Key Points
Gilteritinib maintenance therapy after allogeneic HCT in FLT3-ITD AML is not associated with any difference in HRQOL compared with placebo.
Despite a higher incidence of adverse events, gilteritinib was not associated with any patient-reported impact of side effects.
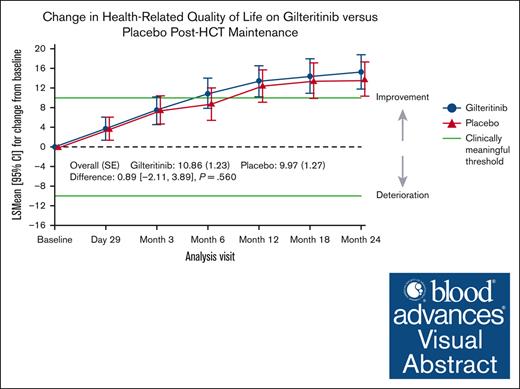
Visual Abstract
The Blood and Marrow Transplant (BMT) Clinical Trials Network conducted a phase 3 randomized trial comparing gilteritinib with placebo after allogeneic hematopoietic cell transplantation (HCT) for FLT3-ITD+ acute myeloid leukemia (AML). The primary analysis demonstrated no statistically significant difference in relapse-free survival (RFS); however, patients with FLT3-ITD measurable residual disease (MRD) peri-HCT had significantly longer RFS with gilteritinib. This analysis investigates the effect of post-HCT gilteritinib vs placebo on health-related quality of life (HRQOL). HRQOL was measured with Functional Assessment of Cancer Therapy-Bone Marrow Transplant (FACT-BMT), FACT-Leukemia (FACT-Leu), and EuroQOL-5 Dimensions (EQ-5D-5L) at post-HCT randomization; day 29; months 3, 6, 12, 18, 24; and/or end of therapy. HRQOL and clinically meaningful differences were summarized using descriptive statistics and compared using mixed model repeated measures to evaluate longitudinal change from baseline and stratified Cox model to evaluate time to improvement. HRQOL completion rate was acceptable (>70%) across all time points and measures. There were no differences in HRQOL scores at any time point between cohorts. Clinically meaningful and time to improvement in HRQOL were similar in both arms. Despite higher treatment-emergent adverse effects with gilteritinib, response to the question of being “bothered by side effects of treatment” did not differ between groups. Subgroup analysis of MRD-positive and negative patients demonstrated no differences in HRQOL between arms. For patients with FLT3-ITD+ AML undergoing HCT, gilteritinib maintenance was not associated with any difference in HRQOL or patient-reported impact of side effects. This trial was registered at www.ClinicalTrials.gov as #NCT02997202.
Introduction
Allogeneic hematopoietic cell transplantation (HCT) is a potentially curative therapy for patients with FLT3-ITD mutated acute myeloid leukemia (AML) and is the standard consolidative postremission treatment in suitable patients.1-3 Several studies investigated the use of FLT3 inhibitors as maintenance therapy after HCT4-6; however, their benefit remained unclear, particularly given risks of toxicity and poor tolerance.7-9 Thus, the Blood and Marrow Transplant Clinical Trials Network (BMT CTN) undertook a randomized, double-blind, placebo-controlled study (BMT CTN 1506 “MORPHO,” identifier: #NCT02997202) to determine whether post-HCT maintenance with the FLT3 inhibitor, gilteritinib, would improve outcomes of patients with FLT3-ITD AML undergoing allogeneic HCT in first complete remission (CR1). The study also investigated whether FLT3-ITD measurable residual disease (MRD) could be used to identify patients more likely to benefit from maintenance therapy. The primary end point of the study was relapse-free survival (RFS) as assessed by a blinded end point review committee. Although the study showed higher RFS in patients randomized to gilteritinib than placebo, the difference was not statistically significant (hazard ratio, 0.679; 95% confidence interval [CI], 0.459-1.005; P = .0518).10 However, among patients with detectable MRD before or after HCT, gilteritinib was associated with a significantly higher RFS (hazard ratio, 0.515; 95% CI, 0.316-0.838; P = .0065). Safety analysis demonstrated that grade 3 or higher treatment-emergent adverse events were higher in patients on gilteritinib than placebo, leading to more drug interruptions (30.9% vs 20.3%), dose reductions (52.2% vs 22%), and withdrawal of therapy (19.7% vs 10.7%), respectively. Although the ideal