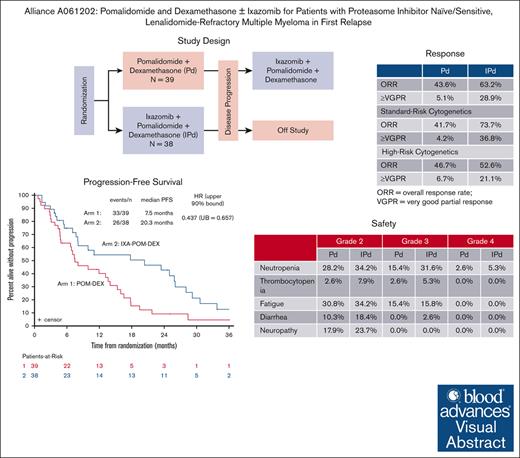
Key Points
IXA added to POM and DEX improved PFS in patients with LEN-refractory multiple myeloma at first relapse.
The safety profile of the IXA-POM-DEX triplet therapy was favorable and support phase 3 study of this all-oral regimen.
Visual Abstract
Optimal therapy for the growing number of patients with lenalidomide (LEN)-refractory multiple myeloma in their first relapse remains poorly defined. We therefore undertook a randomized phase 2 study to evaluate the efficacy and safety of combining the oral proteasome inhibitor ixazomib (IXA) with pomalidomide (POM) and dexamethasone (DEX) in this patient population. The overall response rate (ORR) for POM-DEX was 43.6%, and for IXA-POM-DEX, it was 63.2%. The depth of response, measured by the attainment of at least a very good partial response, favored triplet therapy over doublet therapy (28.9% vs 5.1%; P = .0063). A preplanned interim analysis after 75% of the progression events had occurred demonstrated an improvement in progression-free survival (PFS) that favored IXA-POM-DEX and that crossed the predefined boundary of superiority, leading to release of the study results. With additional follow-up, the median PFS for POM-DEX was 7.5 months (95% confidence interval [CI], 4.8-13.6 months) vs 20.3 months for IXA-POM-DEX (95% CI, 7.7-26.0 months; hazard ratio, 0.437; upper 90% bound = 0.657). The ORR and median PFS for 26 of 30 eligible patients who crossed over from the doublet to the triplet therapy at disease progression was 23.1% and 5.6 months, respectively. Overall survival was similar between the 2 groups. More hematologic toxicities were seen with the triplet therapy, but nonhematologic adverse events were similar between the 2 arms. Our data support further testing of this all-oral triplet therapy in comparison with current standard triplet therapy in the context of phase 3 studies for patients with LEN-refractory disease at first relapse. This trial was registered at www.clinicaltrials.gov as #NCT02004275.
Introduction
Changes in treatment paradigms for patients with newly diagnosed multiple myeloma (MM) have impacted optimal therapy at the time of relapse. Triplet therapy, in which lenalidomide (LEN) and dexamethasone (DEX) are combined with either daratumumab (a CD38 monoclonal antibody), elotuzumab (a signaling lymphocytic activation molecule family 7 monoclonal antibody), or carfilzomib or with the proteasome inhibitor ixazomib, have gained worldwide regulatory approval for patients with relapsed or refractory MM who have received at least 1 previous line of therapy.1-6 However, improvements in progression-free survival (PFS) with continuous LEN in the frontline setting and in overall survival (OS) when used as maintenance therapy after autologous stem cell transplantation (ASCT) or when given with DEX and daratumumab in the nontransplant setting have led to the use of frontline LEN-based therapy until progression of disease (PD) and has become the standard of care in the United States and other parts of the world.7-11 Thus, the utility of LEN-based therapies at first relapse is limited.
Pomalidomide (POM) and DEX have well-established activity in the treatment of patients with relapsed and LEN-refractory MM but these agents have been used largely as later lines of therapy.12-16 In the setting of relapsed or relapsed and/or refractory MM, the addition of either daratumumab or bortezomib to POM plus DEX therapy led to improved PFS, whereas the addition of either isatuximab or elotuzumab to POM plus DEX led to improvements in both PFS and OS.17-22 However, most patients who received POM-based triplet therapy in these randomized studies had received >1 previous line of therapy, and the proportion of patients with LEN-refractory disease was variable. Among patients with relapsed and relapsed and/or refractory MM who have received 1 to 3 previous lines of therapy, the addition of daratumumab and isatuximab to carfilzomib plus DEX improved PFS, but only 32% of patients in each study had LEN-refractory disease.23,24 As such, the optimal therapy for the growing number of patients in their first relapse with PD while on LEN therapy remains unclear.
A previous phase 1 study conducted by the Multiple Myeloma Research Consortium demonstrated that the addition of the oral proteasome inhibitor IXA to POM plus DEX was safe with promising preliminary activity in heavily pretreated patients with relapsed and/or refractory MM.25 The Alliance for Clinical Trials in Oncology (Alliance) A061202 study, which forms part of the National Cancer Institute’s (NCIs) National Clinical Trials Network, is a phase 1 to 2 study of IXA, POM, and DEX that was conducted specifically for patients with LEN and proteasome inhibitor-refractory MM. In the phase 1 portion, a maximum tolerated dose of 4 mg for both POM and IXA was confirmed based on the standard schedule for these agents. The overall response rate (ORR) was 51.7%, the median duration of response (DoR) was 16.8 months, the median PFS was 4.4 months, and the median OS was 34.3 months.26 Given the promising safety and the preliminary activity of this regimen in this patient population, coupled with the recognition of the potential for IXA resistance among those with bortezomib- or carfilzomib-refractory disease, we elected to study the efficacy of this all-oral regimen in the randomized phase 2 portion of our study in patients with proteasome inhibitor-sensitive and -naïve MM at their first relapse with PD while on LEN therapy.
Patients were registered in the phase 2 portion of the study from May 2018 through July 2020. A preplanned interim analysis occurred in October 2021 after 75% of the progression events required to trigger the final analysis had occurred. This analysis found that the O’Brien-Fleming boundary was crossed, thereby showing that the addition of IXA to POM and DEX significantly increased PFS when compared with POM and DEX doublet therapy. The findings were reported to the Alliance Data and Safety Monitoring Board and the NCI’s Cancer Therapy Evaluation Program. The study data were released and provided to the physicians who recruited patients to the trial. Patients on POM and DEX were given the option to add IXA to their regimen immediately rather than at PD. To ensure that the most current data were used to draft the manuscript, a copy of the study database was downloaded and locked on 9 March 2023. The results of the interim analysis and the study results with longer follow-up are presented here.
