Key Points
No association between HU treatment and decreased follicle density in patients with SCD.
Fertility preservation is not necessary before HU treatment.
Visual Abstract
The impact of hydroxyurea (HU) on the ovarian reserve of female patients with sickle cell disease (SCD) remains poorly elucidated. Only direct histological analysis of ovarian follicle density can effectively evaluate HU’s effect on ovarian reserve. By analyzing digitized slides of ovarian tissue from girls and young women with SCD who underwent ovarian tissue cryopreservation (OTC) before hematological stem cell transplantation, we meticulously counted follicles and categorized them based on their growth stage. We then calculated the densities of different follicle types and assessed their correlation with patient characteristics, clinical manifestations, and treatments extracted from medical records. Seventy-six patients with SCD participated in the study, with a median age at OTC of 10.2 years (interquartile range [IQR], 7.5-14.6), and 50 (65.8%) were prepubertal. Of these, 35 patients (46.1%) had received HU, with a median daily dosage of 23.0 mg/kg (IQR, 20.0-25.0) and median exposure time of 44 months (IQR, 24.0-54.0). Primordial follicle density was comparable between the HU and non-HU groups (5.8 follicles per mm2 [IQR, 1.0-13.3] vs 4.2 follicles per mm2 [IQR, 1.1-14.4], respectively; P = .95). However, in the HU group, after adjusting for age, the density of growing follicles was marginally lower than that in the non-HU group (P = .09). Notably, other parameters such as vaso-occlusive crisis did not affect follicular density. In conclusion, exposure to HU did not demonstrate a reduction in ovarian reserve in girls or women with SCD. Therefore, fertility preservation measures before initiating HU treatment do not seem necessary.
Introduction
Sickle cell disease (SCD) is one of the most common monogenic blood disorders worldwide.1 Approximately 300 000 babies are born every year with the disease, most of them in low- and middle-income countries.2 Over the last 4 decades, advances in treatment and supportive care have extended the life expectancy of patients with SCD.
One of the major advances in care for SCD was the introduction of hydroxyurea (HU) in the mid-1990s, which reduced the frequency of vaso-occlusive crisis (VOC), acute chest syndrome (ACS) episodes, and the need for transfusions by increasing the hemoglobin F (HbF) level (and proportionately decreasing the HbS level).3 Despite the proven benefit of HU, concerns have been raised about its side effects. High doses of HU have teratogenic effects in mammals.4,5 Theoretically, HU, an antimetabolite, impairs DNA synthesis and in utero exposure can influence fetal and, subsequently, neonatal development.6,7 There is a paucity of HU ovarian toxicity data in humans. The human ovary contains 2 main types of follicles: primordial, composed of an immature oocyte encircled by a layer of flattened somatic cells, and growing, in which the cells surrounding the oocyte undergo transformation and proliferate, leading to concurrent development of both the oocyte and the follicle. Primordial follicles originate during prenatal development and their quantity is initially established; subsequently, the quantity of primordial follicles exhibits an inverse correlation with a person’s age.8-10 Primordial follicles constitute the majority and signify the ovarian reserve. Wallace et al developed a mathematical model to determine the number of primordial follicles at various ages, drawing from data from 8 histological studies involving 325 individuals.11 Periodically, recruited follicles emerge from this reserve and begin on a growth journey, progressing through multiple stages, starting with the primary follicle and, after puberty, culminating in the preovulatory follicle. These recruited follicles indicate the actively growing follicles. Some case reports have documented the birth of healthy children in women who were exposed to HU during early pregnancy.12 Furthermore, reduction of ovarian reserve in women with SCD receiving HU therapy is not well characterized. In a study by Sampson et al,13 28-day treatment with HU in adult female mice reduced ovarian weight by 50%, with a concomitant reduction in estradiol level and ovulation rate. They concluded that HU could compromise normal folliculogenesis and subsequent embryo development.
There is considerable interindividual variability with respect to the quantitative ovarian reserve even among women of the same age due to varying densities of primordial follicles at birth and follicle loss rates.9 To date, there are no serum markers or imaging modalities that can directly reflect ovarian reserve.
Consequently, only indirect markers of ovarian reserve, such as serum anti-Müllerian hormone (AMH) level and antral follicle count obtained by ultrasound of ovaries, are currently used.14
Histological examination of the ovarian cortex is the only technique for the direct quantification of the primordial follicle pool in ovaries. A few studies have reported follicle density in patients with SCD15-17; however, none of these studies investigated its correlation with HU. To our knowledge, no study has directly investigated the effect of HU on ovarian reserve or growing follicle density.
