | 8 (12.3) |
| Other | 9 (23.7) | 6 (24.0) | 15 (23.1) |
| Prior stem cell transplant (autologous), n (%) | 17 (44.7) | 8 (32.0) | 25 (38.5) |
| . | AITL (n = 38) . | PTCL-NOS∗ (n = 25) . | Total† (N = 65) . |
|---|---|---|---|
| Median age, y (min, max) | 66.27 (41.3, 87.4) | 66.88 (31.3, 87.7) | 66.34 (31.3, 87.7) |
| Sex, n (%) | |||
| Male | 22 (57.9) | 18 (72.0) | 41 (63.1) |
| Female | 16 (42.1) | 7 (28.0) | 24 (36.9) |
| Ethnicity, n (%) | |||
| Hispanic or Latino | 2 (5.3) | 2 (8.0) | 4 (6.2) |
| Not Hispanic or Latino | 36 (94.7) | 23 (92.0) | 61 (93.8) |
| Race, n (%) | |||
| White | 23 (60.5) | 22 (88.0) | 46 (70.8) |
| Black or African American | 1 (2.6) | 1 (4.0) | 3 (4.6) |
| Asian | 12 (31.6) | 0 | 12 (18.5) |
| Other | 2 (5.3) | 2 (8.0) | 4 (6.2) |
| ECOG performance status score, n (%) | |||
| 0 | 18 (47.4) | 8 (32.0) | 26 (40.0) |
| 1 | 13 (34.2) | 16 (64.0) | 31 (47.7) |
| 2 | 7 (18.4) | 1 (4.0) | 8 (12.3) |
| Stage at study entry, n (%) | |||
| I | 1 (1.5) | 0 | 1 (1.5) |
| II | 2 (3.1) | 2 (3.1) | 5 (7.7) |
| III | 11 (16.9) | 6 (9.2) | 17 (26.1) |
| IV | 24 (36.9) | 17 (26.2) | 42 (64.6) |
| Disease status at study entry, n (%)† | |||
| Relapse | 21 (55.3) | 13 (52.0) | 35 (53.8) |
| Refractory | 16 (42.1) | 12 (48.0) | 29 (44.6) |
| Unknown | 1 (2.6) | 0 | 1 (1.5) |
| No. of prior treatments, median (min, max) | 3.0 (1, 7) | 3.0 (1, 8) | 3.0 (1, 8) |
| Prior (any) cancer therapies, n‡(%) | |||
| CHOP + CHOP-like | 22 (57.9) | 15 (60.0) | 37 (56.9) |
| CHOEP + CHOEP-like | 18 (47.4) | 8 (32.0) | 27 (41.5) |
| CHOP-R | 3 (7.9) | 2 (8.0) | 5 (7.7) |
| ICE + ICE-like | 11 (28.9) | 6 (24.0) | 17 (26.2) |
| Single agent‡ | 13 (34.2) | 13 (52.0) | 28 (43.1) |
| Belinostat | 1 (2.6) | 3 (12.0) | 4 (6.2) |
| Brentuximab vedotin | 5 (13.2) | 5 (20.0) | 11 (16.9) |
| Nivolumab | 4 (10.5) | 1 (4.0) | 5 (7.7) |
| Pralatrexate | 1 (2.6) | 4 (16.0) | 5 (7.7) |
| Romidepsin | 8 (21.1) | 7 (28.0) | 17 (26.2) |
| Immediate prior therapy to study drug, n‡(%) | |||
| CHOP-R | 1 (2.6) | 0 (0.0) | 1 (1.5) |
| CHOP + CHOP-like | 7 (18.4) | 4 (16.0) | 11 (16.9) |
| CHOEP + CHOEP-like | 5 (13.2) | 2 (8.0) | 7 (10.8) |
| ICE + ICE-like | 3 (7.9) | 2 (8.0) | 5 (7.7) |
| Single agent | 13 (34.2) | 11 (44.0) | 26 (40.0) |
| Brentuximab vedotin | 4 (10.5) | 4 (16.0) | 8 (12.3) |
| Romidepsin | 5 (13.2) | 1 (4.0) | 8 (12.3) |
| Other | 9 (23.7) | 6 (24.0) | 15 (23.1) |
| Prior stem cell transplant (autologous), n (%) | 17 (44.7) | 8 (32.0) | 25 (38.5) |
ALK, anaplastic lymphoma kinase; CHOEP, cyclophosphamide, doxorubicin, doxorubicin hydrochloride, etoposide, prednisolone, prednisone, vincristine, and vincristine sulfate; CHOEP-like, carmustine, cyclophosphamide, cytarabine, doxorubicin, etoposide, melphalan, methotrexate, prednisone, and vincristine; CHOP, cyclophosphamide, doxorubicin, doxorubicin hydrochloride, prednisolone, prednisone, vincristine, and vincristine sulfate; CHOP-like, cyclophosphamide, calcium folinate, epirubicin, etoposide, dexamethasone, eexrazoxane, doxorubicin, prednisolone, prednisone, pralatrexate, vincristine, vincristine sulfate, and procarbazine; CHOP-R, cyclophosphamide, doxorubicin, etoposide, prednisolone, prednisone, methotrexate, rituximab, vincristine, and vincristine sulfate; ECOG, Eastern Cooperative Oncology Group; ICE, ifosfamide, carboplatin, and etoposide; ICE-like, carboplatin, etoposide, and ifosfamide; ICE-like, carboplatin, cisplatin, dexamethasone, etoposide, ifosfamide, mesna; single agent, alisertib, belinostat, brentuximab vedotin, calcium folinate, chidamide, lambrolizumab, lenalidomide, lirilumab, nivolumab, pralatrexate, rituximab, romidepsin, and ruxolitinib; max, maximum; min, minimum.