Methods
Patients
Individuals, aged 18 years or older, with a histologically confirmed diagnosis of relapsed symptomatic MM were screened for study participation. The eligibility criteria for those who previously received treatment included an upper limit of 1 previous line of systemic therapy; PD while on LEN therapy as part of first-line therapy; POM-naïve disease; and proteasome inhibitor naïve– or sensitive–disease (in which case proteasome inhibitor-sensitive disease was defined as a partial response [PR] or better while on proteasome inhibitor–based therapy that was maintained for at least 60 days after the last dose). Other key eligibility criteria included adequate bone marrow reserves (absolute neutrophil count ≥1.0 × 109/L and platelet count ≥50 × 109/L), creatinine clearance ≥30 mL/minute, adequate hepatic function (aspartate transaminase and alanine transaminase <2.5 times the upper limit of normal, total bilirubin <1.5 times the upper limit of normal), an Eastern Cooperative Oncology Group performance status of 0 to 2, and ≤ grade 2 peripheral neuropathy. Exclusion criteria included receiving medications that are strong inducers or inhibitors of CYP3A4 or CYP1A2 and acute ischemia, > grade 2 corrected QT prolongation, uncontrolled angina or severe ventricular arrhythmias, myocardial infarction within 6 months of registration, or class 3 or higher New York Heart Association congestive heart failure. Women who were pregnant or breast feeding were excluded. A complete listing of eligibility criteria can be found in the protocol supplied as part of the supplemental Data. All patients provided institutional review board (IRB)–approved, protocol-specific written informed consent before enrollment in the study.
Participants were assigned in equal numbers to each treatment arm through the Cancer Trials Support Unit’s Oncology Patient Enrollment Network registration system using a stratified randomization scheme by stage of disease (International Staging System [ISS] 1-2 vs ISS 3), high risk cytogenetic features (yes vs no), and previous treatment with a proteasome inhibitor (yes vs no). High-risk cytogenetics were defined as del(1p), gain of 1q, t(4;14) t(14;16), t(14;20), or del(17p).
Arm 1 treatment (POM-DEX) consisted of 4 mg of POM orally on days 1 to 21 of a 28-day cycle and 40 mg of DEX orally on days 1, 8, 15, and 22 (20 mg for those >75 years of age). Arm 2 treatment (IXA-POM-DEX) had the same treatment dose and schedule for POM and DEX as arm 1, but 4 mg of IXA orally was added on days 1, 8, and 15. Treatment was continued until PD, the emergence of unacceptable toxicity, or patient request to discontinue protocol treatment. Patients assigned to the POM-DEX group could cross over to the 3-drug regimen at PD provided that they met the eligibility criteria required for re-registration. Dose modification guidelines in the protocol are provided in the supplemental data section.
Study procedures
Within 14 days of study registration, patient history was collected and patients underwent a physical examination, assessment of performance status, had blood drawn for the evaluation of complete blood counts and blood chemistries, and underwent an electrocardiogram and toxicity assessment based on the Common Terminology Criteria for Adverse Events (AEs) v. 4.0. Except for the electrocardiograms, these evaluations were repeated on day 1 of each subsequent cycle before the start of treatment and at treatment discontinuation. Tumor assessments were done within 28 days of registration and before each cycle of therapy until PD or treatment discontinuation in accordance with the International Myeloma Working Group uniform response criteria.27 Cytogenetic and fluorescence in situ hybridization evaluations were performed on bone marrow aspirates and biopsies within 28 days of registration and at confirmation of complete response (CR; every other cycle for patients with nonsecretory disease). A skeletal survey was required within 42 days of registration. Additional imaging, including computed tomography, positron emission tomography computed tomography, or magnetic resonance imaging, was mandated for patients with previously documented or suspected extramedullary involvement.
Study design
Phase 2
A randomized phase 2 clinical trial was conducted to assess whether the addition of IXA to POM-DEX would increase PFS for patients with LEN-refractory disease at first relapse. All patients who met the eligibility criteria and who signed a consent form and began treatment were included in the assessment of all clinical endpoints according to the treatment group to which they were randomized, regardless of their actual treatment or duration of treatment.
Statistical methods
The primary endpoint of this trial, PFS, was defined as the time from randomization to the date of PD or death from any cause. The trial was designed under the assumption that the median PFS among those who receive POM-DEX is 6 months and an increase in the median PFS to 12 months with the addition of IXA would be considered promising. Based on a sample size of 70 eligible patients (35 patients per arm) who were enrolled over a 15-month period and who were followed for at least another 15 months after the close of enrollment, and a 1-sided generalized log-rank test with an alpha = 0.10, there would be at least a 90% chance of detecting a 50% decrease in the hazard of PD or death with the addition of IXA to POM-DEX. The number of PD events needed to trigger analysis of the primary endpoint was 57. One interim analysis was planned after 75% of the events occurred. To preserve the type 1 error rate, the Lan-DeMets error spending rate function with O’Brien Fleming boundaries was applied, and the alpha set for this interim analysis was 0.058
Secondary endpoints included ORR, the depth of best response, DoR, OS, and toxicity profile. ORR was defined as the percentage of patients who had a PR, very good PR (VGPR), CR, or stringent CR according to the International Myeloma Working Group uniform response criteria on 2 consecutive evaluations.27 Minimal residual disease status was not assessed in this protocol. DoR was defined as the time from the first noted ≥PR to PD or death from any cause. OS was defined as the time from registration to death from any cause. The distribution of event times was estimated using the Kaplan-Meier method. The censoring rules that were applied when DoR and PFS were estimated were as follows: patients who had no documentation of PD and who had not started nonprotocol treatment were censored at the time of the last disease evaluation; patients who started nonprotocol treatment before documentation of PD were censored at the time of last disease evaluation before the start of new therapy; and patients who died and were progression-free at their last disease evaluation but who had a last disease evaluation was >3 months before their death were censored at the time of their last disease evaluation. A Fisher exact test was used to assess whether the ORR differed between the treatment arms. Conditional Cox modeling (that took into account the 3 randomization stratification factors) was used to obtain an estimate of the hazard ratio (HR) for PD or death. In the 2 subgroups defined according to whether MM had high-risk cytogenetics or not, conditional Cox modeling (with the 2 remaining randomization stratification factors) was used to obtain an estimate of the HR for PD or death. The data lock for this report was 9 March 2023. Statistical analyses were performed using SAS 9.4.
This study was performed after approval from the Cancer Therapy Evaluation Program and NCI Central Institutional Review Board in accordance with assurances filed with and approved by the Department of Health and Human Services. All patients provided IRB–approved, protocol-specific written informed consent before enrollment in the study. Participating centers received IRB approval before participation, and the study was conducted in accordance with the Declaration of Helsinki. Data were collected and analyzed by the Alliance Statistics and Data Management Center and were reviewed by the study chair. This study was monitored semi-annually by the Alliance Data and Safety Monitoring Board. Investigators participated in patient enrollment, interpretation of the data, and preparation of the manuscript.
