Key Points
Nonoptimal rATG exposure increases nonrelapse mortality, relapse, and chronic GVHD and worsens DFS in pediatric AB-TCD haploidentical HCT.
Targeting rATG dosing to a predicted exposure may improve immune reconstitution and preserve graft-versus-leukemia.
Visual Abstract
We hypothesized that the inferior disease-free survival (DFS) seen in older patients who underwent αβ-T-cell/CD19–depleted (AB-TCD) haploidentical hematopoietic cell transplantation (HCT) for hematologic malignancies is caused by excessive exposure to rabbit antithymocyte globulin (rATG; Thymoglobulin). Between 2015 and 2023, 163 patients with a median age of 13 years (range, 0.4-27.4) underwent AB-TCD haploidentical HCT for the treatment of acute lymphoblastic leukemia (n = 98), acute myeloid leukemia/myelodysplastic syndrome (n = 49), or other malignancies (n = 16) at 9 centers in 2 prospective trials. Exposures to rATG before and after HCT were predicted using a validated pharmacokinetic model. Receiver operating characteristic curves were used to identify the optimal target windows for rATG exposure in terms of outcomes. We identified 4 quadrants of rATG exposure, namely quadrant 1 (n = 52) with a high pre-HCT area under curve (AUC; ≥50 arbitrary units [AU] per day per milliliter) and a low post-HCT AUC (<12 AU per day per liter); quadrant 2 (n = 47) with a low pre- and post-HCT AUC; quadrant 3 (n = 13) with a low pre-HCT and a high post-HCT AUC; and quadrant 4 (n = 51) with a high pre- and post-HCT AUC. Quadrant 1 had a 3-year DFS of 86.5%, quadrant 2 had a DFS of 64.6%, quadrant 3 had a DFS of 32.9%, and for quadrant 4 it was 48.2%. An adjusted regression analysis demonstrated additional factors that were associated with an increased hazard for worse DFS, namely minimal residual disease (MRD) positivity and cytomegalovirus (CMV) R+/D− serostatus. Nonoptimal rATG exposure exhibited the strongest effect in unadjusted and adjusted (MRD status or CMV serostatus) analyses. High exposure to rATG after HCT was associated with inferior DFS following AB-TCD haploidentical HCT for pediatric patients with hematologic malignancies. Model-based dosing of rATG to achieve optimal exposure may improve DFS. These trials were registered at www.ClinicalTrials.gov as #NCT02646839 and #NCT04337515.
Introduction
Hematopoietic cell transplantation (HCT) is a potentially curative option for certain hematologic malignancies in children and young adults. The availability of well-matched related and unrelated donors is dependent on the patient’s genetic ancestry.1 The use of haploidentical related donors eliminates this barrier for many patients, but it is limited by bidirectional alloreactivity-mediated graft rejection and graft-versus-host disease (GVHD). The recognition that acute GVHD (aGVHD) is primarily mediated by α-β T-cell receptor (AB-TCR)–positive T cells led to the development of techniques for their selective elimination from the graft, along with CD19+ cells that might serve as a reservoir for Epstein-Barr virus that can stimulate Epstein-Barr virus lymphoproliferative disease.2-5
The Pediatric Transplantation and Cellular Therapy Consortium (PTCTC) ONC1401 trial reported a 2-year disease-free survival (DFS) rate of 75% for pediatric patients with acute leukemia or myelodysplastic syndrome who underwent αβTCR-T-cell/CD19–depleted (AB-TCD) haploidentical HCT.6 However, it was noted that patients aged ≥10 years had significantly worse DFS. Conditioning regimens for AB-TCD HCT in this trial and others have included rabbit antithymocyte globulin (rATG; Thymoglobulin) at a dose of 3 mg/kg per day on days -12 to -10, although variations exist. Models of rATG pharmacokinetics (PK) and pharmacodynamics show lower rates of drug clearance in older children, and in other transplant approaches (T-replete, umbilical cord blood, and CD34 selection), optimal exposure targets have been identified, generally with higher exposure before HCT and lower exposure after HCT.7-10 We hypothesized that the lower rates of DFS seen in older patients may be caused by excessive in vivo T-cell depletion by residual rATG after HCT with abrogation of an adequate graft-versus-leukemia (GVL) effect and slowing of immune reconstitution. We then aimed to identify the optimal rATG exposure for subsequent dose targeting as has been done for T-replete unrelated donors to enhance the likelihood of successful HCT for pediatric malignant disease.11
Methods
Study design and participants
We performed an unplanned, post hoc evaluation of patients with hematologic malignancies who underwent their first HCT using a haploidentical related donor with AB-TCD in 1 of 2 prospective trials of ex vivo T-cell depletion, namely (1) ONC1401 for AB-TCD (NCT02646839; IDE#16412; M.A.P.) and (2) PBMT1902 for AB-TCD (NCT04337515; IDE#19096; C.C.D.) with a data cutoff of 14 August 2023. The primary results of patients who received natural killer inhibitory receptor-favorable HCTs in ONC1401 are published.6 Patients who did not receive rATG (Thymoglobulin) as part of conditioning were excluded. The trials were approved by local institutional review boards, and informed consent was obtained from patients/parents in accordance with the Declaration of Helsinki.
