Key Points
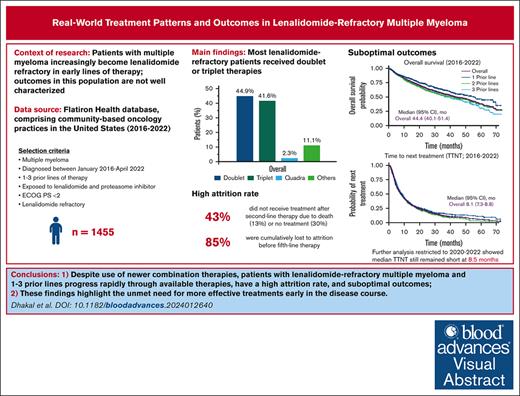
Real-world patients with lenalidomide-refractory MM and 1 to 3 prior LOT progress rapidly through therapies.
Outcomes remain suboptimal, despite greater use of newer combination therapies, highlighting the need for early use of novel treatments.
Visual Abstract
In the context of multiple myeloma (MM), early use of the immunomodulatory drug lenalidomide has led to an increased population of patients with lenalidomide-refractory MM in early-line settings, but their outcomes are not well characterized. Herein, we report treatment patterns, survival outcomes, prognostic variables, and attrition rates for patients with proteasome inhibitor–exposed, lenalidomide-refractory MM, treated with 1 to 3 prior lines of therapy (LOT). From 12 767 patients with MM in the Flatiron Health database between January 2016 and April 2022, 1455 met the inclusion criteria. The most common subsequent treatments were triplet combinations (41.6% of patients); daratumumab/pomalidomide/dexamethasone was the most common treatment regimen (13.2%). Median real-world progression-free survival (RW-PFS) and overall survival (OS) were 6.5 months and 44.4 months, respectively. RW-PFS was similar in patients with 1, 2, or 3 prior LOT. International Staging System stage III, Eastern Cooperative Oncology Group performance status of 1, hemoglobin <12 g/dL, high-risk cytogenetics, and refractoriness to anti-CD38 antibody at baseline were associated with worse RW-PFS and OS. Outcomes remained similar for patients who received National Comprehensive Cancer Network–preferred treatments and those who received treatments after 2020. In 561 patients with 1 prior LOT at inclusion, the cumulative attrition rate from LOT 2 to 5 was 85%, which included 25% patients who died and 60% with no further treatment. Patients with lenalidomide-refractory MM who have received 1 to 3 prior LOT have poor outcomes and progress rapidly through available therapies, highlighting the need for more effective treatments early in the disease course, before patients are lost to attrition.
Introduction
Lenalidomide combination therapy is a preferred treatment recommended by the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) as an NCCN Category 1 and the European Society for Medical Oncology guidelines (strongly/generally recommended) for newly diagnosed multiple myeloma (MM).1-3 It is also recommended for use in combination with other treatments for relapsed/refractory MM (RRMM). In newly diagnosed MM, this recommendation has been made across both transplant-eligible patients (as induction) and transplant-ineligible patients (as main treatment). Its use has become increasingly prevalent in these settings, and as maintenance therapy following stem cell transplantation.4 These practices have led to an increased population of lenalidomide-exposed patients, and because it is often given until progression, there is an increased incidence of lenalidomide-refractoriness as early as the second line of therapy (LOT).4 Once the disease becomes lenalidomide-refractory, choosing a subsequent therapeutic regimen is complex.1-3 Several phase 3 studies have shown improved clinical outcomes with triplet vs doublet therapy for patients with lenalidomide-refractory MM.5-9 However, few studies have documented real-world treatment practices, attrition rates, and outcomes in this population, particularly in the context of treatments that have become available more recently (eg, anti-CD38 monoclonal antibodies).10-12
We sought to characterize real-world data on patients with lenalidomide-refractory MM to define an unmet need in this population, including treatments they receive and their outcomes at the population level. Data were analyzed from the Flatiron Health database, which comprises deidentified electronic health records for patients from primarily community-based oncology practices in the United States.13 We report baseline characteristics, treatment patterns, survival outcomes, and prognostic variables for lenalidomide-refractory patients treated with 1 to 3 prior LOT as they progress from one LOT to the next. Additionally, we report real-world attrition rates in patients with lenalidomide-refractory MM who had received 1 prior LOT.
Methods
Flatiron patient selection
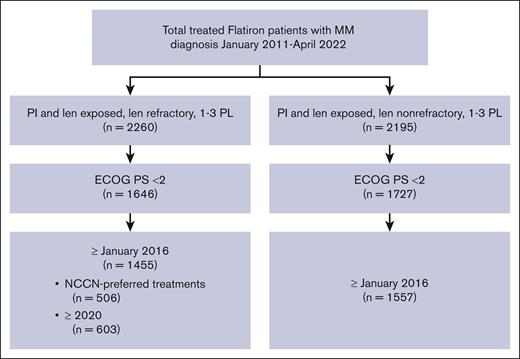
Data were derived from the Flatiron Health deidentified US electronic health records database between January 2016 and April 2022 to reflect the current treatment landscape. Patients included in the primary analysis had a confirmed diagnosis of MM, had received 1 to 3 prior LOT, were exposed to a proteasome inhibitor (PI) and immunomodulatory drug, had lenalidomide-refractory disease, and an Eastern Cooperative Oncology Group performance status (ECOG PS) <2. Patients who were PI- and lenalidomide-exposed but not lenalidomide refractory were separately analyzed for further context. “Lenalidomide refractory” was defined as having a change in treatment within 60 days of last lenalidomide therapy without lenalidomide as a component of the immediate next line, as previously described.14,15 MM was defined as “lenalidomide-exposed-not-refractory” if the patients had previously received lenalidomide therapy for >60 days before a subsequent therapy or had received lenalidomide as a component of the immediate next line at the time they met all other inclusion criteria.14
To better understand the treatment patterns in real-world data as per NCCN Guidelines, additional analyses were conducted on subsets of patients with lenalidomide-refractory MM who met the above inclusion criteria and had been treated with a NCCN-preferred regimen for this patient population (as per NCCN Clinical Practice Guidelines in Oncology [NCCN Guidelines], Multiple Myeloma, 2024).1 Analyses of patients treated since 2020 were also conducted to better understand the current needs of patients with lenalidomide-refractory MM.