Results
Baseline patient, disease, and previous treatment characteristics
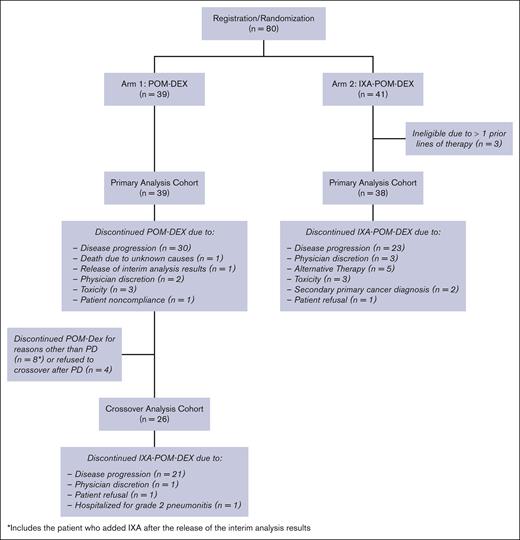
A total of 80 patients were enrolled in this study from May 2018 through July 2020. Three patients randomized to IXA-POM-DEX were ineligible because they received >1 previous line of therapy and were excluded from the analysis (Figure 1). Patient and disease characteristics are presented in Table 1 by treatment arm. Age, gender, race, ethnicity, and Eastern Cooperative Oncology Group performance status were similar between the treatment arms. It should be noted that 93.5% of patients (POM-DEX: 94.9%; IXA-POM-DEX: 92.1%) had received a proteasome inhibitor as part of frontline therapy. None of the patients had previously received a CD38 monoclonal antibody. Patients in the IXA-POM-DEX treatment arm had a higher proportion of high-risk cytogenetic features (50.0% vs 38.5%) and were more likely to have received previous ASCT (52.6% vs 30.8%). In contrast, there were a higher proportion of patients with ISS stage 3 disease at the time of original diagnosis in the POM-DEX arm (15.4% vs 5.3%).
CONSORT diagram for patients on arm 1 (POM-DEX) and arm 2 (IXA-POM-DEX).
Patient demographics, disease characteristics and treatment history at registration
| Characteristic . | Arm 1 POM-DEX (n = 39) . | Arm 2 IXA-POM-DEX (n = 38) . |
|---|---|---|
| Median age at registration (range) | 64 (52-85) | 66 (41-83) |
| Age at registration (y) | ||
| 40-64 | 20 (51.3%) | 14 (36.8%) |
| 65-74 | 8 (20.5%) | 18 (47.4%) |
| 75-85 | 11 (28.2%) | 6 (15.8%) |
| Male | 23 (59.0%) | 19 (50.0%) |
| Race | ||
| White | 32 (82.1%) | 30 (79.0%) |
| Black | 5 (12.8%) | 6 (15.8%) |
| Asian | 0 | 1 (2.6%) |
| Not reported | 2 (5.1%) | 1 (2.6%) |
| Hispanic or Latino | 2 (5.1%) | 1 (2.6%) |
| ECOG performance score | ||
| 0 | 15 (38.5%) | 13 (34.2%) |
| 1 | 21 (53.9%) | 24 (63.2%) |
| 2 | 3 (7.7%) | 1 (2.6%) |
| Time from diagnosis to study entry | ||
| <2 y | 5 (12.8%) | 6 (15.8%) |
| 2.0-4.9 y | 18 (46.2%) | 20 (52.6%) |
| ≥5.0 y | 16 (46.0%) | 13 (31.6%) |
| ISS stage at initial diagnosis | ||
| 1 | 9 (23.1%) | 15 (39.5%) |
| 2 | 14 (35.9%) | 12 (31.6%) |
| 3 | 6 (15.4%) | 2 (5.3%) |
| Unknown | 10 (25.6%) | 9 (23.7%) |
| High-risk cytogenetics∗ | 15 (38.5%) | 19 (50.0%) |
| Pevious ASCT | 12 (30.8%) | 20 (52.6%) |
| Previous proteasome inhibitor | 37 (94.9%) | 35 (92.1%) |
| Previous daratumumab-containing regimen | 0 (0.0%) | 0 (0.0%) |
| LEN refractory yes, progression during treatment | 35 (89.7%) | 34 (89.5%) |
| If yes, progression within 60 d of last dose | 4 (10.3%) | 4 (10.5%) |
| Characteristic . | Arm 1 POM-DEX (n = 39) . | Arm 2 IXA-POM-DEX (n = 38) . |
|---|---|---|
| Median age at registration (range) | 64 (52-85) | 66 (41-83) |
| Age at registration (y) | ||
| 40-64 | 20 (51.3%) | 14 (36.8%) |
| 65-74 | 8 (20.5%) | 18 (47.4%) |
| 75-85 | 11 (28.2%) | 6 (15.8%) |
| Male | 23 (59.0%) | 19 (50.0%) |
| Race | ||
| White | 32 (82.1%) | 30 (79.0%) |
| Black | 5 (12.8%) | 6 (15.8%) |
| Asian | 0 | 1 (2.6%) |
| Not reported | 2 (5.1%) | 1 (2.6%) |
| Hispanic or Latino | 2 (5.1%) | 1 (2.6%) |
| ECOG performance score | ||
| 0 | 15 (38.5%) | 13 (34.2%) |
| 1 | 21 (53.9%) | 24 (63.2%) |
| 2 | 3 (7.7%) | 1 (2.6%) |
| Time from diagnosis to study entry | ||
| <2 y | 5 (12.8%) | 6 (15.8%) |
| 2.0-4.9 y | 18 (46.2%) | 20 (52.6%) |
| ≥5.0 y | 16 (46.0%) | 13 (31.6%) |
| ISS stage at initial diagnosis | ||
| 1 | 9 (23.1%) | 15 (39.5%) |
| 2 | 14 (35.9%) | 12 (31.6%) |
| 3 | 6 (15.4%) | 2 (5.3%) |
| Unknown | 10 (25.6%) | 9 (23.7%) |
| High-risk cytogenetics∗ | 15 (38.5%) | 19 (50.0%) |
| Pevious ASCT | 12 (30.8%) | 20 (52.6%) |
| Previous proteasome inhibitor | 37 (94.9%) | 35 (92.1%) |
| Previous daratumumab-containing regimen | 0 (0.0%) | 0 (0.0%) |
| LEN refractory yes, progression during treatment | 35 (89.7%) | 34 (89.5%) |
| If yes, progression within 60 d of last dose | 4 (10.3%) | 4 (10.5%) |
ECOG, Eastern Cooperative Oncology Group.
High risk cytogenetics: defined as del(1p), gain of 1q, t(4;14) t(14;16), t(14;20), or del(17p).
Treatment course
Arm 1 (POM-DEX)
There were 39 eligible patients, aged 52 to 85 years, who were randomized to arm 1. The median number of cycles administered was 8 (interquartile range, 5-17; range, 1-51+). One patient remained on POM + DEX. The reasons for discontinuing protocol therapy included death from unknown causes during the second cycle of treatment (n = 1); PD (n = 30); AEs (fall with head laceration [n = 1]; hypercalcemia [n = 1], hearing impaired [n = 1]); alternative therapy (1 patient began daratumumab with LEN and DEX after 5 cycles of protocol treatment and 1 patient began daratumumab monotherapy after 11 cycles of protocol treatment); patient noncompliance (n = 1); and opting to cross over without PD after the release of the interim analysis results (n = 1).