Procedures
Conditioning was based on an alkylator backbone of total-body irradiation, busulfan, or melphalan, as previously described.6 Targeted cumulative area under curve (AUC) goal exposure was used for all participants who received busulfan-based regimens. Some patients received model-based dosing of fludarabine to achieve a goal cAUC exposure of 20 mg per hour/L.12-14 ONC1401 recommended administering rATG from day -12 at 3 mg/kg for 3 doses, although different start times were allowed and the protocol did not specify whether actual or (adjusted) ideal body weights should be used.6 The predicted AUCs for rATG in arbitrary units (AU) per day per milliliter before and after HCT were determined using an established PK model (accessible via InsightRx, Inc, San Francisco, CA), which incorporates weight, absolute lymphocyte count (ALC) on admission day, and dose and timing of rATG relative to HCT.7 Both trials recommended the use of granulocyte–colony stimulating factor only after day +14, if needed. Minimal residual disease (MRD) status was determined by best available techniques for the patient’s disease at the time, including multiparameter flow cytometry and next-generation sequencing of the B- and T-cell receptors (Adaptive Biotech).15 Body mass index was analyzed according to the age-based Centers for Disease Control and Prevention guidelines in patients ≥2 years of age and was classified as underweight, normal, overweight, or obese. Posttransplant targeted agents that were administered included dasatinib for patients with t(9;22)(q34;q11) or sorafenib for FLT3-mutated disease.
Outcomes
The main outcome of interest was DFS, defined as the time from HCT to either relapse of hematologic malignancy or death from a cause other than relapse. Patients were censored at their date of last follow-up. Other outcomes included primary graft rejection, defined as failure to establish sustained donor cells with need for a second HCT; nonrelapse mortality (NRM), defined as death from a cause other than relapse with relapse as a competing event; relapse with NRM as a competing event; event-free-survival, defined as freedom from rejection, relapse, or NRM; overall survival (OS); aGVHD and chronic GVHD (cGVHD), which were graded and staged according to standard criteria;16,17 and cGVHD-free relapse-free survival (cGRFS). CD3, CD4, CD8, and CD4/CD45RA T-cell and natural killer cell counts at approximately day 100, 180, 270, and 365 were captured in patients without events or GVHD before that time point.
Statistical analysis
Demographic and disease-related variables were described using frequencies for categorical variables and medians and ranges for quantitative variables. Associations between variables were assessed using Fisher exact tests for categorical variables and Wilcoxon-Mann-Whitney (for 2 groups) or Kruskal-Wallis (for >2 groups) tests for continuous variables. The time-to-event endpoints were predicted using a Kaplan-Meier estimator and log-rank tests for significance. Optimal target cutoff points for rATG exposure associated with DFS were identified using receiver operating characteristic curves with the maximum Youden’s J statistic based on the optimal cutoff points; sensitivity analyses were used to evaluate the effect of altering the exact cutoff point locations. Unadjusted and adjusted regression analyses for the estimation of hazard ratios for DFS events were performed using Cox regression for all covariates with a univariate P value <.1. For NRM and relapse outcomes, we used Fine-Gray competing risk models. For consistency across outcomes, the analyses were adjusted for 1 variable at a time to account for the limited event numbers when analyzing NRM and relapse alone. Thereafter, the impact of predicted rATG exposure was assessed as a categorical variable. Statistical analyses were performed using SPSS Statistics, version 29.0 (IBM, Armonk, NY) and STATA 18.0 (StataCorp, College Station, TX).
Results
Patient and transplant characteristics
The data set included 163 patients who underwent their first haploidentical HCT from 2015 to 2023 at 1 of 9 centers (Table 1). The median follow-up of survivors was 2.6 years (range, 0.2-7.7). The median age at HCT was 13.0 years (range, 0.4-27.4), and the median donor age was 33 years (range, 5-61). Pediatric acute lymphoblastic leukemia was the most common indication for HCT (60.1%), followed by acute myeloid leukemia/myelodysplastic syndrome (30.1%) and other malignant disease (9.8%). The group had racial diversity (only 20% White non-Hispanic), reflecting the geographical locations of the centers and the increased incidence of non-White patients who only had mismatched donors available. The median infused cell doses for CD34 and AB-T cells were 15.2 × 106 cells per kg (range, 2.5 × 106 to 45.8 × 106 cells per kg) and 5.7 × 104 cells per kg (range, 0.1× 104 to 27.6 × 104 per kg), respectively.