Analyses
Time 0 (T0), or index date, was defined as the start of first therapy (index therapy) after a patient met the inclusion criteria. Combinations with 1, 2, or 3 of the following were classified as doublet, triplet, or quadruplet therapy regimens, respectively: PI, immunomodulatory drug, anti-CD38 monoclonal antibodies, steroid, selinexor, belantamab mafodotin, and elotuzumab. Descriptive statistics were used to assess baseline characteristics and treatment patterns in the overall analysis population and were stratified by prior LOT. For each patient, only the first LOT after meeting the inclusion criteria was analyzed.
Time-to-event outcomes (time-to-treatment discontinuation [TTTD], time-to-next treatment [TTNT], real-world progression-free survival [RW-PFS], and overall survival [OS]) were estimated using the Kaplan-Meier method starting at T0. TTTD was defined as the time interval between the start of the first treatment after meeting inclusion criteria and end of that treatment or death, whichever occurred first (if no event occurred, patient was censored at the end of follow-up). TTNT was defined as the time interval between the index date and next treatment or death, whichever occurred first (where there were no events, patients were censored at the end of follow-up). RW-PFS was defined as any event of disease progression, change in antimyeloma treatment, or death, whichever occurred first (if no event occurred, patients were censored at the end of follow-up). OS was defined as the time interval between the index date and all-cause mortality (patients were censored at the end of follow-up). Univariate Cox regression models were used to evaluate prognostic variables for OS and RW-PFS.
The attrition rate was analyzed in patients at LOT 2 (who had only 1 prior LOT) and was defined as the proportion of patients without a record of subsequent MM treatment, including those who were lost to follow-up or who died. Cumulative attrition rates were calculated using the number of patients at LOT 2 as the denominator, and are presented as a Sankey plot. Noncumulative attrition rates were calculated using the number of patients at previous LOT who were on active antimyeloma treatment as the denominator.
Results
Characteristics of patient with lenalidomide-refractory MM
Of the 12 767 patients with MM in the Flatiron database, 1455 PI-exposed patients with lenalidomide-refractory MM and ECOG PS of 0 or 1 were identified (Figure 1). There were 561 patients (38.6%) who had received 1 prior LOT, 566 (38.9%) who had received 2 prior LOT, and 328 (22.5%) who had received 3 prior LOT (Table 1). At inclusion, the median age was 69 years (range, 61-76), with a median time since diagnosis of 1.7 years (interquartile range, 0.8-3.3). The duration of initial treatment in patients with 1 prior LOT was 0.7 (0.4-1.4) years, suggesting inclusion of functionally high-risk patients. Other indicators of disease status include that 19.7% of patients had high-risk cytogenetics (del17p, t[4;14], or t[14;16] mutations), 20.2% were classified as International Staging System (ISS) stage III, and 61.1% had an ECOG PS of 1. The most common treatment before meeting the inclusion criteria (other than lenalidomide) was bortezomib, received by 90.6% of patients. At T0, 71.2% of patients were refractory to a PI; refractoriness to PI increased with increasing numbers of prior LOT (1 prior LOT, 64.9%; 2 prior LOT, 71.0%; 3 prior LOT, 82.3%). Refractoriness to anti-CD38 antibodies (6.7% of patients) also increased with increasing numbers of prior therapies (1 prior LOT, 2.7%; 2 prior LOT, 8.5%; 3 prior LOT, 10.4%).
Baseline characteristics by number of prior LOT in lenalidomide-refractory patients at first index
| Characteristic . | Overall (N = 1455) . | 1 prior LOT (n = 561) . | 2 prior LOT (n = 566) . | 3 prior LOT (n = 328) . |
|---|---|---|---|---|
| Age, median (IQR), y | 69 (61-76) | 69 (61-75) | 70 (61-76) | 68 (60-75) |
| Female, n (%) | 646 (44.4) | 251 (44.7) | 239 (42.2) | 156 (47.6) |
| Race, n (%) | ||||
| White | 916 (63.0) | 347 (61.9) | 355 (62.7) | 214 (65.2) |
| Black | 241 (16.6) | 88 (15.7) | 97 (17.1) | 56 (17.1) |
| Other/unknown | 298 (20.5) | 126 (22.5) | 104 (18.4) | 58 (17.7) |
| Time from diagnosis to T0, median (IQR), y | 1.7 (0.8-3.3) | 0.7 (0.4-1.4) | 2.2 (1.2-3.5) | 3.3 (2.2-4.8) |
| MM subtype, n (%) | ||||
| IgG | 850 (58.4) | 308 (54.9) | 337 (59.5) | 205 (62.5) |
