Quadruplet regimens (anti-CD38 monoclonal antibodies [mAbs] with proteasome inhibitor [PI] and immunomodulatory drugs [IMiDs]) are increasingly being investigated in newly diagnosed multiple myeloma (NDMM). The objective of our study was to conduct a systematic review and meta-analysis to measure the efficacy and toxicity of quadruplet regimens used in NDMM. Embase, MEDLINE, Web of Science, Cochrane Library, clinical trial registries, and meeting libraries from inception to 24 January 2024, in addition to American Society of Clinical Oncology conference abstracts 2024, were searched using terms reflecting multiple myeloma and components of the quadruplet regimen. Included studies were randomized controlled trials (RCTs) that compared backbone regimens consisting of a PI and IMiD vs the same regimen plus an anti-CD38 mAb in NDMM. We identified 7 RCTs including 3716 patients. Compared with triplets, quadruplets increase the overall response rate (ORR; relative risk [RR], 1.03; 95% confidence interval [CI], 1.01-1.05) and progression-free survival (PFS; hazard ratio [HR], 0.55; 95% CI, 0.46-0.66). Quadruplets increase the rates of minimal residual disease (MRD) negativity at 10–5 (RR, 1.39; 95% CI, 1.23-1.58) and 10–6 (RR, 1.62; 95% CI, 1.36-1.94). Quadruplets improve overall survival (OS; HR, 0.65; 95% CI, 0.53-0.79). There was a slight increase in the rates of grade 3 to 4 infections (RR, 1.22; 95% CI, 1.07-1.39) noted with quadruplets compared with triplets. Overall, in this meta-analysis, quadruplets were associated with improved efficacy including ORR, MRD negativity, PFS, and OS, with a slight increase in infection rates. Quadruplet regimens represent a new standard of care, particularly in transplant-eligible NDMM.
Introduction
Triplets (consisting of a proteasome inhibitor [PI], an immunomodulatory drug [IMiD], and dexamethasone) have been considered a standard of care for patients with newly diagnosed multiple myeloma (NDMM) based on randomized controlled trials (RCTs) demonstrating improved depth of response and long-term outcomes, such as progression-free survival (PFS) and overall survival (OS), compared with doublets.1-3 More recently, quadruplet regimens have been investigated for NDMM. Clinical trials evaluating quadruplet regimens containing a PI, IMiD, dexamethasone, and anti-CD38 monoclonal antibody (mAb) have shown improvement in increasing the depth of response, including minimal residual disease (MRD) negativity rates as well as PFS in patients with NDMM.1,4-6 Although RCTs have consistently shown increased response rates and improved PFS with quadruplet therapy, these trials are underpowered for OS or to detect signals of increased toxicity.
A systematic review synthesizing data from all reported RCTs evaluating quadruplet regimens is needed to fully understand the efficacy, including any potential impact on OS as well as toxicity for patients with NDMM. This is particularly important to understand now because these quadruplet regimens are increasingly being used in routine clinical settings. To address this knowledge gap, we conducted a systematic review and meta-analysis to investigate the efficacy and toxicity of quadruplet induction therapy compared with triplets among patients with NDMM.
Methods
We followed the PRISMA (preferred reporting items for systematic reviews and meta-analyses) guidelines7 for the reporting of this systematic review and meta-analysis. This review was prospectively registered on PROspective Systematic REview RegistratiOn (PROSPERO registration number CRD42023491019).
Search strategy
The search strategy was designed with input from all study investigators. Databases electronically searched included Embase, MEDLINE (PubMed), Web of Science Core Collection, and Cochrane Central Register of Controlled Trials database, from inception of each database until January 2024. We used a combination of controlled vocabulary (MeSH [Medical Subject Headings] and Emtree terms) and key words with various synonyms that reflect the following concepts: “multiple myeloma” combined with “newly diagnosed” as well as drug names from each category of PI, IMiD, and anti-CD38. The search result was limited to English language studies, with no filters or hedges for the publication type used. A search of the gray literature was also performed through manual searching of (1) bibliographies of identified randomized clinical trials; (2) trial registries (ClinicalTrials.gov and World Health Organization International Clinical Trials Registry Platform search portal); and (3) conference abstracts from the American Society of Hematology, American Society of Clinical Oncology, the European Hematology Association annual conferences, and the International Myeloma Society Workshop from January 2021 to January 2024. We also searched the abstracts from American Society of Clinical Oncology 2024 because we became aware that the pivotal trial IMROZ8,9 would be presented (details also extracted from the concurrent publication of the results9). Details of the search strategy are provided in the supplemental Data.
Citations were imported into Covidence. Duplicates were then removed. Two independent screeners performed title and abstract review (M.S.E. and R.C.). Disagreements were resolved by a third reviewer (H.M.).
Selection criteria
After preliminary screening, full-text review was done independently by M.S.E and R.C. to confirm whether they were eligible based on the following selection criteria: (1) RCTs of adult patients aged ≥18 years with NDMM; and (2) trials comparing a quadruplet regimen (consisting of at least 1 IMiD, PI, dexamethasone, and anti-CD38) administered for a minimum of 3 cycles with a triplet regimen (consisting of at least 1 IMiD, 1 PI, and dexamethasone). Patients could have received a subsequent autologous stem cell transplant (ASCT) or continued consolidation therapy.