PTCL-NOS = includes all patients regardless of CXCL12 status.
Total = 38 AITL + 25 PTCL-NOS + 2 “other” (ALCL ALK-negative and PTCL-subtype not specified per protocol).
Prior therapies (>5% of patients); patients may have received >1 therapy: CHOEP, CHOEP-like, CHOP, CHOP-like, CHOP-R, ICE, and ICE-like.
Efficacy
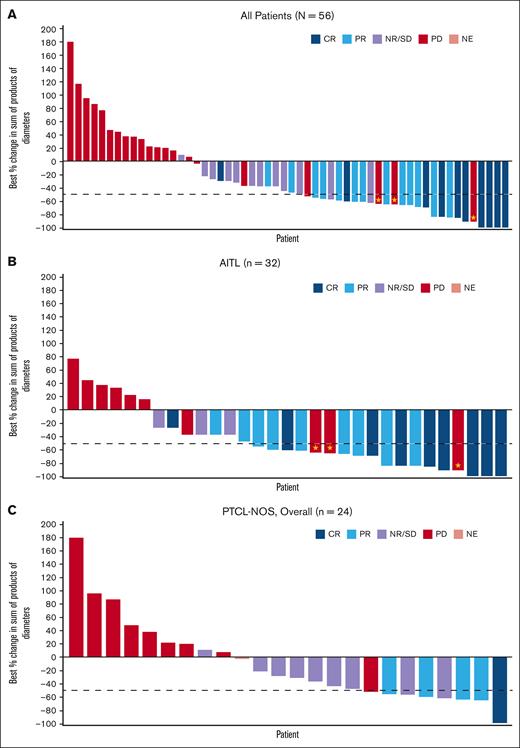
Of 65 enrolled patients, 58 were efficacy evaluable, including 32 with AITL, 24 with PTCL-NOS, and 2 other histologies. A total of 7 patients were not included in the efficacy evaluable analysis set for the following reasons: no baseline data; failure to receive at least 1 dose of tipifarnib; and/or no postbaseline end point data subsequent to at least 1 dose of study drug. In terms of the primary end point, the ORR in the efficacy evaluable analysis set (n = 58) was 39.7% (95% confidence interval [CI], 28.1-52.5) with 22.4% (n = 13) achieving a PR and 17.2% (n = 10) of patients achieving a CR (Table 2; Figure 1A). The secondary efficacy analysis demonstrated that patients with AITL in the efficacy evaluable analysis set (n = 32) had a higher ORR of 56.3% (95% CI, 39.3-71.8), with 28.1% of patients achieving a PR and 28.1% a CR (n = 9 for each) (Table 2; Figure 1B,D). The ORR in the PTCL-NOS subgroup in the efficacy evaluable analysis set (n = 24) was 20.8% (95% CI, 9.2-40.5), including 16.7% (n = 4) with a PR and 4.2% (n = 1) with a CR (Table 2; Figure 1C).