| Light chain | 266 (18.3) | 110 (19.6) | 88 (15.5) | 68 (20.7) |
| Others | 339 (23.3) | 143 (25.5) | 141 (24.9) | 55 (16.8) |
| Cytogenetic risk, n (%) | ||||
| High∗ | 286 (19.7) | 105 (18.7) | 114 (20.1) | 67 (20.4) |
| Standard | 807 (55.5) | 318 (56.7) | 318 (56.2) | 171 (52.1) |
| Unknown | 362 (24.9) | 138 (24.6) | 134 (23.7) | 90 (27.4) |
| ISS stage, n (%) | ||||
| I | 339 (23.3) | 136 (24.2) | 134 (23.7) | 69 (21.0) |
| II | 322 (22.1) | 137 (24.4) | 117 (20.7) | 68 (20.7) |
| III | 294 (20.2) | 125 (22.3) | 105 (18.6) | 64 (19.5) |
| Unknown | 500 (34.4) | 163 (29.1) | 210 (37.1) | 127 (38.7) |
| ECOG PS, n (%) | ||||
| 0 | 566 (38.9) | 241 (43.0) | 219 (38.7) | 106 (32.3) |
| 1 | 889 (61.1) | 320 (57.0) | 347 (61.3) | 222 (67.7) |
| Hemoglobin at T0, n (%) | ||||
| ≥12 g/dL | 537 (36.9) | 214 (38.1) | 195 (34.5) | 128 (39.0) |
| <12 g/dL | 918 (63.1) | 347 (61.9) | 371 (65.5) | 200 (61.0) |
| Prior stem cell transplant, n (%) | 542 (37.3) | 170 (30.3) | 227 (40.1) | 145 (44.2) |
| Prior treatments, n (%) | ||||
| Bortezomib | 1318 (90.6) | 528 (94.1) | 498 (88.0) | 292 (89.0) |
| Carfilzomib | 226 (15.5) | 24 (4.3) | 100 (17.7) | 102 (31.1) |
| Daratumumab | 166 (11.4) | 21 (3.7) | 89 (15.7) | 56 (17.1) |
| Ixazomib | 133 (9.1) | 11 (2.0) | 64 (11.3) | 58 (17.7) |
| Pomalidomide | 82 (5.6) | 2 (0.4) | 33 (5.8) | 47 (14.3) |
| Thalidomide | 14 (1.0) | 0 | 6 (1.1) | 8 (2.4) |
| Elotuzumab | 37 (2.5) | 0 | 18 (3.2) | 19 (5.8) |
| Isatuximab | 0 | 0 | 0 | 0 |
| Time to progression on last line, n (%) | ||||
| ≤4 mo | 338 (23.2) | 132 (23.5) | 133 (23.5) | 73 (22.3) |
| >4 mo | 1117 (76.8) | 429 (76.5) | 433 (76.5) | 255 (77.7) |
| Refractory status, n (%) | ||||
| PI | 1036 (71.2) | 364 (64.9) | 402 (71.0) | 270 (82.3) |
| Anti-CD38 antibody | 97 (6.7) | 15 (2.7) | 48 (8.5) | 34 (10.4) |
| Characteristic . | Overall (N = 1455) . | 1 prior LOT (n = 561) . | 2 prior LOT (n = 566) . | 3 prior LOT (n = 328) . |
|---|---|---|---|---|
| Age, median (IQR), y | 69 (61-76) | 69 (61-75) | 70 (61-76) | 68 (60-75) |
| Female, n (%) | 646 (44.4) | 251 (44.7) | 239 (42.2) | 156 (47.6) |
| Race, n (%) | ||||
| White | 916 (63.0) | 347 (61.9) | 355 (62.7) | 214 (65.2) |
| Black | 241 (16.6) | 88 (15.7) | 97 (17.1) | 56 (17.1) |
| Other/unknown | 298 (20.5) | 126 (22.5) | 104 (18.4) | 58 (17.7) |
| Time from diagnosis to T0, median (IQR), y | 1.7 (0.8-3.3) | 0.7 (0.4-1.4) | 2.2 (1.2-3.5) | 3.3 (2.2-4.8) |
| MM subtype, n (%) | ||||
| IgG | 850 (58.4) | 308 (54.9) | 337 (59.5) | 205 (62.5) |
| Light chain | 266 (18.3) | 110 (19.6) | 88 (15.5) | 68 (20.7) |
| Others | 339 (23.3) | 143 (25.5) | 141 (24.9) | 55 (16.8) |
| Cytogenetic risk, n (%) | ||||
| High∗ | 286 (19.7) | 105 (18.7) | 114 (20.1) | 67 (20.4) |
| Standard | 807 (55.5) | 318 (56.7) | 318 (56.2) | 171 (52.1) |
| Unknown | 362 (24.9) | 138 (24.6) | 134 (23.7) | 90 (27.4) |
| ISS stage, n (%) | ||||
| I | 339 (23.3) | 136 (24.2) | 134 (23.7) | 69 (21.0) |
| II | 322 (22.1) | 137 (24.4) | 117 (20.7) | 68 (20.7) |
| III | 294 (20.2) | 125 (22.3) | 105 (18.6) | 64 (19.5) |
| Unknown | 500 (34.4) | 163 (29.1) | 210 (37.1) | 127 (38.7) |
| ECOG PS, n (%) | ||||
| 0 | 566 (38.9) | 241 (43.0) | 219 (38.7) | 106 (32.3) |
| 1 | 889 (61.1) | 320 (57.0) | 347 (61.3) | 222 (67.7) |
| Hemoglobin at T0, n (%) | ||||
| ≥12 g/dL | 537 (36.9) | 214 (38.1) | 195 (34.5) | 128 (39.0) |
| <12 g/dL | 918 (63.1) | 347 (61.9) | 371 (65.5) | 200 (61.0) |
| Prior stem cell transplant, n (%) | 542 (37.3) | 170 (30.3) | 227 (40.1) | 145 (44.2) |
| Prior treatments, n (%) | ||||
| Bortezomib | 1318 (90.6) | 528 (94.1) | 498 (88.0) | 292 (89.0) |
| Carfilzomib | 226 (15.5) | 24 (4.3) | 100 (17.7) | 102 (31.1) |
| Daratumumab | 166 (11.4) | 21 (3.7) | 89 (15.7) | 56 (17.1) |
| Ixazomib | 133 (9.1) | 11 (2.0) | 64 (11.3) | 58 (17.7) |
| Pomalidomide | 82 (5.6) | 2 (0.4) | 33 (5.8) | 47 (14.3) |
| Thalidomide | 14 (1.0) | 0 | 6 (1.1) | 8 (2.4) |
| Elotuzumab | 37 (2.5) | 0 | 18 (3.2) | 19 (5.8) |
| Isatuximab | 0 | 0 | 0 | 0 |
| Time to progression on last line, n (%) | ||||
| ≤4 mo | 338 (23.2) | 132 (23.5) | 133 (23.5) | 73 (22.3) |
| >4 mo | 1117 (76.8) | 429 (76.5) | 433 (76.5) | 255 (77.7) |
| Refractory status, n (%) | ||||
| PI | 1036 (71.2) | 364 (64.9) | 402 (71.0) | 270 (82.3) |
| Anti-CD38 antibody | 97 (6.7) | 15 (2.7) | 48 (8.5) | 34 (10.4) |
IgG, immunoglobulin G; IQR, interquartile range.