Of the 30 patients who discontinued POM-DEX because of PD, 26 chose to add IXA to their regimen. The median number of cycles administered after crossover was 5 (range, 2-28). Two patients remain on IXA-POM-DEX. The reasons for discontinuing protocol therapy included PD (n = 21); refusal (n = 1); physician discretion (n = 1); and other medical issue (n = 1).
Arm 2 (IXA-POM-DEX)
There were 38 eligible patients aged 41 to 83 years who were randomized to arm 2. The median number of cycles administered was 9.5 (interquartile range, 5-26; range, 1-52+). One patient remains on protocol treatment. The reasons for discontinuing IXA-POM-DEX were PD (n = 22); physician concerns about worsening disease (3 patients experienced PD shortly after discontinuing protocol treatment and had not received any subsequent treatment; 1 patient began daratumumab + POM-DEX); alternative therapy (4 patients underwent ASCT after 5-7 cycles of protocol treatment and 1 patient began daratumumab monotherapy after 10 cycles of protocol treatment); AEs (tremors [n = 1]; acute kidney injury [n = 1], upper gastrointestinal hemorrhage [n = 1]); second primary cancer (testicular cancer [n = 1], gallbladder cancer [n = 1]), and refusal (1 patient had difficulty traveling to registering site and began IXA-POM-DEX closer to home).
Results of the planned interim analysis
After 43 (75%) of the required 57 events for the final efficacy analysis was recorded, PFS was found to be increased with the addition of IXA to POM-DEX (1-sided stratified log rank test value = 5.8371; P = .0157 [less than the P value boundary of 0.058 for rejecting null hypothesis]). The adjusted HR for PFS was estimated to be 0.451 (upper 90% bound = 0.694).
Clinical outcomes at data lock
Safety profile
In arm 1, there were 20 (51.3%) patients who required at least 1 POM dose reduction. The most common reasons for POM dose reductions were fatigue (n = 4), peripheral neuropathy (n = 3), and pneumonia (n = 2). The most common severe (grade 3-4) AEs reported, regardless of attribution, were lymphopenia (23.1%), neutropenia (17.9%), fatigue (15.4%), and anemia (10.3%) (Table 2). For the 26 patients who elected to crossover to IXA-POM-DEX at PD, the most common severe (grade 3-4) AEs reported were neutropenia (23.1%), lymphopenia (11.5%), thrombocytopenia (11.5%), and anemia (11.5%).
Most common grade 2 to 4 AEs reported (regardless of attribution)
| Toxicity . | Arm . | Grade 2 . | Grade 3 . | Grade 4 . | Total . |
|---|---|---|---|---|---|
| Anemia | 1 | 10.3% | 10.3% | 0 | 20.5% |
| 2 | 18.4% | 15.8% | 0 | 34.2% | |
| 1 x-over | 19.2% | 11.5% | 0 | 30.8% | |
| Lymphocyte count decrease | 1 | 12.8% | 17.9% | 5.1% | 35.9% |
| 2 | 23.7% | 28.9% | 10.5% | 63.2% | |
| 1 x-over | 0 | 7.7% | 3.8% | 11.5% | |
| Neutrophil count decrease | 1 | 28.2% | 15.4% | 2.6% | 46.2% |
| 2 | 34.2% | 31.6% | 5.3% | 71.1% | |
| 1 x-over | 26.9% | 23.1% | 0 | 50.0% | |
| Platelet count decrease | 1 | 2.6% | 2.6% | 0 | 5.1% |
| 2 | 7.9% | 5.3% | 0 | 13.2% | |
| 1 x-over | 7.7% | 7.7% | 3.8% | 19.2% | |
| White blood cell count decrease | 1 | 23.1% | 2.6% | 0 | 25.6% |
| 2 | 28.9% | 2.6% | 10.5% | 42.1% | |
| 1 x-over | 23.1% | 0 | 0 | 23.1% | |
| Anxiety | 1 | 10.3% | 0 | 0 | 10.3% |
| 2 | 7.9% | 0 | 0 | 7.9% | |
| 1 x-over | 3.8% | 0 | 0 | 3.8% | |
| Atrial fibrillation | 1 | 0 | 0 | 0 | 0 |
| 2 | 7.9% | 2.6% | 0 | 10.5% | |
| 1 x-over | 7.7% | 3.8% | 0 | 11.5% | |
| Back Pain | 1 | 12.8% | 2.6% | 0 | 15.4% |
| 2 | 10.5% | 2.6% | 0 | 13.2% | |
| 1 x-over | 19.2% | 0 | 0 | 19.2% | |
| Chronic kidney disease | 1 | 10.3% | 0 | 0 | 10.3% |
| 2 | 2.6% | 0 | 0 | 2.6% | |
| 1 x-over | 7.7% | 0 | 0 | 7.7% | |
| Constipation | 1 | 15.4% | 0 | 0 | 15.4% |
| 2 | 7.9% | 0 | 0 | 7.9% | |
| 1 x-over | 11.5% | 0 | 0 | 11.5% | |
| Dehydration | 1 | 2.6% | 0 | 0 | 2.6% |
| 2 | 7.9% | 2.6% | 0 | 10.5% | |
| 1 x-over | 0 | 3.8% | 0 | 3.8% | |
| Diarrhea | 1 | 10.3% | 0 | 0 | 10.3% |
| 2 | 18.4% | 2.6% | 0 | 21.1% | |
| 1 x-over | 19.2% | 0 | 0 | 19.2% | |
| Fatigue | 1 | 30.8% | 15.4% | 0 | 46.2% |
| 2 | 34.2% | 15.8% | 0 | 50.0% | |
| 1 x-over | 42.3% | 3.8% | 0 | 46.2% | |
| Generalized muscle weakness | 1 | 10.3% | 2.6% | 0 | 12.8% |
| 2 | 2.6% | 0 | 0 | 2.6% | |
| 1 x-over | 3.8% | 0 | 0 | 3.8% | |
| Hyperglycemia | 1 | 10.3% | 2.6% | 0 | 12.8% |
| 2 | 10.5% | 10.5% | 0 | 21.1% | |
| 1 x-over | 7.7% | 3.8% | 0 | 11.5% | |
| Hypertension | 1 | 2.6% | 7.7% | 0 | 10.3% |
| 2 | 5.3% | 2.6% | 0 | 7.9% | |
| 1 x-over | 11.5% | 3.8% | 0 | 15.4% | |
| Hypophosphatemia | 1 | 7.7% | 7.7% | 0 | 15.4% |
| 2 | 7.9% | 10.5% | 0 | 18.4% | |