Patient demographics and transplant characteristics by disease
| Patient or transplant factor . | Overall . | ALL∗ . | AML/MDS† . | Other‡ . |
|---|---|---|---|---|
| 163 | 98 | 49 | 16 | |
| Age | ||||
| <10 y (%) | 59 (36.2) | 104 (63.8) | 21 (42.9) | 6 (37.5) |
| ≥10 y (%) | 104 (63.8) | 104 (63.8) | 28 (57.1) | 10 (62.5) |
| Sex | ||||
| Male (%) | 97 (59.5) | 56 (57.1) | 30 (61.2) | 11 (68.7) |
| Female (%) | 66 (40.5) | 42 (42.9) | 19 (38.8) | 5 (31.3) |
| Race and ethnicity | ||||
| White non-Hispanic (%) | 33 (20.2) | 16 (16.4) | 13 (26.6) | 4 (25) |
| White Hispanic (%) | 73 (44.7) | 50 (51) | 17 (34.7) | 6 (37.4) |
| Black (%) | 19 (11.7) | 9 (9.2) | 6 (12.2) | 4 (25) |
| Asian (%) | 19 (11.7) | 12 (12.2) | 6 (12.2) | 1 (6.3) |
| Other or >1 (%) | 19 (11.7) | 11 (11.2) | 7 (14.3) | 1 (6.3) |
| Weight, median (range), kg | 50.6 (6.3-148.4) | 51.9 (6.3-148.4) | 39.6 (6.6-102.1) | 55.9 (8.5-116) |
| Body mass index (per CDC) | ||||
| Not done (<2 y) (%) | 15 (9.2) | 6 (6.1) | 7 (18.4) | 2 (12.5) |
| Underweight (%) | 10 (6.1) | 6 (6.1) | 2 (4.1) | 2 (12.5) |
| Healthy weight (%) | 79 (48.5) | 40 (40.8) | 31 (63.2) | 8 (50) |
| Overweight (%) | 24 (14.7) | 15 (15.3) | 6 (12.2) | 3 (18.7) |
| Obese (%) | 35 (21.5) | 31 (31.7) | 3 (6.1) | 1 (6.3) |
| Remission status | ||||
| Active disease (%) | 9 (5.5) | 1 (1) | 7 (14.3) | 1 (6.3) |
| CR1 (%) | 81 (49.7) | 41 (41.8) | 30 (61.2) | 10 (62.5) |
| CR2 (%) | 58 (35.6) | 42 (42.9) | 12 (24.5) | 4 (25) |
| CR3+ (%) | 15 (9.2) | 14 (14.3) | 0 | 1 (6.3) |
| MRD status | ||||
| Negative by NGS (%) | 54 (33.1) | 65 (39.9) | 4 (8.2) | 4 (25) |
| Negative by flow (%) | 65 (39.9) | 39 (23.9) | 32 (65.3) | 3 (18.7) |
| Positive (by either method) (%) | 39 (23.9) | 5 (3.1) | 12 (24.5) | 5 (31.3) |
| Not done (%) | 5 (3.1) | 0 | 1 (2) | 4 (25) |
| Admission ALC (×109/L), median (range) | 0.95 (0.01-5.11) | 0.88 (0.01-3.12) | 1.05 (0.01-4.51) | 1.23 (0.33-5.11) |
| Donor | ||||
| Father (%) | 51 (31.3) | 26 (26.5) | 20 (40.8) | 5 (31.3) |
| Mother (%) | 56 (34.4) | 34 (34.7) | 17 (34.7) | 5 (31.2) |
| Sibling (full or half) (%) | 52 (31.9) | 36 (36.7) | 10 (20.4) | 6 (37.5) |
| Other§ (%) | 4 (2.4) | 2 (2.1) | 2 (4.1) | 0 |
| CMV serostatus | ||||
| R−/D− (%) | 29 (17.8) | 14 (14.3) | 9 (18.4) | 6 (37.5) |
| R−/D+ (%) | 29 (17.8) | 19 (19.4) | 10 (20.4) | 0 |
| R+/D+ (%) | 79 (48.5) | 51 (52) | 19 (38.8) | 9 (56.2) |
| R+/D− (%) | 26 (15.9) | 14 (14.3) | 11 (22.4) | 1 (6.3) |
| Conditioning | ||||
| Melphalan based (%) | 65 (39.9) | 31 (31.7) | 22 (44.9) | 12 (75) |
| Busulfan based (%) | 31 (19) | 6 (6.1) | 23 (46.9) | 2 (12.5) |
| TBI based (%) | 67 (41.1) | 61 (62.2) | 4 (8.2) | 2 (12.5) |
| ATG timing | ||||
| Proximal (day -10 or closer) (%) | 26 (16) | 15 (15.3) | 10 (20.4) | 1 (6.3) |
| Distal (day -11 or further) (%) | 127 (84) | 83 (84.7) | 39 (79.6) | 15 (93.7) |
| Targeted agent after HCT|| (%) | 19 (11.7) | 9 (9.2) | 5 (10.2) | 5 (31.3) |
| Patient or transplant factor . | Overall . | ALL∗ . | AML/MDS† . | Other‡ . |
|---|---|---|---|---|
| 163 | 98 | 49 | 16 | |
| Age | ||||
| <10 y (%) | 59 (36.2) | 104 (63.8) | 21 (42.9) | 6 (37.5) |
| ≥10 y (%) | 104 (63.8) | 104 (63.8) | 28 (57.1) | 10 (62.5) |
| Sex | ||||
| Male (%) | 97 (59.5) | 56 (57.1) | 30 (61.2) | 11 (68.7) |