Defined as the presence of ≥1 del17p, t(4;14), or t(14;16) mutation.
Real-world treatment patterns in patients with lenalidomide-refractory MM
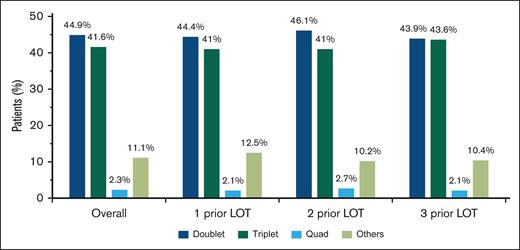
For index therapies, doublet and triplet treatment regimens were the most common treatment approaches regardless of the number of prior LOT (Figure 2); 44.9% of patients received doublet regimens, 41.6% received triplet regimens, and 2.3% received quadruplet index therapy regimens. This distribution remained consistent across numbers of prior LOT.
Most common treatment combinations in patients with lenalidomide-refractory RRMM after index date. Combinations with 1, 2, or 3 of the following were classified as doublet, triplet, or quadruplet therapy regimens, respectively: PI, immunomodulatory drug, anti-CD38 monoclonal antibodies, steroid, selinexor, belantamab mafodotin, and elotuzumab. Other treatment regimens included combinations that did not fall into any of the above categories.
Most common treatment combinations in patients with lenalidomide-refractory RRMM after index date. Combinations with 1, 2, or 3 of the following were classified as doublet, triplet, or quadruplet therapy regimens, respectively: PI, immunomodulatory drug, anti-CD38 monoclonal antibodies, steroid, selinexor, belantamab mafodotin, and elotuzumab. Other treatment regimens included combinations that did not fall into any of the above categories.
Overall, 34.8% of patients received an index treatment that is an NCCN-preferred regimen for patients with lenalidomide-refractory MM with 1 to 3 prior LOT (Table 2).1 Daratumumab/pomalidomide/dexamethasone (DPd), received by 13.2% of patients, was most common. Daratumumab monotherapy and pomalidomide/dexamethasone, which are not NCCN-preferred treatments for patients with lenalidomide-refractory MM, were the second (8.3%) and third (7.7%) most frequently used regimens, respectively. The recommended carfilzomib (K)-based regimens carfilzomib/pomalidomide/dexamethasone (7.1%) and carfilzomib/dexamethasone (7.0%) were also commonly used, as were daratumumab/carfilzomib/dexamethasone (DKd) (3.8%) and isatuximab/carfilzomib/dexamethasone (0.2%), to a lower extent. Other treatment regimens not indicated for patients with lenalidomide-refractory MM by NCCN Guidelines1 were received by 26.4% of patients. Treatment patterns evolved throughout the study period. For example, use of daratumumab (monotherapy or any combination) increased each year (2016-2022, 15.2%, 20.4%, 35.5%, 38.8%, 45.7%, 51.0%, and 58.6%).
Index treatment regimens received by patients with lenalidomide-refractory RRMM previously treated with 1 to 3 prior lines of treatment
| Treatment regimen, n (%) . | Overall . | 1 prior LOT (LOT 2) . | 2 prior LOT (LOT 3) . | 3 prior LOT (LOT 4) . |
|---|---|---|---|---|
| (N = 1455) . | (n = 561) . | (n = 566) . | (n = 328) . | |
| NCCN-preferred regimens for lenalidomide-refractory 1-3 PL RRMM∗ | ||||
| DPd | 192 (13.2) | 58 (10.3) | 80 (14.1) | 54 (16.5) |
| KPd | 104 (7.1) | 53 (9.4) | 34 (6.0) | 17 (5.2) |
| DVd | 95 (6.5) | 48 (8.6) | 32 (5.7) | 15 (4.6) |
| DKd | 56 (3.8) | 24 (4.3) | 25 (4.4) | 7 (2.1) |
| PVd | 39 (2.7) | 23 (4.1) | 11 (1.9) | 5 (1.5) |
| IxaPd | 14 (1.0) | 4 (0.7) | 6 (1.1) | 4 (1.2) |
| IsaKd | 3 (0.2) | 1 (0.2) | 2 (0.4) | 0 |
| IsaPd | 3 (0.2) | 0 | 2 (0.4) | 1 (0.3) |
| Total | 506 (34.8) | 211 (37.6) | 192 (33.9) | 103 (31.4) |
| Other regimens recommended by NCCN for 1-3 PL RRMM (not specific for lenalidomide refractory)† | ||||
| Kd | 102 (7.0) | 38 (6.8) | 39 (6.9) | 25 (7.6) |
| CyBorD | 57 (3.9) | 43 (7.7) | 11 (1.9) | 3 (0.9) |
| CyKd | 26 (1.8) | 12 (2.1) | 10 (1.8) | 4 (1.2) |
| EloPd | 26 (1.8) | 2 (0.4) | 15 (2.7) | 9 (2.7) |
| PCyd | 5 (0.3) | 2 (0.4) | 2 (0.4) | 1 (0.3) |
| IxaCyd | 3 (0.2) | 0 | 2 (0.4) | 1 (0.3) |
| Vdoxo | 3 (0.2) | 3 (0.5) | 0 | 0 |
| DCyVd | 3 (0.2) | 3 (0.5) | 0 | 0 |
| EloVd | 1 (0.1) | 0 | 1 (0.2) | 0 |
| SVd | 1 (0.1) | 0 | 0 | 1 (0.3) |