Data extraction
Two reviewers (M.S.E. and R.C.) independently extracted included study characteristics (first author, year of publication, study design, sample size, treatment regimens, and duration of follow-up) and the baseline characteristics of the participants (age, stage, and cytogenetic risk [high-risk cytogenetics were classified as per the original RCT from which the data were extracted]). Data were also extracted for efficacy and safety outcomes as described below. If multiple publications were available from the same study, we used the publication with the longest follow-up for data extraction.
Definition of outcomes
Efficacy
Overall response rate (ORR), rate of complete response or better (CR+), and rate of stringent complete response (sCR) were defined based on International Myeloma Working Group criteria as reported in the trials.10 MRD negativity was defined as MRD at 2 thresholds of both 10–⁵ and 10–⁶ cells, if available. Sustained MRD negativity was defined as MRD-negative disease for at least 1 year. PFS was defined from the time of randomization to the index regimen (quadruplet or triplet) until death or progression (whichever came first). OS was defined as the time from the date of randomization to death from any cause.
Toxicity
Serious adverse events (SAEs), grade 3 to 4 neutropenia and thrombocytopenia, and grade 3 to 4 infections were captured as reported in the original trial, graded using the National Cancer Institute Common Terminology Criteria for Adverse Events. Data regarding rates of secondary malignancies, defined as any malignancy diagnosed after index regimen initiation, were also collected. For RCTs in which ASCT was planned, data were also collected on the median stem cell yield and the proportion of patients requiring stem cell rescue mobilization, as defined in the original trial.
Subgroup analysis
We conducted a subgroup analysis comparing standard-risk multiple myeloma vs high-risk (HRMM) groups as defined in the individual studies. The instrument for assessing the credibility of effect modification analyses tool was used to assess subgroup credibility only for subgroup effect modification that was statistically significant.11
Assessment of risk of bias
Two reviewers independently assessed the risk of bias (M.S.E. and R.C.) using the Cochrane Risk of Bias tool, version 2.12 We assessed the risk of bias for each outcome and from each study, including selection bias, performance bias, detection bias, attrition bias, and reporting bias. Any disagreements were resolved by discussion and adjudication by a third reviewer (H.M.).
Statistical analysis
The statistical analysis was conducted according to the prespecified protocol. We reported relative risk (RR) for ORR, CR+, sCR, progression or death, and MRD negativity, with 95% confidence intervals (CIs). For PFS and OS, we pooled effect estimates as log hazard ratio (HR). For toxicity outcomes, we reported RR with 95% CIs. We used the Mantel-Haenszel random-effect model for all outcomes, with a DerSimonian-Laird random-effects model to pool the log of precalculated HRs across trials, using an inverse variance-weighted approach. We used RevMan (version 5.3.5; Copenhagen: The Nordic Cochrane Center, The Cochrane Collaboration, 2014) for all analyses. All P values were 2-sided, and the results were deemed statistically significant at P < .05.
We used the GRADE (Grades of Recommendation, Assessment, Development, and Evaluation) approach to assess the certainty of evidence. We prepared the evidence profile using GRADEpro software (GRADEpro GDT: GRADEpro Guideline Development Tool [Software], McMaster University, 2020), using the GRADE narrative summary document.13 We assessed study-level heterogeneity between studies visually as well as using I2 and Cochran Q statistics.
Results
Study selection
We identified 5438 citations, and after excluding duplicates, 3326 were reviewed at titles and abstract screening. We reviewed 56 studies for full-text review and included 14 publications of 7 different RCTs that included 3716 patients.4-6,14-23 These 7 RCTs included 1 phase 2 study (GRIFFIN5,15,22) and 6 phase 3 studies, including CASSEIOPEIA,4,16,19,20,23 IsKia,14 GEM2017FIT,17 PERSEUS,6 GMMG-HD7,18,21 and IMROZ.8,9 The PRISMA flow diagram is presented in Figure 1.
Study characteristics
Major characteristics of each study are summarized in Table 1. Quadruplet regimens included a combination of either daratumumab or isatuximab (anti-CD38 mAbs), either lenalidomide or thalidomide (IMiDs), either carfilzomib or bortezomib (PIs), and dexamethasone. The PI backbone was carfilzomib for 2 studies in our review: IsKia for patients undergoing ASCT and GEM2017FIT for nontransplant patients, with the rest using bortezomib. Isatuximab was the added anti-CD38 to the triplet regimen for 3 of the RCTs included in our review (IsKia, GMMG-HD7, and IMROZ), with the rest using daratumumab. In all RCTs except 2,8,17 patients were planned to proceed to high-dose chemotherapy followed by autologous hematopoietic cell transplant and maintenance. All studies defined high-risk multiple myeloma (HRMM) as having ≥1 of the following: translocation(t) 4;14, t(14;16), or deletion 17p, except for Cassiopeia,4,16 which defined it as having at least 1 of t(4;14) or deletion 17p [without including t(14;16)].