| Female (%) | 66 (40.5) | 42 (42.9) | 19 (38.8) | 5 (31.3) |
| Race and ethnicity | ||||
| White non-Hispanic (%) | 33 (20.2) | 16 (16.4) | 13 (26.6) | 4 (25) |
| White Hispanic (%) | 73 (44.7) | 50 (51) | 17 (34.7) | 6 (37.4) |
| Black (%) | 19 (11.7) | 9 (9.2) | 6 (12.2) | 4 (25) |
| Asian (%) | 19 (11.7) | 12 (12.2) | 6 (12.2) | 1 (6.3) |
| Other or >1 (%) | 19 (11.7) | 11 (11.2) | 7 (14.3) | 1 (6.3) |
| Weight, median (range), kg | 50.6 (6.3-148.4) | 51.9 (6.3-148.4) | 39.6 (6.6-102.1) | 55.9 (8.5-116) |
| Body mass index (per CDC) | ||||
| Not done (<2 y) (%) | 15 (9.2) | 6 (6.1) | 7 (18.4) | 2 (12.5) |
| Underweight (%) | 10 (6.1) | 6 (6.1) | 2 (4.1) | 2 (12.5) |
| Healthy weight (%) | 79 (48.5) | 40 (40.8) | 31 (63.2) | 8 (50) |
| Overweight (%) | 24 (14.7) | 15 (15.3) | 6 (12.2) | 3 (18.7) |
| Obese (%) | 35 (21.5) | 31 (31.7) | 3 (6.1) | 1 (6.3) |
| Remission status | ||||
| Active disease (%) | 9 (5.5) | 1 (1) | 7 (14.3) | 1 (6.3) |
| CR1 (%) | 81 (49.7) | 41 (41.8) | 30 (61.2) | 10 (62.5) |
| CR2 (%) | 58 (35.6) | 42 (42.9) | 12 (24.5) | 4 (25) |
| CR3+ (%) | 15 (9.2) | 14 (14.3) | 0 | 1 (6.3) |
| MRD status | ||||
| Negative by NGS (%) | 54 (33.1) | 65 (39.9) | 4 (8.2) | 4 (25) |
| Negative by flow (%) | 65 (39.9) | 39 (23.9) | 32 (65.3) | 3 (18.7) |
| Positive (by either method) (%) | 39 (23.9) | 5 (3.1) | 12 (24.5) | 5 (31.3) |
| Not done (%) | 5 (3.1) | 0 | 1 (2) | 4 (25) |
| Admission ALC (×109/L), median (range) | 0.95 (0.01-5.11) | 0.88 (0.01-3.12) | 1.05 (0.01-4.51) | 1.23 (0.33-5.11) |
| Donor | ||||
| Father (%) | 51 (31.3) | 26 (26.5) | 20 (40.8) | 5 (31.3) |
| Mother (%) | 56 (34.4) | 34 (34.7) | 17 (34.7) | 5 (31.2) |
| Sibling (full or half) (%) | 52 (31.9) | 36 (36.7) | 10 (20.4) | 6 (37.5) |
| Other§ (%) | 4 (2.4) | 2 (2.1) | 2 (4.1) | 0 |
| CMV serostatus | ||||
| R−/D− (%) | 29 (17.8) | 14 (14.3) | 9 (18.4) | 6 (37.5) |
| R−/D+ (%) | 29 (17.8) | 19 (19.4) | 10 (20.4) | 0 |
| R+/D+ (%) | 79 (48.5) | 51 (52) | 19 (38.8) | 9 (56.2) |
| R+/D− (%) | 26 (15.9) | 14 (14.3) | 11 (22.4) | 1 (6.3) |
| Conditioning | ||||
| Melphalan based (%) | 65 (39.9) | 31 (31.7) | 22 (44.9) | 12 (75) |
| Busulfan based (%) | 31 (19) | 6 (6.1) | 23 (46.9) | 2 (12.5) |
| TBI based (%) | 67 (41.1) | 61 (62.2) | 4 (8.2) | 2 (12.5) |
| ATG timing | ||||
| Proximal (day -10 or closer) (%) | 26 (16) | 15 (15.3) | 10 (20.4) | 1 (6.3) |
| Distal (day -11 or further) (%) | 127 (84) | 83 (84.7) | 39 (79.6) | 15 (93.7) |
| Targeted agent after HCT|| (%) | 19 (11.7) | 9 (9.2) | 5 (10.2) | 5 (31.3) |
ALL, acute lymphoblastic leukemia; AML, acute myeloblastic leukemia; CDC, Centers for Disease Control and Prevention; CR, complete remission; MDS, myelodysplastic syndrome; NGS, next-generation sequencing; R+/D−, recipient seropositive and donor seronegative, TBI, total-body irradiation.
Pre-B (n = 80), Ph+ Pre-B (n = 9), and T (n = 9).
AML (n = 32), FLT3-interal tandem duplication AML (n = 5), MDS (n = 7), and secondary MDS/AML (n = 5).
Mixed phenotype acute leukemia (MPAL; n = 5), Ph+ MPAL (n = 3), non-Hodgkin Lymphoma (n = 5), juvenile myelomonocytic leukemia (n = 2), and chronic myelogenous leukemia accelerated phase (n = 1).
Other donors included: uncle (n = 2), cousin (n = 1), and daughter (n = 1).
Targeted agents included dasatinib (n = 13) and sorafenib (n = 6).