| 1 x-over | 19.2% | 3.8% | 0 | 23.1% | |
| Insomnia | 1 | 17.9% | 2.6% | 0 | 20.5% |
| 2 | 13.2% | 0 | 0 | 13.2% | |
| 1 x-over | 19.2% | 0 | 0 | 19.2% | |
| Limb edema | 1 | 7.7% | 0 | 0 | 7.7% |
| 2 | 10.5% | 0 | 0 | 10.5% | |
| 1 x-over | 7.7% | 0 | 0 | 7.7% | |
| Lung Infection | 1 | 0 | 7.7% | 0 | 7.7% |
| 2 | 2.6% | 7.9% | 0 | 10.5% | |
| 1 x-over | |||||
| Maculo-papular rash | 1 | 7.7% | 2.6% | 0 | 10.3% |
| 2 | 7.9% | 2.6% | 0 | 10.5% | |
| 1 x-over | |||||
| Nausea | 1 | 5.1% | 0 | 0 | 5.1% |
| 2 | 10.5% | 0 | 0 | 10.5% | |
| 1 x-over | 15.4% | 3.8% | 0 | 19.2% | |
| Peripheral sensory neuropathy | 1 | 17.9% | 0 | 0 | 17.9% |
| 2 | 23.7% | 0 | 0 | 23.7% | |
| 1 x-over | 15.4% | 0 | 0 | 15.4% | |
| Upper respiratory infection | 1 | 20.5% | 0 | 0 | 20.5% |
| 2 | 18.4% | 0 | 0 | 18.4% | |
| 1 x-over | 3.8% | 0 | 3.8% | 7.7% | |
| Weight gain | 1 | 0 | 0 | 0 | 0 |
| 2 | 13.2% | 0 | 0 | 13.2% | |
| 1 x-over | 7.7% | 0 | 0 | 7.7% |
| Toxicity . | Arm . | Grade 2 . | Grade 3 . | Grade 4 . | Total . |
|---|---|---|---|---|---|
| Anemia | 1 | 10.3% | 10.3% | 0 | 20.5% |
| 2 | 18.4% | 15.8% | 0 | 34.2% | |
| 1 x-over | 19.2% | 11.5% | 0 | 30.8% | |
| Lymphocyte count decrease | 1 | 12.8% | 17.9% | 5.1% | 35.9% |
| 2 | 23.7% | 28.9% | 10.5% | 63.2% | |
| 1 x-over | 0 | 7.7% | 3.8% | 11.5% | |
| Neutrophil count decrease | 1 | 28.2% | 15.4% | 2.6% | 46.2% |
| 2 | 34.2% | 31.6% | 5.3% | 71.1% | |
| 1 x-over | 26.9% | 23.1% | 0 | 50.0% | |
| Platelet count decrease | 1 | 2.6% | 2.6% | 0 | 5.1% |
| 2 | 7.9% | 5.3% | 0 | 13.2% | |
| 1 x-over | 7.7% | 7.7% | 3.8% | 19.2% | |
| White blood cell count decrease | 1 | 23.1% | 2.6% | 0 | 25.6% |
| 2 | 28.9% | 2.6% | 10.5% | 42.1% | |
| 1 x-over | 23.1% | 0 | 0 | 23.1% | |
| Anxiety | 1 | 10.3% | 0 | 0 | 10.3% |
| 2 | 7.9% | 0 | 0 | 7.9% | |
| 1 x-over | 3.8% | 0 | 0 | 3.8% | |
| Atrial fibrillation | 1 | 0 | 0 | 0 | 0 |
| 2 | 7.9% | 2.6% | 0 | 10.5% | |
| 1 x-over | 7.7% | 3.8% | 0 | 11.5% | |
| Back Pain | 1 | 12.8% | 2.6% | 0 | 15.4% |
| 2 | 10.5% | 2.6% | 0 | 13.2% | |
| 1 x-over | 19.2% | 0 | 0 | 19.2% | |
| Chronic kidney disease | 1 | 10.3% | 0 | 0 | 10.3% |
| 2 | 2.6% | 0 | 0 | 2.6% | |
| 1 x-over | 7.7% | 0 | 0 | 7.7% | |
| Constipation | 1 | 15.4% | 0 | 0 | 15.4% |
| 2 | 7.9% | 0 | 0 | 7.9% | |
| 1 x-over | 11.5% | 0 | 0 | 11.5% | |
| Dehydration | 1 | 2.6% | 0 | 0 | 2.6% |
| 2 | 7.9% | 2.6% | 0 | 10.5% | |
| 1 x-over | 0 | 3.8% | 0 | 3.8% | |
| Diarrhea | 1 | 10.3% | 0 | 0 | 10.3% |
| 2 | 18.4% | 2.6% | 0 | 21.1% | |
| 1 x-over | 19.2% | 0 | 0 | 19.2% | |
| Fatigue | 1 | 30.8% | 15.4% | 0 | 46.2% |
| 2 | 34.2% | 15.8% | 0 | 50.0% | |
| 1 x-over | 42.3% | 3.8% | 0 | 46.2% | |
| Generalized muscle weakness | 1 | 10.3% | 2.6% | 0 | 12.8% |
| 2 | 2.6% | 0 | 0 | 2.6% | |
| 1 x-over | 3.8% | 0 | 0 | 3.8% | |
| Hyperglycemia | 1 | 10.3% | 2.6% | 0 | 12.8% |
| 2 | 10.5% | 10.5% | 0 | 21.1% | |
| 1 x-over | 7.7% | 3.8% | 0 | 11.5% | |
| Hypertension | 1 | 2.6% | 7.7% | 0 | 10.3% |
| 2 | 5.3% | 2.6% | 0 | 7.9% | |
| 1 x-over | 11.5% | 3.8% | 0 | 15.4% | |
| Hypophosphatemia | 1 | 7.7% | 7.7% | 0 | 15.4% |
| 2 | 7.9% | 10.5% | 0 | 18.4% | |
| 1 x-over | 19.2% | 3.8% | 0 | 23.1% | |
| Insomnia | 1 | 17.9% | 2.6% | 0 | 20.5% |
| 2 | 13.2% | 0 | 0 | 13.2% | |
| 1 x-over | 19.2% | 0 | 0 | 19.2% | |
| Limb edema | 1 | 7.7% | 0 | 0 | 7.7% |
| 2 | 10.5% | 0 | 0 | 10.5% | |
| 1 x-over | 7.7% | 0 | 0 | 7.7% | |
| Lung Infection | 1 | 0 | 7.7% | 0 | 7.7% |
| 2 | 2.6% | 7.9% | 0 | 10.5% | |
| 1 x-over | |||||
| Maculo-papular rash | 1 | 7.7% | 2.6% | 0 | 10.3% |
| 2 | 7.9% | 2.6% | 0 | 10.5% | |
| 1 x-over | |||||
| Nausea | 1 | 5.1% | 0 | 0 | 5.1% |
| 2 | 10.5% | 0 | 0 | 10.5% | |
| 1 x-over | 15.4% | 3.8% | 0 | 19.2% | |
| Peripheral sensory neuropathy | 1 | 17.9% | 0 | 0 | 17.9% |
| 2 | 23.7% | 0 | 0 | 23.7% | |
| 1 x-over | 15.4% | 0 | 0 | 15.4% | |
| Upper respiratory infection | 1 | 20.5% | 0 | 0 | 20.5% |
| 2 | 18.4% | 0 | 0 | 18.4% | |
| 1 x-over | 3.8% | 0 | 3.8% | 7.7% | |
| Weight gain | 1 | 0 | 0 | 0 | 0 |
| 2 | 13.2% | 0 | 0 | 13.2% | |
| 1 x-over | 7.7% | 0 | 0 | 7.7% |
In arm 2, there were 19 (50.0%) patients who required at least 1 POM dose modification and 11 (28.9%) patients who required at least 1 IXA dose reduction. The most common reasons for these dose reductions were peripheral neuropathy (n = 5), fatigue (n = 4), and neutropenia (n = 3). The most common severe (grade 3-4) AEs reported, regardless of attribution, were lymphopenia (39.4%), neutropenia (36.8%), leukopenia (13.2%), anemia (15.8%), fatigue (15.8%), hyperglycemia (10.5%), and hypophosphatemia (10.5%) (Table 2).
