Key Points
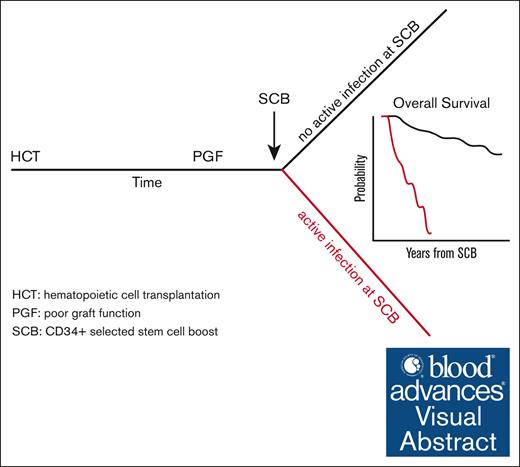
CD34+ selected SCB can restore hematopoiesis after PGF after allogeneic hematopoietic stem cell transplant.
An active infection at the time of CD34+ selected SCB is the strongest predictor of treatment failure and poor overall survival.
Visual Abstract
The use of CD34+ selected stem cell boost (SCB) after allogeneic hematopoietic cell transplant (allo-HCT) has been increasing. Predictors of treatment failure after SCB, both in the context of poor graft function (PGF) or other settings, are not well characterized. We report among the largest single-center retrospective experiences of the use of SCB and evaluate potential predictors of response and outcomes. A total of 58 patients who underwent HCT between 2015 and 2022 and who received SCB, were identified. The indication for SCB was predominantly PGF, defined as the presence of ≥2 cytopenias for at least 2 consecutive weeks beyond day +14 after allo-HCT in the presence of ≤30% bone marrow cellularity and ≥90% donor myeloid chimerism in the absence of morphologic disease. The median dose of infused CD34+ selected SCB products was 3.88 × 106 CD34+ cells per kg (range, 0.99 × 106 to 9.92 × 106). The median 2-year overall survival and nonrelapse mortality after SCB was 47% and 38%, respectively. The cumulative incidences of 6-month grade 3 to 4 acute and 2-year moderate-severe chronic graft-versus-host disease after SCB were 3.4% and 12%, respectively. Overall response (complete response + partial response) was attained in 36 of 58 patients (62%) and in 69% of patients with PGF. On multivariable analysis, an active infection at the time of SCB was the greatest predictor of poor response and survival (P = .013) after SCB. SCB can restore hematopoiesis in the majority of patients, particularly for those with PGF and in whom there is no active infection at the time of infusion.
Introduction
The use of CD34+ selected stem cell boost (SCB) after an allogeneic hematopoietic cell transplant (allo-HCT) has been increasing in the last 20 years.1-16 The most common indication has been for the treatment of poor graft function (PGF),1-5,7-16 defined as the development of at least 2 cytopenias in the presence of hypoplastic-aplastic bone marrow (BM) with full donor chimerism.1 In a systematic review and meta-analysis of studies describing the use of SCB for PGF with a median follow-up of 42 months after SCB, the overall survival (OS) rate was 54%.8
Reports of the use of CD34+ selected SCB in the setting of mixed donor chimerism suggest the therapy may have some efficacy in the context, although less so, than in the fully donor chimeric setting.6 Predictors of response or a lack thereof to SCB in patients after allo-HCT, either in patients with PGF or in those with cytopenias who do not meet criteria for PGF, are not well characterized. Whether transplant factors such as graft source or the time from post–allo-HCT engraftment to the development of cytopenias requiring SCB, the presence of acute or chronic graft-versus-host disease (GVHD) or therapy with myelosuppressive medications to treat GVHD, or product factors such as CD34 dose or cryopreservation play a role in predicting response to SCB and long-term survival is unknown.
We sought to address these current gaps in knowledge through analysis of a large single-center retrospective experience of the use of SCB after allo-HCT, with particular focus on potential predictors of response and OS after SCB.
