Key Points
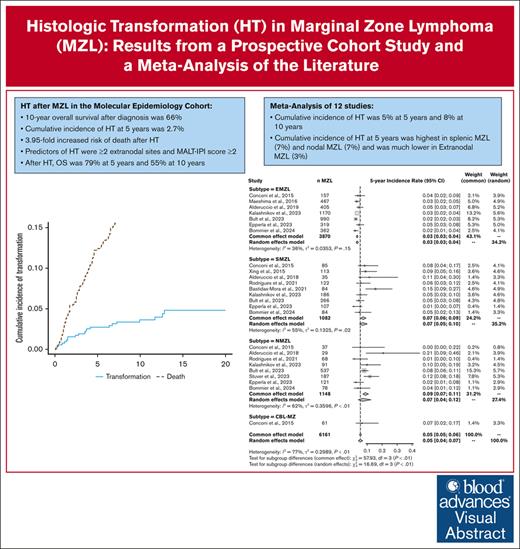
The cumulative incidence of transformation in MZL is 5% at 5 years and 8% at 10 years after diagnosis.
Transformation is associated with a 3.95-fold increased risk of death.
Visual Abstract
Marginal zone lymphoma (MZL) includes extranodal MZL (EMZL), splenic MZL (SMZL), and nodal MZL (NMZL) subtypes. Histologic transformation (HT) to large B-cell lymphomas is well documented but with a large variability in published cumulative incidence rates. We report results from the Molecular Epidemiology Resource (MER) cohort on the cumulative incidence of HT (with death as competing risk) and the associated risk factors and outcomes. We also conducted a meta-analysis of available studies on the cumulative incidence of HT. From 2002 to 2015, 529 patients with MZL were enrolled in the MER study (69% EMZL, 16% SMZL, and 15% NMZL). The 10-year overall survival (OS) from diagnosis was 66%. HT occurred in 21 patients with a 5-year and 10-year cumulative incidence of 2.7% (95% confidence interval [CI], 0.02-0.05) and 3.6% (95% CI, 0.02-0.06), respectively. HT was associated with an increased risk for death (subdistribution hazard ratio [HR], 3.95; 95% CI, 2.06-7.55). Predictors of HT were ≥2 extranodal sites and mucosa-associated lymphoid tissue International Prognostic Index score ≥2. The OS was 79% at 5 years and 55% at 10 years after HT. In the meta-analysis of 12 studies (6161 patients), the 5- and 10-year cumulative incidence of HT across all subtypes were 5% (95% CI, 0.05-0.06) and 8% (95% CI, 0.07-0.09), respectively. These rates were lower in EMZL (3% and 5%) than in SMZL (7% and 13%) and NMZL (9% and 13%). Although HT is relatively uncommon in the first decade after MZL diagnosis, it is associated with an inferior outcome and needs new approaches for prevention and management.
Introduction
Marginal zone lymphoma (MZL) comprises a group of low-grade B-cell lymphomas subclassified into extranodal MZL (EMZL), splenic MZL (SMZL), and nodal MZL (NMZL).1 Although MZL typically exhibits an indolent course, histologic transformation (HT) to large B-cell lymphomas (under which we include diffuse large B-cell lymphoma [DLBCL] not otherwise specified [NOS] and high-grade large B-cell lymphoma NOS), hereafter referred to as transformed MZL (tMZL), can occur. Managing patients with MZL with HT remains a clinical challenge, because tMZL is associated with a higher risk for death and existing prognostic scores at baseline are not tailored to predict it.2 In addition, the accumulating literature reports a variety of cumulative incidence rates, risk factors, and outcomes,3-13 which makes clinical implementation challenging. For example, the current literature provides 5-year cumulative incidence rates for HT that vary from 1% to 11% in SMZL and from 0% to 21% in NMZL.
Research on tMZL faces many obstacles. First, estimating the cumulative incidence of tMZL and the predictors thereof require systematic and prolonged follow-up, generally from inception cohort studies or population-based registries. Second, centralized pathology review is not always feasible, and detailed clinical or biologic data are not always available, particularly for many regional or national registries. Third, the low incidence of tMZL often leads to underpowered analyses because of small study sizes or too short study duration. Fourth, because MZL has an older median age at diagnosis, the competing risk of death from other causes needs to be addressed when evaluating the cumulative incidence. Fifth, MZL and its associated subtypes have varying incidence rates across different populations (eg, geographic variation in the incidence of Helicobacter pylori–driven gastric EMZL14-16), and it is unknown whether the wide range in the cumulative incidence of tMZL recorded in the literature is explained by the populations studied or by the quality of data collection.
Our study had 2 aims. First, this study aimed to evaluate the cumulative incidence of tMZL, the overall survival (OS) after HT, and the prognostic factors in a well-characterized prospective cohort of patients with MZL from the Midwest in the United States; and second, to provide robust estimates of the cumulative incidence of tMZL at 5 and 10 years after MZL diagnosis by conducting a systematic review and meta-analysis of the existing literature.