Grade 2 to 4 AEs that were more commonly seen with IXA-POM-DEX than with POM-DEX included neutropenia (71.1% vs 46.2%), lymphopenia (63.2% vs 35.9%), thrombocytopenia (13.2% vs 5.1%), anemia (34.2% and 20.5%), nausea (10.5% vs 5.1%), and diarrhea (21.1% vs 10.3%). Grade 2 peripheral neuropathy occurred in 23.7% and 17.9% of patients on IXA-POM-DEX and POM-DEX, respectively, but no 1 in either arm experienced grade 3 peripheral neuropathy. Rates of grade 2 to 4 fatigue (IXA-POM-DEX, 50.0% vs POM-DEX, 46.2%) and rash (IXA-POM-DEX, 10.5% vs POM-DEX, 10.3%) were similar in both arms (Table 2).
Efficacy profile
There were 15 PRs, 1 VGPR, and 1 stringent CR with a median DoR of 12.3 months (95% confidence interval [CI], 5.7-15.6 months) in the POM-DEX arm. Thus, the ≥VGPR rate and the ORR were 5.1% (95% CI, 0.6-17.3) and 43.6% (95% CI, 27.8-60.4), respectively (Table 3).
Clinical response by initial treatment
| Response . | POM + DEX (n = 39) . | POM + DEX + IXA (n = 38) . | P value . |
|---|---|---|---|
| ORR, all patients (95% CI) | 43.6% (27.8-60.4) | 63.2% (46.0-78.2) | P = .1113∗ |
| ≥VGPR rate, all patients (95% CI) | 5.1% (0.6-17.3) | 28.9% (15.4-45.9) | P = .0063∗ |
| ORR, standard-risk cytogenetics (95% CI) | 41.7% (22.1-63.4) | 73.7% (48.8-90.9) | |
| ≥VGPR rate, standard-risk cytogenetics (95% CI) | 4.2% (0.1-21.1) | 36.8% (16.3-61.6) | |
| ORR, high-risk cytogenetics (95% CI) | 46.7% (21.3-73.4) | 52.6% (28.9-75.6) | |
| ≥VGPR rate, high-risk cytogenetics (95% CI) | 6.7% (0.2-32.0) | 21.1% (6.1-45.6) | |
| Median duration of ≥ PR | 12.3 mo | 23.7 mo | P = .1727† |
| Response . | POM + DEX (n = 39) . | POM + DEX + IXA (n = 38) . | P value . |
|---|---|---|---|
| ORR, all patients (95% CI) | 43.6% (27.8-60.4) | 63.2% (46.0-78.2) | P = .1113∗ |
| ≥VGPR rate, all patients (95% CI) | 5.1% (0.6-17.3) | 28.9% (15.4-45.9) | P = .0063∗ |
| ORR, standard-risk cytogenetics (95% CI) | 41.7% (22.1-63.4) | 73.7% (48.8-90.9) | |
| ≥VGPR rate, standard-risk cytogenetics (95% CI) | 4.2% (0.1-21.1) | 36.8% (16.3-61.6) | |
| ORR, high-risk cytogenetics (95% CI) | 46.7% (21.3-73.4) | 52.6% (28.9-75.6) | |
| ≥VGPR rate, high-risk cytogenetics (95% CI) | 6.7% (0.2-32.0) | 21.1% (6.1-45.6) | |
| Median duration of ≥ PR | 12.3 mo | 23.7 mo | P = .1727† |
Fisher’s exact test result.
log rank test results.
There were 13 PRs, 10 VGPRs, and 1 CR with a median DoR of 23.7 months (95% CI: 9.9-26.9 months) in the IXA-POM-DEX arm. The ≥VGPR rate and the ORR were 28.9% (95% CI, 15.4-45.9) and 63.2% (95% CI, 46.0-78.2), respectively (Table 3).
The ORR was somewhat higher with IXA-POM-DEX, but it was not statistically significant. However, the ≥VGPR rate was significantly higher with IXA-POM-DEX than with POM-DEX (P = .0063).
Among the 26 patients on POM-DEX who added IXA at time of progression, there were 1 PR, 4 VGPRs, and 1 CR. The ≥VGPR rate and ORR were 19.2% (95% CI, 6.6-39.4) and 23.1% (95% CI, 9.0-43.7), respectively.
The median length of follow-up among the 53 patients alive at last follow-up was 3.1 years (range, 4 months to 4.2 years). PFS events included 58 patients (POM-DEX, 32; IXA-POM-DEX, 26) who progressed before the start of nonprotocol therapy or crossover treatment and 1 patient (POM-DEX) who died without disease progression.
The median PFS was estimated to be 7.5 months (95% CI, 4.8-13.6 months) with POM-DEX and 20.3 months (95% CI, 7.7-26.0 months) with IXA-POM-DEX. Conditional Cox modeling found a reduction in the hazard of disease progression or death of nearly 57% with the addition of IXA to POM-DEX (HR, 0.437; upper 90% bound = 0.657; Figure 2A).
PFS and OS for arm 1 (POM-DEX) vs arm 2 (IXA-POM-DEX). (A) PFS for all patients. (B) PFS for patients with standard-risk cytogenetic abnormalities. (C) PFS for patients with high-risk cytogenetic abnormalities. (D) OS. High-risk cytogenetic abnormalities were defined as the presence of at least 1 of the following: del(1p), del(17p), t(14;16), t(14;20), or gain/amp of 1q21.
PFS and OS for arm 1 (POM-DEX) vs arm 2 (IXA-POM-DEX). (A) PFS for all patients. (B) PFS for patients with standard-risk cytogenetic abnormalities. (C) PFS for patients with high-risk cytogenetic abnormalities. (D) OS. High-risk cytogenetic abnormalities were defined as the presence of at least 1 of the following: del(1p), del(17p), t(14;16), t(14;20), or gain/amp of 1q21.
An exploratory analysis found a tendency toward prolongation of PFS with the addition of IXA to POM-DEX for those with standard risk cytogenetic abnormalities (HR, 0.452; 95% CI, 0.21-0.99; P = .0464; Figure 2B) and for those with high-risk cytogenetic abnormalities (HR, 0.417; 95% CI, 0.17-1.06; Figure 2C). However, these data should be interpreted cautiously given the small sample size.