maintenance regimen should mitigate risk of post-HCT treatment failure, it should also not add significant toxicity affecting patients’ quality of life (QOL). To the best of our knowledge, no studies have examined the impact of postallogeneic HCT maintenance treatment on QOL in patients with AML. Patient-reported outcomes (PROs) are increasingly recognized as meaningful measures of clinical benefit, as reflected by their routine incorporation into clinical trials and the recognition of PROs as a valid measure of clinical benefit for new drug approvals.11 Health-related QOL (HRQOL) measurement in clinical trials is critical to the assessment of treatment benefit or risk from the patients’ perspective. Herein we report the prespecified exploratory analysis of HRQOL and patient-reported symptoms in HCT recipients in the BMT CTN 1506 trial (“MORPHO”), comparing patients receiving maintenance gilteritinib with placebo.
Methods
Study design
Study enrollment occurred between August 2017 and July 2020 at 110 centers internationally. Eligibility included adult patients with a diagnosis of FLT3-ITD mutant AML in CR1 suitable to undergo allogeneic HCT from any donor and graft source, with any conditioning regimen and graft-versus-host disease prophylaxis. Between days 30 and 90 after HCT, participants were randomized to gilteritinib or placebo for 24 months. Further details regarding study design are reported in the primary publication.10 Patients enrolled completed the following QOL assessments: Functional Assessment of Cancer Therapy (FACT)-Bone Marrow Transplantation (BMT) measure, FACT-Leukemia (FACT-Leu) measure, and the EuroQOL-5 Dimensions (EQ-5D-5L). These were completed by patients before HCT (within 30 days of start of conditioning), at randomization (days 30-90 after HCT, baseline), and at months 1, 3, 6, 12, 18, and 24, or end of treatment. QOL assessments were given to patients electronically on provided tablets. The primary objective of this exploratory QOL analysis was to describe and compare the changes over time from baseline (randomization) in measures of HRQOL between treatment arms, using the FACT-BMT, FACT-Leu, and EQ-5D-5L utility score.
Institutional review boards at each site approved the trial protocol.
HRQOL instruments
The FACT-BMT version 4.0 instrument is a 37-item scale comprising the Functional Assessment of Cancer Therapy-General (FACT-G), a 27-item general core questionnaire, which evaluates the HRQOL of patients receiving treatment for cancer, and a specific BMT-module, BMT Concerns (10 items), which addresses disease- and treatment-related questions specific to BMT. Symptoms and HRQOL are assessed on a 5-point Likert-type scale. The FACT-G (score range, 0-108) consists of 4 subscales developed and normalized in patients with cancer: physical well-being (PWB) (range, 0-28), social/family well-being (SWB) (range, 0-28), emotional well-being (EWB) (range, 0-24), and functional well-being (FWB) (range, 0-28). FACT-BMT trial outcome index (TOI) is a composite of the physical, functional, and transplantation-specific subscales that is meant to capture domains, particularly sensitive to the effects of different medical treatments. The TOI does not include the social and emotional subscales. The FACT-BMT total score is the grand total of all items (range, 0-148) and is used as an outcome measure in summarizing FACT-BMT data. Each subscale is positively scored, with higher scores indicating better functioning and better QOL.12,13
The FACT-Leu also includes the FACT-G, as well as a specific Leu subscale.14 THE FACT-G was administered as part of the FACT-BMT and was not repeated with Leu. The Leu-specific subscale (17 items) assesses patient concerns relating to Leu-specific items. The FACT-Leu total score (range, 0-176) is the total of all items in the FACT-G and FACT-Leu modules and was used as the outcome measures summarizing FACT-Leu data. Higher scores indicate better QOL.