Methods
Cohort analyses
Risk of HT
Patients aged ≥18 years with newly diagnosed MZL were prospectively enrolled in the University of Iowa/Mayo Clinic Molecular Epidemiology Resource (MER) cohort from 2002 to 2015; the full details are provided elsewhere.17 Briefly, clinical and treatment data were abstracted from medical records using a standard protocol. Participants were actively followed (every 6 months for the first 3 years and annually thereafter) for retreatment, transformation, and death through December 2022, and all events were validated against medical records. Pathology was classified according to the World Health Organization (WHO) 4/4R1, and transformations were all centrally reviewed by expert hematopathologists. For the analysis of HT, only patients with a pathologically confirmed diagnosis of tMZL to large B-cell lymphoma (ie, DLBCL NOS and high-grade large B-cell lymphoma NOS) upon re-review were included in the study; other subtypes remained in the at-risk cohort. Patients with MZL with concomitant low-grade and aggressive lymphomas at diagnosis were excluded for the cumulative incidence estimation but were included for all other analyses. The institutional review boards of the Mayo Clinic and the University of Iowa approved this study, and all participants provided written, informed consent.
Outcomes after HT
To evaluate predictors of OS after HT, we supplemented the MER tMZL cases (n = 21) with additional patients aged ≥18 years with newly diagnosed tMZL from 1995 to 2002 who were identified in the Mayo Clinic Lymphoma Database (n = 11) using the same definition and protocol used for the MER cohort to define tMZL. Because the latter cases were not part of an inception cohort, they could not be included in the cumulative incidence analysis.
Statistical analyses
The baseline characteristics, including demographics, the International Prognostic Index (IPI) score, follicular lymphoma IPI (FLIPI), mucosa-associated lymphoid tissue (MALT) IPI (MALT-IPI), and initial management, were summarized using descriptive statistics. The median follow-up was estimated using the reverse Kaplan-Meier method.
The cumulative incidence of tMZL was determined using death as a competing risk. The time to HT was defined as the time from the date of MZL diagnosis to the date of HT. We evaluated potential predictors of HT by fitting Fine and Gray’s proportional subdistribution hazards model18 and derived subdistribution hazard ratios (SHRs) and 95% confidence intervals (CIs). Multivariate models were derived using stepwise selection among candidate variables with a P value cutoff of ≤.10 required to enter in the model. We also evaluated the time from the initial MZL diagnosis to death from any cause. An estimate of the impact of HT on OS was derived from a Fine and Gray’s model, which takes into account competing risk data and estimates the magnitude of the relative change in the subdistribution hazard function associated with death among patients with MZL, irrespective of if they experienced HT.18
We used the Kaplan-Meier method to evaluate the time from HT to death from any cause. Univariate and multivariate analyses using a Cox proportional hazards regression were conducted to evaluate the effect of potential prognostic variables at the time of HT on OS.
Meta-analysis
Study selection
On 13 September 2023, a literature search was conducted in the MedLine database using the following keywords: marginal zone lymphoma, incidence or cumulative incidence, and transformation. Only studies published after 1995 (year of the Revised European American Classification of Lymphoid Neoplasms) were considered. Studies that restricted their analysis to a subgroup of patients with MZL (eg, only treated patients) were excluded to avoid biasing the estimate of the cumulative incidence rate of HT. Studies that were not published in English were excluded. Interventional or observational studies that reported the cumulative incidence of pathology-defined HT to large B-cell lymphoma in a population of patients with MZL using competing risk of death models were included in this analysis.
Data extraction
We extracted the reported cumulative incidence of HT for each MZL subtype at 5 and 10 years, along with the main study characteristics and the follow-up duration. When the cumulative incidence rate was not reported for each subtype in the original article, the investigators were contacted to provide the missing values.
Statistical analysis
The outcome of interest was the cumulative incidence rate at 5 and 10 years based on competing risk (of death) models for MZL overall and for each of the subtypes. To compute the pooled estimate of the cumulative incidence of HT, both common- and random-effects models19 were used for the overall population and for each of the 3 subtypes. A forest plot was generated based on the random intercept Poisson regression model with the computed 95% CI. For each model, a test of heterogeneity (Q-statistic) was calculated using a restricted maximum likelihood estimator.20,21 The total heterogeneity (τ2) and the total heterogeneity relative to the variance (I2) were also calculated.22 Although thresholds for interpreting I2 can be misleading because the importance of inconsistency depends on several factors, it is generally considered that values <40% and 40% to 60% refer to insubstantial and moderate heterogeneity, respectively.23
All data analyses were performed using R Statistics (version 3.6.1; R Core Team, 2019).