Methods
Adult patients who underwent SCB after allo-HCT at the Dana-Farber Cancer Institute during the period from 1 January 2015 to 1 July 2022 were identified using an institutional database. Institutional practice is to generally consider SCB based on at least 2 cytopenias after initial engraftment, absence of gross relapse, and total unfractionated peripheral blood or marrow donor chimerism of >70%. CD34+ cell selection was performed according to procedures established based on the CliniMACS Users Operating Manual. Apheresis collection goal was at least 2 × 106 CD34+ cells per kg of recipient body weight after system processing, with standard recommendation to use prophylaxis against acute GVHD if >1 × 105 cells per kg of recipient body weight are infused. Detailed review of patient records was undertaken to evaluate the indication for SCB, including data from the peripheral blood and BM to evaluate for the presence of PGF. The latter was defined as the presence of at least 2 cytopenic lines for at least 2 consecutive weeks beyond day +14 after allo-HCT (with all patients in this data set attaining criteria for engraftment after allo-HCT), with cytopenias defined as hemoglobin (Hb) <10 g/dL, absolute neutrophil count (ANC) <1 × 109 cells per μL, and platelet count <30 × 109 cells per μL and/or the presence of transfusion requirement, in the presence of hypoplastic-aplastic marrow with full donor chimerism (patients with a hypoplastic-aplastic marrow had ≤30% cellularity, and full donor chimerism referred to ≥90% myeloid chimerism in the absence of active morphologic disease). Complete response (CR) to SCB was defined as the attainment of Hb >10 g/dL, ANC >1 × 109 cells per liter, and platelet count >30 × 109 cells per liter. Partial response (PR) was defined as transfusion independence without CR if previously transfusion dependent.1 Treatment failure was defined as not attaining CR or PR after boost. An active infection at the time of SCB was defined as one or more of: (1) positive blood culture or positive culture from a biopsy site preceding the SCB and necessitating antimicrobial coverage that continued on the day of SCB (in other words, the antimicrobial coverage course was not completed prior to or on the day of SCB), or (2) the clinical suspicion of active infection by the treating team that necessitated antimicrobial coverage that continued on the day of SCB.
Flow cytometry
All patients treated at the Dana-Farber Cancer Institute and included in this study provided consent for a standard institutional review board–approved protocol for post–allo-HCT correlative sample collection. Longitudinal peripheral blood samples after allo-HCT were collected at the discretion of the treating clinician. Peripheral blood mononuclear cells were isolated by Ficoll centrifugation and cryopreserved in 10% dimethyl sulfoxide until the time of analysis. A panel of antibodies customized to identify T-cell, natural-killer–cell (NK-cell), and B-cell subsets was applied to the collected samples as previously described.17 All flow cytometry cell staining was performed as previously described.17 Data were acquired on a BD LSR Fortessa flow cytometer and analyzed using FlowJo (Tree Star) software.
Statistical analysis
Baseline and transplant characteristics were reported descriptively. Univariable Cox regression analysis was performed to investigate clinical factors that were associated with response to SCB. The Kaplan-Meier method was used to estimate OS and progression-free survival (PFS), whereas cumulative incidences of nonrelapse mortality (NRM) and relapse were estimated in the context of a competing risks framework treating relapse and NRM as a competing event, respectively. OS was defined from the initiation of SCB to death from any cause or censored at the last clinical evaluation. Log-rank test was used for group comparison of OS and PFS, whereas Gray test18 was used for the comparison of cumulative incidence of NRM and relapse. To identify risk factors for survival outcomes after SCB, univariable Cox regression analysis for OS and PFS and Fine and Gray regression analysis19 for cumulative incidences of NRM and relapse were performed. Due to the limited event size, variables with a P value < .05 from univariable analysis were included in the multivariable analysis. The effect of response to SCB on survival outcomes was assessed treating time to response as a time-dependent variable in these models. Before modeling, the proportional hazards and linearity assumptions were examined. The linearity assumption for continuous variables was examined using the methods of restricted cubic spline function on relative hazard20 and classification and regression tree for survival data.21 Cutoff values for continuous variables were assessed using these methods. Immunologic parameters were analyzed primarily descriptively and compared using the Mann-Whitney U test for group comparison. All P value were 2-sided at a significance level of .05, and multiplicity was not considered. All calculations were performed using SAS 9.4 (SAS Institute, Inc, Cary, NC) and R version 3.5.1.
Results
A total of 58 patients received SCB during the study period, with a median time from post–allo-HCT engraftment to SCB of 164 days (range, 19-1728; Table 1). The median dose of infused CD34+ selected SCB products was 3.88 × 106 CD34+ cells per kg (range, 0.99 × 106 to 9.92 × 106). The majority of CD34+ selected SCB products were infused fresh, with 6 of 58 products (10%) cryopreserved before infusion. None of the latter were collected at the time of the original stem cell donation for transplant. Thirty-one patients (53%) had previously received a stem cell transplant from an HLA-matched unrelated donor, of whom 9 of 31 (29%) were from a single-antigen mismatched donor, whereas 27 of 58 (47%) received a transplant from a related donor, with 18 of 27 (66%) of these having received a haploidentical graft. Acute and chronic GVHD occurred in 11 (19%) and 5 of 58 patients (9%), respectively, before SCB. Disease relapse occurred in 13 of 58 patients (22%) before SCB, but all patients were in morphologic disease-free state at the time of SCB. All patients who received SCB had ≥1 cytopenia, with a median ANC and platelet count at the time of SCB of 0.86 × 109 cells per μL (range, 0 to 8.23 × 109) and 21.5 × 109 cells per μL (range, 2 × 109 to 1024 × 109), respectively, and a median Hb at the time of SCB of 8.2 g/dL (range, 6.6-12.8). The median myeloid chimerism at the time of SCB was 100% (range, 30%-100%), with 48 of 56 patients (83%) having >90% myeloid chimerism. The median T-cell chimerism at the time of SCB was 99% (range, 0%-100%), with 31 of 42 (74%) having >90% T-cell chimerism.