The EQ-5D-5L is an international, standardized instrument assessing HRQOL. It consists of 2 parts: the EQ-5D-5L descriptive system and the visual analog scale (VAS). The EQ-5D-5L descriptive system comprises 5 dimensions of health: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression.15 The instrument incudes 1 single question per dimension and each dimension has 5 levels of response corresponding to increasing level of impairment: no problems, slight problems, moderate problems, severe problems, and extreme problems (1-5, respectively). The VAS score records the respondent’s self-rated health status on a vertical graduated scale (0-100), where the 2 end points are labeled as 0 = “worst imaginable state” and 100 = “best imaginable health state,” with higher scores indicating better HRQOL.
Statistical analysis
PRO analysis was conducted on the intent-to-treat (ITT) analysis set. Pre-HCT PRO assessments were collected and described, but the baseline assessment was defined as the measurement on or before the first dose of randomized drug or placebo. Change from baseline for each of the 8 FACT-BMT and FACT-Leu scores, as well as for the 2 EQ-5D-5L scores, was defined as the postbaseline value minus the baseline value and was calculated for each assessment. Clinically important threshold values were based on values previously reported in the literature, and the upper end of the reported threshold ranges was used to define the improvement or deterioration for the primary analysis.12,13,16-18 Specific individual threshold values for clinically meaningful change are as follows: 10 points for FACT-BMT, 12 points for FACT-Leu, and 12 points for EQ-5D-5L VAS.
Descriptive statistics were used to report continuous (eg, mean, standard deviation, median) and categorical data (eg, number, percentage). Statistical comparisons were conducted using 2-sided tests at the 5% significance level. Adjustments for multiple comparisons were not made given the exploratory nature of this analysis. All calculated P values were nominal.
Instrument completion rate at each time point was reported for each instrument on the ITT population. Both unadjusted and adjusted completion rates were calculated by treatment group per time point. An adjusted completion rate at each visit was calculated among patients who are expected to have PRO assessments. Beyond baseline PROs, an assessment was expected if the patient was alive and on treatment at each subsequent time point. As with PRO analyses, missing data were expected and reported between groups for pattern (monotone, meaning if a patient has missing data at 1 visit, data will be missing at subsequent visits; vs nonmonotone or intermittent, when a patient skips 1 visit, but returns for evaluation at subsequent visit[s]). Change from baseline in PRO scores was analyzed using a restricted maximum likelihood–based mixed models repeated measures approach, accounting for missing observations that are assumed to be missing at random. To address nonrandom missing data, sensitivity analysis using a pattern mixture model with sequential modeling using multiple imputation and delta adjustment was done. Although the analysis was performed on the ITT population, only patients with a baseline and at least 1 postbaseline assessment were included. Time to clinically meaningful improvement based on the defined threshold values was analyzed using 2 definitions: time to first clinically meaningful improvement and time to confirmed clinically meaningful improvement (TTCI). TTCI further requires that improvement is confirmed at a subsequent visit. Time to event outcomes were described in each arm using Kaplan-Meier estimates and compared between arms using a stratified log-rank test, using the same strata as used in the randomization. Hazard ratios were estimated with 95% CI from a stratified Cox model. All analysis was performed using SAS version 9.4.