Summary of baseline characteristics of RCTs
| Study . | Griffin . | Cassiopeia∗ . | IsKia† . | GEM2017FIT‡ . | PERSEUS . | GMMG-HD7 . | IMROZ . |
|---|---|---|---|---|---|---|---|
| First author, y | Voorhees 202015 and 20235 | Moreau 201916 and 20214 | Gay 202314 | Mateos 202317 | Sonneveld 20246 | Goldschmidt 202218 | Facon 20249 |
| Phase | 2 | 3 | 3 | 3 | 3 | 3 | 3 |
| Sample size (intervention:control) | 104:103 | 543:542 | 151:151 | 153:154 | 355:354 | 331:329 | 265:181 |
| Median age (intervention:control) | 59:61 | 59:58 | 61:60 | NR | 59:60 | 61:59 | NR |
| Intervention | Dara-VRd | Dara-VTd | Isa-KRd | Dara-KRd | Dara-VRd | Isa-VRd | Isa-VRd |
| Control | VRd | VTd | KRd | KRd | VRd | VRd | VRd |
| HRMM definition | del 17p, t(4;14), t(14;16) | del 17p, t(4;14) | del 17p, t(4;14), t(14;16) | del 17p, t(4;14), t(14;16) | del 17p, t(4;14), t(14;16) | del 17p, t(4;14), t(14;16) | NR |
| HRMM participants, n (%), (intervention:control) | 16 (16):14 (14) | 82 (15):86 (16) | 27 (18):29 (19) | NR | 76 (21):78 (22) | 58 (18):66 (20) | NR |
| Median follow-up | 49.6 mo | 80.1 mo | 20 mo | NR | 47.5 mo | 125 d | 59.7 mo |
| Stem cell transplant intent | Yes | Yes | Yes | No | Yes | Yes | No |
| Maintenance regimen | Lenalidomide for all daratumumab added for the intervention arm only | Second randomization to daratumumab or observation | NA | No maintenance Control arm received 4 cycles DRd consolidation | Lenalidomide for all Daratumumab added for the intervention arm only | NA | NA |
| Study . | Griffin . | Cassiopeia∗ . | IsKia† . | GEM2017FIT‡ . | PERSEUS . | GMMG-HD7 . | IMROZ . |
|---|---|---|---|---|---|---|---|
| First author, y | Voorhees 202015 and 20235 | Moreau 201916 and 20214 | Gay 202314 | Mateos 202317 | Sonneveld 20246 | Goldschmidt 202218 | Facon 20249 |
| Phase | 2 | 3 | 3 | 3 | 3 | 3 | 3 |
| Sample size (intervention:control) | 104:103 | 543:542 | 151:151 | 153:154 | 355:354 | 331:329 | 265:181 |
| Median age (intervention:control) | 59:61 | 59:58 | 61:60 | NR | 59:60 | 61:59 | NR |
| Intervention | Dara-VRd | Dara-VTd | Isa-KRd | Dara-KRd | Dara-VRd | Isa-VRd | Isa-VRd |
| Control | VRd | VTd | KRd | KRd | VRd | VRd | VRd |
| HRMM definition | del 17p, t(4;14), t(14;16) | del 17p, t(4;14) | del 17p, t(4;14), t(14;16) | del 17p, t(4;14), t(14;16) | del 17p, t(4;14), t(14;16) | del 17p, t(4;14), t(14;16) | NR |
| HRMM participants, n (%), (intervention:control) | 16 (16):14 (14) | 82 (15):86 (16) | 27 (18):29 (19) | NR | 76 (21):78 (22) | 58 (18):66 (20) | NR |
| Median follow-up | 49.6 mo | 80.1 mo | 20 mo | NR | 47.5 mo | 125 d | 59.7 mo |
| Stem cell transplant intent | Yes | Yes | Yes | No | Yes | Yes | No |
| Maintenance regimen | Lenalidomide for all daratumumab added for the intervention arm only | Second randomization to daratumumab or observation | NA | No maintenance Control arm received 4 cycles DRd consolidation | Lenalidomide for all Daratumumab added for the intervention arm only | NA | NA |
Dara-KRd, Daratumumab with carfilzomib, lenalidomide, and dexamethasone; Dara-VRd, daratumumab with bortezomib, lenalidomide, and dexamethasone; DRd, daratumumab, lenalidomide, and dexamethasone; Isa-KRd, isatuximab with carfilzomib, lenalidomide, and dexamethasone; Isa-VRd, isatuximab with bortezomib, lenalidomide, and dexamethasone; KRd, carfilzomib, lenalidomide, and dexamethasone; NA, not applicable; NR, not reported; VRd, bortezomib, lenalidomide, and dexamethasone; VTd, bortezomib, thalidomide, and dexamethasone.
Cassiopeia 2021 included second randomization to maintenance daratumumab or observation but data included by induction regimen.
Abstract only.