ATG before and after HCT exposures
The median ALC at admission before initiation of rATG was 0.95 × 109/L (range, 0.0 × 109 to 5.11 × 109/L). The median day of rATG initiation was day -12 (range, −14 to −5), and the median total rATG dose was 9 mg/kg (range, 2.7-15) based on actual body weight. The median predicted pre-HCT and post-HCT rATG exposures were 59.1 AU per day per milliliter (range, 10.1-109) and 9.7 AU per day per milliliter (range, 1.1-42.5), respectively. Patients <10 years of age (n = 59) had a lower median pre-HCT rATG exposure of 41.9 AU per day per milliliter (range, 10.1-84.1) when compared with 71.9 AU per day per milliliter (range, 23.9-109) among those ≥10 years of age (n = 104; P < .001). The median post-HCT rATG was also lower among those <10 years of age, namely 6.2 AU per day per milliliter (range, 1.9-34.2) as opposed to 14.6 AU per day per milliliter (range, 1.1-42.5) among those ≥10 years of age (P < .001). Patients who received proximal rATG dosing (starting day -10 or closer to HCT; n = 26) had a lower median pre-HCT AUC of 40.4 AU per day per milliliter (range, 10.1-83.9) as opposed to 62.8 AU per day per milliliter (range, 16.4-109) among those who received distal dosing from day -11 or further from HCT (n = 127; P < .001). Conversely, patients who received proximal rATG dosing had a higher median post-HCT AUC of 16.9 AU per day per milliliter (range, 3.1-42.5) as opposed to 8.7 AU per day per milliliter (range, 1.1-34.9) among those who received distal dosing (P = .003).
Engraftment and GVHD
The 100-day cumulative incidence of rejection was 9.3% (95% confidence interval [CI], 4.8-13.8); 14 of 15 patients with rejection had a successful engraftment with a subsequent HCT, and 2 of those subsequently died (from relapse and GVHD, respectively), such that 80% were long-term survivors. Lower rates of rejection were seen among patients who received total-body irradiation–based conditioning (1.5%; 95% CI, 0.1-4.4; n = 67) as opposed to busulfan (16.3%; 95% CI, 3.2-29.4; n = 31) or melphalan (13.9%; 95% CI, 5.5-22.3; n = 65; P = .02; supplemental Table 1). Rejection was more common among patients with a pre-rATG ALC of ≥1.2 × 109/L (n = 58), namely 19% (95% CI, 8.8-29.2) vs 3.9% (95% CI, 0.2-7.6) among those with low ALCs (n = 105; P = .002).
The 100-day cumulative incidence of grades 2 to 4 and 3 to 4 aGVHD were 25% (95% CI, 17.6-32.4) and 8.5% (95% CI, 4-13), respectively. The 3-year cumulative incidence of cGVHD was 19.2% (95% CI, 11.4-27).
NRM and relapse
The 3-year cumulative incidence of NRM and relapse was 14.4% (95% CI, 7.9-20.9) and 23.8% (95% CI, 16.2-31.4), respectively. The median time to relapse was 0.49 (range, 0.09-2.55) years; 90.3% of relapses occurred before 18 months. There was no difference in the 3-year relapse incidence whether a patient was MRD negative when measured by flow cytometry (15.9%; 95% CI, 5.5-26.3; n = 65) or by next-generation sequencing (18.3%; 95% CI, 5.2-31.4; n = 54); therefore, these were combined into a single MRD-negative category for subsequent analyses. The 3-year event-free survival, DFS, and OS were 57.9% (95% CI, 49.5-66.3), 65.1% (95% CI, 56.9-73.3), and 74.9% (95% CI, 67.3-82.5), respectively. Of the patients who relapsed, 13 of 31 (41.9%) were alive at the data cutoff. The causes of death, separated by conditioning regimen, are listed in supplemental Table 2.
In the univariate analysis, no patient-related factors significantly impacted the 3-year NRM (supplemental Table 3). However, transplant factors impacted the 3-year NRM and included having an R+/D− cytomegalovirus (CMV) serostatus (38.9%; 95% CI, 13.6-64.2; n = 26) as opposed to a CMV serostatus of R−/D− (4.2%; 95% CI, 0.1-12.2; n = 29), R−/D+ (14.9%; 95% CI, 0.1-30.6; n = 29), or R+/D+ (11.8%; 95% CI, 3-20.6; n = 79; P = .003; supplemental Table 4). Furthermore, having a haploidentical sibling donor was associated with higher NRM (27%; 95% CI, 13.5-40.5; n = 52) as opposed to a paternal donor (2%; 95% CI, 0.1-5.9; n = 56) or a maternal donor and (13.6%; 95% CI, 1.8-25.4; n = 51; P = .01). Imbalances in other characteristics within the donor groups may have influenced this finding (supplemental Table 5); patients with sibling haploidentical donors were more likely to be ≥10 years of age (80.8%) than those with father or mother donors (51% or 57.1%; P = .004), and the donors were younger (median age of 18 years vs 40 or 36 years for fathers and mothers; P < .001).