For patients in arm 1 who chose to add IXA to their regimen at PD, the median PFS from start of IXA-POM-DEX at crossover was 5.6 months (95% CI, 3.0-12.9 months).
There have been 24 deaths, including 9 patients in the POM-DEX arm who crossed over to IXA-POM-DEX, 4 patients in the POM-DEX arm who did not crossover, and 11 patients in the IXA-POM-DEX arm. The causes of death were PD (n = 8), cardiac arrest (n = 2), COVID-19 (n = 1), and unknown (n = 2) in the POM-DEX arm and PD (n = 7), sepsis secondary to rapid progression of adenocarcinoma of the gallbladder and extrahepatic bile duct (n = 1), plasma cell leukemia (n = 1), and unknown (n = 2) in the IXA-POM-DEX arm. The median OS time has not been reached in either treatment arm. The 2-year OS rate was 79.5% (95% CI, 67.8-93.2) in the POM-DEX arm and 78.4% (95% CI, 66.2-92.8) in the IXA-POM-DEX arm. No significant differences have been observed in OS between the treatment arms (HR, 0.774; 95% CI, 0.33-1.80; P = .5522; Figure 2D).
Discussion
There is a need to develop treatment options for patients with MM who have LEN-refractory disease at the time of first relapse. Our phase 2 study was a randomized trial designed to specifically address this patient need. We demonstrated both an improvement in the depth of response, defined by achievement of a VGPR or better, and a significant reduction in the risk for PD or death (56.3%), yielding a median PFS of 20.3 months with IXA-POM-DEX treatment in comparison with the median PFS of 7.5 months with POM-DEX treatment. Improvement in PFS with the addition of IXA to POM-DEX was seen in patients with standard- and high-risk cytogenetics, much in line with what was seen in the study of IXA-LEN-DEX for patients with relapsed and relapsed and/or refractory MM who had received 1 or more previous therapies.28 IXA was readily incorporated into the POM-DEX backbone with acceptable safety. Although neutropenia was more common with IXA-POM-DEX, it led to dose reductions in only 3 patients, likely because of the relatively low rates of grade 4 neutropenia seen with the triplet treatment. This contrasts with higher rates of overall and high-grade neutropenia seen when POM-DEX is combined with CD38 monoclonal antibodies. Moreover, there was no significant difference in the rates of respiratory tract infections between the 2 arms of the trial. Given the small sample size, the length of follow-up (∼1-third of those enrolled have died), the crossover design, and the fact that the trial was not designed to assess whether the addition of IXA to POM-DEX improves OS, it is not surprising that no significant improvement in OS was found. One limitation of our study is the fact that a higher proportion of patients who received IXA-POM-DEX discontinued protocol therapy for reasons other than PD or death, leading to a higher rate of censoring in that arm. Given the small sample size, this could have had an impact on the primary outcome. Nonetheless, our findings support pursuit of a larger, confirmatory phase 3 study in this patient population. Given the fact that POM-DEX is no longer considered an optimal standard of care for relapsed patients, a phase 3 study would need to incorporate a modern triplet regimen as the control arm.
The issue of optimal therapy sequencing remains of vital interest in MM; specifically, the question is whether it is more effective to add newer therapeutics into early lines of existing MM regimens or to administer them at relapse. In this study, all patients who received POM-DEX had access to IXA immediately at PD, and 26 of 30 eligible patients with PD in the POM-DEX arm elected to cross over to IXA-POM-DEX. Unfortunately, the ORR of IXA-POM-DEX for patients who had PD on POM-DEX therapy was only 23.1%, and the median PFS was 5.6 months (on top of the suboptimal median PFS of 7.5 months experienced on initial POM-DEX). When compared with the median PFS of 20.3 months with the IXA-POM-DEX triplet therapy used from the outset, our results suggest that there is a potential clinical synergy between IXA and POM and support the use of the agents together from the start of treatment rather than giving the treatments sequentially. Certainly, these observations are currently hypothesis generating and would need to be confirmed in a larger phase 3 study but are nonetheless informative.
The treatment landscape for MM is in rapid flux with the approval of newer agents in earlier lines of therapy impacting treatment options at relapse. Frontline treatment with daratumumab with LEN-DEX until PD has become a worldwide standard of care based on its significant improvement in PFS and OS when compared with LEN-DEX alone in newly diagnosed, transplant-ineligible patients.11,29 Furthermore, the addition of daratumumab to LEN-DEX and bortezomib has been shown to improve the depth of response and PFS for transplant-eligible patients, leading to a global acceptance of daratumumab-based quadruplet therapy as a new standard of care for this patient population.30-32 A limitation of our study is that no patient received CD38 monoclonal antibodies as part of frontline therapy given that patients were enrolled in a time33 period where daratumumab had yet to be incorporated into upfront therapy as standard of care. As such, it remains to be seen if the addition of IXA to POM-DEX would improve PFS in this patient population. If a phase 3 study of IXA-POM-DEX were pursued in patients at first relapse with PD while on LEN therapy, stratification by previous CD38 monoclonal antibody and proteasome inhibitor exposure would be critical.
Our findings align with the results of other studies that evaluated POM-proteasome inhibitor triplets in this patient population. A subset analysis from the phase 3 OPTIMMISM study that assessed the addition of POM to bortezomib and DEX combination therapy in patients with LEN-refractory disease at first relapse demonstrated a PFS advantage with the addition of POM to the bortezomib-DEX backbone (median PFS, 9.84 months with bortezomib-DEX vs 17.84 months with bortezomib-POM-DEX) and a 45% reduction in the risk for PD or death with the triplet.33 Similarly, the European Myeloma Network conducted a phase 2 study that evaluated 8 cycles of carfilzomib with POM-DEX in patients at first relapse with PD on LEN maintenance, followed by POM-DEX or POM maintenance. In that study, the median PFS was 17 months for those who did not receive a salvage ASCT as part of their second-line therapy.34 Our results also compare favorably with the median PFS of 23.7 months seen when daratumumab was added to POM-DEX in patients with LEN-refractory MM at first and second relapse.35 Nonetheless, our findings, although practice informing in the United States, ideally would need to be replicated in the context of a phase 3 study to change the international standard of care for these patients.
Although a PFS benefit was seen when IXA was added to LEN-DEX for patients with relapsed or relapsed and/or refractory MM who had received ≥1 previous therapy and as maintenance after ASCT or nontransplant-based induction, 2 more recent IXA-based studies generated negative results.6,36-39 Specifically, the addition of IXA to LEN-DEX for newly diagnosed patients with transplant-ineligible MM did not improve PFS. Similarly, a study conducted in Spain demonstrated no improvement in PFS when IXA was added to LEN-DEX as maintenance therapy after ASCT. The study populations and disease settings of these studies differed from our study in which all patients had received 1 previous line of therapy, most of whom had received a proteasome inhibitor as part of their initial induction. As such, most patients on our study had known proteasome inhibitor-sensitive disease going into randomization. This selection of patients with proteasome inhibitor-sensitive disease may have impacted the notable reduction in the risk for PD or death seen in our trial, and we would not recommend the use of this regimen in patients with proteasome inhibitor-refractory disease. Given the small number of patients with proteasome inhibitor–naïve disease enrolled in this study, we cannot comment on the safety or activity of the regimen in that patient population.