‡Abstract only. Third arm with bortezomib, melphalan, and prednisone followed by lenalidomide excluded.
Risk of bias
Details of the risk of bias for each outcome from each study are available in supplemental Figure 1A for efficacy outcomes and supplemental Figure 1B for safety outcomes. The risk of bias from most studies for the majority of outcomes was low, except for the outcomes related to MRD negativity, for which we identified issues relating to missing data.
Meta-analysis for the association of quadruplet regimens with efficacy and toxicity outcomes
A summary of findings is presented in Table 2 for efficacy and Table 3 for toxicity, with both effect size and the certainty of evidence.
GRADE summary of findings for efficacy outcomes
| Quadruplet vs triplet therapy for patients with NDMM . | ||||||
|---|---|---|---|---|---|---|
| Outcomes . | Anticipated absolute effects∗ (95% CI) . | Relative effect (95% CI) . | No. of participants (studies) . | Certainty of the evidence (GRADE) . | Comments . | |
| Risk with triplets . | Risk with quadruplets . | |||||
| ORR | 897/1000 | 924/1000 (906-942) | RR, 1.03 (1.01-1.05) | 3405 (6 studies) | ⨁⨁⨁⨁ High† | Induction quadruplet therapy increases ORR slightly, compared with triplet induction therapy. |
| CR+ | 516/1000 | 625/1000 (563-692) | RR, 1.21 (1.09-1.34) | 3047 (6 studies) | ⨁⨁⨁⨁ High†,‡ | Induction quadruplet therapy increases the rate of CR+ compared with triplet induction therapy. |
| sCR | 363/1000 | 476/1000 (374-607) | RR, 1.31 (1.03-1.67) | 2294 (4 RCTs) | ⨁⨁⨁ˆ Moderate†,§ | Induction quadruplet therapy likely increases the rate of stringent CR compared with triplet induction therapy. |
| MRD 10–5 negativity | 462/1000 | 642/1000 (568-730) | RR, 1.39 (1.23-1.58) | 3716 (7 studies) | ⨁⨁⨁ˆ Moderate‡,|| | Induction quadruplet therapy likely results in an increase in MRD 10–5 negativity rates compared with triplet induction therapy. |
| MRD 10–6 negativity | 347/1000 | 562/1000 (472-674) | RR, 1.62 (1.36-1.94) | 2857 (6 studies) | ⨁⨁⨁ˆ Moderate|| | Induction quadruplet therapy likely results in a large increase in MRD 10–6 negativity rates compared with triplet induction therapy. |
| Sustained MRD negativity | 276/1000 | 561/1000 (453-691) | RR, 2.03 (1.64-2.50) | 2447 (4 studies) | ⨁⨁⨁ˆ Moderate‡,|| | Induction quadruplet therapy likely results in a large increase in sustained MRD 10–5 negativity rates compared with triplet induction therapy. |
| PFS | NA | NA | HR, 0.55 (0.46-0.66) | 2447 (4 studies) | ⨁⨁⨁⨁ High | Induction quadruplet therapy results in large increase in PFS compared with triplet induction therapy. |
| OS | NA | NA | HR, 0.65 (0.53-0.79) | 2447 (4 studies) | ⨁⨁ˆˆ Low¶ | Induction quadruplet therapy may result in an increase in OS compared with triplet induction therapy. |
| Quadruplet vs triplet therapy for patients with NDMM . | ||||||
|---|---|---|---|---|---|---|
| Outcomes . | Anticipated absolute effects∗ (95% CI) . | Relative effect (95% CI) . | No. of participants (studies) . | Certainty of the evidence (GRADE) . | Comments . | |
| Risk with triplets . | Risk with quadruplets . | |||||
| ORR | 897/1000 | 924/1000 (906-942) | RR, 1.03 (1.01-1.05) | 3405 (6 studies) | ⨁⨁⨁⨁ High† | Induction quadruplet therapy increases ORR slightly, compared with triplet induction therapy. |
| CR+ | 516/1000 | 625/1000 (563-692) | RR, 1.21 (1.09-1.34) | 3047 (6 studies) | ⨁⨁⨁⨁ High†,‡ | Induction quadruplet therapy increases the rate of CR+ compared with triplet induction therapy. |
| sCR | 363/1000 | 476/1000 (374-607) | RR, 1.31 (1.03-1.67) | 2294 (4 RCTs) | ⨁⨁⨁ˆ Moderate†,§ | Induction quadruplet therapy likely increases the rate of stringent CR compared with triplet induction therapy. |
| MRD 10–5 negativity | 462/1000 | 642/1000 (568-730) | RR, 1.39 (1.23-1.58) | 3716 (7 studies) | ⨁⨁⨁ˆ Moderate‡,|| | Induction quadruplet therapy likely results in an increase in MRD 10–5 negativity rates compared with triplet induction therapy. |
| MRD 10–6 negativity | 347/1000 | 562/1000 (472-674) | RR, 1.62 (1.36-1.94) | 2857 (6 studies) | ⨁⨁⨁ˆ Moderate|| | Induction quadruplet therapy likely results in a large increase in MRD 10–6 negativity rates compared with triplet induction therapy. |
| Sustained MRD negativity | 276/1000 | 561/1000 (453-691) | RR, 2.03 (1.64-2.50) | 2447 (4 studies) | ⨁⨁⨁ˆ Moderate‡,|| | Induction quadruplet therapy likely results in a large increase in sustained MRD 10–5 negativity rates compared with triplet induction therapy. |
| PFS | NA | NA | HR, 0.55 (0.46-0.66) | 2447 (4 studies) | ⨁⨁⨁⨁ High | Induction quadruplet therapy results in large increase in PFS compared with triplet induction therapy. |
| OS | NA | NA | HR, 0.65 (0.53-0.79) | 2447 (4 studies) | ⨁⨁ˆˆ Low¶ | Induction quadruplet therapy may result in an increase in OS compared with triplet induction therapy. |
OR, odds ratio.