Results
MER cohort analysis
Patients’ characteristics at MZL diagnosis
From 2002 to 2015, 524 patients with biopsy-proven MZL were enrolled in the MER cohort (2002-2005: n = 97; 2006-2010: n = 227; 2011-2015: n = 200), including 362 (69%) with EMZL, 84 (16%) with SMZL, and 78 (15%) with NMZL. The clinical characteristics of these patients are provided in Table 1. The median age at diagnosis was 63 years (range, 18-92); 233 were men (45%), and the vast majority of patients were White (n = 459, 95%). Although limited (stage I-II, n = 251, 49%) and advanced (stage III-IV, n = 264, 51%) stage disease was balanced overall, patients with the SMZL (n = 75, 91%) and NMZL (n = 52, 69%) subtypes were more likely to have advanced disease than those with EMZL (n = 137, 38%). Bone marrow involvement at diagnosis was observed in 161 (32%) patients, and patients with the SMZL subtype had a higher involvement rate (n = 72, 86%). Most patients (n = 493, 94%) had an Eastern Cooperative Oncology Group performance status of 0 to 1. Lactate dehydrogenase (LDH) was elevated in 78 patients (18%), and hemoglobin was less than 12 g/dL in 133 (30%). The initial management approaches at enrollment included observation (n = 192, 37%), radiation (n = 93, 18%), surgery (n = 43, 8%), systemic therapy (n = 174, 34%), and all other (n = 22, 4%).
Descriptive characteristics and univariable analysis for risk of HT in the MER cohort
| Variable . | Category . | No. (%) . | SHRs (95% CI) . | P value . |
|---|---|---|---|---|
| Total | 524 (100) | |||
| Age | >60 | 300 (57.3) | 1.70 (0.68-4.22) | .25 |
| >70 | 151 (28.8) | 0.72 (0.24-2.14) | .55 | |
| Sex | Male | 233 (44.5) | 1.19 (0.51-2.80) | .69 |
| Race | White | 459 (95) | (Not modeled) | |
| Ethnicity | Hispanic/Latino | 7 (1.4) | (Not modeled) | |
| ECOG performance status | ≥2 | 31 (6.0) | 1.10 (0.15-8.16) | .98 |
| Ann Arbor stage | III-IV | 264 (51.3) | 3.43 (1.26-9.38) | .016 |
| Number of extranodal sites | ≥2 | 122 (23.4) | 3.13 (1.33-7.39) | .009 |
| Mucosal sites∗ | ≥2 | 32 (10.8) | 2.72 (0.75-9.89) | .13 |
| LDH | Greater than normal | 78 (18.0) | 2.59 (0.97-6.90) | .057 |
| Hemoglobin | <12 g/dL | 133 (30.1) | 1.00 (0.38-2.81) | .99 |
| Lymphocytes | <1000 per mm3 | 99 (20.9) | 0.74 (0.22-2.55) | .64 |
| Platelets | <100 109/L | 25 (5.1) | 0.99 (0.13-7.37) | .99 |
| IPI Group | Low (0-1) | 275 (52.5) | 1.00 (reference) | |
| Low intermediate (2) | 149 (28.4) | 7.55 (2.10-27.10) | .002 | |
| High intermediate (3) | 81 (15.5) | 8.57 (2.14-34.30) | .002 | |
| High (4-5) | 19 (3.6) | 6.65 (0.69-64.21) | .10 | |
| MALT-IPI group | Low (0) | 169 (32.3) | 1.00 (reference) | |
| Intermediate (1) | 230 (43.9) | 3.03 (0.84-10.86) | .08 | |
| High (2-3) | 125 (23.9) | 4.28 (1.10-16.60) | .036 | |
| FLIPI group | Low (0-1) | 281 (53.6) | 1.00 (reference) | |
| Intermediate (2) | 149 (28.4) | 1.86 (0.67-5.14) | .23 | |
| High (3-5) | 94 (17.9) | 2.78 (0.96-8.03) | .058 | |
| Initial management | Observation | 192 (37) | - | |
| Surgery | 43 (8.2) | - | ||
| Radiation therapy | 93 (18) | - | ||
| Systemic therapy | 174 (33.5) | - | ||
| Other (including antibiotics) | 22 (4.2) | - | ||
| Systemic therapy | Yes | 174 (33.5) | 1.03 (0.42-2.56) | .94 |
| Variable . | Category . | No. (%) . | SHRs (95% CI) . | P value . |
|---|---|---|---|---|
| Total | 524 (100) | |||
| Age | >60 | 300 (57.3) | 1.70 (0.68-4.22) | .25 |
| >70 | 151 (28.8) | 0.72 (0.24-2.14) | .55 | |
| Sex | Male | 233 (44.5) | 1.19 (0.51-2.80) | .69 |
| Race | White | 459 (95) | (Not modeled) | |
| Ethnicity | Hispanic/Latino | 7 (1.4) | (Not modeled) | |
| ECOG performance status | ≥2 | 31 (6.0) | 1.10 (0.15-8.16) | .98 |
| Ann Arbor stage | III-IV | 264 (51.3) | 3.43 (1.26-9.38) | .016 |
| Number of extranodal sites | ≥2 | 122 (23.4) | 3.13 (1.33-7.39) | .009 |
| Mucosal sites∗ | ≥2 | 32 (10.8) | 2.72 (0.75-9.89) | .13 |
| LDH | Greater than normal | 78 (18.0) | 2.59 (0.97-6.90) | .057 |
| Hemoglobin | <12 g/dL | 133 (30.1) | 1.00 (0.38-2.81) | .99 |
| Lymphocytes | <1000 per mm3 | 99 (20.9) | 0.74 (0.22-2.55) | .64 |
| Platelets | <100 109/L | 25 (5.1) | 0.99 (0.13-7.37) | .99 |
| IPI Group | Low (0-1) | 275 (52.5) | 1.00 (reference) | |
| Low intermediate (2) | 149 (28.4) | 7.55 (2.10-27.10) | .002 | |
| High intermediate (3) | 81 (15.5) | 8.57 (2.14-34.30) | .002 | |
| High (4-5) | 19 (3.6) | 6.65 (0.69-64.21) | .10 | |
| MALT-IPI group | Low (0) | 169 (32.3) | 1.00 (reference) | |
| Intermediate (1) | 230 (43.9) | 3.03 (0.84-10.86) | .08 | |
| High (2-3) | 125 (23.9) | 4.28 (1.10-16.60) | .036 | |
| FLIPI group | Low (0-1) | 281 (53.6) | 1.00 (reference) | |
| Intermediate (2) | 149 (28.4) | 1.86 (0.67-5.14) | .23 | |
| High (3-5) | 94 (17.9) | 2.78 (0.96-8.03) | .058 | |
| Initial management | Observation | 192 (37) | - | |
| Surgery | 43 (8.2) | - | ||
| Radiation therapy | 93 (18) | - | ||
| Systemic therapy | 174 (33.5) | - | ||
| Other (including antibiotics) | 22 (4.2) | - | ||
| Systemic therapy | Yes | 174 (33.5) | 1.03 (0.42-2.56) | .94 |
ECOG, Eastern Cooperative Oncology Group.