| Total | 227 (15.6) | 103 (18.4) | 80 (14.1) | 44 (13.4) |
| Regimens listed by NCCN as useful in certain circumstances (not specific for lenalidomide refractory) | ||||
| D monotherapy | 121 (8.3) | 43 (7.7) | 44 (7.8) | 34 (10.4) |
| Pd | 112 (7.7) | 26 (4.6) | 54 (9.5) | 32 (9.8) |
| Vd | 97 (6.7) | 51 (9.1) | 32 (5.7) | 14 (4.3) |
| Sd | 1 (0.1) | 0 | 0 | 1 (0.3) |
| KCyTd | 1 (0.1) | 1 (0.2) | 0 | 0 |
| Ven + d | 2 (0.1) | 2 (0.4) | 0 | 0 |
| Total | 334 (23.0) | 123 (21.9) | 130 (23.0) | 81 (24.7) |
| NCCN-recommended for later-line relapses (not specific for lenalidomide-refractory RRMM) | ||||
| Bendamustine | 3 (0.2) | 0 | 1 (0.2) | 2 (0.6) |
| Cyclophosphamide | 1 (0.1) | 1 (0.2) | 0 | 0 |
| Total | 4 (0.3) | 1 (0.2) | 1 (0.2) | 2 (0.6) |
| Regimens not recommended by NCCN | ||||
| Other MM treatment‡ | 137 (9.4) | 27 (4.8) | 65 (11.5) | 45 (13.7) |
| Other D regimens | 56 (3.8) | 18 (3.2) | 23 (4.1) | 15 (4.6) |
| Ixad | 42 (2.9) | 20 (3.6) | 18 (3.2) | 4 (1.2) |
| Total | 235 (16.2) | 65 (11.6) | 106 (18.7) | 64 (19.5) |
| Clinical trials | 48 (3.3) | 16 (2.9) | 19 (3.4) | 13 (4.0) |
| Other treatments§ | 101 (6.9) | 42 (7.5) | 38 (6.7) | 21 (6.4) |
| Treatment regimen, n (%) . | Overall . | 1 prior LOT (LOT 2) . | 2 prior LOT (LOT 3) . | 3 prior LOT (LOT 4) . |
|---|---|---|---|---|
| (N = 1455) . | (n = 561) . | (n = 566) . | (n = 328) . | |
| NCCN-preferred regimens for lenalidomide-refractory 1-3 PL RRMM∗ | ||||
| DPd | 192 (13.2) | 58 (10.3) | 80 (14.1) | 54 (16.5) |
| KPd | 104 (7.1) | 53 (9.4) | 34 (6.0) | 17 (5.2) |
| DVd | 95 (6.5) | 48 (8.6) | 32 (5.7) | 15 (4.6) |
| DKd | 56 (3.8) | 24 (4.3) | 25 (4.4) | 7 (2.1) |
| PVd | 39 (2.7) | 23 (4.1) | 11 (1.9) | 5 (1.5) |
| IxaPd | 14 (1.0) | 4 (0.7) | 6 (1.1) | 4 (1.2) |
| IsaKd | 3 (0.2) | 1 (0.2) | 2 (0.4) | 0 |
| IsaPd | 3 (0.2) | 0 | 2 (0.4) | 1 (0.3) |
| Total | 506 (34.8) | 211 (37.6) | 192 (33.9) | 103 (31.4) |
| Other regimens recommended by NCCN for 1-3 PL RRMM (not specific for lenalidomide refractory)† | ||||
| Kd | 102 (7.0) | 38 (6.8) | 39 (6.9) | 25 (7.6) |
| CyBorD | 57 (3.9) | 43 (7.7) | 11 (1.9) | 3 (0.9) |
| CyKd | 26 (1.8) | 12 (2.1) | 10 (1.8) | 4 (1.2) |
| EloPd | 26 (1.8) | 2 (0.4) | 15 (2.7) | 9 (2.7) |
| PCyd | 5 (0.3) | 2 (0.4) | 2 (0.4) | 1 (0.3) |
| IxaCyd | 3 (0.2) | 0 | 2 (0.4) | 1 (0.3) |
| Vdoxo | 3 (0.2) | 3 (0.5) | 0 | 0 |
| DCyVd | 3 (0.2) | 3 (0.5) | 0 | 0 |
| EloVd | 1 (0.1) | 0 | 1 (0.2) | 0 |
| SVd | 1 (0.1) | 0 | 0 | 1 (0.3) |
| Total | 227 (15.6) | 103 (18.4) | 80 (14.1) | 44 (13.4) |
| Regimens listed by NCCN as useful in certain circumstances (not specific for lenalidomide refractory) | ||||
| D monotherapy | 121 (8.3) | 43 (7.7) | 44 (7.8) | 34 (10.4) |
| Pd | 112 (7.7) | 26 (4.6) | 54 (9.5) | 32 (9.8) |
| Vd | 97 (6.7) | 51 (9.1) | 32 (5.7) | 14 (4.3) |
| Sd | 1 (0.1) | 0 | 0 | 1 (0.3) |
| KCyTd | 1 (0.1) | 1 (0.2) | 0 | 0 |
| Ven + d | 2 (0.1) | 2 (0.4) | 0 | 0 |
| Total | 334 (23.0) | 123 (21.9) | 130 (23.0) | 81 (24.7) |
| NCCN-recommended for later-line relapses (not specific for lenalidomide-refractory RRMM) | ||||
| Bendamustine | 3 (0.2) | 0 | 1 (0.2) | 2 (0.6) |
| Cyclophosphamide | 1 (0.1) | 1 (0.2) | 0 | 0 |
| Total | 4 (0.3) | 1 (0.2) | 1 (0.2) | 2 (0.6) |
| Regimens not recommended by NCCN | ||||
| Other MM treatment‡ | 137 (9.4) | 27 (4.8) | 65 (11.5) | 45 (13.7) |
| Other D regimens | 56 (3.8) | 18 (3.2) | 23 (4.1) | 15 (4.6) |
| Ixad | 42 (2.9) | 20 (3.6) | 18 (3.2) | 4 (1.2) |
| Total | 235 (16.2) | 65 (11.6) | 106 (18.7) | 64 (19.5) |
| Clinical trials | 48 (3.3) | 16 (2.9) | 19 (3.4) | 13 (4.0) |
| Other treatments§ | 101 (6.9) | 42 (7.5) | 38 (6.7) | 21 (6.4) |
No patients received CAR T-cell therapy, bispecific therapy, bendamustine + Kd, bendamustine + Vd, SDd, SKd, SPd, DCEP (dexamethasone, cyclophosphamide, etoposide, and cisplatin), or VTDPACE (bortezomib [Velcade], thalidomide, dexamethasone, platinum [cisplatin], Adriamycin [doxorubicin], cyclophosphamide, and etoposide).