This study assessed the direct impact of HU exposure on ovarian reserve and growing follicle density in girls or women with SCD who underwent ovarian tissue cryopreservation (OTC) before hematological stem cell transplantation (HSCT) to preserve fertility.
Methods
Data sources
Ovarian tissue samples and medical data were collected prospectively after obtaining written consent from April 1998 to November 2020. Histological examination and data analysis for this study were conducted retrospectively from June to November 2021 using data obtained from medical records and digitized histological samples from 76 of the included patients.
This study was approved by the ethical committee of Avicenne Academic Hospital, APHP, France (CLEA-2021-195) and performed in compliance with the principles enshrined in the Declaration of Helsinki. Written informed consent was provided by patients or parents/participants’ legal guardians.
Selection of patients
Consecutive girls or women with SCD who underwent OTC for fertility preservation before HSCT were included in this study. They were recruited from different French reference centers in Paris (Centre Hospitalier Intercommunal de Créteil [n = 43], Robert Debré Academic Hospital [n = 17], Necker Enfants Malades Academic Hospital [n = 12], Mondor Academic Hospital in Créteil [n = 1], and others [n = 3]). Of the 76 patients, who underwent OTC, 72 had a transplantation in the following transplant units: St-Louis Academic Hospital (n = 33); Robert Debré Academic Hospital (n = 25); Necker Enfants Malades Academic Hospital (n = 12); others (n = 2). Before the OTC, all patients received standardized fertility preservation counseling from the same team of reproductive specialists.
Specific data related to the disease, applied treatments (exact number of applied red blood cell units transfused per blood bank records, HU dose, and exposure time), and clinical manifestations were collected from medical records. Biological data (Hb, leukocyte, neutrophil, reticulocyte count, and mean corpuscular volume [MCV]) were recorded at the time of OTC. The indication for starting HU treatment included severe VOC, recurrent ACS, and severe anemia (<7 g/dL). Furthermore, time interval between HU interruption and time point of cryopreservation (washout period) was analyzed in months. The HU-naïve patients served as the control group because there is no indication to administer HU before OTC.
OTC
OTC was performed between April 1998 and November 2020. The tissue was collected laparoscopically under general anesthesia simultaneously with the placement of the central line. In all cases, the entire ovary was removed and transported on ice to the laboratory. Upon receipt at the laboratory, the ovary specimens were immediately prepared for freezing. The ovarian cortex was isolated from the medulla and cut into fragments. Each fragment was placed in a cryotube containing 1 mL of freezing solution containing dimethyl sulfoxide as a cryoprotectant before being subjected to a slow freezing protocol, as described previously.18 The ovarian fragments were equilibrated for 30 minutes in a freezing solution, then frozen and stocked at the temperature of liquid nitrogen. At the time of OTC, 1 randomly selected sample of the ovarian cortex from each patient was fixed in formaldehyde and sent to the department of pathology. The OTC procedure and histological examination did not change over the 22-year collection period.
Histological evaluation of follicular density
After fixation, samples were paraffin-embedded and sectioned at a thickness of 3 μm. The sections were stained with hematein-eosin-saffron stain. Digital slides were prepared by scanning the full surface of the tissue section at 40× magnification and a resolution of 0.25 μm/pixel using an Aperio ScanScope XT scanner (Aperio Leica Biosystems Inc, Wetzlar, Germany). The digitized slides were uploaded to a server (HewelBox, Hewel, and SAS ICM, Paris, France). The HewelBox system broadcasts through its independent Wi-Fi network that is accessible for group use, regardless of geographical distance, using secure access. The digitized slides were observed on a digital tablet with a new visualization system and analyzed using specific software (HistoWel, Hewel ICM). This system allows different users to classify the number of follicles in the same fields by type (primordial, primary, secondary, or tertiary). Analysis, visualization, and annotations can be shared among users. Each histological slide was retrievable from a table and reviewed by 2 independent investigators (T.D.-F. and C.P.) on the digital system. All follicles in the ovarian cortex were counted and classified according to the modified Oktay classification19 and recorded. The surface of each histological fragment (mm2) was measured automatically. Follicular density refers to the number of follicles divided by the surface area. The density of primordial follicles represents the ovarian reserve. The density of growing follicles was also measured.
Evaluated parameters
Age at OTC, treatments administered before OTC (eg, number of transfusions, administration of HU, median HU dose, and exposure in months), and SCD-related complications before OTC (eg, history of severe reported VOC and spleen sequestration) were recorded.