As defined by Alderuccio et al.7
Risk of HT
The median follow-up from enrollment was 8.9 years (range, 0.4-18.5). The 10-year OS from diagnosis was 66% overall (95% CI, 0.62-0.71) and varied across subtypes with an OS of 69% (95% CI, 0.63-0.74) for EMZL, 53% (95% CI, 0.42-0.66) for SMZL, and 72% (95% CI, 0.61-0.85) for NMZL.
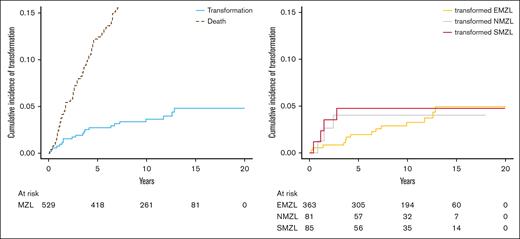
Of the 524 patients who were enrolled in the MER cohort and who were followed through 2022, tMZL was observed in 21 patients (14 EMZL, 4 SMZL, and 3 NMZL). Because many of the tMZLs occurred before fluorescence in situ hybridization was routinely performed in this setting, we only had fluorescence in situ hybridization data for 5 patients; we did not exclude any DLBCL cases with MYC and BCL2 and/or BCL6 rearrangements. Among the 21 patients with HT, 9 (43%) were still untreated at HT, which occurred within 24 months after diagnosis for 5 of them. Characteristics of these 9 cases at diagnosis included 44% female sex (n = 4); median age of 66 years (range 32-86); 56% EMZL (n = 5), 33% SMZL (n = 3), and 11% NMZL (n = 1); 89% Ann Arbor stage IV (n = 8); 89% MALT-IPI score 1 (n = 8); and 89% FLIPI score 0 to 2 (n = 8). The 5- and 10-year overall cumulative incidence of HT was 2.7% (95% CI, 0.02-0.05) and 3.6% (95% CI, 0.02-0.06), respectively. The 5-year cumulative incidence of HT was 2% (95% CI, 0.01-0.04) in patients with EMZL, 5% (95% CI, 0.02-0.13) in SMZL, and 4% (95% CI, 0.01-0.12) in NMZL; the 10-year cumulative incidence increased in EMZL (4%; 95% CI, 0.02-0.06) but plateaued in NMZL and SMZL after 5 years of follow-up (Figure 1). The time to HT (range, 3.1-154 months) was much longer in patients with EMZL with a median of 42 months (interquartile range [IQR], 16-81) than in patients with SMZL with a median of 16 months (IQR, 11-22) or in patients with NMZL with a median of 18 months (IQR, 14-23). Our competing risk analysis showed that HT was associated with a fourfold higher risk for death (SHR, 3.95; 95% CI, 2.06-7.55; P < .001). In a sensitivity analysis that accounted for HT as first event only, the cumulative incidence rates were 1.6% (EMZL, 0.9%; SMZL, 2.4%; NMZL, 4.0%) and 1.8% (EMZL, 1.2%; SMZL, 2.4%; NMZL, 4.0%) after 5 and 10 years, respectively. Given the long enrollment period and the associated changes in clinical practice (particularly positron emission tomography and computed tomography scanning), we conducted a sensitivity analysis to compare the cumulative incidence rates when stratified by the diagnosis year 2010 (supplemental Figure 1). Before 2010, the cumulative incidence rates for HT were 2.2% (EMZL, 2.0%; SMZL, 2.1%; NMZL, 4.2%) and 3.8% (EMZL, 4.1%; SMZL, 2.1%; NMZL, 4.2%) after 5 and 10 years, respectively; on or after 2010, the cumulative incidence rates were 2.5% (EMZL, 1.3%; SMZL, 5.6%; NMZL, 4.0%) and 2.9% (EMZL, 1.3%; SMZL, 8.5%; NMZL, 4.0%) after 5 and 10 years, respectively.