Bor, bortezomib; CAR, chimeric antigen receptor; Cy, cyclophosphamide; d, dexamethasone; D, daratumumab; doxo, doxorubicin; DVd, daratumumab, bortezomib, and dexamethasone; Elo, elotuzumab; Isa, isatuximab; Ixa, ixazomib; K, carfilzomib; P, pomalidomide; S, selinexor; T, tamoxifen; V, Velcade/bor; Ven, venetoclax.
NCCN Guidelines v3.2023, dated 8 December 2022, were current at the time of the analysis.
Regimens listed as “other recommended” or “useful in certain circumstances” in NCCN Guidelines v3.2023 for RRMM, although not specifically for lenalidomide-refractory RRMM.
Other MM combinations, including ≥1 PI, immunomodulatory drug, and/or anti-CD38 indicated for RRMM that does not reflect NCCN recommendations. See NCCN Guidelines for Multiple Myeloma v4.2024 for current recommendations.
Other treatment regimens primarily consisted of chemotherapies, the vast majority of which were used by <10 patients; includes some lenalidomide-containing regimens, as lenalidomide-refractory patients could be rechallenged with lenalidomide in a later LOT.
Outcomes in patients with lenalidomide-refractory MM
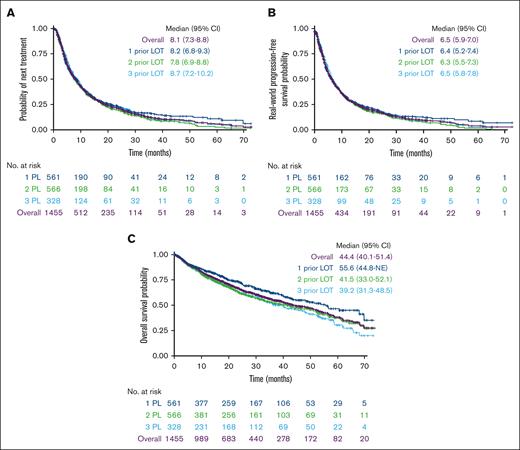
At a median follow-up of 29.9 months, median TTTD was 7.1 months (95% confidence interval [CI], 6.4-7.7) for the overall population. Median TTNT was 8.1 months (95% CI, 7.3-8.8) overall and was similar for patients with 1, 2, and 3 prior LOT (Figure 3A). RW-PFS was also similar for patients with 1, 2, or 3 prior LOT, with an overall median of 6.5 months (95% CI, 5.6-7.0) (Figure 3B). Median OS was 44.4 months (95% CI, 40.1-51.4) (Figure 3C). When stratified by prior LOT, median OS numerically decreased with increasing numbers of prior LOT, ranging from 39 to 56 months.
Outcomes in patients with lenalidomide-refractory RRMM by number of LOT. (A) TTNT, (B) RW-PFS, and (C) OS.
Outcomes in patients with lenalidomide-refractory RRMM by number of LOT. (A) TTNT, (B) RW-PFS, and (C) OS.
Prognostic variables in patients with lenalidomide-refractory MM
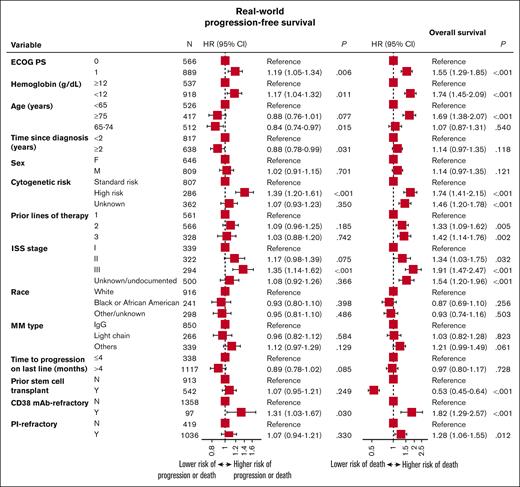
Prognostic variables for worse RW-PFS and OS outcomes included ISS stage III, ECOG PS of 1, hemoglobin <12 g/dL at T0, anti-CD38 refractoriness, and high-risk cytogenetics (Figure 4). Age ≥75 years and not having a prior stem cell transplant were additional prognostic variables for worse OS outcomes.
Prognostic variables for RW-PFS and OS for patients with lenalidomide-refractory RRMM. HR, hazard ratio.
Prognostic variables for RW-PFS and OS for patients with lenalidomide-refractory RRMM. HR, hazard ratio.
Outcomes in patients with lenalidomide-refractory MM treated with NCCN Guideline–recommended approaches
In the subgroup of patients who received NCCN-preferred treatments (n = 506), 211 (41.7%) received 1 prior LOT, 192 (37.9%) received 2 prior LOT, and 103 (20.3%) received 3 prior LOT. Median TTTD was 7.1 months (95% CI, 6.2-8.6), median TTNT was 8.5 months (95% CI, 7.2-9.7), median RW-PFS was 6.7 months (95% CI, 5.9-7.6), and median OS was 40.8 months (95% CI, 35.4-56.6) (supplemental Figure 1).