In the univariate analysis, factors that impacted the 3-year relapse incidence included (1) being MRD positive before HCT (46.8%; 95% CI, 19.4-64.2; n = 39 vs 16.9%; 95% CI, 8.9-24.9; n = 119 for MRD-negative patients P < .001) and (2) the use of posttransplant targeted agents (0%; 95% CI, 0-19.8; n = 19 vs 27.2%; 95% CI, 18.8-35.6; n = 127 without targeted agents P = .03). Patients who received posttransplant targeted agents had a median follow-up of 3.2 years (range, 0.8-6.8) as opposed to 2.4 years (range, 0.2-7.7 years) for those without targeted agents. In the univariate analysis, factors that impacted the 3-year DFS included (1) being MRD positive (50%; 95% CI, 32.9-67.1 vs 70% for MRD negative 95% CI, 60.8-79.2; P = .01) and (2) CMV serostatus (80.9%; 95% CI, 65.8-96 for R−/D− vs 63.7%; 95% CI, 42.5-84.9 for R−/D+ vs 70%; 95% CI, 59-81 for R+/D+ vs 30.9%; 95% CI, 9.5-52.3 for R+/D−; P < .001).
ATG exposure and outcomes
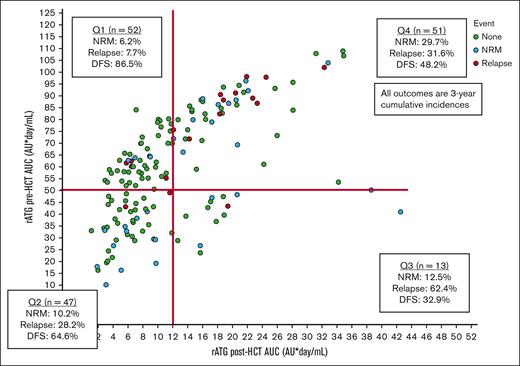
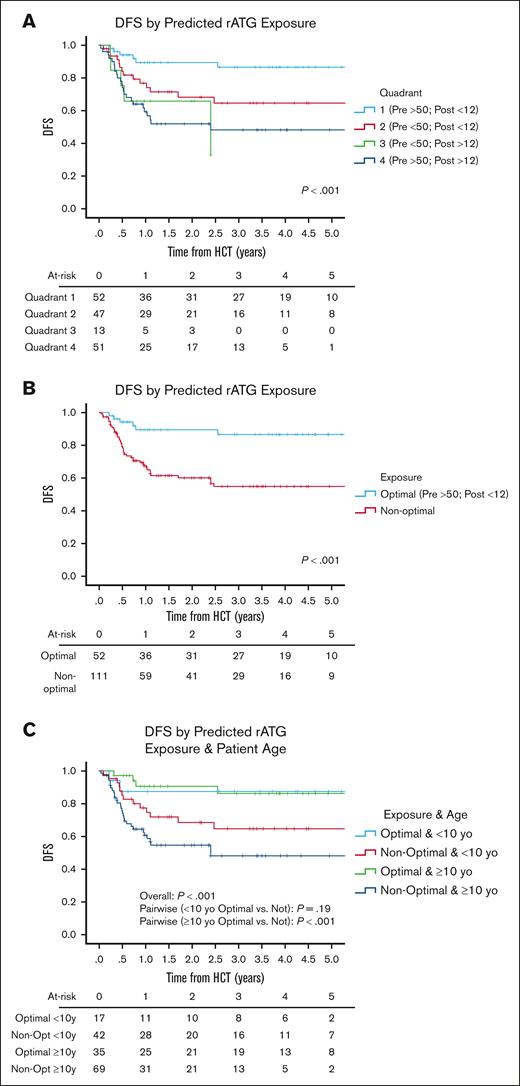
We identified 4 quadrants (Qs) of predicted pre- and post-HCT ATG exposure associated with outcomes (Figure 1). Q1 (n = 52) had a high pre-HCT AUC (≥50 AU per day per milliliter) and a low AUC after HCT (<12 AU per day per liter); Q2 (n = 47) had a low pre-HCT AUC (<50 AU per day per milliliter) and a low post-HCT AUC (<12 AU per day per liter); Q3 (n = 13) had a low pre-HCT AUC (<50 AU per day per milliliter) and a high post-HCT AUC (≥12 AU per day per liter); and Q4 (n = 51) had a high pre-HCT AUC (≥50 AU per day per milliliter) and a high AUC after HCT (≥12 AU per day per liter). Q4 had the highest 3-year NRM rates of 29.7% (95% CI, 13.4-46) when compared with Q1 (6.2%; 95% CI, 0.1-13.1), Q2 (10.2%; 95% CI, 0.1-22.2), or Q3 (12.5%; 95% CI, 0.1-35.4) (P = .02). Q1 had the lowest 3-year relapse incidence of 7.7% (95% CI, 0.1-16.1) when compared with Q2 (28.2%; 95% CI, 13.9-42.5), Q3 (62.4%; 95% CI, 8.9-99.9), or Q4 (31.6%; 95% CI, 16.9-46.3) (P = .01). The lower NRM and relapse translated into Q1 having the best 3-year DFS of 86.5% (95% CI, 76.3-96.7) when compared with Q2 (64.6%; 95% CI, 49.1-80.1), Q3 (32.9%; 95% CI, 0.1-80.5), or Q4 (48.2%; 95% CI, 22.1-63.3) (P < .001; Figure 2A). When combining Q2 to Q4 into a nonoptimal exposure group, a significantly worse 3-year DFS of 54.8% (95% CI, 44.2-65.4; n = 111) was obtained when compared with the optimal exposure of Q1 for which the 3-year DFS was 86.5% (95% CI, 76.3-96.7; n = 52; P < .001; Figure 2B). This was mainly driven by the lower relapse rates in Q1 (P = .002), although there was also a trend toward lower NRM in Q1 (P = .08; supplemental Table 3). The optimal exposure group had superior 3-year OS of 91.4% (95% CI, 83.4-99.4) as opposed to 66.6% (95% CI, 56.2-77) in the nonoptimal exposure group (P = .007).