Cumulative incidence of transformation in the MER cohort. Cumulative incidence of transformation with competing risk of death for all MZL (left) and according to each subtype (right).
Cumulative incidence of transformation in the MER cohort. Cumulative incidence of transformation with competing risk of death for all MZL (left) and according to each subtype (right).
At MZL diagnosis, variables that met the stepwise selection criterion (ie, P ≤ .10) in the univariable competing risk analysis were ≥2 extranodal sites (SHR, 3.13; 95% CI, 1.33-7.39; P = .009), Ann Arbor stage III to IV (SHR, 3.43; 95% CI, 1.26-9.38; P = .016), elevated LDH (SHR, 2.59; 95% CI ,0.97-6.90; P = .057), IPI score ≥2 (HR, 7.55; 95% CI, 2.10-27.10; P = .002), MALT-IPI score ≥2 (SHR, 4.28; 95% CI, 1.10-16.60; P = .036), and FLIPI score ≥3 (SHR, 2.78; 95% CI, 0.96-8.03; P = .058). The univariable Fine and Gray proportional subdistribution hazards models for HT are listed in Table 1. Moving to the multivariable modeling, redundant variables (eg, elevated LDH and MALT-IPI score or stage IV and ≥2 extranodal sites) could not be included in the same model. Therefore, we fit the following 4 multivariable models: (1) LDH + ≥2 extranodal sites; (2) MALT-IPI score + ≥2 extranodal sites; (3) FLIPI score + ≥2 extranodal sites; and (4) LDH + Ann Arbor III to IV. In the multivariable analyses, the strongest predictors of HT were ≥2 extranodal sites (SHR, 2.88; 95% CI, 1.15-7.18; P = .023 in model 1; SHR, 2.72; 95% CI, 1.03-7.21; P = .044 in model 3) and MALT-IPI score ≥2 (SHR, 1.99; 95% CI, 0.51-7.85; P = .033 in model 2).
Survival after HT
To maximize our sample size, we combined the tMZL cases from the MER cohort (n = 21, initial MZL diagnosis from 2002-2015) with additional tMZL cases from the Mayo Clinic Lymphoma Database (n = 11, initial MZL diagnosis from 1995-2002) to obtain a total cohort of 32 patients with tMZL. Patients who were treated for their initial MZL received either anti-CD20 antibody therapy (monotherapy or combined with CHOP [cyclophosphamide, doxorubicin, vincristine, prednisone] or CVP [cyclophosphamide, vincristine, and prednisone], n = 8), chlorambucil (n = 2), fludarabine (n = 1), or had an initial splenectomy (n = 1). The clinical characteristics at HT are described in Table 2. Of those included, 6 (19%) patients were not eligible for systemic therapy and only received palliative care. Furthermore, 26 (81%) were treated with systemic therapy, which primarily included a CHOP backbone (R-CHOP [rituximab-cyclophosphamide, vincristine, and prednisone], n = 13; CHOP, n = 4; R-EPOCH [rituximab, etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin], n = 3; lenalidomide-R-CHOP, n = 1). Among the 26 treated patients, the overall response rate was 85% (n = 22/26). Three of the 4 nonresponders died within 1 year. Among all patients, the median follow-up after HT was 9.6 years (IQR, 8.7-14.6). The 2- and 5-year OS from HT were 79% (95% CI, 0.62-0.93) and 55% (95% CI, 0.39-0.76), respectively, and the median time survival time was 11.6 years (95% CI, 2.8 to not reached). The 2-year OS was higher in transformed EMZL (tEMZL, 85%; 95% CI, 0.71-1.00) than in transformed SMZL (tSMZL, 60%; 95% CI, 0.29-1.00) and transformed NMZL (tNMZL, 50%; 95% CI, 0.19-1.00; P values were not computed because of the low number of patients in each subtype).