Severe reported VOC were defined as VOC episodes that led to hospitalization. The primary outcome of the study was to evaluate the effect of HU on primordial follicle density (ovarian reserve) in patients with SCD. Secondary outcomes included the correlation of primordial follicle density with the occurrence of VOC and age; the correlation of the number of severe VOC with primordial follicle density; the impact of HU on growing follicle (sum of primary, secondary, and tertiary follicles) density after correcting for age; and the number of red blood cell units administered before OTC with and without correction for HU.
Statistical analysis
Baseline and follow-up patient characteristics were summarized as frequency (%) for categorical variables and median (interquartile range [IQR]) for continuous variables. Between-group differences were assessed for statistical significance using the χ2 or Fisher test for categorical variables and the Wilcoxon rank sum test for continuous variables. The primary analysis compared the effect of HU on follicle density using the Wilcoxon rank sum test.
In the secondary analysis, a linear regression model was fitted to evaluate the effect of the presence of VOC on suitably normal transformed follicle density, after adjusting for age.
Three additional analyses of covariance were performed on transformed primary, secondary, and tertiary follicle densities to analyze the impact of the 2 treatment groups, after adjusting for age. Furthermore, linear regression analysis was performed to evaluate the correlation between the log-transformed number of red blood cell units and follicle density, adjusting for HU. Finally, multiple linear regression analysis was performed with primary follicle density and age as predictors and suitably normal transformed follicle density as the outcome.
Data count and follicle density calculation were independently performed by 2 investigators. Cronbach alpha test was performed to verify the interobserver consistency.
All analyses were based on cases with complete data only. A P value <.05 was considered indicative of statistical significance. The analysis was performed using the statistical software R (version 4.0.3, R Development Core Team, Vienna, Austria).20
Results
Seventy-six female patients with SCD and homozygosity for HbS were enrolled in this study. The patient characteristics are summarized in Table 1 and supplemental Table 1. The median age at OTC was 10.2 years (IQR, 7.5-14.6; range, 4.0-28.3). The median age of patients in the HU group at baseline was significantly greater than that in the group not exposed to HU (12.2 vs 9.6 years; P = .035). Fifty patients (65.8%) were prepubertal at the time of OTC. Thirty-five patients (46.0%) had undergone HU treatment before OTC; the median daily dosage was 23.0 mg/kg (IQR, 20.0-25.0) and the median exposure time was 44.0 months (IQR, 24.0-54.0). The median age for starting HU was 6.7 years (IQR, 5.1-9.2).
Clinical and demographic characteristics of patients stratified by HU exposure (n = 76)
| . | Cohort (N = 76) . | HU = no (N = 41) . | HU = yes (N = 35) . | P value† (2 vs 5) . | P value† (3 vs 6) . | P value† (4 vs 7) . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total (1) . | Prepubertal (2) n = 32∗ . | Postpubertal (3) n = 9∗ . | Total (4) N = 41 . | Prepubertal (5) n = 18∗ . | Postpubertal (6) n = 17∗ . | Total (7) N = 35 . | ||||