Summary of tumor response, PFS, DOR, and OS in the efficacy analysis set
| Cohort . | AITL (n = 32) . | PTCL-NOS (n = 24) . | Other∗ (n = 2) . | Total† (N = 58) . |
|---|---|---|---|---|
| Primary: ORR (CR + PR), % (n; 95% CI; P value) | 56.3 (18; 39.3, 71.8; P < .001) | 20.8 (5; 9.2, 40.5; P = .077) | 0 (0; 0.0, 84.2; P = 1.000) | 39.7 (23; 28.1, 52.5; P < .001) |
| CR, n (%) | 9 (28.1) | 1 (4.2) | 0 | 10 (17.2) |
| PR, n (%) | 9 (28.1) | 4 (16.7) | 0 | 13 (22.4) |
| Stable disease, n (%) | 3 (9.4) | 9 (37.5) | 0 | 12 (20.7) |
| Progressive disease, n (%) | 11 (34.4) | 9 (37.5) | 2 (100) | 22 (37.9) |
| Nonevaluable‡, n (%) | 0 | 1 (4.2) | 0 | 1 (1.7) |
| Secondary: mPFS (mo) (n; 95% CI) | 3.6 (32; 1.9-8.3) | 3.5 (24; 1.8-5.3) | 1.4 (2; 1.1-1.6) | 3.5 (58; 2.1-4.4) |
| mDOR (mo) (n; 95% CI) | 7.8 (18; 2.0-16.3) | 2.0 (5; 1.0 to NA) | NA (0; NA to NA) | 3.7 (23; 2.0-15.3) |
| Exploratory: mOS (mo) (n; 95% CI) | 32.8 (32; 16.7 to NA) | 21.7 (24; 7.0 to NA) | NA (2; 4.7 to NA) | 32.8 (58; 14.4 to NA) |
| Cohort . | AITL (n = 32) . | PTCL-NOS (n = 24) . | Other∗ (n = 2) . | Total† (N = 58) . |
|---|---|---|---|---|
| Primary: ORR (CR + PR), % (n; 95% CI; P value) | 56.3 (18; 39.3, 71.8; P < .001) | 20.8 (5; 9.2, 40.5; P = .077) | 0 (0; 0.0, 84.2; P = 1.000) | 39.7 (23; 28.1, 52.5; P < .001) |
| CR, n (%) | 9 (28.1) | 1 (4.2) | 0 | 10 (17.2) |
| PR, n (%) | 9 (28.1) | 4 (16.7) | 0 | 13 (22.4) |
| Stable disease, n (%) | 3 (9.4) | 9 (37.5) | 0 | 12 (20.7) |
| Progressive disease, n (%) | 11 (34.4) | 9 (37.5) | 2 (100) | 22 (37.9) |
| Nonevaluable‡, n (%) | 0 | 1 (4.2) | 0 | 1 (1.7) |
| Secondary: mPFS (mo) (n; 95% CI) | 3.6 (32; 1.9-8.3) | 3.5 (24; 1.8-5.3) | 1.4 (2; 1.1-1.6) | 3.5 (58; 2.1-4.4) |
| mDOR (mo) (n; 95% CI) | 7.8 (18; 2.0-16.3) | 2.0 (5; 1.0 to NA) | NA (0; NA to NA) | 3.7 (23; 2.0-15.3) |
| Exploratory: mOS (mo) (n; 95% CI) | 32.8 (32; 16.7 to NA) | 21.7 (24; 7.0 to NA) | NA (2; 4.7 to NA) | 32.8 (58; 14.4 to NA) |
ALK, anaplastic lymphoma kinase; mDOR, median DOR; mo, months; mOS, median overall survival; mPFS, median PFS; NA, not applicable; PD, progressive disease; SD, stable disease.
Other includes ALCL ALK-negative and PTCL-subtype not specified per protocol.
Total = 32 AITL + 24 PTCL-NOS + 2 other. A total of 7 patients were not included in the efficacy analysis set because of lack of postbaseline data subsequent to at least 1 dose of study drug.
Nonevaluable: patient was on study for 1 cycle; postdose scan at end of treatment visit was not collected for all lesions.
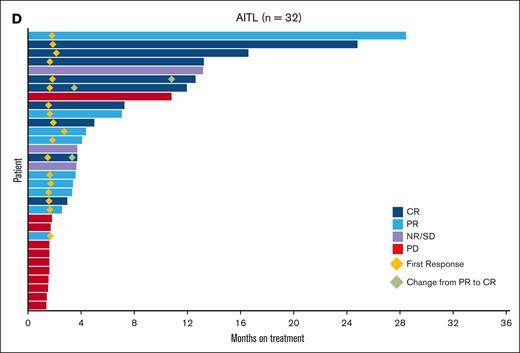
Response to tipifarnib. A. Waterfall plot. Best percent change from baseline in sum of tumor diameters in the efficacy analysis set (all participants cohort). N = 56: 2 patients were excluded because they did not have postbaseline index lesion measurements. Sum of product of longest diameter and shortest diameter of all tumors assessed in each visit in square mm. ⋆ Best % change in SPD of >50% but new lesion(s) reported at the same visit. (B) Waterfall plot. Best percent change from baseline in sum of tumor diameters in the efficacy analysis set (AITL cohort). N = 31: 1 patient did not have posttreatment measurements because of obvious deterioration; patient had a best overall response of PD. ⋆ Best % change in SPD of >50% but new lesion(s) reported at the same visit. (C) Waterfall plot. Best percent change from baseline in sum of tumor diameters in the efficacy analysis set (PTCL-NOS, overall cohort). N = 24: 1 patient did not have posttreatment measurements because of obvious deterioration; patient had a best overall response of PD. (D) Swimmer plot. DOR and time to response in patients in the AITL cohort receiving tipifarnib. NE, nonevaluable; NR/SD, no response or stable disease; PD, progressive disease; SD, stable disease; SPD, sum of products of diameters.