Baseline characteristics of patients receiving CD34+ selected SCB
| Total . | N . | % . |
|---|---|---|
| Patients | 58 | 100 |
| Recipient age at HCT, median (range) | 62 (18-76) | |
| Donor age at HCT, median (range), y | 34 (16-65) | |
| Donor type, n (%) | ||
| MRD | 9 | 16 |
| MUD | 22 | 38 |
| MMUD | 9 | 16 |
| Haplo | 18 | 31 |
| Disease at HCT, n (%) | ||
| AML/ALL | 23 | 40 |
| MDS/MPN | 20 | 34 |
| Other | 15 | 26 |
| Cell source at HCT, n (%) | ||
| BM | 16 | 28 |
| PBSC | 42 | 62 |
| PGF at boost, n (%) | ||
| Yes | 32 | 55 |
| No | 26 | 45 |
| Time from engraftment to boost, median (range), d | 164 (19-1728) | |
| CD34 per kg at boost, median (range) | 3.88 (0.99-9.92) | |
| CD34 product at boost, n (%) | ||
| Fresh | 50 | 86 |
| Cryopreserved | 6 | 10 |
| Data not available | 2 | 4 |
| Infection at boost, n (%) | ||
| Any | 15 | 26 |
| Fungal | 9 | 16 |
| Bacterial | 10 | 17 |
| Viral | 3 | 5 |
| Myelosuppressive medications∗at boost, n (%) | ||
| Yes | 24 | 41 |
| No | 34 | 59 |
| Active disease at boost†, n (%) | ||
| Yes | 10 | 17 |
| No | 48 | 83 |
| Myeloid chimerism at SCB, n (%) | ||
| ≥90% | 48 | 83 |
| <90% | 8 | 14 |
| NA | 2 | 3 |
| T-cell chimerism at SCB, n (%) | ||
| ≥90% | 31 | 53 |
| <90% | 11 | 19 |
| NA | 16 | 28 |
| BM fibrosis at boost, n (%) | ||
| Yes | 9 | 16 |
| No | 40 | 68 |
| NA | 9 | 16 |
| BM hemosiderin at boost, n (%) | ||
| Yes | 31 | 53 |
| No | 19 | 33 |
| NA | 8 | 14 |
| Total . | N . | % . |
|---|---|---|
| Patients | 58 | 100 |
| Recipient age at HCT, median (range) | 62 (18-76) | |
| Donor age at HCT, median (range), y | 34 (16-65) | |
| Donor type, n (%) | ||
| MRD | 9 | 16 |
| MUD | 22 | 38 |
| MMUD | 9 | 16 |
| Haplo | 18 | 31 |
| Disease at HCT, n (%) | ||
| AML/ALL | 23 | 40 |
| MDS/MPN | 20 | 34 |
| Other | 15 | 26 |
| Cell source at HCT, n (%) | ||
| BM | 16 | 28 |
| PBSC | 42 | 62 |
| PGF at boost, n (%) | ||
| Yes | 32 | 55 |
| No | 26 | 45 |
| Time from engraftment to boost, median (range), d | 164 (19-1728) | |
| CD34 per kg at boost, median (range) | 3.88 (0.99-9.92) | |
| CD34 product at boost, n (%) | ||
| Fresh | 50 | 86 |
| Cryopreserved | 6 | 10 |
| Data not available | 2 | 4 |
| Infection at boost, n (%) | ||
| Any | 15 | 26 |
| Fungal | 9 | 16 |
| Bacterial | 10 | 17 |
| Viral | 3 | 5 |
| Myelosuppressive medications∗at boost, n (%) | ||
| Yes | 24 | 41 |
| No | 34 | 59 |
| Active disease at boost†, n (%) | ||
| Yes | 10 | 17 |
| No | 48 | 83 |
| Myeloid chimerism at SCB, n (%) | ||
| ≥90% | 48 | 83 |
| <90% | 8 | 14 |
| NA | 2 | 3 |
| T-cell chimerism at SCB, n (%) | ||
| ≥90% | 31 | 53 |
| <90% | 11 | 19 |
| NA | 16 | 28 |
| BM fibrosis at boost, n (%) | ||
| Yes | 9 | 16 |
| No | 40 | 68 |
| NA | 9 | 16 |
| BM hemosiderin at boost, n (%) | ||
| Yes | 31 | 53 |
| No | 19 | 33 |
| NA | 8 | 14 |
Other includes patients with non-Hodgkin lymphoma, BM failure, chronic lymphocytic leukemia, and T-cell prolymphocytic leukemia. AML/ALL, acute myeloid leukemia or acute lymphoblastic leukemia; Haplo, haploidentical; MDS/MPN, myelodysplastic syndrome and/or myeloproliferative neoplasm; MMUD, single-antigen mismatched unrelated donor; MRD, HLA-matched related donor; MUD, HLA-matched unrelated donor; NA, not available; PBSC, peripheral blood stem cell.