| Age at OTC, y∗ | 10.2 (7.5-14.6) | 7.7 (5.4-10.4) | 17.3 (16.0-18.7) | 9.6 (6.3-12.5) | 8.7 (8.0-9.8) | 15.5 (14.4-17.5) | 12.2 (8.7-15.0) | .38 | .08 | .035 |
| History of hospitalized VOC, n (%)‡ | ||||||||||
| No | 11 (14.5) | 8 (25.0) | 0 (0.0) | 8 (19.5) | 3 (16.7) | 0 (0.0) | 3 (8.6) | .72 | NA | .15 |
| Yes | 61 (80.3) | 22 (68.8) | 8 (88.9) | 30 (73.2) | 14 (77.8) | 17 (100.0) | 31 (88.6) | |||
| Cerebral vasculopathy, n (%)‡ | ||||||||||
| No | 22 (28.9) | 6 (18.8) | 1 (11.1) | 7 (17.1) | 9 (50.0) | 6 (35.3) | 15 (42.9) | .02 | .36 | .018 |
| Yes | 50 (65.8) | 24 (75.0) | 7 (77.8) | 31 (75.6) | 8 (44.4) | 11 (64.7) | 19 (54.3) | |||
| History of splenic sequestration, n (%)‡ | ||||||||||
| No | 62 (81.6) | 24 (75.0) | 8 (88.9) | 32 (78.0) | 16 (88.9) | 14 (82.4) | 30 (85.7) | .40 | .53 | .74 |
| Yes | 10 (13.2) | 6 (18.8) | 0 (0.0) | 6 (14.6) | 1 (5.6) | 3 (17.6) | 4 (11.4) | |||
| HbF max on HU, g/dL∗ | 16.4 (9.4-19.5) | NA | NA | NA | 16.2 (15.0-16.6) | 18.5 (3.5-18.5) | 16.4 (9.4-19.5) | |||
| Missing n (%) | 66 (86.8) | 13 (37.1) | 12 (34.3) | 25 (71.4) | ||||||
| MCV max on HU, fL∗ | 96.5 (89.0-105.6) | NA | NA | NA | 100.6 (92.4-100.9) | 91.0 (86.0-107.1) | 96.5 (89.0-105.6) | |||
| Missing n (%) | 66 (86.8) | 13 (37.1) | 12 (34.3) | 25 (71.4) | ||||||
| Ferritin at OTC, μg/L∗ | 969.5 (299.3-1765.3) | 969.5 (416.3-1678.3) | 870.0 (582.0-n940.0) | 923.0 (301.0-1760.0) | 846.5 (213.3-1357.8) | 1285.0 (313.0-1816.0) | 1277.0 (299.0-1776.5) | .67 | .99 | .88 |
| Missing n (%) | 40 (52.6) | 18 (56.3) | 6 (66.7) | 24 (58.5) | 8 (44.4) | 8 (47.1) | 16 (45.7) | |||
| Transfusion = yes, n (%) | 74 (97.4) | 32 (100) | 8 (88.9) | 40 (97.6) | 17 (94.4) | 17 (100.0) | 34 (97.1) | .36 | .35 | .99 |
| No. of transfusions, n∗ | 23.5 (13.5-47.5) | 28.0 (17.3-40.8) | 18.0 (4.0-207.0) | 27.0 (15.0-53.0) | 18.5 (12.5-35.8) | 23.0 (12.0-53.0) | 19.0 (12.0-46.0) | .25 | .85 | .40 |
| HU dose (mg/kg) | NA | NA | NA | NA | 23.0 (21.5-25.0) | 23.0 (20.8-29.5) | 23.0 (20.0-25.0) | |||
| Missing n (%) | 3 (6.7) | 3 (17.6) | 6 (17.1) | |||||||
| HU exposure (mo) | NA | NA | NA | NA | 30.0 (20.0-44.0) | 56.5 (41.5-73.8) | 44.0 (24.0-54.0) | |||
| Missing n (%) | 1 (5.6) | 1 (5.9) | 2 (5.7) | |||||||
| Primordial follicle density∗ (follicles per mm2) | 5.6 (1.1-14.2) | 7.4 (1.4-17.2) | 1.0 (0.0-1.9) | 4.2 (1.1-14.4) | 10.9 (6.9-23.5) | 2.2 (0.4-5.5) | 5.8 (1.0-13.3) | .61 | .21 | .95 (.39§) |
| Growing follicle density∗ (follicles per mm2) | 0.3 (0.1-0.7) | 0.5 (0.2-1.2) | 0.2 (0.0-0.4) | 0.5 (0.2-1.0) | 0.3 (0.0-0.7) | 0.2 (0.0-0.3) | 0.2 (0.0-0.4) | .09 | .72 | .016 (.09§) |
| . | Cohort (N = 76) . | HU = no (N = 41) . | HU = yes (N = 35) . | P value† (2 vs 5) . | P value† (3 vs 6) . | P value† (4 vs 7) . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total (1) . | Prepubertal (2) n = 32∗ . | Postpubertal (3) n = 9∗ . | Total (4) N = 41 . | Prepubertal (5) n = 18∗ . | Postpubertal (6) n = 17∗ . | Total (7) N = 35 . | ||||
| Age at OTC, y∗ | 10.2 (7.5-14.6) | 7.7 (5.4-10.4) | 17.3 (16.0-18.7) | 9.6 (6.3-12.5) | 8.7 (8.0-9.8) | 15.5 (14.4-17.5) | 12.2 (8.7-15.0) | .38 | .08 | .035 |