Response to tipifarnib. A. Waterfall plot. Best percent change from baseline in sum of tumor diameters in the efficacy analysis set (all participants cohort). N = 56: 2 patients were excluded because they did not have postbaseline index lesion measurements. Sum of product of longest diameter and shortest diameter of all tumors assessed in each visit in square mm. ⋆ Best % change in SPD of >50% but new lesion(s) reported at the same visit. (B) Waterfall plot. Best percent change from baseline in sum of tumor diameters in the efficacy analysis set (AITL cohort). N = 31: 1 patient did not have posttreatment measurements because of obvious deterioration; patient had a best overall response of PD. ⋆ Best % change in SPD of >50% but new lesion(s) reported at the same visit. (C) Waterfall plot. Best percent change from baseline in sum of tumor diameters in the efficacy analysis set (PTCL-NOS, overall cohort). N = 24: 1 patient did not have posttreatment measurements because of obvious deterioration; patient had a best overall response of PD. (D) Swimmer plot. DOR and time to response in patients in the AITL cohort receiving tipifarnib. NE, nonevaluable; NR/SD, no response or stable disease; PD, progressive disease; SD, stable disease; SPD, sum of products of diameters.
The median PFS rate in the efficacy evaluable analysis set (n = 58) was 3.5 months (95% CI, 2.1-4.4) and was similar for AITL (3.6 months [95% CI, 1.9-8.3]) and PTCL-NOS (3.5 months [95% CI, 1.8-5.3]) subtypes (Table 2; supplemental Figure 2). The median DOR was 3.7 months (95% CI, 2.0-15.3), with the AITL cohort experiencing the longest DOR at 7.8 months (95% CI, 2.0-16.3). The time to response was rapid, with most responders (18 responders of 32 patients) achieving a response within 4 months (Table 2; Figure 1D). The median OS was 32.8 months (95% CI, 14.4 to not available; Table 2; supplemental Figure 3). A total of 63 (96.9%) patients discontinued study treatment: 41 (63.1%) because of disease progression, and a total of 13 (20.0%) patients had treatment-emergent adverse events (AEs) leading to study drug discontinuation. Two patients remained on treatment until the end of the trial.
Safety
All 65 enrolled patients were evaluable for safety. The mean treatment duration was 5.9 cycles (range, 1-37) in all patients, with no significant differences between AITL and PTCL-NOS subtypes (supplemental Table 3). The mean number of study drug interruptions was 3.12 (range, 1-14), and the mean number of reductions per patient for those experiencing a disruption (defined as drug interruptions, or dose reductions or increases) was 1.33 (range, 1-3; supplemental Table 4). Concomitant colony stimulating factors were used in 27 (41.5%) of all patients. Most patients (57 [87.7%]) experienced at least 1 treatment-related AE (TRAE; as defined by the Medical Dictionary for Regulatory Activities Terminology), with neutropenia (43.1%), thrombocytopenia (36.9%), anemia (30.8%), nausea (29.2%), and diarrhea (27.7%) being the most common (supplemental Table 5). Forty-three patients (66.2%) experienced at least 1 grade ≥3 TRAE, of which 56.9% were related to blood and lymphatic system disorders, including neutropenia (38.5%), thrombocytopenia (32.3%), anemia (21.5%), leukopenia (16.9%), and febrile neutropenia (15.4%; Table 3). Despite these AEs, tipifarnib with the permitted dose reductions was well tolerated, with 7.7% of patients who experienced at least 1 TRAE discontinuing the study drug (Table 3).
Incidence of TRAEs grade 3 or more and TEAEs leading to discontinuation in safety analysis set
| . | AITL (n = 38) . | PTCL-NOS∗ (n = 25) . | Total† (N = 65) . |
|---|---|---|---|
| Total number of grade ≥3 TRAEs | 114 | 66 | 182 |
| Patients with at least 1 grade ≥3 TRAE, n (%) | 26 (68.4) | 16 (64) | 43 (66.2) |
| Blood and lymphatic system disorders, n (%) | 22 (57.9) | 14 (56) | 37 (56.9) |
| Neutropenia | 11 (28.9) | 14 (56) | 25 (38.5) |
| Thrombocytopenia | 13 (34.2) | 8 (32) | 21 (32.3) |
| Anemia | 10 (26.3) | 4 (16) | 14 (21.5) |
| Leukopenia | 4 (10.5) | 6 (24) | 11 (16.9) |
| Febrile neutropenia | 4 (10.5) | 5 (20) | 10 (15.4) |