Myelosuppressive medications include sirolimus, valganciclovir, ruxolitinib, cellcept, and tyrosine kinase inhibitor.
Refers to patients who had measurable residual disease in the BM or else were receiving therapy for relapse immediately before receiving CD34-selected SCB.
Survival outcomes after CD34+ selected SCB
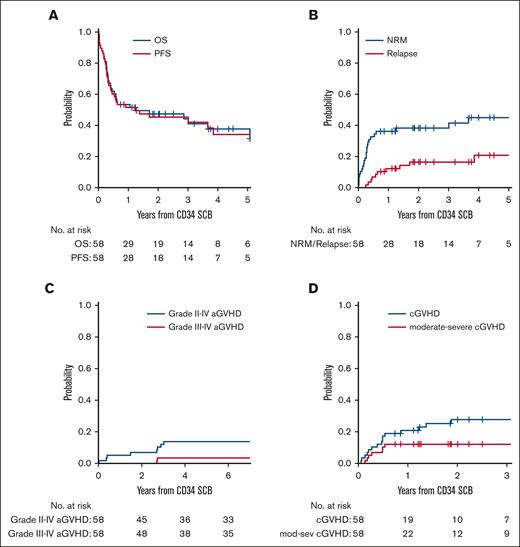
For the entire cohort, the median follow-up time among survivors was 28.5 months (range, 9-81). Patients had a median 2-year OS of 47% and 2-year PFS of 45% after SCB, with a median 2-year NRM of 38% and 2-year relapse of 16.5% after SCB (Figure 1; supplemental Table 1). The cumulative incidence of grade 2 to 4 acute GVHD at 6 months after SCB was 14%, with grade 3 to 4 acute GVHD of 3.4%. The cumulative incidence of chronic GVHD at 2 years after SCB was 28%, with 2-year moderate-severe chronic GVHD of 12% (supplemental Table 1).
Clinical outcomes after SCB. (A) OS and PFS of patients treated with SCB from the time of receiving the boost. (B) Cumulative incidence of NRM and relapse in patients who received SCB. (C) Cumulative incidence of grade 2 to 4 and grade 3 to 4 acute GVHD after SCB. (D) Cumulative incidence of cGVHD and mod-sev cGVHD after SCB. aGVHD, acute GVHD; cGVHD, chronic GVHD; mod-sev, moderate-severe.
Clinical outcomes after SCB. (A) OS and PFS of patients treated with SCB from the time of receiving the boost. (B) Cumulative incidence of NRM and relapse in patients who received SCB. (C) Cumulative incidence of grade 2 to 4 and grade 3 to 4 acute GVHD after SCB. (D) Cumulative incidence of cGVHD and mod-sev cGVHD after SCB. aGVHD, acute GVHD; cGVHD, chronic GVHD; mod-sev, moderate-severe.
PGF is associated with a trend for improved OS after CD34+ selected SCB
The indication for SCB was predominantly PGF as defined by Larocca et al1 in 32 of 58 patients (55%), with the remaining patients not meeting criteria for PGF most commonly because of incomplete donor chimerism (10/58 [17%]), absence of BM hypoplasia (8/58 [14%]), or presence of measurable nonmorphologic BM disease at the time of SCB (10/58 [17%]). Among patients with PGF, the overall response rate (ORR; CR + PR) was 69%, compared with 54% in patients without PGF. Patients with PGF treated with SCB had 2-year OS and NRM of 59% and 32%, respectively, whereas patients without PGF treated with SCB had 2-year OS and NRM of 29% and 45%, respectively (supplemental Figure 1). There was a trend for improved 2-year OS in patients with PGF compared with those without PGF treated with SCB (P = .08). In univariable analysis, the hazard ratio (HR) for OS was 1.72 (P = .12) for patients without PGF, and the subdistribution HR (sHR) for NRM was 1.42 for patients without PGF (P = .4; supplemental Table 2).
CR to CD34+ selected SCB is associated with OS
CR to SCB was attained in 26 of 58 patients (45%), with a median time to ANC response of 19 days (range, 3-153), median time to platelet response of 58 days (range, 13-1343), and median time to Hb response of 70 days (range, 19-343). PR was attained in an additional 10 of 58 patients (17%), with 22 (38%) not responding to SCB. To assess the effect of achieving CR on survival outcome, we treated response as a time-dependent variable in univariable and multivariable analysis. In univariable analysis, the HR for OS was 0.25 (P = .008) for CR, and the sHR for NRM was 0.25 for CR (P = .045; supplemental Table 2). In multivariable analysis, the HR for OS was 0.36 (P = .10) for CR, and the sHR for NRM was 0.34 for CR (P = .15; supplemental Table 3).