Results
Patient characteristics and PRO completion
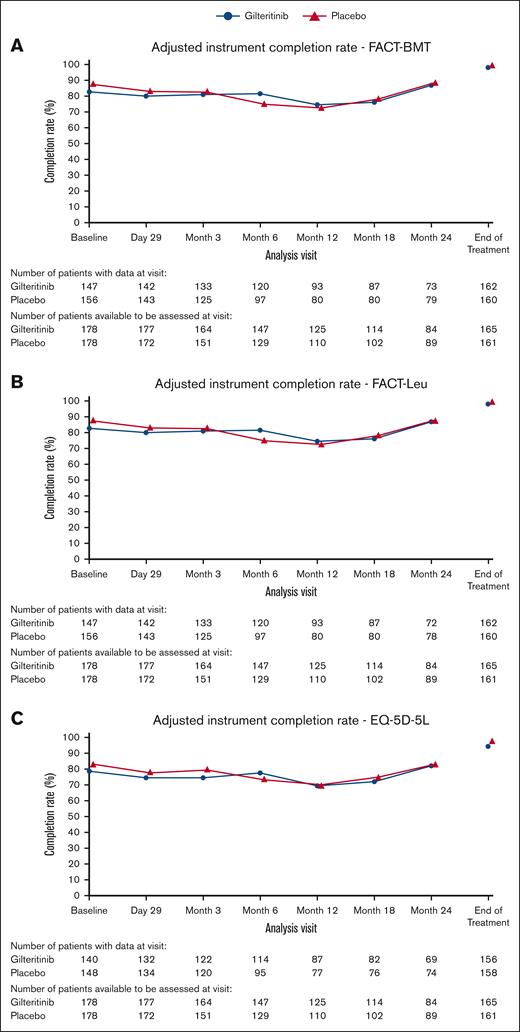
Patient, disease, and transplant characteristics are detailed in the primary publication.10 Briefly, 356 patients were randomized, 178 to placebo and 178 to gilteritinib. Time to randomization (initiation of maintenance therapy) was similar between groups. More than half of patients in both cohorts (52.8% in gilteritinib arm and 53.9% in placebo arm) completed 24 months of treatment on protocol. Median age at time of randomization was 53 years for both cohorts (range, 20-78 with gilteritinib and 18-76 with placebo). Baseline characteristic in the 2 treatment arms were similar. In the gilteritinib arm, adjusted completion rates (patients remaining on study thus expected to have a PRO assessment) for the FACT-BMT and FACT-Leu were 82.6% at baseline and >74.4% at each subsequent time point (day 29 to 24 months, and/or end of treatment). Completion rate for EQ-5D-5L was slightly lower at baseline (78.7%) and >69.6% at subsequent time points. Similar adjusted completion rates were seen in the placebo arm for FACT-BMT and FACT-Leu, 87.6% at baseline and >72.7% at all time points, and for EQ-5D-5L, 83.1% at baseline and >70.0% at all time points (Figure 1).
Adjusted completion rate of HRQOL measures in gilteritinib vs placebo. (A) Adjusted completion rate of FACT-BMT measure in gilteritinib vs placebo groups across each time point. (B) Adjusted completion rate of FACT-Leu measure in gilteritinib vs placebo groups across each time point. (C) Adjusted completion rate of EQ-5D-5L measure in gilteritinib vs placebo groups across each time point.
Adjusted completion rate of HRQOL measures in gilteritinib vs placebo. (A) Adjusted completion rate of FACT-BMT measure in gilteritinib vs placebo groups across each time point. (B) Adjusted completion rate of FACT-Leu measure in gilteritinib vs placebo groups across each time point. (C) Adjusted completion rate of EQ-5D-5L measure in gilteritinib vs placebo groups across each time point.
Missing data occurred in FACT-BMT total scores at a frequency of 39.9% (n = 71) in the gilteritinib arm and 29.8% (n = 53) in the placebo arm. “Monotone” missing data (missed data at 1 time point and subsequent remaining time points) were relatively low for both gilteritinib and placebo, respectively: 2.8% and 4.5% from day 29 onward, 5.1% and 9.6% from 3 months onward, 4.5% and 12.4% from 6 months onward, 8.4% and 5.1% from 12 months onward, 3.9% and 3.4% from 18 months onward, and 5.1% and 2.2% from 24 months onward. Similar missingness patterns were seen with FACT-BMT subscales, FACT-Leu, and EQ-5D-5L scores (data not shown). There were no differences in mean scores of any of the QOL assessments over time among those who completed therapy or discontinued for whatever reason, including adverse events, death, progression, or other (eg, loss to follow-up, noncompliance, protocol deviation; supplemental Figure 1).