| History of hospitalized VOC, n (%)‡ | ||||||||||
| No | 11 (14.5) | 8 (25.0) | 0 (0.0) | 8 (19.5) | 3 (16.7) | 0 (0.0) | 3 (8.6) | .72 | NA | .15 |
| Yes | 61 (80.3) | 22 (68.8) | 8 (88.9) | 30 (73.2) | 14 (77.8) | 17 (100.0) | 31 (88.6) | |||
| Cerebral vasculopathy, n (%)‡ | ||||||||||
| No | 22 (28.9) | 6 (18.8) | 1 (11.1) | 7 (17.1) | 9 (50.0) | 6 (35.3) | 15 (42.9) | .02 | .36 | .018 |
| Yes | 50 (65.8) | 24 (75.0) | 7 (77.8) | 31 (75.6) | 8 (44.4) | 11 (64.7) | 19 (54.3) | |||
| History of splenic sequestration, n (%)‡ | ||||||||||
| No | 62 (81.6) | 24 (75.0) | 8 (88.9) | 32 (78.0) | 16 (88.9) | 14 (82.4) | 30 (85.7) | .40 | .53 | .74 |
| Yes | 10 (13.2) | 6 (18.8) | 0 (0.0) | 6 (14.6) | 1 (5.6) | 3 (17.6) | 4 (11.4) | |||
| HbF max on HU, g/dL∗ | 16.4 (9.4-19.5) | NA | NA | NA | 16.2 (15.0-16.6) | 18.5 (3.5-18.5) | 16.4 (9.4-19.5) | |||
| Missing n (%) | 66 (86.8) | 13 (37.1) | 12 (34.3) | 25 (71.4) | ||||||
| MCV max on HU, fL∗ | 96.5 (89.0-105.6) | NA | NA | NA | 100.6 (92.4-100.9) | 91.0 (86.0-107.1) | 96.5 (89.0-105.6) | |||
| Missing n (%) | 66 (86.8) | 13 (37.1) | 12 (34.3) | 25 (71.4) | ||||||
| Ferritin at OTC, μg/L∗ | 969.5 (299.3-1765.3) | 969.5 (416.3-1678.3) | 870.0 (582.0-n940.0) | 923.0 (301.0-1760.0) | 846.5 (213.3-1357.8) | 1285.0 (313.0-1816.0) | 1277.0 (299.0-1776.5) | .67 | .99 | .88 |
| Missing n (%) | 40 (52.6) | 18 (56.3) | 6 (66.7) | 24 (58.5) | 8 (44.4) | 8 (47.1) | 16 (45.7) | |||
| Transfusion = yes, n (%) | 74 (97.4) | 32 (100) | 8 (88.9) | 40 (97.6) | 17 (94.4) | 17 (100.0) | 34 (97.1) | .36 | .35 | .99 |
| No. of transfusions, n∗ | 23.5 (13.5-47.5) | 28.0 (17.3-40.8) | 18.0 (4.0-207.0) | 27.0 (15.0-53.0) | 18.5 (12.5-35.8) | 23.0 (12.0-53.0) | 19.0 (12.0-46.0) | .25 | .85 | .40 |
| HU dose (mg/kg) | NA | NA | NA | NA | 23.0 (21.5-25.0) | 23.0 (20.8-29.5) | 23.0 (20.0-25.0) | |||
| Missing n (%) | 3 (6.7) | 3 (17.6) | 6 (17.1) | |||||||
| HU exposure (mo) | NA | NA | NA | NA | 30.0 (20.0-44.0) | 56.5 (41.5-73.8) | 44.0 (24.0-54.0) | |||
| Missing n (%) | 1 (5.6) | 1 (5.9) | 2 (5.7) | |||||||
| Primordial follicle density∗ (follicles per mm2) | 5.6 (1.1-14.2) | 7.4 (1.4-17.2) | 1.0 (0.0-1.9) | 4.2 (1.1-14.4) | 10.9 (6.9-23.5) | 2.2 (0.4-5.5) | 5.8 (1.0-13.3) | .61 | .21 | .95 (.39§) |
| Growing follicle density∗ (follicles per mm2) | 0.3 (0.1-0.7) | 0.5 (0.2-1.2) | 0.2 (0.0-0.4) | 0.5 (0.2-1.0) | 0.3 (0.0-0.7) | 0.2 (0.0-0.3) | 0.2 (0.0-0.4) | .09 | .72 | .016 (.09§) |
HbF max on HU, HbF during HU treatment; MCV max on HU, MCV during HU treatment; NA, not adapted.
Median [IQR]; n (%).
Wilcoxon rank sum test; Fisher exact test.
For counts, missing values can be obtained by difference.
P value age adapted.
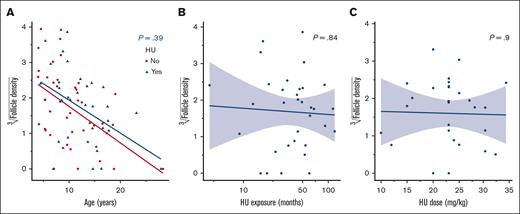
The maximum MCV reported in 10 of 35 patients (28.6%) under HU treatment was 96.5 fL (IQR, 89.0-105.6) (supplemental Table 1). The subanalysis of follicle density of primordial follicles vs MCV max under treatment and HbF max under treatment did not show a significant impact (P values of .5 and .8, respectively). There were no associations between applied dose of HU (P = .4), nor HU exposure time (P = .9), and follicle density (Figure 1; supplemental Table 2).