Boldface values indicate statistical significance and relevance to the overall findings of the study.
The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
Some concern with missing information especially from abstracts but not enough to rate down.
Despite heterogeneity, all studies were on the side of benefit.
Visual inconsistency and statistical tests for heterogeneity were significant.
Risk of bias from missing data was significant.
Small number of events.
GRADE summary of findings for toxicity outcomes
| Quadruplet vs triplet therapy for patients with NDMM . | ||||||
|---|---|---|---|---|---|---|
| Outcomes . | Anticipated absolute effects∗ (95% CI) . | Relative effect (95% CI) . | No. of participants (studies) . | Certainty of the evidence (GRADE) . | Comments . | |
| Risk with triplets . | Risk with quadruplets . | |||||
| SAEs | 464/1000 | 482/1000 (450-520) | RR, 1.04 (0.97-1.12) | 3077 (5 studies) | ⨁⨁⨁ˆ Moderate† | Quadruplet therapy likely results in little to no difference in serious adverse effects. |
| Grade 3-4 neutropenia | 244/1000 | 441/1000 (346-563) | RR, 1.81 (1.42-2.31) | 3686 (7 studies) | ⨁⨁⨁⨁ High‡ | Quadruplet therapy increases grade 3-4 neutropenia. |
| Grade 3-4 thrombocytopenia | 124/1000 | 162/1000 (133-197) | RR, 1.30 (1.07-1.58) | 3686 (7 studies) | ⨁⨁⨁⨁ High | Quadruplet therapy increases grade 3-4 thrombocytopenia slightly. |
| Grade 3-4 infections | 188/1000 | 229/1000 (201-261) | RR, 1.22 (1.07-1.39) | 3384 (6 studies) | ⨁⨁⨁⨁ High | Quadruplet therapy increases grade 3-4 infections slightly. |
| Secondary malignancy | 53/1000 | 73/1000 (52-101) | RR, 1.37 (0.98-1.90) | 2229 (4 studies) | ⨁ˆˆˆ Very low§,|| | Quadruplet therapy likely results in little to no difference in rate of secondary malignancy. |
| Rescue stem cell mobilization | 132/1000 | 240/1000 (149-386) | RR, 1.82 (1.13-2.93) | 1810 (3 RCTs) | ⨁⨁⨁ˆ Moderate‡,§ | Quadruplet therapy likely increases the need for rescue stem cell mobilization. |
| Median stem cell yield | The mean median stem cell yield was 7.01 × 106. | MD 2.22 × 106 lower (2.98 lower to 1.47 lower) | NA | 1745 (2 RCTs) | ⨁⨁⨁ˆ Moderate‡ | Quadruplet therapy likely reduces median stem cell yield. |
| Quadruplet vs triplet therapy for patients with NDMM . | ||||||
|---|---|---|---|---|---|---|
| Outcomes . | Anticipated absolute effects∗ (95% CI) . | Relative effect (95% CI) . | No. of participants (studies) . | Certainty of the evidence (GRADE) . | Comments . | |
| Risk with triplets . | Risk with quadruplets . | |||||
| SAEs | 464/1000 | 482/1000 (450-520) | RR, 1.04 (0.97-1.12) | 3077 (5 studies) | ⨁⨁⨁ˆ Moderate† | Quadruplet therapy likely results in little to no difference in serious adverse effects. |
| Grade 3-4 neutropenia | 244/1000 | 441/1000 (346-563) | RR, 1.81 (1.42-2.31) | 3686 (7 studies) | ⨁⨁⨁⨁ High‡ | Quadruplet therapy increases grade 3-4 neutropenia. |
| Grade 3-4 thrombocytopenia | 124/1000 | 162/1000 (133-197) | RR, 1.30 (1.07-1.58) | 3686 (7 studies) | ⨁⨁⨁⨁ High | Quadruplet therapy increases grade 3-4 thrombocytopenia slightly. |
| Grade 3-4 infections | 188/1000 | 229/1000 (201-261) | RR, 1.22 (1.07-1.39) | 3384 (6 studies) | ⨁⨁⨁⨁ High | Quadruplet therapy increases grade 3-4 infections slightly. |
| Secondary malignancy | 53/1000 | 73/1000 (52-101) | RR, 1.37 (0.98-1.90) | 2229 (4 studies) | ⨁ˆˆˆ Very low§,|| | Quadruplet therapy likely results in little to no difference in rate of secondary malignancy. |
| Rescue stem cell mobilization | 132/1000 | 240/1000 (149-386) | RR, 1.82 (1.13-2.93) | 1810 (3 RCTs) | ⨁⨁⨁ˆ Moderate‡,§ | Quadruplet therapy likely increases the need for rescue stem cell mobilization. |
| Median stem cell yield | The mean median stem cell yield was 7.01 × 106. | MD 2.22 × 106 lower (2.98 lower to 1.47 lower) | NA | 1745 (2 RCTs) | ⨁⨁⨁ˆ Moderate‡ | Quadruplet therapy likely reduces median stem cell yield. |
MD, mean difference; NA, not available.