Treatment patterns and outcomes in patients with lenalidomide-refractory MM treated since 2020
A total of 603 patients in the Flatiron database met the inclusion criteria since 2020, including 252 (41.8%) with 1 prior LOT, 229 (38.0%) with 2 prior LOT, and 122 (20.2%) with 3 prior LOT. Most received triplet regimens (48.1%) followed by doublet regimens (38.0%), and this was generally consistent across LOT (supplemental Table 1). Daratumumab-based regimens were used most frequently, including DPd (17.2%); daratumumab monotherapy (9.3%); daratumumab, bortezomib, and dexamethasone (8.9%); and DKd (7.5%). Despite the use of these highly effective treatments during this time period, overall patient outcomes remained poor, with a median TTTD of 7.8 months (95% CI, 6.9-9.2), median TTNT of 8.5 months (95% CI, 7.4-9.9), and median RW-PFS of 7.1 months (95% CI, 6.0-8.1) (supplemental Figure 2). Median OS was not reached at a median follow-up of 13.6 months.
Treatment attrition rate in patients with lenalidomide-refractory MM
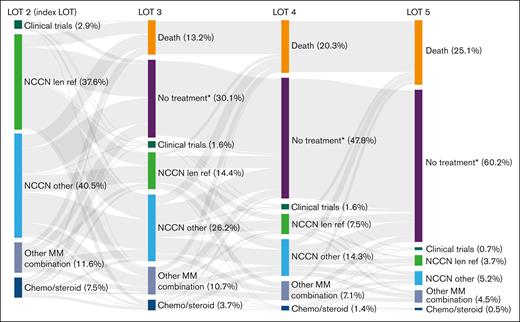
To determine attrition rates, patients with 1 prior LOT were observed over the course of their next 4 LOT (LOT 2-5). Of the 561 patients who received LOT 2 at index date, 318 patients proceeded to receive LOT 3, 179 received LOT 4, and 82 received LOT 5. The attrition rate between LOTs 2 and 3 was 43%, including 13% of patients who died and 30% of patients with no subsequent treatment or who were lost to follow-up (Figure 5). Noncumulative attrition rates after LOTs 3 and 4 were 44% and 54%, respectively, including mortality rates of 13% and 15% at each LOT, and an additional 31% and 39% of patients with no subsequent treatment or who were lost to follow-up. Cumulatively, 479 patients (85%) were lost to attrition by LOT 5, including 141 (25%) who died and 338 (60%) who received no subsequent treatment or who were lost to follow-up.
Cumulative attrition rates and treatments used in successive LOTs in patients with lenalidomide-refractory RRMM and 1 prior LOT. See Table 2 for treatment regimens included in each category. ∗No treatment includes patients who received an active antimyeloma treatment and had ≥1 follow-up assessment but were subsequently lost to follow-up. len ref, lenalidomide refractory.
Cumulative attrition rates and treatments used in successive LOTs in patients with lenalidomide-refractory RRMM and 1 prior LOT. See Table 2 for treatment regimens included in each category. ∗No treatment includes patients who received an active antimyeloma treatment and had ≥1 follow-up assessment but were subsequently lost to follow-up. len ref, lenalidomide refractory.
Outcomes in patients with lenalidomide-exposed-not-refractory MM
A total of 1577 PI-exposed patients with lenalidomide-exposed-not-refractory MM were identified, including 1119 (71.0%) who had received 1 prior LOT, 363 (23.0%) who had received 2 prior LOT, and 95 (6.0%) who had received 3 prior LOT. At index date, the median age was 67 years (range, 60-74), with a median time since diagnosis of 1.5 years (range, 0.8-2.9); 17.6% of patients had high-risk cytogenetics; 18.7% of patients were classified as ISS stage III; and 55.7% of patients had an ECOG PS of 1 (supplemental Table 2).
At a median follow-up of 31.6 months, median TTTD was 10.8 months (95% CI, 9.6-11.7) and median TTNT was 12.2 months (95% CI, 11.2-13.5). Median RW-PFS was 9.2 months (95% CI, 8.5-10.6) and median OS was 64.3 months (95% CI, 58.6-69.0) for patients with lenalidomide-exposed-not-refractory MM (supplemental Figure 3).
Discussion
Given the increased use of lenalidomide in earlier-line settings in recent years,4 it is important to understand real-world treatments and outcomes in patients with PI-exposed, lenalidomide-refractory RRMM who have received 1 to 3 prior LOT. Most patients with lenalidomide-refractory MM in our analysis received doublet (44.9%) or triplet (41.6%) therapy regimens regardless of the number of prior LOT. These treatment approaches are typically administered until disease progression, implying an ongoing treatment burden. Substantial use of doublet regimens reflects that NCCN Guidelines for this patient population are not fully translated into clinical practice; however, use of triplet regimens did increase incrementally from 44.9% in the overall data set to 48.1% from 2020 to 2022. Only 34.8% of patients overall received NCCN-preferred regimens for early-line lenalidomide-refractory disease. Of the 8 NCCN-preferred treatments, DPd was used most frequently, and its use increased with each LOT. Use of the daratumumab-based NCCN-recommended regimens DPd; daratumumab, bortezomib, and dexamethasone; and DKd increased from 2020 to 2022 compared with the overall data from 2016 to 2022. Interestingly, a substantial proportion of patients (26.4%) were treated with various regimens not recommended by the NCCN. The diversity of therapy shown in this analysis was consistent with observations in real-world studies in heavily pretreated patients (≥3 prior LOT) and reflects the complexity of choosing treatment for patients with lenalidomide-refractory MM, even at early stages of their treatment course for MM.7,16,17
A study using an older real-world data set showed that median PFS was ≤12 months in patients with lenalidomide-refractory MM receiving 1 to 3 prior LOT.11 In our study, similar trends were noted in a data set that reflects the current treatment landscape. We demonstrated a median RW-PFS of 6.5 months in this population, and subgroup analyses focusing on the most recent data did not show marked improvements over the full study population. Outcomes in patients with lenalidomide-refractory MM after early-line treatment were comparable to those of more heavily pretreated patients with ≥3 prior LOT, who demonstrated a median RW-PFS of ∼5 months in a recent report.17 Furthermore, outcomes analyzed by number of prior LOT in our analysis showed minimal differences, highlighting that patients progress rapidly through currently available therapies, regardless of the extent of prior treatments. These observations may possibly be explained by 2 factors: (1) patients were included at the point when they became refractory to lenalidomide, ensuring that the disease stage was similar in all patients at T0; and (2) the population may have been enriched for patients with functionally high-risk disease, as evidenced by the short duration from diagnosis to T0 in patients with 1 prior LOT and the large fraction of the overall population (71.2%) who were also refractory to a PI. Suboptimal outcomes were also observed in patients with lenalidomide-exposed-not-refractory MM, which could be due to refractoriness to other classes of therapies. These data indicate an unmet need for treatments that can meaningfully extend PFS in patients with lenalidomide-refractory MM in earlier-line settings.