Active infection at the time of CD34+ selected SCB is the strongest predictor of treatment failure and poor survival
Fifteen patients (26%) had an active infection at the time of SCB. Among these, only 3 of 15 patients (20%; 2 CR and 1 PR) with active infection attained CR/PR, whereas 33 of 43 patients (77%) without active infection at the time of SCB attained CR/PR (P = .0001; supplemental Table 4). The diagnosis of PGF, the time from post-bone marrow transplant engraftment, the CD34+ per kg dose, the presence of splenomegaly, BM fibrosis, BM hemosiderin, presence of measurable residual disease, myeloid chimerism <90%, or cytomegalovirus reactivation at the time of SCB were not associated with treatment failure. The use of myelosuppressive medication such as valganciclovir, sirolimus, Jakafi, cellcept, or tyrosine kinase inhibitor at the time of SCB had no association with treatment failure (supplemental Table 5).
Thirteen of 15 patients with active infection, including 1 patient who achieved PR, died within 8 months of SCB, and 2 patients who achieved CR have survived for >5 years (Figure 2). On univariable analysis to evaluate potential predictors of survival after SCB, an active infection at the time of SCB had HR of 3.35 (95% confidence interval [CI], 1.65-6.8; P = .008) for OS and 4.61 (95% CI, 1.98-10.72; P = .0004) for NRM. In multivariable analysis, an active infection at the time of SCB was the strongest predictor of mortality, with an HR of 3.25 (95% CI, 1.28-8.22; P = .013) for OS and a sHR of 11.73 (95% CI, 2.36-58.3; P = .0026) for NRM (supplemental Table 3).
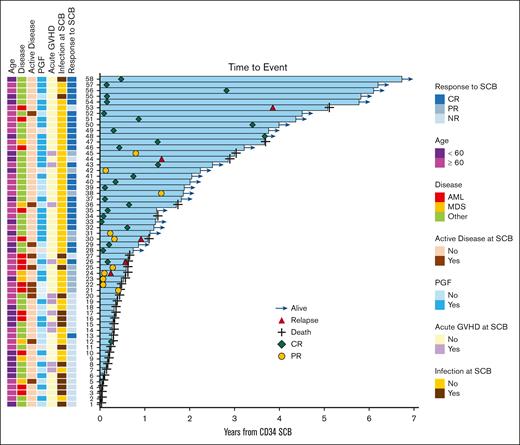
Swimmer plot of outcomes after SCB in all treated patients. Selected features evaluated as potential predictors of response to SCB (left). Clinical course of treated patients with outcomes (right). Response refers to hematologic response. Active disease refers to presence of nonmorphologic residual disease. Acute GVHD refers to the presence of any acute GVHD that onset before administering the SCB. AML, acute myeloid leukemia; MDS, myelodysplastic syndrome; NR, no response.
Swimmer plot of outcomes after SCB in all treated patients. Selected features evaluated as potential predictors of response to SCB (left). Clinical course of treated patients with outcomes (right). Response refers to hematologic response. Active disease refers to presence of nonmorphologic residual disease. Acute GVHD refers to the presence of any acute GVHD that onset before administering the SCB. AML, acute myeloid leukemia; MDS, myelodysplastic syndrome; NR, no response.
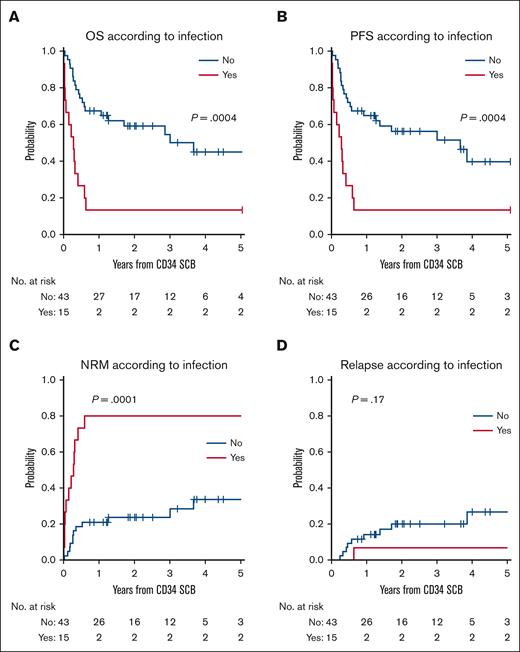
The 2-year OS after SCB among patients with active infection was 13% (range, 2%-35%), compared with 59% (P = .0004) for patients without infection. The 2-year cumulative incidence of NRM was 80% (95% CI, 46%-94%) for patients with infection vs 24% (P = .0001) for those without infection (Figure 3; supplemental Table 6). Among patients with active infection at the time of SCB, 8 of 15 (53%) had evidence of bacterial infection, and 9 of 15 (60%) had evidence of a fungal infection. The 3 patients with active infection who attained CR/PR after SCB had relatively mild infections, with 1 patient in CR having had Candida albicans infection responding to fluconazole, another CR having had suspected respiratory syncytial virus pneumonia while being covered with broad spectrum antimicrobials, and the third with PR having had enterococcus bacteremia treated with vancomycin but without any systemic symptoms. The latter patient died from a secondary malignancy. Among the patients with active infections who did not respond to SCB, the cause of death was related to infection in 50% of patients, whereas the remaining causes of death were related to relapse of original disease, acute respiratory distress syndrome, and idiopathic pneumonia syndrome.