Boldface values indicate statistical significance and relevance to the overall findings of the study.
The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
Narrow CI, but absolute risk difference is clinically significant.
Heterogeneity testing statistically significant, but all RCTs favor triplets.
Small number of events.
Indirect outcome.
Efficacy outcomes
Six studies reported the ORR and CR+ rates. In our meta-analysis, based on high certainty evidence, quadruplets increased the ORR (RR, 1.03; 95% CI, 1.01-1.05) and rates of CR+ (RR, 1.21; 95% CI ,1.09-1.34; supplemental Figure 2A and B, respectively). Four trials reported on sCR rates. Based upon a moderate certainty of evidence, quadruplets likely increased the rate of sCR compared with triplets (RR, 1.31; 95% CI, 1.03-1.67; supplement Figure 2C).
Seven studies reported MRD negativity at the 10–5 and 6 studies at the 10–6 thresholds. Based on a moderate certainty of evidence, quadruplets likely increased MRD negativity at the 10–5 threshold (RR, 1.39; 95% CI, 1.23-1.58) as well as at the 10–6 threshold (RR, 1.62; 95% CI, 1.36-1.94; supplemental Figure 3A and B, respectively). Similarly, based on moderate certainty, quadruplets likely lead to an increase in sustained MRD negativity at the 10–5 threshold for ≥1 year compared with triplets (RR, 2.03; 95% CI, 1.64-2.50; supplemental Figure 3C). As noted in supplemental Figures 2 and 3, these improvements in responses and MRD negativity rates were seen regardless of the type of PI triplet back bone (carfilzomib vs bortezomib) or the type of anti-CD38 added the backbone (isatuximab vs daratumumab). Additionally, these improvements in outcomes were observed for both transplant-eligible and transplant-ineligible patients included in the studies.
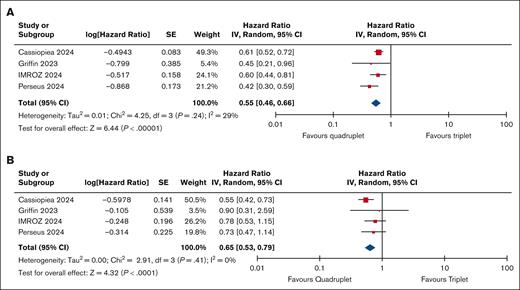
With regard to long-term outcomes, based upon a high certainty of evidence, quadruplets resulted in an improvement in PFS (HR, 0.55; 95% CI, 0.46-0.66; Figure 2A). Based on low certainty evidence, quadruplets may lead to an improvement in OS (HR, 0.65; 95% CI, 0.53-0.79; Figure 2B).
Survival outcomes. (A) PFS for quadruplet vs triplet therapy for NDMM. (B) OS for quadruplet vs triplet therapy for NDMM. SE, standard error.
Survival outcomes. (A) PFS for quadruplet vs triplet therapy for NDMM. (B) OS for quadruplet vs triplet therapy for NDMM. SE, standard error.
Safety outcomes
Five studies reported SAEs with 3077 patients and 1470 events. Based on moderate certainty evidence, quadruplet therapy likely resulted in little to no difference in the rate of SAEs (RR, 1.04; 95% CI, 0.97-1.12; supplemental Figure 4A). For grade 3 to 4 infections, 6 studies were included with 3384 patients and 708 events. Based on high certainty evidence, quadruplet therapy increased grade 3 to 4 infections, (RR, 1.22; 95% CI, 1.07-1.39), grade 3 to 4 neutropenia (RR, 1.81; 95% CI, 1.42-2.31), and grade 3 to 4 thrombocytopenia (RR, 1.30; 95% CI, 1.07-1.58; supplemental Figure 4B-D).
For secondary malignancies, 4 RCTs were included with 2229 patients and 142 secondary cancers diagnosed. Based on very low certainty evidence, the impact of quadruplet regimens on secondary malignancies is uncertain (RR, 1.37; 95% CI, 0.98-1.90; supplemental Figure 5).