Characteristics at HT of the 32 patients with transformed MZL and univariable analysis for death from any cause
| Characteristic . | Category . | No. (%) . | HR (CI 95%) . | P value . |
|---|---|---|---|---|
| Age at transformation | ≥70 y | 12 (37.5) | 3.57 (1.34-9.48) | .011 |
| Time to HT | <1 y | 8 (25.0) | 1.10 (0.40-3.00) | .86 |
| Ann Arbor stage | III-IV | 24 (80.0) | 0.58 (0.20-1.71) | .32 |
| Missing data | 2 | |||
| Bone marrow involvement | Yes | 9 (39.1) | 0.37 (0.09-1.41) | .14 |
| Missing data | 9 | |||
| ECOG performance status | ≥2 | 10 (34.5) | 2.22 (0.81-6.08) | .12 |
| Missing data | 3 | |||
| LDH | Greater than normal | 11 (57.9) | 1.14 (0.18-7.16) | .89 |
| Missing data | 13 | |||
| Hemoglobin | <12 g/dL | 12 (54.5) | 2.90 (0.75-11.26) | .13 |
| Missing data | 10 | |||
| Lymphocytes | <1000 per mm3 | 8 (36.4) | 0.38 (0.08-1.86) | .23 |
| Missing data | 10 | |||
| Platelets | <100 109/L | 3 (13.6) | - | |
| Missing data | 10 |
| Characteristic . | Category . | No. (%) . | HR (CI 95%) . | P value . |
|---|---|---|---|---|
| Age at transformation | ≥70 y | 12 (37.5) | 3.57 (1.34-9.48) | .011 |
| Time to HT | <1 y | 8 (25.0) | 1.10 (0.40-3.00) | .86 |
| Ann Arbor stage | III-IV | 24 (80.0) | 0.58 (0.20-1.71) | .32 |
| Missing data | 2 | |||
| Bone marrow involvement | Yes | 9 (39.1) | 0.37 (0.09-1.41) | .14 |
| Missing data | 9 | |||
| ECOG performance status | ≥2 | 10 (34.5) | 2.22 (0.81-6.08) | .12 |
| Missing data | 3 | |||
| LDH | Greater than normal | 11 (57.9) | 1.14 (0.18-7.16) | .89 |
| Missing data | 13 | |||
| Hemoglobin | <12 g/dL | 12 (54.5) | 2.90 (0.75-11.26) | .13 |
| Missing data | 10 | |||
| Lymphocytes | <1000 per mm3 | 8 (36.4) | 0.38 (0.08-1.86) | .23 |
| Missing data | 10 | |||
| Platelets | <100 109/L | 3 (13.6) | - | |
| Missing data | 10 |
ECOG, Eastern Cooperative Oncology Group.
Among all patients with HT, an age at HT of ≥70 years was the only variable associated with OS after HT (HR, 3.57; 95% CI, 1.34-9.48; P = .011). Because only 3 patients had a platelet count <100 109/L at HT, this variable could not be evaluated as a predictor of OS (Table 2).
Meta-analysis
Cohort characteristics
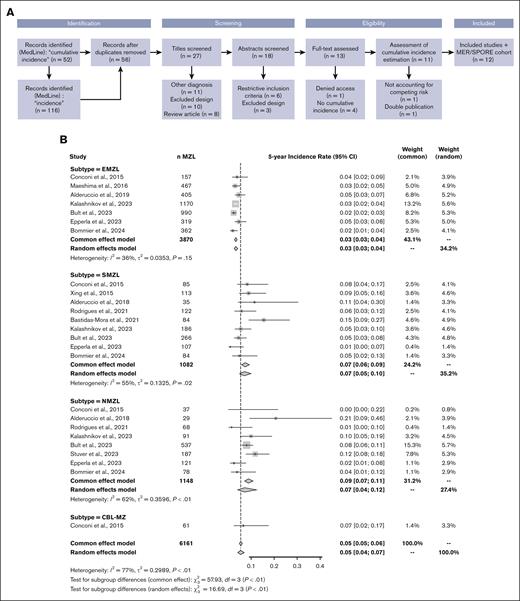
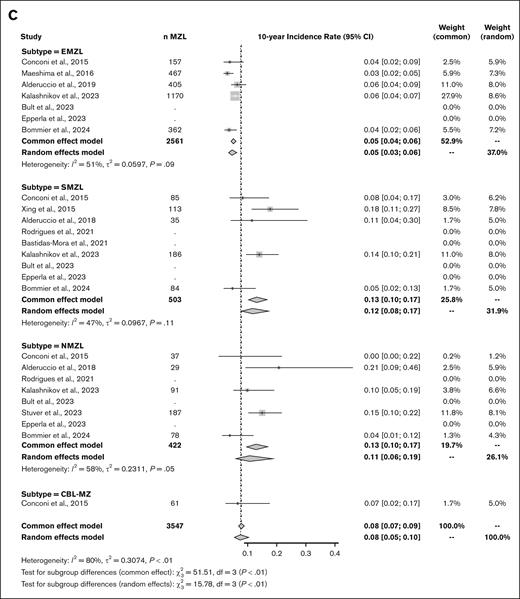
A total of 158 articles were initially identified in the MedLine database. After the removal of duplicates, 56 studies were further evaluated. During the eligibility assessment, 1 article was excluded because the estimated cumulative incidence did not take into account death as a competing risk. After assessment by reviewers, 11 studies, in addition to our own study, were included in the final analysis,3-13 which ultimately included data on 6161 patients (EMZL, n = 3870; SMZL, n = 1082; NMZL, n = 1148; clonal B lymphocytosis-marginal zone, n = 61) at 5 years and 3547 patients (EMZL, n = 2561; SMZL, n = 503; NMZL, n = 422; clonal B lymphocytosis-marginal zone, n = 61) at 10 years. A flowchart is provided in Figure 2A. The 12 studies were published between 2015 and 2024, and except for 1 study that started accrual in 1985, the accrual period spanned from 1995 to 2020 (studies are summarized in Supplemental Table 1). Leveraging the prolonged follow-up for many of these studies (up to 24 years), there were a total of 348 transformations among 6161 patients with an initial MZL diagnosis.