Identification of predicted rATG pre- and post-HCT exposure quadrants.P values were determined using the 2-sided log-rank test.
Identification of predicted rATG pre- and post-HCT exposure quadrants.P values were determined using the 2-sided log-rank test.
DFS. DFS as predicted by rATG (A) exposure quadrants, (B) optimal exposure, and (C) optimal exposure and age. P values were determined using the 2-sided log-rank test.
DFS. DFS as predicted by rATG (A) exposure quadrants, (B) optimal exposure, and (C) optimal exposure and age. P values were determined using the 2-sided log-rank test.
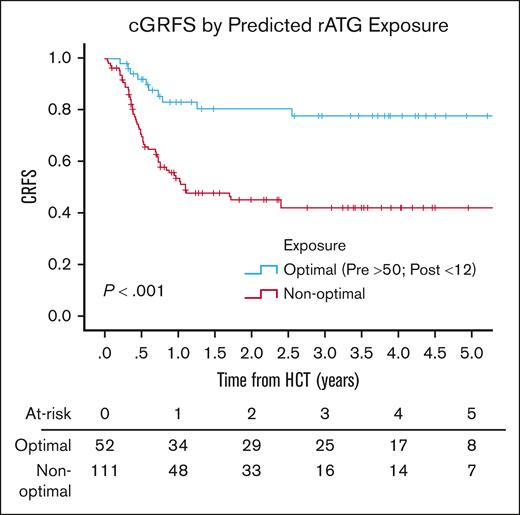
There was no difference in the day 100 incidence of grade 2 to 4 (P = .6) or grade 3 to 4 (P = .52) aGVHD by predicted ATG exposure (supplemental Table 6). The 3-year incidence of cGVHD was higher in patients with a low pre-HCT AUC; when comparing an AUC before HCT of ≥50 with an AUC of <50 AU per day per milliliter, the 3-year incidence of cGVHD was 12.6% (95% CI, 4.8-20.4) vs 34.1% (95% CI, 16.3-51.9; P = .01). Q1 had superior 3-year cGRFS of 77.8% (95% CI, 65.5-90.1) when compared with grouped Q2 to 4 (42.3%; 95% CI, 32.1-52.5; P < .001; Figure 3). CD3 and CD4 reconstitution were worse at day 100 for patients in Q3 and Q4, driven by exposure after HCT of ≥12 AU per day per liter (supplemental Tables 7 and 8).
Chronic GVHD-free relapse-free survival by predicted rATG optimal exposure.P value was determined using the 2-sided log-rank test.
Chronic GVHD-free relapse-free survival by predicted rATG optimal exposure.P value was determined using the 2-sided log-rank test.
Of note, in the sensitivity analyses, certain other cutoff points for determining optimal exposure performed similar in terms of 3-year DFS as the chosen exposure before HCT cutoff of ≥50 AU per day per milliliter and the after HCT cutoff of <12 AU per day per milliliter, especially at a before HCT cutoff of ≥45 AU per day per milliliter and an after HCT cutoff of <10 AU per day per milliliter (supplemental Table 11). However, the chosen cutoff points represent an ideal balance between optimizing 3-year DFS, while also being an exposure into which a large number of patients naturally fell with standard mg/kg dosing and is thus a potentially targetable exposure.
Factors that impacted ATG exposure and outcomes
The association of various patient and transplant characteristics with rATG exposures and outcomes are reported in supplemental Tables 9 and 10. The PK of rATG demonstrated higher clearance in younger children; the model predicted that patients <10 years of age had rATG exposures most often found in Q2 (both the pre- and post-HCT AUCs were low), whereas those ≥10 years of age were most often found in Q4 (both pre- and post-HCT AUCs were high) (supplemental Figure 1A). Patients with overweight or obesity were overrepresented in exposure Q4 (supplemental Figure 1B), although 29% of patients with overweight and 37% of patients with obesity received an rATG dose of ≤2.5 mg/kg per dose, which adjusted some of them into Q1 exposure. It should also be noted that all patients <2 years of age were in exposure Q2. The timing of administration of rATG impacted the predicted exposure. Patients who started rATG more proximal to HCT (day -10 or closer) were never in Q1 (high pre-HCT AUC, low post-HCT AUC) and most often in Q3 (low pre-HCT AUC, high post-HCT AUC; supplemental Figure 1C). As expected, patients with high admission ALCs (≥1200 × 109/L) were primarily found in Q1 and Q2, whereas patients with low ALCs were most often found in Q4 (supplemental Figure 1D).