With regard to stem cell mobilization, 2 studies with 1745 patients were included for median stem cell yield meta-analysis. Based on moderate certainty evidence, quadruplets likely reduced the median stem cell yield during mobilization, with a mean difference of –2.22 × 106 (95% CI, –2.98 × 106 to –1.47 ×106; supplemental Figure 6A). Three RCTs with 1810 patients had 346 events of stem cell mobilization difficulty requiring rescue mobilization. Based on moderate certainty evidence, quadruplet therapy likely increased the need for stem cell mobilization rescue (RR, 1.82; 95% CI, 1.13-2.93; supplemental Figure 6B).
Subgroup analysis
Subgroup analysis based on standard-risk and high-risk cytogenetics was available only for the outcomes of the rates of sCR and MRD status at 10–5 and are presented in supplemental Figure 7A and B, respectively. A credible subgroup effect was seen, with quadruplet therapy favored in patients with standard-risk multiple myeloma but not in HRMM for the outcome of sCR. With regard to MRD at 10–5, consistent with the overall analysis, quadruplet therapy deepened the responses in both high-risk (RR, 1.38; 95% CI, 1.16-1.64) and standard-risk cytogenetics subgroups (RR, 1.59; 95% CI, 1.38-1.82).
Discussion
This systematic review and meta-analysis of 7 RCTs with 3716 patients with NDMM provides high certainty evidence that patients with NDMM treated with a quadruplet regimen containing an anti-CD38 mAb, PI, and IMiD backbone have overall improved outcomes, including response rates, MRD negativity rates, PFS, and OS, with some added toxicity lending to infections. These findings are important and of direct clinical relevance because they position quadruplet therapies as a potential standard of care among patients with NDMM.
Triplet therapies for the treatment of NDMM have long been the standard of care, with improved outcomes noted both in clinical trials and real world.24-26 Anti-CD38–based regimens have been a cornerstone of relapsed refractory treatment for the past few years, with benefits seen for both PFS and OS in multiple studies.27-29 As anti-CD38 mAbs enter the newly diagnosed setting with quadruplet combinations, understanding their benefit for both transplant-eligible and transplant-ineligible patients is essential.
Our systematic review and meta-analysis highlight the progressively higher differences noted in the risk ratios of MRD at 10–5 (RR, 1.39), MRD 10–6 (RR, 1.62), and sustained MRD negativity (RR, 2.03) between quadruplet and triplet regimens. MRD is an important individual-level surrogate for long-term outcomes such as PFS and OS; MRD negativity may supersede the importance of achieving a CR based on conventional International Myeloma Working Group response criteria.30 Although the effect was less pronounced for MRD negativity at 10–5 for patients with high cytogenetic risk compared with standard risk, the high-risk group continued to benefit from quadruplets. This is consistent with other studies that have also shown improved outcomes for patients with HRMM with intensive induction, including quadruplets in single-cohort prospective studies,31,32 further justifying the role of quadruplet therapy in patients with high-risk cytogenetics. It was also interesting to note that quadruplet regimens continue to do better than triplets regardless of the PI (carfilzomib and bortezomib) backbone and regardless of which anti-CD38 agent (isatuximab and daratumumab) is used.
With respect to PFS and OS, our study highlights improvement in both, with outcomes with quadruplet vs triplet regimens with pooled HRs of 0.55 and 0.65, respectively. Although the certainty of evidence, particularly with the OS estimate, is limited due to the small event rate with relatively short follow-up, when coupled with the increase noted in MRD negativity at a greater depth at 10–6 including sustained MRD negativity, our meta-analysis results suggest that quadruplets may increase OS compared with triplets. Confirming OS benefit is increasingly challenging in the newly diagnosed setting, given the long follow-up period required to see the impact of therapy. Furthermore, the availability of effective salvage regimens including those with an anti-CD38 component and T-cell redirecting immunotherapies in the relapsed/refractory setting will require further longitudinal analyses to answer questions about optimal sequencing of therapy and to find the potential “cure” fraction, because only a single frontline intervention may lead to very long-term disease control.
Our meta-analysis also highlights the increased rates of toxicity noted with the quadruplet regimens including infection and cytopenias (particularly neutropenia). These potential increases in side effects may limit the usability of these regimen, particularly among those at the highest risk of toxicity, including frail older adults. Because our meta-analysis only captured 2 studies among transplant-ineligible patients, GEM2017FIT RCT and IMROZ, both of which excluded patients aged >80 years, additional studies may be needed particularly among frail older adults to understand the toxicity and tolerability of this regimen. In these frail patient population groups, which are often excluded from clinical trial,33 our meta-analysis is not able to provide robust clinical guidance because these patients were not included. Conversely, the identification of older patients who are “fit” enough for a quad but “frail” enough for a stem cell transplant remains an evolving field. Among this patient population group, selection remains challenging, given the lack of robust frailty tools that have been validated specifically for this outcome. Assessing fitness to select older patients for quadruplet regimens remains an unmet need requiring further studies. Stem cell mobilization is another potential concern that may affect physician choice in choosing regimens. In our analysis, there was a lower median stem cell yield and increased need for stem cell mobilization rescue with quadruplets vs triplets. This may warrant consideration of the upfront use of rescue agents such as plerixafor in select setting and potentially avoiding delayed collection by individual clinicians.