Meta-analysis of the 5-year and 10-year cumulative incidence rates of MZL transformation into higher grade lymphoma. (A) Study selection process. Adapted from the Preferred Reporting Items for Systematic Reviews and Meta-Analyses flowchart 2009. (B) Forest plot of the 5-year cumulative incidence rate. (C) Forest plot of the 10-year cumulative incidence rate. τ2, total heterogeneity; I2, total heterogeneity relative to the variance.
Meta-analysis of the 5-year and 10-year cumulative incidence rates of MZL transformation into higher grade lymphoma. (A) Study selection process. Adapted from the Preferred Reporting Items for Systematic Reviews and Meta-Analyses flowchart 2009. (B) Forest plot of the 5-year cumulative incidence rate. (C) Forest plot of the 10-year cumulative incidence rate. τ2, total heterogeneity; I2, total heterogeneity relative to the variance.
The 5- and 10-year cumulative incidence of HT
The pooled 5-year and 10-year cumulative incidence rates of HT across all subtypes were 5% (95% CI, 0.05-0.06) and 8% (95% CI, 0.07-0.09), respectively. When using a random-effects model, the values were similar at 5% (95% CI, 0.04-0.07) and 8% (95% CI, 0.05-0.10), respectively. These rates varied according to subtypes with lower rates in patients with EMZL (3% and 5%) than in patients with SMZL (7% and 13%) or NMZL (9% and 13%). Despite the geographic diversity of the included studies, heterogeneity between studies within each subtype was deemed as moderate (I2 < 60%). The corresponding forest plots are provided in Figure 2B-C.
Discussion
Based on the prospective MER cohort study initiated in 2002 and with long follow-up, we estimated the cumulative incidence of HT, and then, in a meta-analysis of our study and 11 others, we found that the 5- and 10-year cumulative incidence of HT across all subtypes were 5% and 8%, respectively, with lower rates in EMZL relative to SMZL and NMZL. In the MER cohort, we also found that the strongest predictors of HT were ≥2 extranodal sites and a MALT-IPI score ≥2 at diagnosis and that HT was associated with a significantly higher risk for death. At HT, being ≥70 years old was associated with a poor OS.
To our knowledge, this is the first meta-analysis of HT in patients with MZL. Leveraging multiple recent publications in the modern treatment era from various geographic regions, we were able to provide a robust estimate of the 5-year and 10-year cumulative incidence of HT from MZL diagnosis and found modest heterogeneity in the HT estimates within MZL subtypes and important heterogeneity between MZL subtypes. Strengths of the meta-analysis include a comprehensive approach to the ascertainment and extraction of data from published studies, the estimation of HT for each MZL subtype, and the use of both fixed- and random-effects models. There are also limitations. First, a meta-analysis is always limited compared with studies pooling individual patient-level data in terms of data harmonization, subgroup assessment, and analytic approaches, among other issues. Second, the 12 included studies represent only a portion of all the studies that reported follow-up of patients with MZL. Indeed, many studies were excluded either because they did not account for patients managed with observation alone or because they reported a crude rate rather than a proper cumulative incidence rate of HT.24-26 Third, even the included studies varied in terms of data quality because of differences in study design (retrospective vs prospective), data origin (population-based vs local registries or cohorts), central or expert pathology review of tMZL cases to confirm HT, or the duration of follow-up. Indeed, retrospective collection of nonreviewed cases and the use of local databases could lead to the overestimation of tMZL cumulative incidence. Furthermore, the pooled analysis did not allow for the assessment of cumulative incidence by potentially relevant subgroups, such as age, sex, race/ethnicity, and treatment approach (particularly use of rituximab). Finally, we did not include a recent study based on the Surveillance, Epidemiology and End Result (SEER) database from 2000 to 2018 (because it would overlap with some of the US-based studies), which found that the cumulative incidence rate of HT at 10 years was much lower in patients with MZL overall (2.23%) and for EMZL (1.5%), NMZL (2.7%), and SMZL (5.8%) when compared with the rates found in our meta-analysis.27 The lower rates may be because of the distribution of subtypes (eg, SEER has lower percentage of SMZL), definition of HT (DLBCL), and follow-up of patients (SEER would only identify a HT to DLBCL if the MZL patient was still a resident in a SEER region).
Across all published series for patients with transformed MZL, the median time to transformation varied by subtype and ranged from 15 to 36 months and frequently occurred at the time of first relapse or disease progression. This suggests the presence of a subset of patients who are predisposed to this type of progression. When comparing the transformation rate with that of other indolent B-cell lymphomas, it seems to be close to what is observed in chronic lymphocytic leukemia in which the cumulative incidence of Richter syndrome at 10 years is estimated to be 5% to 10%.28,29 As a comparison, the 10-year cumulative incidence of HT in follicular lymphoma is estimated to be 15% to 20%.30-32 However, in these 3 indolent lymphomas, the curves tend to reach a plateau, suggesting that a majority of patients will never experience transformation.