HRQOL after allogeneic HCT and comparison between treatment cohorts
Mean scores for FACT-BMT subscales and total score, FACT-Leu, and EQ-5D-5L utility score and VAS are shown for gilteritinib vs placebo in supplemental Table 1. Baseline scores decreased from pre-HCT to baseline, although scores were overall similar across time points for each measurement and there were no differences between treatment arms. However, the relative change from baseline scores returned to pre-HCT levels by month 3 and continued to increase over time in several HRQOL measures and subscales, particularly those focused on physical functioning domains including: the FACT-G PWB subscale, FWB subscale, FACT-BMT additional concerns subscale, FACT-BMT TOI, and FACT-Leu TOI. Change in summary scores from baseline, such as FACT-G total score, FACT-BMT total score, FACT-Leu total score and TOI, and EQ-5D-5L VAS score also increased over time, which reflected predominant contribution of physical functioning domains. Clinically meaningful improvement as measured by change from baseline scores (defined by ±10 for FACT-BMT, ±12 for FACT-Leu and EQ-5D-5L VAS) evaluated longitudinally by mixed model repeated measures was seen at several time points after transplant and continued to increase from 6 months to 24 months for several subscales (PWB, FWB, TOI, FACT-G, FACT-BMT, and FACT-Leu total scores). Figure 2 demonstrates representative change in scores from baseline for FACT-BMT, FACT-Leu, and the EQ-5D-5L VAS. There was no difference between gilteritinib and placebo with regard to time to first clinically meaningful improvement (supplemental Figures 2 and 3). Interestingly, although the median time to first clinically meaningful improvements in domains of physical functioning were typically in the 3 to 6 months’ range, any improvement in domains of SWB and EWB occurred much longer after transplant (range, 12-18 months). As representative of physical functioning domains, the median time to first improvement in FACT-BMT TOI was 3.02 months for both gilteritinib (95% CI, 2.83-5.85) and placebo (95% CI, 2.92-5.82; P = .767), and FACT-G was 4.99 (95% CI, 2.99-5.98) and 3.09 months (95% CI, 2.92-5.88; P = 1.0), respectively. In contrast, the median time to first improvement for the EWB subscale was 11.63 months (95% CI, 5.95-18.20) for gilteritinib and 11.76 months (95% CI, 5.72-17.81) for placebo (P = .687), and for the SWB subscale was 12.42 (95% CI, 5.85-23.95) for gilteritinib and 18.37 (95% CI, 12.09 to not calculable) for placebo (P = .107; supplemental Figure 3). Similar patterns were seen in TTCI.
Change in HRQOL scores from baseline to 24 months after transplant. (A) Change in FACT-BMT total score in gilteritinib vs placebo groups across time. Clinically meaningful change is defined as a change of 10 points for FACT-BMT. (B) Change in FACT-Leu total score in gilteritinib vs placebo groups across time. Clinically meaningful change is defined as a change of 12 points for FACT-Leu. (C) Change in EQ-5D-5L VAS score in gilteritinib vs placebo groups across time. Clinically meaningful change is defined as a change of 12 points for EQ-5D-5L. SE, standard error.
Change in HRQOL scores from baseline to 24 months after transplant. (A) Change in FACT-BMT total score in gilteritinib vs placebo groups across time. Clinically meaningful change is defined as a change of 10 points for FACT-BMT. (B) Change in FACT-Leu total score in gilteritinib vs placebo groups across time. Clinically meaningful change is defined as a change of 12 points for FACT-Leu. (C) Change in EQ-5D-5L VAS score in gilteritinib vs placebo groups across time. Clinically meaningful change is defined as a change of 12 points for EQ-5D-5L. SE, standard error.