Active infection at the time of SCB is associated with survival outcomes. OS (A) and PFS (B) with respect to the presence of active infection at the time of SCB. Cumulative incidence of NRM (C) and relapse (D) with respect to infection status at the time of SCB.
Active infection at the time of SCB is associated with survival outcomes. OS (A) and PFS (B) with respect to the presence of active infection at the time of SCB. Cumulative incidence of NRM (C) and relapse (D) with respect to infection status at the time of SCB.
Immune reconstitution before SCB is not associated with treatment response
We sought to evaluate whether immune reconstitution after allo-HCT has any association with the development of PGF needing SCB. Longitudinal correlative sample data on immune reconstitution were available in 29 of 58 patients in this retrospective cohort. Because the median time to SCB after allo-HCT was 6 months, we evaluated 2 time points preceding SCB: at 3 months after allo-HCT; and within 3 months of needing SCB. We found no association between immune reconstitution of T cells, B cells, or NK cells and the development of PGF needing SCB (supplemental Figure 2). We noted a trend for reduced T-cell counts before SCB in patients who developed an active infection at the time of SCB compared with those who did not (median absolute CD3+, 60 cells per μL vs 188 cells per μL; CD4+ conventional T cells, 29.3 cells per μL vs 69.5 cells per μL; CD4+ regulatory T cells [Treg], 5.1 cells per μL vs 9.1 cells per μL; CD8+, 19.6 cells per μL vs 65.5 cells per μL, respectively), but the differences did not meet statistical significance (supplemental Figure 3). We then compared immune reconstitution after allo-HCT in patients who responded to SCB with those who did not and found that there was no association between quantities of CD4+ conventional T cells, CD4+ Treg, CD8+ T, NK, or B lymphocytes at any of the evaluated time points and treatment response (supplemental Figure 2).
Discussion
The development of ≥1 cytopenia after allo-HCT is the result of numerous potential etiologies.22 CD34 selected SCB is a safe and effective treatment for patients with post–allo-HCT cytopenias, with most published data in the context of PGF.1-5,7-16 Predictors of response to SCB or a lack thereof have been difficult to evaluate because the number of patients reported in individual studies has been relatively small, and a pooled analysis of studies was limited by heterogeneity.8 Published studies with a sufficient number of patients in which a univariable and/or multivariable analysis of risk factors for lack of response to SCB could be performed are shown in Table 2, with smaller studies shown in supplemental Table 7. Although there is a range of OS and CR rates after SCB, it is important to point out that not all studies had the same indications for administering SCB (supplemental Table 8). The experience at our center is generally similar to the published experience at other centers, with an important consideration being that the definitions of PGF and response outcomes were not identical to the corresponding definitions in some of the other centers.
Outcomes after SCB in the published literature
| Publication . | No. of patients . | Indication for SCB∗ . | Median CD34+ 106/kg at SCB . | Best response† . | Median time to best response (range), d . | Median OS . | Active infection at SCB‡ . | Predictors of treatment failure after SCB on univariable or multivariable analysis . | Ref . |
|---|---|---|---|---|---|---|---|---|---|
| Al-Ramahi et al, 2023 | 51 | PGF | 4.8 | CR, 37/51 (72.5%), PR: 5/51 (9.8%) | 27.5 d (22-41) | 6 mo | 6/51 (12%) viral infections, 9/51 (18%) bacterial infections, 2/51 (4%) fungal infections | Poor Karnofsky performance status, prior acute GVHD, active hospitalization, bacterial infection at SCB | 12 |
| Gaffet et al, 2023 | 55 | PGF | 5.9 | 12-mo CR, 23/49 (47%), 12-mo PR, 5/49 (10%) | 19/23 (39%) attained CR at 30 d post SCB | 2-y OS 60% | 0/51 (0%) | Lower ANC at the time of SCB | 10 |
| Cuadrado et al, 2020 | 62 | PGF | 3.2 | CR, 39/62 (63%), PR, 8/62 (13%) | ANC, 29 d (6-1182), Plts, 18 d (5-600), Hb, 25 d (6-511) | All, 5.4 y, if in CR: 5-y OS 74% | 24/62 (39%) | Donor or recipient CMV seropositive, donor/recipient sex mismatch, active infection at time of boost | 7 |
| Mainardi et al, 2017 | 50 | PGF | 3.2 | CR, 18/50 (36.5%), PR, 21/50 (42.3%) | ANC, 64% attained response at 56 d post SCB, Plt: transfusion indep attained in 58.8% at 56 d post SCB, Hb: transfusion indep in 47% at 56 d post SCB | 5-y OS 38% | Not available | Donor age >40 at HCT | 13 |