| Total number of TEAEs leading to permanent study drug discontinuation (%) | 11 | 12 | 23 |
| Patients with at least 1 TEAE leading to permanent study drug discontinuation | 9 (23.7) | 4 (16) | 13 (20.0) |
| Blood and lymphatic system disorders | 3 (7.9) | 3 (12) | 6 (9.2) |
| Anemia | 1 (2.6) | 1 (4) | 2 (3.1) |
| Thrombocytopenia | 1 (2.6) | 1 (4) | 2 (3.1) |
| Cytopenia | 0 | 1 (4) | 1 (1.5) |
| Hemolytic anemia | 1 (2.6) | 0 | 1 (1.5) |
| Histiocytosis hematophagic | 0 | 1 (4) | 1 (1.5) |
| Pancytopenia | 1 (2.6) | 0 | 1 (1.5) |
| Total number TRAEs leading to permanent study drug discontinuation | |||
| Patients with at least 1 TRAE leading to permanent study drug discontinuation | 4 (10.5) | 1 (4) | 5 (7.7) |
| Blood and lymphatic system disorders | 2 (5.3) | 0 | 2 (3.1) |
| Anemia | 1 (2.6) | 0 | 1 (1.5) |
| Pancytopenia | 1 (2.6) | 0 | 1 (1.5) |
| . | AITL (n = 38) . | PTCL-NOS∗ (n = 25) . | Total† (N = 65) . |
|---|---|---|---|
| Total number of grade ≥3 TRAEs | 114 | 66 | 182 |
| Patients with at least 1 grade ≥3 TRAE, n (%) | 26 (68.4) | 16 (64) | 43 (66.2) |
| Blood and lymphatic system disorders, n (%) | 22 (57.9) | 14 (56) | 37 (56.9) |
| Neutropenia | 11 (28.9) | 14 (56) | 25 (38.5) |
| Thrombocytopenia | 13 (34.2) | 8 (32) | 21 (32.3) |
| Anemia | 10 (26.3) | 4 (16) | 14 (21.5) |
| Leukopenia | 4 (10.5) | 6 (24) | 11 (16.9) |
| Febrile neutropenia | 4 (10.5) | 5 (20) | 10 (15.4) |
| Total number of TEAEs leading to permanent study drug discontinuation (%) | 11 | 12 | 23 |
| Patients with at least 1 TEAE leading to permanent study drug discontinuation | 9 (23.7) | 4 (16) | 13 (20.0) |
| Blood and lymphatic system disorders | 3 (7.9) | 3 (12) | 6 (9.2) |
| Anemia | 1 (2.6) | 1 (4) | 2 (3.1) |
| Thrombocytopenia | 1 (2.6) | 1 (4) | 2 (3.1) |
| Cytopenia | 0 | 1 (4) | 1 (1.5) |
| Hemolytic anemia | 1 (2.6) | 0 | 1 (1.5) |
| Histiocytosis hematophagic | 0 | 1 (4) | 1 (1.5) |
| Pancytopenia | 1 (2.6) | 0 | 1 (1.5) |
| Total number TRAEs leading to permanent study drug discontinuation | |||
| Patients with at least 1 TRAE leading to permanent study drug discontinuation | 4 (10.5) | 1 (4) | 5 (7.7) |
| Blood and lymphatic system disorders | 2 (5.3) | 0 | 2 (3.1) |
| Anemia | 1 (2.6) | 0 | 1 (1.5) |
| Pancytopenia | 1 (2.6) | 0 | 1 (1.5) |
ALK, anaplastic lymphoma kinase; TEAE, treatment-emergent adverse event; other cohorts, other includes ALCL ALK-negative and PTCL-subtype not specified per protocol.
PTCL-NOS = includes all patients regardless of CXCL12 status.
Total = 38 AITL + 25 PTCL-NOS + 2 “other” (ALCL ALK-negative and PTCL-subtype not specified per protocol).
Serious TRAEs were reported in 18 (27.7%) patients. The most frequent serious TRAEs included febrile neutropenia (10.8%), pancytopenia (anemia, thrombocytopenia, and leukopenia; 4.6%), and neutropenia (3.1%). Six patients (9.2%) died within 30 days of the last dose of study drug (or immediately before the administration of another anticancer treatment); reasons for death were lung infection, dehydration, death not otherwise specified, cardiopulmonary failure, sepsis, and respiratory failure. None of the deaths were considered related to study drug, except for lung infection (n = 1).
Biomarker data
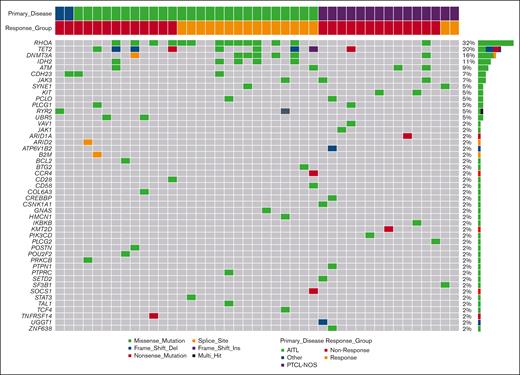
Forty-three patients had baseline samples with sufficient tumor tissue for mutational analysis by whole-exome sequencing (AITL, n = 26; PTCL-NOS, n = 15; and “other,” n = 2). The investigation of baseline mutation profiles focused on known or likely pathogenic mutations in 300 genes related to PTCL.8-12,32-34 Oncoplots showing the distribution of mutations according to the disease subtype are shown in Figure 2 and supplemental Figures 4 and 5. Known or likely pathogenic somatic mutations in baseline tumor tissue were identified in the RhoA, TET2, DNMT3A, and IDH2 genes in 32% (14/43), 20% (9/43), 16% (7/43), and 11% (5/43) of patients, respectively (Figure 2). The AITL subgroup had a higher prevalence of mutations in RhoA (AITL, 50% [13/26] vs PTCL-NOS, 6.7% [1/15]), TET2 (AITL, 31% [8/26] vs PTCL-NOS, 6.7% [1/15]), DNMT3A (AITL, 23% [6/26] vs PTCL-NOS, 6.7% [1/15]), and IDH2 (AITL 19% [5/26] vs PTCL-NOS, 0% [0/15]; supplemental Figure 4).