Median OS in this study (44 months) was consistent with previous clinical trials and real-world evidence studies, demonstrating shorter OS with each additional LOT and an especially poor prognosis for patients with lenalidomide-refractory MM.10,11,18,19 These data can provide a benchmark against which to evaluate novel MM treatments in current and future clinical trials.13,20-23
Our analysis showing high attrition rates (43%-54%) in patients with lenalidomide-refractory RRMM and 1 prior LOT is consistent with a previous retrospective analysis of real-world data from the United States in patients with newly diagnosed MM, which demonstrated high attrition rates after frontline therapy in nontransplant patients (43%-57%) and transplant patients (21%-37%).24 We acknowledge alternative methods for calculating attrition,25,26 and the potential for overestimating attrition rates, given that reasons for not receiving subsequent therapy are difficult to capture in administrative databases. Nevertheless, OS in our data set confirms the poor outcomes in this population.
The Flatiron database offers a community-based, comprehensive source of clinical data with validated end points, allowing for a robust analysis of current treatment patterns and outcomes for patients with RRMM. Real-world data, however, may underestimate incidence of disease progression; therefore, we define RW-PFS as a progression event or a change in antimyeloma treatment. There is also the possibility in real-world data sets of misclassifying a lenalidomide-refractory patient as nonrefractory, which would select for a lenalidomide-refractory group that is particularly high-risk. The definition of lenalidomide refractory was not completely based on International Myeloma Working Group Criteria because response data are lacking in the Flatiron database. In addition, this analysis did not include characterization of extramedullary disease status, comorbidities, or other variables to better understand the clinical realities of this population. Selection bias may occur because of the exclusion of variables where several data points are missing, including a potential bias toward the inclusion of high-risk patients, as suggested by the short duration of initial treatment in patients with 1 prior LOT. Furthermore, this US-based data set is not reflective of practices outside of the United States and only represents the commercially insured and Medicare-eligible US population. Finally, our data set reflects the evolution of MM treatment over the 2016 to 2022 time frame, which has shown tremendous expansion of available therapies and their uptake (eg, daratumumab). Thus, the overall data set is not a snapshot of the most current treatment patterns.
Overall, these real-world data demonstrate that patients whose disease becomes lenalidomide refractory in early LOT have suboptimal outcomes and high attrition rates despite available treatment options. These results reflect the shortcomings of currently available therapies for patients with RRMM who have previously received 1 to 3 LOT, highlighting an unmet need for new, safe, and effective treatment regimens. The most effective regimens should be used earlier in treatment before the patients are lost to attrition instead of reserving them for later LOT.
Acknowledgments
This study was funded by Janssen Global Services, LLC. Medical writing support was provided by Sarika Pathak Sharma of Eloquent Scientific Solutions, and funded by Janssen Global Services.
Authorship
Contribution: B.D., H.E., J.M.S., W.D., N.L., A.S., C.L., A.K., P.C., S.V., and K.Y. contributed to conceptualization, investigation, and analysis of the study; and all authors participated in writing the manuscript.
Conflict-of-interest disclosure: B.D. has served in a consulting or advisory role for Amgen, GlaxoSmithKline, Janssen, Natera, Sanofi, and Takeda; has received honoraria from Celgene, GlaxoSmithKline, Karyopharm Therapeutics, and Sanofi; and has received research funding from Amgen, GlaxoSmithKline, and Janssen. H.E. has served in a consulting or advisory role for Amgen, Bristol Myers Squibb, Celgene, Janssen, Novartis, and Takeda; has received travel funding from Amgen, Bristol Myers Squibb, Celgene, Janssen, and Takeda; has received honoraria from Amgen, Bristol Myers Squibb, Celgene, Janssen, Novartis, and Takeda; and has received research funding from Amgen, Bristol Myers Squibb, Celgene, and Janssen. J.M.S. is employed by and owns stock in Janssen. W.D. is employed by, owns stock in, and has received patent royalties from Janssen. N.L. is employed by and owns stock in Janssen. A.S. is employed by and owns stock in Janssen. C.L. is employed by Janssen. S.N. is employed by Janssen. J.H. is employed by and owns stock in Janssen and has an immediate family member employed by Bristol Myers Squibb. A.K. is a previous Janssen employee. P.C. is employed by and owns stock in Janssen. S.V. is a previous Janssen employee. K.Y. has served in a consulting or advisory role for Janssen-Cilag; has participated in speakers’ bureaus for Amgen, Sanofi, and Takeda; has received honoraria from Amgen, Janssen-Cilag, Sanofi, and Takeda; and has received research funding from Amgen, Autolus, Janssen-Cilag, Sanofi, and Takeda.
The current affiliation for S.V. is Bristol Myers Squibb, Princeton, NJ.
Correspondence: Binod Dhakal, Division of Hematology and Oncology, Medical College of Wisconsin, 8800 West Doyne Ave, Milwaukee, WI 53226; email: bdhakal@mcw.edu.
References
Author notes
The data-sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. These data were made available by Flatiron Health, Inc, and used under license for the present study and are not publicly available. Other researchers should contact https://flatiron.com.
The full-text version of this article contains a data supplement.