Correlation between HU dose, HU exposure, and follicle density. (A) Follicle density in correlation with HU and age (years). (B) Follicle density according to HU exposure (months). (C) Follicle density according to HU dose (mg/kg).
Correlation between HU dose, HU exposure, and follicle density. (A) Follicle density in correlation with HU and age (years). (B) Follicle density according to HU exposure (months). (C) Follicle density according to HU dose (mg/kg).
Blood transfusions were administered to 74 of 76 patients (97%). The median number of numbers of red blood cell units was 23.5 (IQR, 13.5-47.5). Chronic transfusion was defined as monthly transfusions for at least 6 months. Such transfusions were applied in 56 of 76 patients (74%). Indications for chronic transfusion included cerebral vasculopathy (n = 50) or HU failure (n = 4) to prevent ACS or frequent crises. The median duration of chronic transfusion was 2.2 years (IQR, 1.1-3.8). Most patients were on chronic transfusion for at least 3 months or had undergone exchange transfusion before OTC to lower HbS levels to <30% and avoid further disease-related complications.
At the time of OTC, no patients were taking HU. The median HU washout period was 2.4 months (IQR, 1.2-13.8), and in 9 of 35 patients the median HU washout period exceeded 1 year. History of hospitalized VOC were reported in 61 of 76 patients (80%) in the cohort, 51% (31 of 61) of whom were undergoing HU treatment. Median number of VOC in 61 of 76 patients (80.3%) was 3 (IQR, 2.0-8.0).
For histological evaluation, a match between the 2 independent investigators was determined. Cronbach alpha test showed a concordance of 96.5% between the 2 investigators. For the study, values from T.D.-F. were used. Histological evaluation was performed on 76 patients. For the primary analysis, the median follicle density of primordial follicles was not significantly lower in the HU group (n = 35) and the non-HU (n = 41) group (median 5.8 follicles per mm2 [IQR, 1.0-13.3] vs median 4.2 follicles per mm2 [IQR, 1.1-14.4]; P = .95) (Table 1). After correcting for age, analyses of covariance revealed that the Box-Cox–transformed (ie, cube root) follicle density was not significantly different between the 2 groups (P = .39) (Table 1). After dividing the patients into prepubertal and postpubertal groups, no significant differences were found in both groups concerning follicle density of primordial follicles (P = .61 and .21 in prepubertal and postpubertal, respectively). In the HU group, the follicle density of growing follicles without age correction exhibited a decrease (P = .016).
After age adjustment, the density of the growing follicles was marginally lower in the HU group than in the HU-naïve group (P = .09; Table 1).
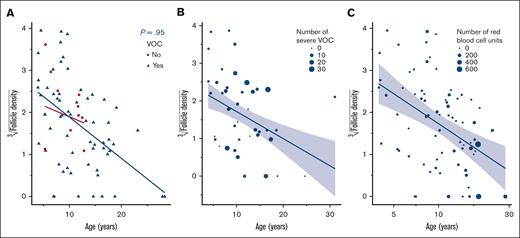
Sixty-one of 76 patients (80%) showed a history of severe VOC. Primordial follicle density was associated with age (P < .001), with presence of VOC not being significant at the 5% level in adjusted models (P = .95; Figure 2A). No association between follicle density and the number of VOC in prepubertal or postpubertal patients was observed (P = .95; Figure 2B). No association was found between primordial follicle density and transfusion doses before OTC (P = .13; Figure 2C).
Correlation between VOC, transfusion, and follicle density. (A) Correlation between the presence of VOC and primordial follicle density. (B) Correlation between the number of VOC and primordial follicle density. (C) Correlation between primordial follicle density and administered transfusion doses.
Correlation between VOC, transfusion, and follicle density. (A) Correlation between the presence of VOC and primordial follicle density. (B) Correlation between the number of VOC and primordial follicle density. (C) Correlation between primordial follicle density and administered transfusion doses.
Supplemental Table 3 summarizes the univariate and multivariate analyses performed with primordial follicle density as outcome, and age, HU, VOC, and growing follicles as predictors.
Discussion
The present study is the first, to our knowledge, to directly evaluate the effect of HU on primordial and growing follicle densities in girls or women with SCD. Our data indicate that there was no significant difference between follicle density in HU-exposed and HU-naïve patients.