Based on our real-world prospective data from the upper Midwest in the United States, ≥2 extranodal sites, Ann Arbor stage III to IV, and elevated LDH levels at diagnosis were single variables that were associated with HT, and this aligns with what was previously reported by Alderuccio et al6 (Florida), Bult et al12 (the Netherlands), Conconi et al3 (Northern Italy and Southern Switzerland), Maeshima et al5 (Japan), and Bastidas-Mora et al9 (Spain). Radiomics could not be assessed in our study as potential predictors of HT, because disease assessment was done according to standard of care and evolved over the time frame of the study. More particularly, for data that were prospectively collected from 2002 onward, positron emission tomography and computed tomography data were not documented in the MER study.
Although we only had limited data on 32 tMZL cases for the outcome analysis, this still represents one of the larger series in the literature. HT was associated with an increased risk for death, which concurs with the existing literature. Indeed, Meyer et al26 (Germany) were the first to demonstrate that patients with MZL who developed a second malignancy or HT had significantly shorter survival. Conconi et al found that the 2-year OS rate after transformation was 57%.3 According to Kalashnikov et al10 (Finland), the risk for death after transformation was increased fivefold (HR, 5.18; 95% CI, 3.58-7.50) and the 5- and 10-year OS rates were 76% and 59%, respectively. However, the poorer outcome associated with HT does not necessarily mean that HT is always fatal. In our series, the median OS from HT was 11.6 years (95% CI, 2.8 to not reached), similar to the 10.8 years reported by Alderuccio et al (95% CI, 4.6 to not reached). The 2-year OS after HT was 79% in our series with a higher rate in tEMZL (85%) than in tSMZL (60%) and tNMZL (50%). This is in line with the 2-year OS rates published by Bastidas-Mora et al (63% in tSMZL),9 Bult et al (67% across all subtypes),12 Alderuccio et al (80% across all subtypes),6 and Maeshima et al (97% in tEMZL).5 In our series, we found that age at HT was the only factor that was associated with OS after HT. However, we did not assess the genetic biomarkers, and there is now growing evidence that certain molecular abnormalities could predict survival outcomes, such as TBL1XR1 mutations across all subtypes33 or KLF2 mutations and complex karyotypes in transformed SMZL.9,34
In conclusion, our study provides robust estimates of the 5- and 10-year cumulative incidence rates of HT in MZL at 5% and 8%, respectively, and supports a lower risk for HT among patients with EMZL than among those with the other subtypes. We also confirmed that HT is associated with an overall increased risk for death despite the favorable overall response rate to chemoimmunotherapy at HT and that patients with tEMZL exhibit a better outcome in terms of OS after HT. Although disseminated disease and elevated LDH at diagnosis are predictors of HT, neither of these markers offer an opportunity to change the initial clinical management. A better understanding of the molecular features that underlie HT may lead to the development of novel prevention or management approaches that will improve survival in patients with tMZL.
Acknowledgments
The authors acknowledge Tanner W. Reicks, Rachel L. Benson, Stephaney A. Klein, and Sara K. Borgschatz for help with retrieving pathology slides and blocks. The authors also acknowledge investigators who accepted to share unpublished results allowing the performance of the meta-analysis, including Emanuele Zucca (Oncology Institute of Southern Switzerland, Bellinzona) and Gianluca Gaidano (Division of Hematology of the Amedeo Avogadro University of Eastern Piedmont, Novara).
The Molecular Epidemiology Resource study was funded through the following grants from the National Cancer Institute (P50 CA97274 and U01 CA195568) and from the Predolin Foundation. The Mayo Clinic Lymphoma Database was supported by the Division of Hematology, Mayo Clinic, Rochester, Minnesota. C.B. was supported by Inserm/Aviesan Multi-Organization Institute for Cancer through a doctoral grant and was awarded the Bertrand Coiffier Prize by Lymphoma Study Association/European Lymphoma Institute. C.B. also received funding from the Philippe Foundation and Institut Servier (CT0101951) for travel support.
Authorship
Contribution: C.B., B.K.L., M.J.M., T.M.H., R.L.K., and J.R.C. designed the research concept; B.K.L., A.K., Y.W., C.A.T., G.S.N., and T.M.H. included patients in the Molecular Epidemiology Resource cohort; T.M.H. contributed patients from the Mayo Clinic Lymphoma Database; A.L.F. and R.L.K. centrally reviewed the pathology of every case; M.J.M., M.C.L., and B.J.G. structured the data set; C.B. conducted the analysis; C.B. and J.R.C. performed the systematic review and wrote the first draft of the manuscript; J.P.A., I.S.L., N.E., and A.C. provided unpublished results for the meta-analysis; and all authors contributed to the final version of the manuscript.
Conflict-of-interest disclosure: All authors declare no competing financial interests.
Correspondence: James R. Cerhan, Department of Quantitative Health Sciences, Mayo Clinic, 200 First St SW, Rochester, MN 55905; email: cerhan.james@mayo.edu.
References
Author notes
The data used in the study are not publicly available. Data sharing policies and the process to request the data that support the findings of this study can be found on the Lymphoma Epidemiology of Outcomes Cohort website at https://leocohort.org/.
The full-text version of this article contains a data supplement.