The combination of the CD38 monoclonal antibodies isatuximab and daratumumab with carfilzomib and DEX have become important global standards of care for the treatment of patients with relapsed MM who have received 1 to 3 previous lines of therapy.23,24 Although the PFS data from these studies compare favorably with what we saw with IXA-POM-DEX, these regimens can be onerous, requiring 3 trips to an infusion center with each 4-week cycle. Furthermore, as the use of CD38 monoclonal antibodies as frontline therapy becomes more frequent, these regimens will become less relevant in early relapse, with the possible exception of the patient who is CD38 monoclonal antibody exposed but not refractory. For all these reasons, we feel that there is a role for IXA-POM-DEX at first relapse, particularly among those who cannot access an infusion center easily and those with cardiovascular comorbidities that would make carfilzomib-based therapy less tenable.
Recently, phase 3 studies that compared the chimeric antigen receptor (CAR) T-cell therapies idecabtagene vicleucel and ciltacabtagene autoleucel with existing standards of care have been conducted in patients with relapsed and relapsed and/or refractory MM who have received ≥2 and 1 to 3 previous lines of therapy, respectively.40,41 Patients in these studies had LEN-refractory disease. Both studies demonstrated a significant PFS advantage with CAR T-cell therapy, paving the way for regulatory approvals of these agents in early relapse, including ciltacabtagene autoleucel at first relapse. We certainly would not suggest that IXA-POM-DEX is more active than ciltacabtagene autoleucel at first relapse. However, an important limitation of CAR T-cell therapy is availability and tolerability in older, frailer patients. In fact, characterization of optimal therapy for frail patients with MM remains poorly defined in general. It should be noted that 22% of the patients in our study were ≥75 years of age. The similar rates of ≥grade 3 nonhematologic AEs and dose modifications seen in the 2 arms of our study would suggest that IXA-POM-DEX might be well suited for a frailer patient population. Moreover, IXA-POM-DEX could be a convenient and safe strategy to control disease in the leadup to leukapheresis and as bridging therapy during the manufacturing process for those patients who have access to CAR T-cell therapy at early relapse. Lastly, the use of an all-oral therapy remains a highly attractive option for those who are not candidates for T-cell redirecting therapies, who are not able to access these therapies or the infusion services needed for their supportive care, or those who simply decide against this treatment approach for socioeconomic reasons and others. It should be noted that many of the patients in this trial were impacted by the COVID-19 pandemic, and the ability to receive care virtually without the need for infusion services was invaluable in limiting their exposure risk, thereby minimizing the COVID-19 mortality seen.
To conclude, the addition of IXA to the POM-DEX backbone significantly improved the DoR and PFS for patients with MM with LEN-refractory disease at first relapse with a favorable safety profile. Our results buttress the inclusion of this all-oral regimen in the National Comprehensive Cancer Network guidelines for relapsed MM and will help to inform clinical practice of these patients in the United States. Furthermore, our results support a phase 3 study of this regimen to confirm our results and to potentially change the standard of care globally.
Acknowledgments
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under award numbers U10CA180821 (principal investigator Suzanne George) and U10CA180882 (principal investigator Sumithra Mandrekar). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: P.V., V.S., P.M., and P.R. designed the research study; P.V., Y.E., N.R., S.T., C.R., J.L., and P.G.R. recruited patients to the research study; M.B.-S. and D.C. provided critical support for protocol and data integrity; P.V., V.S., S.U., and P.G.R. analyzed the data; and P.V. and V.S. wrote the manuscript with contributions from all authors.
Conflict-of-interest disclosure: P.V. reports serving as a consultant for Karyopharm and Regeneron; serving as a consultant for and receiving research funding from Janssen and GlaxoSmithKline; serving as a consultant, on an advisory committee, and on the data safety and monitoring board for Bristol Myers Squibb; serving on an advisory committee for Sanofi, Adaptive Biotechnologies, Lava Therapeutics, and AstraZeneca; receiving research funding from Nervianos Medical Sciences; serving as a consultant and on an advisory committee for and receiving research funding from AbbVie; serving as a consultant and on an advisory committee for Takeda Pharmaceuticals; and serving on an advisory committee for and receiving research funding from Oncopeptides and TeneoBio. Y.E. reports receiving honoraria from and serving on the speakers' bureau for Janssen, Sanofi, Adaptive, and Pfizer, and serving on the chair adjudication committee for Orca Bio and Takeda. N.R. reports serving as a consultant and as a member on an advisory committee for and receiving research funding from GlaxoSmithKline, Sanofi, AbbVie, Immuneel, Bristol Myers Squibb, Pfizer, Janssen, Caribou Biosciences, and K36 Therapeutics. S.T. reports receiving honoraria from and serving on the advisory board for Sanofi, Janssen, and GlaxoSmithKline, and receiving research funding from Karyopharm, Sanofi, Caelum, AbbVie, and Bristol Myers Squibb. C.R. reports serving on an advisory committee for Janssen, Takeda, Bristol Myers Squibb, Amgen, and Karyopharm Therapeutics. J.L. reports receiving honoraria from Lignancies. S.U. reports serving on an advisory committee for and receiving research funding from Janssen, GlaxoSmithKline, Gilead Sciences, Seattle Genetics, Sanofi, SkylineDX, Takeda, Celgene, Bristol Meyers Squibb, Amgen, and AbbVie; serving on an advisory committee for EdoPharma, Genetech, K36 Therapeutics, Moderna, Oncopeptides, Novartis, SecuraBio, and TeneoBio; and receiving research funding from Merch, Array Biopharma, and Pharmacyclics. P.M. reports receiving honoraria from and serving on the Alliance Data and Safety Monitoring Board for Bristol Myers Squibb and Karyopharm, and receiving honoraria from GlaxoSmithKline. P.G.R. reports serving on advisory committees for and receiving research funding from Bristol Myers Squibb, GlaxoSmithKline, and Celgene Corporation; serving as a consultant for Sanofi; and serving as a consultant for and receiving research funding from Takeda Pharmaceuticals Inc and Oncopeptides. The remaining authors declare no competing financial interests.
Correspondence: Peter Voorhees, Levine Cancer Institute, Atrium Health – Wake Forest University School of Medicine, 1021 Morehead Dr, Charlotte, NC 28204; email: peter.voorhees@atriumhealth.org.
References
Author notes
The data that support the findings of this study are available in the National Clinical Trials Network (NCTN)/National Cancer Institute Community Oncology Research Program (NCORP) data archive (https://nctn-data-archive.nci.nih.gov).
The full-text version of this article contains a data supplement.