Four previous studies have indirectly evaluated the effect of HU on ovarian reserve by analyzing only AMH levels in patients with SCD with and without HU treatment.21-24 They concluded that the low AMH levels observed in the HU group indicate a diminished ovarian reserve because of HU treatment. In the ovary, AMH is expressed by the granulosa cells of growing follicles from the primary up to the small antral stages. Additionally, AMH secretion is gonadotropin independent and cycle independent, which allows for AMH determination at any time.14 An AMH level below 1 ng/mL may indicate a low ovarian reserve. Kopieka et al evaluated a cohort of 50 reproductive-age patients with SCD and found that AMH levels were significantly lower in the sickle cell group than those in the age-matched healthy controls.23 Pecker et al analyzed 3 studies investigating the potential link between AMH levels and exposure to HU.21 The findings were consistent with those of Elchuri et al, showing that ∼25% of HU-exposed, postpubescent women under 31 years of age had reduced AMH levels.22
No histological analysis was performed in these publications. There are 3 case reports reporting follicle density in patients with SCD (n = 6) without performing a correlation to HU treatment.15,17,25
Contemporary guidelines in United States recommend offering HU to all infants with SCD beginning from the age of 9 months regardless of severity of the disease (with an initial starting dose of 20 mg/kg and increasing to 35 mg/kg, if tolerated).26 However, at time of the study, in France HU was initiated only in children who experienced at least 2 VOC per year or had experienced 2 episodes of ACS during their lifetime, or had baseline anemia with Hb levels <7 g/dL. In our population, the median exposure time was 44 months, which is still short considering that some patients may live >20 years before conceiving. There remains a lack of long-term safety data, and further studies are necessary.
HU was discontinued at least 1 month before HSCT (mean duration 0.23 year before OTC and subsequent condition regimen). This discontinuation period varied between 1 and 3 months, in accordance with the guidelines of the respective local institutions. Moreover, chronic or exchange transfusion were initiated 3 months before OTC.
Follicle density and age are inversely correlated.27 Through computer simulation, Schenck et al demonstrated that interfollicular signals inhibit the loss of follicles at short distances, resulting in the development of clustered follicle density.28 The median age in our study population was 10.2 years, and 65.8% of the patients were prepubertal at the time of OTC. Consequently, we assumed that, in this period of life, follicle density is still homogeneously distributed in the ovary. However, this is also why in our study some obtained fragments did not contain any follicles (n = 8). The mean age of these 8 patients was 16.4 years with a median of 15.4 years (IQR, 10.6-21.0).
We found no significant difference in the number of primordial follicles between HU-exposed patients and the control group, the HU-naïve patients, indicating that HU did not affect ovarian reserve. Importantly, results of ovarian tissue transplantation correlate with ovarian reserve. A higher follicular density is associated with a higher cumulative incidence of ovarian function recovery and pregnancy.16
However, HU, which is a DNA synthesis inhibitor, inhibits the maturation of growing/activated follicles by increasing apoptosis and oxidative stress.29,30 In our cohort, after adjusting for age, only the Box-Cox–transformed densities of growing follicles (primary follicles) were marginally reduced in the HU group. In our study, we did not observe the direct impact of HU treatment on the number of growing follicles, which would explain the greater homogeneous decline in AMH levels in patients with SCD than in the control group.23 We hypothesize that a low AMH level reflects altered function of growing follicles and not a quantitative decline in their number. Two-thirds of our population were prepubertal at the time of OTC. Matched sibling donor (MSD) HSCT should be considered for all individuals at risk of neurologic injury, with recurrent vaso-occlusive pain crises, or a history of recurrent ACS. Furthermore, when feasible, HSCT should be undertaken at the earliest age possible to obtain a better outcome.26,31 The 5-year overall survival rate in patients with SCD with a matched sibling donor is currently over 95%.32 The predominant conditioning regimen administered in our population was myeloablative with busulfan and cyclophosphamide. These 2 alkylating agents are very toxic to ovarian tissue, disrupting both endocrine and exocrine functions, leading to premature ovarian insufficiency and infertility. Treatment of SCD is mainly supportive and may include infection prophylaxis and pain management, especially in high-risk patients who require chronic blood exchange transfusions.33-35 Iron overload is a significant clinical issue in patients with SCD, with the reported incidence ranging between 30% and 45%.36,37 Typically, iron is accumulated primarily in hepatocytes and, in the advanced stage, in cardiomyocytes.38