| Publication . | No. of patients . | Indication for SCB∗ . | Median CD34+ 106/kg at SCB . | Best response† . | Median time to best response (range), d . | Median OS . | Active infection at SCB‡ . | Predictors of treatment failure after SCB on univariable or multivariable analysis . | Ref . |
|---|---|---|---|---|---|---|---|---|---|
| Al-Ramahi et al, 2023 | 51 | PGF | 4.8 | CR, 37/51 (72.5%), PR: 5/51 (9.8%) | 27.5 d (22-41) | 6 mo | 6/51 (12%) viral infections, 9/51 (18%) bacterial infections, 2/51 (4%) fungal infections | Poor Karnofsky performance status, prior acute GVHD, active hospitalization, bacterial infection at SCB | 12 |
| Gaffet et al, 2023 | 55 | PGF | 5.9 | 12-mo CR, 23/49 (47%), 12-mo PR, 5/49 (10%) | 19/23 (39%) attained CR at 30 d post SCB | 2-y OS 60% | 0/51 (0%) | Lower ANC at the time of SCB | 10 |
| Cuadrado et al, 2020 | 62 | PGF | 3.2 | CR, 39/62 (63%), PR, 8/62 (13%) | ANC, 29 d (6-1182), Plts, 18 d (5-600), Hb, 25 d (6-511) | All, 5.4 y, if in CR: 5-y OS 74% | 24/62 (39%) | Donor or recipient CMV seropositive, donor/recipient sex mismatch, active infection at time of boost | 7 |
| Mainardi et al, 2017 | 50 | PGF | 3.2 | CR, 18/50 (36.5%), PR, 21/50 (42.3%) | ANC, 64% attained response at 56 d post SCB, Plt: transfusion indep attained in 58.8% at 56 d post SCB, Hb: transfusion indep in 47% at 56 d post SCB | 5-y OS 38% | Not available | Donor age >40 at HCT | 13 |
CMV, cytomegalovirus; indep, independence; Plt, platelet; Ref, reference.
∗†‡Definitions of poor graft function, criteria for response, and definition of active infection used in the individual studies are presented in supplemental Table 8.
In these retrospective single-center data of the use of SCB, we demonstrate that responses (CR + PR) could be attained in 62% of treated patients. Among patients with PGF, the ORR was 69%, consistent with published data.7-16 Patients without PGF who received SCB all had ≥1 cytopenia but did not meet the criteria for PGF1 based on either donor chimerism, BM cellularity data, or presence of active disease. SCB was well tolerated and associated with a low incidence of grade 2 to 4 acute or moderate-severe chronic GVHD, consistent with the high CD34+ purity of the infused products. The median time to response differed based on the affected cell line, with the ANC generally meeting the response cutoff earlier than the platelets and the red blood cells taking the longest to recover, consistent with the expected courses of differentiation of these cell lines from CD34+ progenitors.
We evaluated numerous potential patient and graft characteristics as predictors of response to SCB, including the CD34 dose, whether the product was infused fresh or cryopreserved, the donor source, the timing from allo-HCT, among several others, and none were associated with the attainment of response. Although the number of treated patients limits the identification of predictors of response to SCB in this study as it has in others, the presence of active infection at the time of SCB was identified as the strongest predictor of treatment failure and eventual mortality. This is consistent with the largest published experiences of SCB, in which an active infection at the time of SCB was a negative predictor of outcome.7,12 Not all infections are necessarily associated with poor outcomes in the context of SCB, as exemplified in our cohort in which patients whose infections were controlled with antimicrobial therapy were able to attain hematologic responses and longer survival. Fungal infections tended to be associated with poor responses to SCB, consistent with the increased NRM associated with these infections.23 The diagnosis of PGF was not predictive of the attainment of response or longer survival in this study, but this may be because of the confounding effect of concurrent infection because the latter was seen in 50% of patients with PGF who did not attain a response.
Sepsis is associated with the development of pancytopenia, in part because of the suppression of hematopoiesis. In addition, excess inflammatory cytokines, as may be seen in severe infections, may lead to functional defects and apoptosis of hematopoietic progenitors.24-26 Hematopoietic progenitors infused in the context of SCB may be suppressed in the same way, potentially explaining why an active infection was associated with poor outcomes in our data. Consistent with our data, infections were also the leading cause of death reported in several multicenter retrospective cohorts of the use of SCB for the treatment of PGF after allo-HCT.7,10,12 However, infection was an exclusion criterion in some published studies of the use of SCB for PGF,5,10,16 and in most others, there was no report of active infection at the time of SCB.2-4,6,13-15 More data are needed to definitively associate an active infection with treatment failure or with poor survival outcomes after SCB.