Patient-reported fatigue, QOL, anxiety/depression, and side effects of treatment
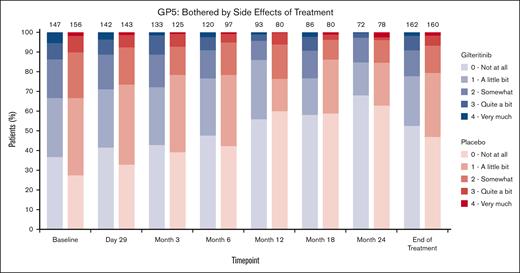
Responses to specific individual questions focusing on fatigue (eg, “I get tired easily,” and “I am able to do my usual activities”), symptoms (“I am bothered by skin problems”), general QOL (eg, “I am content with the quality of my life right now”), anxiety and depression (eg, “I feel anxious,” “I feel sad”), and social roles (eg, “I feel close to my friends”) were similarly observed for gilteritinib and placebo groups at any time point. Responses generally indicated improvement over 24 months regardless of treatment arm (supplemental Figure 4). Responses to a specific question regarding side effects of therapy (“I am bothered by side effects of treatment”) were similar for gilteritinib and placebo arms. Most patients reported “a little bit” or “not at all,” and the proportion of those reporting “not at all” increased over the 24-month time period (Figure 3) in both arms.
Patient-reported side effects of treatment. Comparison of responses to a specific question regarding side effects of therapy (“I am bothered by side effects of treatment”) between gilteritinib and placebo arms.
Patient-reported side effects of treatment. Comparison of responses to a specific question regarding side effects of therapy (“I am bothered by side effects of treatment”) between gilteritinib and placebo arms.
HRQOL in MRD-positive vs MRD-negative patients
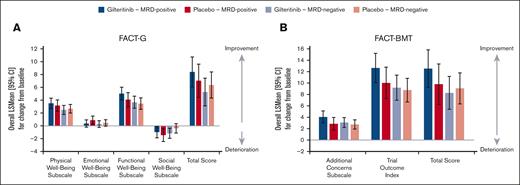
Subgroup analyses of MRD-positive (≥1 × 10–6) and MRD-negative groups demonstrated similar patterns across all domains as with the entire cohort. Positive change/improvement in PWB and FWB from baseline to post-HCT occurred, whereas minimal changes in EWB and SWB were noted across MRD-positive and MRD-negative subsets. Although change from baseline scores across QOL measures was higher in the gilteritinib arm than placebo in the MRD-positive cohort, these differences were not statistically significant (Figure 4).
HRQOL in MRD-positive and MRD-negative patients. (A) Change in baseline FACT-G domains and total scores in MRD-positive and MRD-negative patients. (B) Change in baseline FACT-BMT domains and total scores in MRD-positive and MRD-negative patients.
HRQOL in MRD-positive and MRD-negative patients. (A) Change in baseline FACT-G domains and total scores in MRD-positive and MRD-negative patients. (B) Change in baseline FACT-BMT domains and total scores in MRD-positive and MRD-negative patients.
Discussion
BMT CTN 1506 (“MORPHO”) demonstrated that gilteritinib maintenance after allogeneic HCT conferred a RFS benefit for patients with AML in CR1 with detectable peri-HCT FLT3-ITD MRD. Despite a higher rate of treatment-emergent adverse effects in the gilteritinib arm, this exploratory analysis showed no worsening impact of gilteritinib maintenance on HRQOL than placebo. HRQOL in both arms continued to improve over the 24-month period of post-HCT maintenance regardless of treatment. The lack of association between the higher treatment-emergent adverse effects with gilteritinib and HRQOL is likely due to the fact that most reported adverse events requiring dose adjustment or discontinuation were changes in laboratory parameters (eg, neutropenia and thrombocytopenia) that would not necessarily add to a patient’s symptom burden. These findings emphasize the need to specifically assess QOL and tolerability from the patient perspective.