By combining multiple studies with similar trial designs, this meta-analysis has increased power to assess the efficacy and safety of quadruplet regimens. The selection of studies as well as the method used are robust; however, there remains some heterogeneity in the study populations, as well as in the timing and follow-up of the outcome measurements. The duration of both the induction quadruplet and subsequent maintenance treatment differed among the included trials, which could have affected the results. In the PERSEUS and GRIFFIN RCTs, dual maintenance was used; however, the duration of it was different even among the 2 studies, with PERSEUS using an MRD-directed approach for maintenance cessation. Additionally, in the included Cassiopeia RCT, in which patients with either triplet or quadruplet regimens underwent second randomization to daratumumab (anti-CD38 mAb) or observation, this could affect the results. Similar results were seen with a quadruplet including a PI, IMiD, and anti-CD38 mAb in the BENEFIT/IFM2020-05 study,34 which did not meet our inclusion criteria, with the control arm containing an anti-CD38 mAb. The addition of daratumumab for transplant-ineligible patients with NDMM has also been shown to improve OS and outcomes in a recently published Cochrane systematic review and meta-analysis.35
Strengths of our meta-analysis include the comprehensive search strategy, inclusion of the most recent data available via abstracts, inclusion of only consistent quadruplets with all 3 classes (PIs, IMiDs, and anti-CD38 mAbs) with dexamethasone, and following methodological protocols. Limitations of our meta-analysis include inclusion of RCTs with outcome assessments at different time points (ie, after induction, consolidation, or during maintenance). Although we hypothesize that efficacy benefits should be apparent early during treatment, the degree of benefit may change during the course of therapy with longer follow-up. Another limitation specifically for the MRD outcomes is the high-risk of bias from missing information, with all the studies counting missing data as MRD positivity but not reporting the number of patients with missing MRD assessments. Due to the risk of bias, the certainty of evidence was downgraded. Another limitation is that the duration of follow-up may not be sufficient to determine with confidence the effect of quadruplet regimens on survival. Lastly, our results have limited generalizability to transplant-ineligible patients with NDMM, especially those aged ≥80 years, with only 2 RCTs including transplant-ineligible patients with an upper age limit of 80 years. Results from dedicated studies in this population are eagerly awaited. Furthermore, our study cannot provide any guidance on which older adults benefit from a quadruplet induction followed by transplant vs a nontransplant quadruplet regimen alone. Lastly, clinical decision-making for using quadruplet regimens does not rely solely on efficacy and toxicity but also on the quality-of-life consideration. For the purposes of this review, quality-of-life data were not included but may affect patient-clinician shared decision-making.
Conclusion
In our systematic review and meta-analysis of quadruplets containing a PI, an IMiD, and an anti-CD38 mAb, we showed superior efficacy compared with triplets for the treatment of patients with NDMM. Quadruplets should be considered a new standard of care for first-line therapy in NDMM, particularly in the transplant-eligible setting. Further studies are needed, especially with a focus on HRMM and transplant-ineligible patients with NDMM to further refine which populations incur the greatest magnitude of benefit.
Authorship
Contribution: M.S.E. and H.M. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis; M.S.E. and R.C. drafted the manuscript; M.S.E. and B.R. performed statistical analysis; H.M. provided administrative, technical, or material support; B.R., R.C., and H.M. provided supervision; and all authors contributed to concept and design, acquisition, analysis, or interpretation of data, and critical revision of the manuscript for important intellectual content.
Conflict-of-interest disclosure: R.C. reports consultancy for Janssen, Sanofi Pasteur, and Adaptive Biotechnologies. A.V. reports consultancy/honoraria from Janssen, Sanofi, and Apotex. C.P.V. reports honoraria from Takeda, Bristol Myers Squibb (BMS), Janssen, Pfizer, Sanofi, GlaxoSmithKline (GSK), AbbVie, and FORUS Therapeutics. I.S. reports honoraria from Celgene/BMS, Gilead/Kite, GSK, Sanofi, Janssen, BeiGene, FORUS Therapeutics, and Pfizer, and stock with IllumiSonics Inc. A.M. reports honoraria from Celgene, Janssen, Amgen, Takeda, Sanofi, and GSK. T.F. reports consultancy for Janssen, bms, Roche, and Takeda, and speakers' bureau fees from Janssen and BMS. M.-V.M. reports consultancy fees/honoraria from Janssen, Celgene/BMS, GSK, Sanofi, Amgen, Menarini-Stemline, AbbVie, and Pfizer. H.M. reports consultancy fees from Janssen, Celgene/BMS, GSK, Sanofi, Amgen, AbbVie, and Pfizer; research funding from Janssen and Pfizer; and is supported by an Early Career Award from Hamilton Health Sciences. The remaining authors declare no competing financial interests.
Correspondence: Hira Mian, McMaster University, 1280 Main St West, Hamilton, ON L8S 4L8, Canada; email: hira.mian@medportal.ca.
References
Author notes
The full-text version of this article contains a data supplement.