The role of immune effector cells after allo-HCT in the development of poor hematopoiesis is not well understood, and the potential role of T cells either directly via a graft vs marrow response or indirectly via reduced Tregs and resulting inflammatory cytokine suppression of hematopoietic stem cells have been proposed.27 There is the possibility that a predisposition to active infection at the time of SCB, for example, due to quantitative differences in immune reconstitution after allo-HCT, could contribute to the association of an active infection with SCB treatment failure. We sought to address this with available longitudinal immune reconstitution data, finding a trend toward lower T-cell counts in patients who developed an active infection at SCB than in those who did not. We found no association between the absolute peripheral blood quantities of T cells, NK cells, and B cells after allo-HCT and the development of PGF needing SCB or response to therapy. Although this analysis is limited by numbers and selection bias due to solely evaluating patients needing SCB, it suggests that quantitative differences in the evaluated immune effector populations before SCB do not account for variation in treatment outcome. Further studies are needed to evaluate potential qualitative differences in these immune effector populations that could account for either the development of a BM microenvironment leading to PGF or for the poor response to SCB that is seen in some of these patients irrespective of whether they have an active infection at the time of treatment.
This study has important limitations, mostly due to its retrospective nature, limited sample size, and heterogeneous patient population. These limitations affect the evaluation of factors that are predictive of response to SCB. However, to our knowledge, this is among the largest single-center studies evaluating the use of SCB with comprehensively collected clinical, laboratory, and BM data and is an important addition to the published experience of the use of SCB. The association of an active infection at the time of SCB with treatment failure and poor survival is consistent with other retrospective studies and raises important questions about whether the boost should be reserved for patients in the absence of active infection.
In summary, we demonstrate in this retrospective experience with CD34+ selected SCB that it is effective for the treatment of post–allo-HCT cytopenias, both for PGF as defined in this study and outside this specific context. We further show that an active infection at the time of SCB is a predictor for treatment failure and poor survival. In patients who develop cytopenias after allo-HCT without loss of myeloid donor chimerism or active morphologic relapse of their original disease, prospective studies evaluating the use of early SCB, before the development of severe infection as opposed to later SCB, are required.
Acknowledgments
The authors are grateful for the patient volunteers who gave their time and provided the samples for all correlative studies. The authors acknowledge the Dana-Farber Cancer Institute (DFCI)/Harvard Cancer Center clinical oncology and bone marrow transplantation support staff for continued care of these patients. The authors are also grateful to the bone marrow transplant and research coordinators at the DFCI.
Authorship
Contribution: R.M.S., H.T.K., R.J.S., and S.N. designed the study; R.M.S. collected the data and wrote the manuscript; R.D., K.P., C.A., and C.G. collected the immune reconstitution data; H.T.K. performed the statistical analysis; R.D., J.S.L., R.J.S., C.S.C., C.J.W., J.R., J.K., R.R., and J.H.A. contributed critical revisions to the manuscript; S.N., H.M.G., and J.R. oversaw the production and generation of all stem cell boost (SCB) products; D.L. contributed clinical outcome data; and R.M.S., V.T.H., C.S.C., J.K., M.G., J.H.A., A.H.K., R.R., and R.J.S. contributed clinical data for patients treated with SCB.
Conflict-of-interest disclosure: R.M.S. consults for or has served on the advisory board for Hansa Biopharma. R.D. reports research funding from Ligue Contre le Cancer, Arthur Sachs, Monahan Foundation, Servier Foundation, Philippe Foundation, DCP Assistance Publique–Hôpitaux de Paris; honoraria from Novartis and Takeda; and nonfinancial support from Kite Pharma/Gilead and Sanofi. C.S.C. provides consultancy for or is on the advisory board of Incyte, Kadmon, Jazz, Medsenic, Generon, and Mesoblast. J.K. reports research support from Amgen, Equillium, Bristol Myers Squibb, Miltenyi Biotec, Regeneron, and Clinigen; consulting income from Amgen, Equillium, and Moderna Therapeutics; and is a scientific advisory board member for Cugene and Therakos. S.N. reports ad hoc advisory boards for Kite/Gilead, GlaxoSmithKline, Iovance, A2 Bio, and Sobi. C.J.W. holds equity in BioNTech and receives research funding from Pharmacyclics. J.R. received research funding from Amgen, Equillium, Kite Pharma, and Novartis, and served on advisory boards for Akron, Avrobio, Clade, Garuda, Immunitas, LifeVault, Rheos, Talaris, and TScan. R.R. receives funding from CRISPR Therapeutics and Skyline Therapeutics, and is on the advisory board of Glycostem. R.J.S. serves on the board of directors for Be The Match/National Marrow Donor Program; provided consulting for Vor Biopharma, Neovii, CSL Behring, BlueSphere Bio, Cugene, Jasper, and Smart Immune; and is on the data safety monitoring board for Juno Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Roman M. Shapiro, Department of Medical Oncology, Dana-Farber Cancer Institute, 450 Brookline Ave, Boston, MA 02215; email: roman_shapiro@dfci.harvard.edu.
References
Author notes
Original data are available on request from the corresponding author, Roman M. Shapiro (roman_shapiro@dfci.harvard.edu).
The full-text version of this article contains a data supplement.