In cancer care, a key challenge remains balancing treatment benefit with therapy-related toxicity and subsequent impact on HRQOL. Patients eligible for maintenance therapy are often asymptomatic and are seeking to maintain their HRQOL in addition to extending remission.19-21 Thus, safety and QOL are critical considerations of maintenance therapy, particularly after the typically intensive and highly toxic treatment modalities used for AML. To our knowledge, there have been no studies evaluating the impact of maintenance therapy on HRQOL after allogeneic HCT. A recent survey of 84 patients with AML and 145 physicians assessing preferences regarding maintenance therapies demonstrated that the most important attribute valued by patients was QOL, followed by duration of hospitalization and chance of 2-year RFS, whereas physicians primarily valued chance of RFS, followed by QOL and risk of serious infections.22
In multiple myeloma, where maintenance after autologous HCT is a standard approach to improve progression-free survival, HRQOL is an important secondary end point in most contemporary trials.21,23,24 A small survey of patients investigating patients’ preferences about maintenance therapy also suggests that QOL is an important but patient preference may be variable in regard to willingness to accept longer progression-free survival at the cost of reduced QOL and may also be dependent on where they are in their disease course,24 highlighting the importance of the patient’s perspective in making clinical decisions.
We found no significant differences in HRQOL between gilteritinib and placebo across any measures. Importantly, a specific question assessing how bothered a patient was by side effects of treatment demonstrated no differences between active maintenance therapy with gilteritinib and placebo, which improved (patients became less bothered by treatment) over time after HCT regardless of treatment arm. The fact that more than half of patients answered “not at all” or “a little bit” at baseline and day 29 is reassuring, particularly given the toxicity burden typically experienced by transplant recipients early in the postengraftment period. Similarly, there were no differences in other singular questions assessing specific symptoms and domains (eg, sleep, fatigue, anxiety, depression, and skin problems) between gilteritinib and placebo.
As previously reported, we demonstrate a decrease in QOL in the acute period after HCT, returning to pre-HCT levels by 3 months after HCT. There was a continued consistent trajectory of improvement over 24 months in HRQOL, specifically in physical functioning domains, which was the primary contributor to the improvement in summary scores. Although emotional and social well-being domains were already relatively high (indicating good health status in line with cancer and general population norms25) at baseline, there were no significant or clinically meaningful changes in these measures in the 24-month period after transplant.
We acknowledge a number of limitations to this analysis. This was a prespecified exploratory analysis of HRQOL and adjustments for multiple comparisons were not performed. Missing data generally occurred at low frequency; however, missing data may introduce selection bias given that patients had to be well enough to fill out a QOL assessment. Overall completion rates were acceptable and higher than previously conducted BMT CTN studies assessing QOL. This may be due to electronic delivery of PRO administered during clinic visits. Given the exploratory nature of this analysis, HRQOL was not analyzed in regard to posttransplant events including graft-versus-host disease. In particular, although HRQOL was collected at the end of treatment time point, no additional QOL data were collected after relapse or withdrawal from study for other reasons including toxicity, which may have confounded the lack of differences in HRQOL between arms. However, the number of patients withdrawing from study for any reason was small and similar between groups and thus not likely to be significant.
Conclusion
To the best of our knowledge, this is the first QOL analysis of maintenance therapy after allogeneic HCT. Our findings that gilteritinib after HCT does not adversely affect HRQOL are reassuring and support the use of gilteritinib as maintenance therapy after HCT in patients with peri-HCT detectable FLT3-ITD MRD.
Acknowledgments
Support for this study was provided by grants from the National Heart, Lung, and Blood Institute and the National Cancer Institute (grants U10HL069294 and U24HL138660) to the Blood and Marrow Transplant Clinical Trials Network and by funding from Astellas Pharma Global Development Inc.
Authorship
Contribution: B.K.H. contributed to analysis and interpretation of data and wrote the manuscript; B.J.P., C.I., D.E., D.C., L.C., and C.P. contributed to collection, assembly and analysis of data, and critically reviewed the manuscript; and M.H., Y.-B.C., M.J.L., M.U.O., M.R.L., R.J.S., C.U., A.E.P., A.K.S., N.G., N.H., M.R., M.M.H., and B.L. contributed to conception and design of study, collection of data, data analysis and interpretation, and critical review of the manuscript.