Oncoplot of known somatic and possible somatic variants in All PTCL cohort (n = 43). Only genes with at least 1 mutation are on the oncoplot. The y-axis on the right of the histogram displays frequency and number of samples for each gene; the x-axis displays the type of mutation. No obvious distribution pattern of mutations within the response groups.
Oncoplot of known somatic and possible somatic variants in All PTCL cohort (n = 43). Only genes with at least 1 mutation are on the oncoplot. The y-axis on the right of the histogram displays frequency and number of samples for each gene; the x-axis displays the type of mutation. No obvious distribution pattern of mutations within the response groups.
Within the AITL subset, 61.5% (16/26) of patients had at least 1 mutation in RhoA, TET2, DNMT3A, or IDH2, and 67% (10/15) had a response. In contrast, 42% (11/26) of tumors did not have a mutation in any of the 4 key genes and the ORR for that AITL subset was 33% (5/15). Compared with nonresponders, AITL responders had a higher frequency of mutations in RhoA (60% [9/15] vs 36% [4/11]), DNMT3A (33% [5/15] vs 9% [1/11]) and IDH2 (27% [4/15] vs 9% [1/11]). No obvious correlation was observed for TET2 mutations between responders and nonresponders.
In the PTCL-NOS cohort, mutations in ATM (12%; 2/15), JAK3 (12%; 2/15), and KIT (12%; 2/15) were most common, whereas only individual patients harbored a mutation in either RhoA, TET2, or DNMT3A (supplemental Figure 5).
Discussion
The results from this international phase 2 trial suggest that in heavily pretreated relapsed/refractory PTCL, tipifarnib 300 mg orally twice daily on days 1 to 21 on 28-day cycles has antitumor efficacy with a manageable safety profile. For all 58 efficacy-evaluable patients, the ORR (CR + PR) was 39.7%, with 23 patients achieving a CR (17.2%) or PR (22.4%) and a median PFS and DOR of 3.5 and 3.7 months, respectively. These results are similar to an earlier study by Witzig et al in which single-agent tipifarnib in a similar sample of patients primarily in their 60s with mainly stage IV, heavily pretreated T-cell and Hodgkin lymphoma, produced an ORR of 31%, a median DOR of 7.5 months (95% CI, 3.2-29.8), and an OS of 19.7 months (95% CI, 9-60), with manageable toxicities.27
Tipifarnib was highly active in the AITL subtype, with an ORR of 56.3%, a DOR of 7.8 months, and an OS of 32.8 months. In this trial, the ORR in the AITL subset (56.3%) was higher than responses observed in single-agent phase 2 studies in similar relapsed/refractory PTCL populations with brentuximab vedotin (54%), belinostat (45.5%), romidepsin (30%; PTCL indication withdrawn from the US market), and pralatrexate (8%).35-38 In the PTCL-NOS cohort in this trial, the ORR (20.8%) was comparable with brentuximab vedotin (33%), belinostat (23.4%), romidepsin (29%), and pralatrexate (32%; pralatrexate study not designed to evaluate ORR in specific subsets).35-38
Toxicities observed in this trial were consistent with the known safety profile of tipifarnib. The most common treatment-emergent AEs included hematologic events, gastrointestinal disturbances, and fatigue. Decreased blood counts were anticipated in this study given the liberal eligibility criteria for absolute neutrophil count and platelet counts in these heavily pretreated patients (supplemental Table 1). The most common grade ≥3 TRAEs were hematologic in nature; however, because myelosuppression was manageable with treatment interruption, dose reduction, and/or growth factor support, very few patients (3.1%) discontinued because of hematologic TRAEs. It is noteworthy that after study closeout, 3 patients with a CR (2 AITL, and 1 PTCL-NOS) transitioned to the tipifarnib compassionate use program and have remained on treatment between 2 and 4 years. All 3 patients are currently active in the program, confirming the tolerability, safety, and durability of response with long-term tipifarnib treatment.