In contrast to patients with thalassemia, iron overload–associated endocrinopathy is uncommon in patients with SCD aged <20 years.39 Hagag et al investigated the correlation of iron overload with gonadal hormone levels in 40 girls with SCD and found a negative correlation between gonadal hormones (follicle-stimulating hormone, luteinizing hormone, and estrogen) and ferritin levels.40 In our study, we found no significant correlation between the number of applied transfusion doses administered and follicle density. Joseph et al found that, in males, transfusions appeared to benefit spermatogenesis.41 Severe vaso-occlusive events cause significant morbidity and lead to hospitalization in 95% of cases.42 Recurrent episodes have a significant impact on the quality of life and can cause multiorgan dysfunction and premature mortality.43-45 Causes of VOC are multifactorial, with the principal cause being microvascular occlusion, which leads to increased inflammation and ischemic tissue injury.45,46 Different studies support the hypothesis that microvascular injury may accelerate follicle apoptosis in SCD, resulting in a sharper decline in AMH level.23,47-49 In our population, a direct correlation was observed between history of hospitalized VOC and follicle density. However, after adjusting VOC for age, our analysis revealed that the decisive factor is age, with VOC being insignificant (in models including age as well). In the study by Mamsen et al, follicle morphology and density in SCD were within the range of those in the age-matched healthy control group.15 Other studies have also found patients with SCD with normal ovarian reserve.23,50 To the best of our knowledge, no other studies have investigated the direct effect of VOC on ovarian reserve. Overall, these findings indicate that the primordial follicle density is not affected by HU and support fertility preservation before HSCT. These findings question the necessity of discontinuing HU, particularly concerning ovarian reserve. In our cohort, most patients had cerebral vasculopathy and were receiving chronic transfusions, whereas the remainder underwent stem cell transplantation because of inadequate response to HU therapy. Therefore, discontinuing HU in these cases was a measure taken to prevent further toxicities.
Due to the risk of gonadotoxicity associated with planned conditioning regimen, pretransplant counseling and fertility preservation are crucial interventions for these patients.
Strengths of the study
A key strength of our study is the use of histological samples for the evaluation of the effective number of follicles in the ovarian tissue, which allowed a direct evaluation of both ovarian reserve and the density of growing follicles. Furthermore, the use of a digital system reduces the likelihood of calculation errors.
In addition, the sample size in our study is higher than that in previously reported studies.15,17,25 Most previous studies have included fewer patients (mean = 26). Another strength of this study is the direct evaluation of ovarian tissue samples, which enabled an objective analysis of the effect of HU on follicle density.
Limitations of the study
One crucial limitation of this study is the absence of AMH level at the time of OTC, thus impeding the correlation with follicle density. Consequently, the value of AMH level as a marker of ovarian reserve is still unclear. For some statistical analyses (eg, VOC), a significant imbalance was observed in the number of records between the treatment groups (11 vs 61), reducing the statistical power of analysis. OTC was performed after a particularly long period of HU washout, especially in those with cerebral vasculopathy, making it challenging to draw conclusions about the impact on growing follicles. Lastly, for the moment, it is premature to conclude that follicle density in patients with SCD is normal because there was no healthy control group for comparison.
Conclusion
To our knowledge, this study is the first to demonstrate that HU may not affect ovarian reserve in patients with SCD. Furthermore, transfusion history, the presence of VOC, or the frequency of VOC was not found to significantly influence ovarian reserve and the density of growing follicles in SCD. Overall, these findings indicate that ovarian reserve in SCD was not reduced by HU treatment, transfusion, or VOC. Therefore, OTC should be considered as a fertility preservation option before gonadotoxic treatment for this population.
Acknowledgments
The authors thank Lilia Grira, Corinne Journo, and Anne Gloaguen for their technical support.
This study was supported by the foundation Stiftung für krebskranke Kinder, Regio Basiliensis, Basel, Switzerland and the Schweizerischer Nationalfonds (SNF) Scientific Exchange, Bern, Switzerland (Stiftung: #2021F014; SNF: IZSEZ0201317/1).
Authorship
Contribution: C.P., T.D.-F., and J.-H.D. conceived and designed the study; C.P., T.D.-F., C.S., and A.A. were responsible for the analysis and interpretation of data; T.D.-F. and C.P. wrote the manuscript; F.B. and J.-H.D. critically revised the manuscript for intellectual content; F.B., C.P., N.D., B.N., F.P., G.L., I.B., and B.T. collected the data; and the final version of the manuscript was approved by all authors.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Tamara Diesch-Furlanetto, Division of Pediatric Oncology/Hematology, University Children’s Hospital of Basel, Spitalstrasse 33, 4056 Basel, Switzerland; email: tamara.diesch@ukbb.ch.
References
Author notes
The data set used in this study is held securely in coded form at the University Children’s Hospital of Basel. The data are available on reasonable request from the corresponding author, Tamara Diesch-Furlanetto (tamara.diesch@ukbb.ch), after granting prespecified criteria for confidential access.
The full-text version of this article contains a data supplement.