Conflict-of-interest disclosure: B.K.H. reports consulting/advisory roles with Sanofi, Incyte, Rigel, Maat Pharma, and ACI Group; has served on the data safety monitoring board for Angiocrine; and has served on the adjudication committee for Commonwealth Serum Laboratories Behring. B.J.P. reports employment with Astellas Pharma. C.I. reports employment with IQVIA. D.E. reports employment with Astellas Pharma; stock and other ownership interests in Astellas Pharma; and research funding from Astellas Pharma. M.H. reports honoraria from Celgene; consulting or advisory roles with Incyte, Antibody Drug Conjugates Therapeutics, Puma Biotechnology, Verastem, Kite/Gilead, MorphoSys, Omeros, Novartis, Gamida Cell, Seagen, Genmab, Myeloid Therapeutics, BeiGene, AstraZeneca, Sanofi, Bristol Myers Squibb (BMS)/Celgene, CRISPR Therapeutics, Caribou Biosciences, AbbVie, and Genentech; speakers' bureau activities with Genzyme, AstraZeneca, BeiGene, Antibody Drug Conjugates Therapeutics, and Kite/Gilead; and research funding from Takeda, Spectrum Pharmaceuticals, Otsuka, Astellas Pharma, and Genzyme. Y.-B.C. reports a leadership role at ImmunoFree; stock and other ownership interests in ImmunoFree; and consulting or advisory roles with Magenta Therapeutics, Incyte, Novo Nordisk, Editas Medicine, Alexion Pharmaceuticals, Astellas Pharma, Takeda, Pharmacosmos, and Vor Biopharma. M.J.L. reports consulting or advisory roles with Daiichi Sankyo, Amgen, Fujifilm, Astellas Pharma, Menarini, BMS, AbbVie/Genentech, GlaxoSmithKline, Jazz Pharmaceuticals; and research funding from Astellas Pharma and Fujifilm. M.R.L. reports honoraria from BeiGene Shanghai and Amgen; participation in speakers' bureau for BeiGene Shanghai and Amgen; research funding from Amgen, Astellas Pharma, Actinium Pharmaceuticals, and Syndax; and other relationship roles with BioSight. R.J.S. reports a leadership at Be the Match/National Marrow Donor Program; consulting or advisory roles with Astellas, Amgen, Vor Biopharma, Smart Immune, Neovii, Bluesphere Bio, and Jasper; and has served on the data and safety monitoring board for BMS. C.U. reports employment with Takeda and Blueprint Medicines; honoraria from Novartis and Blueprint Medicines; and participation in speakers' bureau for Novartis. A.E.P. reports honoraria from Astellas Pharma and Daiichi Sankyo; consulting or advisory roles with Astellas Pharma, Actinium Pharmaceuticals, Daiichi Sankyo, AbbVie, Forma Therapeutics, Sumitomo Dainippon, Celgene/BMS, Syndax, Genentech, BerGenBio, ImmunoGen, Foghorn Therapeutics, Rigel, and Curis; and research funding from Astellas Pharma, Bayer, Daiichi Sankyo, Fujifilm, AbbVie, and Syndax. N.H. reports employment with Astellas Pharma and research funding from Astellas Pharma. M.R. reports employment with, stock and other ownership interests in, and research funding from Astellas Pharma. L.C. and C.P. report employment with IQIVIA. M.M.H. reports consulting or advisory roles with Medac, and research funding from Jazz Pharmaceuticals, Novartis, Sanofi, Astellas Pharma, Xenikos, and Gamida Cell. The remaining authors declare no competing financial interests.
Correspondence: Betty K. Hamilton, Blood and Marrow Transplant Program, Department of Hematology and Medical Oncology, Taussig Cancer Institute, Cleveland Clinic, 9500 Euclid Ave CA60, Cleveland, OH 44195; email: hamiltb2@ccf.org.
References
Author notes
Presented as an oral abstract at the annual meeting of the American Society of Transplantation and Cellular Therapy, San Antonio, TX, 22 February 2024.
Researchers may request access to anonymized participant-level data, trial level data, and protocols from Astellas sponsored clinical trials at www.clinicalstudydatarequest.com. The Astellas criteria on data sharing is available at https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Astellas.aspx.
The full-text version of this article contains a data supplement.