Biomarker analysis is consistent with previous data demonstrating frequent mutations in TET2 and DNMT3A genes in these and other T-cell lymphomas arising from TFH cells.8-12,33,35,39-41 When the top 300 previously identified mutated genes in PTCL were interrogated in our biomarker correlative studies, RhoA, TET2, and DNMT3A were highly mutated. Specifically, mutations in the RhoA, TET2, DNMT3A, and IDH2 genes were most prevalent in the AITL subtype compared with the PTCL-NOS subtype. In this study, and in accordance with published reports, the majority (80%) of IDH2 mutations co-occurred with TET2 mutations.10,11,39-42 Although TET2 mutations were evenly distributed between responders and nonresponders, the biomarker analyses revealed that AITL responders in this study were more likely to harbor a mutation in RhoA. RhoA mutations are common in T-cell lymphomas,43 especially TFH cells that characterize AITL, and are usually G17V resulting in a loss of function.42 They are typically associated with a mutation in another epigenetic regulator. Indeed, in Figure 2, in all but 1 case, the RhoA mutations were associated with a mutation in IDH2, DNMT3A, or TET2. In mouse models of RhoA G17V-mutant AITL,44 the tumor cells show increased proliferation and activation of the phosphoinositide-3 kinase–protein kinase B– mammalian target of rapamycin (mTOR) and mitogen-activated protein kinase (MAPK) pathways.
Tipifarnib’s mechanism of action in lymphoma has previously been shown to be due to MAPK inhibition,45 which provides a potential explanation as to why tumors with RhoA mutations have a higher response rate. Notably, 1 patient with CR with AITL currently enrolled in tipifarnib’s compassionate use program carries mutations in RhoA and in DNMT3A (no mutational data available for the other patient with AITL in the compassionate use program) and has remained on treatment for 2 years. Cell-based screening demonstrated that AITL cell lines harboring mutations in DNMT3A and/or TET2 were sensitive to tipifarnib, but growth of AITL cells with wild-type DNMT3A and TET2 was not inhibited by tipifarnib.26 Patients with tumors that had mutations in any of the DNMT3A, IDH12, and RhoA genes had an ORR of 75%, whereas a lack of any 1 of those mutations led to a decreased ORR of 30%. These data are intriguing in that they suggest increased tipifarnib activity in TFH cells may be associated with the mutational landscape in these tumors, and that mutational data could potentially be used as selection criteria for patient selection for therapies with FTIs such as tipifarnib.40-42
RhoA mutations render T cells particularly susceptible to T-cell receptor–mediated activation of the extracellular signal-regulated kinase (ERK) and protein kinase B pathways.42 A previous study demonstrated that signaling through the ERK pathway is affected when tipifarnib interrupts signaling downstream of the RAS guanine nucleotide exchange factor RasGRP1,45 an ERK pathway activator that is uniquely expressed in lymphoid cells, especially T cells. In addition, signaling through the phosphoinositide-3 kinase/mTOR pathway is affected when tipifarnib interrupts the farnesylation of the small guanosine triphosphate hydrolase Ras homolog enriched in the brain (Rheb), which is required for mTOR activation.46 Thus, tipifarnib has the ability to simultaneously inhibit the 2 main pathways activated when RhoA is mutated in AITL. Simultaneous inhibition of the 2 major activated pathways in RhoA-mutated AITLs could be lethal to lymphoma cells. Various combinations have been proposed and might be worth consideration, such as combinations with standard-of-care regimens, immunotherapy, histone deacetylase inhibitors, and brentuximab. Recent studies suggested that combination therapy with different epigenetic modulatory agents may be synergistic in patients with relapsed/refractory PTCL.47-49 In an exploratory analysis conducted by Bachy et al, previously untreated patients with AITL and other T-cell lymphomas with TFH phenotype demonstrated prolonged PFS when treated with romidepsin-CHOP (n = 103; median PFS, 19.5 months) compared to CHOP (n = 98; median PFS, 10.6 months)49; however, the Bachy study was not designed to assess PFS benefit in this group.49,50 In another study, the combination of romidepsin and oral 5-azacytidine was shown to be synergistic and to have an ORR of 61% (80% in TFH subtype) in a phase 1/2 study in patients with relapsed/refractory PTCL.48
Although preliminary results for the trial included the use of CXCL12 single-nucleotide polymorphism as a biomarker, mature data do not support the use of CXCL12 as a biomarker of clinical response to tipifarnib in PTCL. Correlation of higher CXCL12 expression levels is stronger in other cancers.51
The correlative observations for mutation profile and clinical response to tipifarnib are useful because this study used only 1 drug (tipifarnib), allowing for easier interpretation of drug–response relationship compared with a combination regimen. Because this was a proof-of-concept study, investigator-initiated trials that fine-tune combinations would be encouraged. These analyses need to be tested in future studies but are quite promising and relevant given the increasing clinical application of mutational profiling in patients with lymphomas in general and T-cell types specifically. These data should stimulate further research into the biology of PTCL and how these specific mutations lead to susceptibility with farnesyltransferase inhibition. Future studies should incorporate mutation analysis and explore potentially synergistic combinations of tipifarnib with other active agents in relapsed PTCL as well as its potential role as an alternative to multiagent chemotherapy regimens as a bridging agent to stem cell transplant or chimeric antigen receptor T-cell approaches.
Acknowledgments
The authors thank Cynthia Gioiello and Courtney Breuel of MedVal Scientific Information Services, LLC, for medical writing and editorial assistance, which were funded by Kura Oncology, Inc. This manuscript was prepared according to the International Society for Medical Publication Professionals’ “Good Publication Practice for Communicating Company-Sponsored Medical Research: GPP3.”
This study was funded by Kura Oncology, Inc.
Authorship
Contribution: T.W., F.F., A.S., N.B., J.M., and M.L. conceptualized and designed the study, and provided administrative support; T.W., L.S., W.S.K., F.d.l.C.V., A.M.G.-S., R.A., J.M.R.V., R.d.O.N., A.M.-N., A.R.I., M.J.T., E.D.-D., and F.F. provided study materials or recruited patients; T.W., L.S., W.S.K., F.d.l.C.V., A.M.G.-S., R.A., J.M.R.V., R.d.O.N., A.M.-N., A.R.I., M.J.T., E.D.-D., and F.F. collected and assembled data; all authors analyzed and interpreted data, wrote the manuscript and approved the final manuscript; and are accountable for all aspects of the work.
Conflict-of-interest disclosure: T.W. received clinical trial support from Kura Oncology, Inc. L.S. reports advisory board role with Kyowa-Kirin Pharma, Secura Bio, CRISPR Therapeutics, Daiichi Sankyo, and EUSA Pharma; consulted for Dren Biol; and received research support from Kyowa-Kirin Pharma and EUSA Pharma. W.S.K. received grants or contracts from Sanofi, Boryong, and Kyowa-Kyrin; received consulting fees from Celltrion; and received honoraria from Takeda. F.d.l.C.V. received personal fees and nonfinancial support from Takeda, Roche, AbbVie, and Janssen; as well as personal fees from AstraZeneca, Kyowa Kirin, EUSA Pharma, and BeiGene outside the submitted work. A.M.G.-S. reports consulting fees, honoraria, or research grants from Janssen, Roche, Celgene/Bristol Myers Squibb, Kyowa Kirin, Clinigen, EUSA Pharma, Novartis, Gilead/Kite, Incyte, Lilly, Takeda, ADC Therapeutics America, Miltenyi, Ideogen, and AbbVie. R.A. received consulting fees, honoraria, or research grants from Kura, Merck, Epizyme, Daiichi, Morphosys, BeiGene; and reports participation on a data safety monitoring board or advisory board of Genentech/Roche, Sanofi, and ADC Therapeutics. A.M.-N. reports participation on a data safety monitoring board or advisory board of Kiowa Kirin, Takeda, Janssen, AbbVie, Kite, AstraZeneca, Roche, and Lilly; and received honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Takeda, Janssen, Kite, Lilly, and AbbVie. E.D.-D. reports participation on a data safety monitoring board or advisory board of Takeda. A.S., N.B., J.M., and M.L., are employees of Kura Oncology, Inc. F.F. received consulting fees, honoraria, or research grants from Seagen and Acrotech; received honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Seagen and Acrotech; reports participation on a data safety monitoring board or advisory board of Crisper and Astex; reports leadership or fiduciary role in other board, society, committee, or advocacy group, paid or unpaid of United States Cutaneous Lymphoma Consortium (officer) and the Cutaneous Lymphoma Foundation (advisory committee member). The remaining authors declare no competing financial interests.
Correspondence: Francine Foss, Medical Oncology, Yale Hematology, Smilow Cancer Hospital at Yale New Haven, 35 Park St, New Haven, CT 06511; email: francine.foss@yale.edu; and Thomas Witzig, Division of Hematology, Department of Medicine, Mayo Clinic, 200 1st St SW, Rochester, MN 55905; email: witzig.thomas@mayo.edu.
References
Author notes
Presented at the 59th American Society of Hematology annual meeting and exposition, Atlanta, GA, 9 to 12 December 2017; the 60th American Society of Hematology annual meeting and exposition, San Diego, CA, 1 to 4 December 2018; the 61st American Society of Hematology annual meeting and exposition, Orlando, FL, 7 to 10 December, 2019; the 63rd American Society of Hematology annual meeting and exposition, Atlanta, GA, 11 to 14 December 2021; the 22nd European Hematology Association congress, Madrid, Spain, 22 to 25 June 2017; the 24th European Hematology Association congress, Amsterdam, The Netherlands, 13 to 16 June 2019; European Hematology Association annual meeting, virtual, 11 to 14 June 2020; the 14th International Conference on Malignant Lymphoma, Lugano, Switzerland, 14 to 17 June 2017; and the 15th International Conference on Malignant Lymphoma, Lugano, Switzerland, 18 to 22 June 2019.
Individual participant data will not be shared.
The full-text version of this article contains a data supplement.