Bivariate and subgroup analyses
In the bivariate analyses, after controlling for ATG exposure quadrant (optimal [Q1] vs nonoptimal [Q2-4]), being CMV-seropositive with a seronegative donor (P = .002) and having a haploidentical sibling (P = .007) were associated with an increased hazard ratio (HR) for NRM (Table 2). When controlling for age, donor type, and CMV serostatus, ATG exposure quadrant was not associated with an increased HR for NRM (Table 3). However, for patients with haploidentical sibling grafts, those with optimal rATG exposure (Q1) had a 3-year NRM of 0% (95% CI, 0-23.9; n = 15) as opposed to 40% (95% CI, 20.8-59.2; n = 37) for those with suboptimal rATG exposure (Q2-4) (P = .01; supplemental Figure 2). In the bivariate analysis that controlled for ATG exposure quadrant (optimal vs nonoptimal), being MRD positive (P < .001) and having CMV R+/D− serostatus (P = .02) were associated with an increased HR for relapse (Table 2). When controlling for MRD status and CMV serostatus, ATG exposure quadrant was significantly associated with an increased HR for relapse (P = .003 and P = .007, respectively; Table 3).
Bivariate analysis of factors associated with NRM, relapse, and 1-DFS controlling for ATG exposure quadrant (optimal vs nonoptimal)
| Variable and categories . | n . | NRM . | Relapse . | 1-DFS . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n events . | SHR estimate∗ (95% CI) . | P value . | n events . | SHR estimate∗ (95% CI) . | P value . | n events . | HR estimate† (95% CI) . | P value . | ||
| Age | ||||||||||
| <10 y | 59 | 3 | 1.0 | .055 | 12 | — | — | 15 | — | — |
| ≥10 y | 104 | 15 | 3.38 (0.98-11.71) | 19 | 34 | |||||
| MRD status‡ | ||||||||||
| Negative | 119 | 15 | — | — | 15 | 1.0 | <.001 | 30 | 1.0 | .003 |
| Positive | 39 | 2 | 16 | 4.45 (2.19-9.05) | 18 | 2.45 (1.36-4.41) | ||||
| Donor§ | ||||||||||
| Parent | 107 | 6 | 1.0 | .007 | 25 | — | — | 31 | — | — |
| Full or half sibling | 52 | 12 | 3.89 (1.46-10.38) | 5 | 17 | |||||
| CMV serostatus | ||||||||||
| All others | 137 | 11 | 1.0 | .002 | 23 | 1.0 | .02 | 34 | 1.0 | <.001 |
| R+/D− | 26 | 7 | 4.68 (1.79-12.27) | 8 | 2.62 (1.16-5.91) | 15 | 3.33 (1.8-6.16) | |||
| CD34 dose | ||||||||||
| <15 × 106/kg | 75 | 9 | — | — | 19 | — | — | 28 | 1.0 | .08 |
| ≥15 × 106/kg | 88 | 9 | 12 | 18 | 0.6 (0.34-1.06) | |||||
| Variable and categories . | n . | NRM . | Relapse . | 1-DFS . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n events . | SHR estimate∗ (95% CI) . | P value . | n events . | SHR estimate∗ (95% CI) . | P value . | n events . | HR estimate† (95% CI) . | P value . | ||
| Age | ||||||||||
| <10 y | 59 | 3 | 1.0 | .055 | 12 | — | — | 15 | — | — |
| ≥10 y | 104 | 15 | 3.38 (0.98-11.71) | 19 | 34 | |||||
| MRD status‡ | ||||||||||
| Negative | 119 | 15 | — | — | 15 | 1.0 | <.001 | 30 | 1.0 | .003 |
| Positive | 39 | 2 | 16 | 4.45 (2.19-9.05) | 18 | 2.45 (1.36-4.41) | ||||
| Donor§ | ||||||||||
| Parent | 107 | 6 | 1.0 | .007 | 25 | — | — | 31 | — | — |
| Full or half sibling | 52 | 12 | 3.89 (1.46-10.38) | 5 | 17 | |||||
| CMV serostatus | ||||||||||
| All others | 137 | 11 | 1.0 | .002 | 23 | 1.0 | .02 | 34 | 1.0 | <.001 |
| R+/D− | 26 | 7 | 4.68 (1.79-12.27) | 8 | 2.62 (1.16-5.91) | 15 | 3.33 (1.8-6.16) | |||
| CD34 dose | ||||||||||
| <15 × 106/kg | 75 | 9 | — | — | 19 | — | — | 28 | 1.0 | .08 |
| ≥15 × 106/kg | 88 | 9 | 12 | 18 | 0.6 (0.34-1.06) | |||||
Boldface values are considered to be statistically significant.
R+/D−, recipient seropositive and donor seronegative.
Determined using Fine-Gray competing risk regression.
Determined using Cox regression proportional hazard models.
Omitted unknown MRD status (n = 4).
Omitted other haploidentical donors (n = 4).
Bivariate analysis of ATG exposure association with NRM, relapse, and DFS with controlling for other factors
| Variable and categories . | n . | NRM . | SHR estimate∗ (95% CI) . | P value . | Relapse . | SHR estimate∗ (95% CI) . | P value . | 1-DFS . | HR estimate† (95% CI) . | P value . |
|---|---|---|---|---|---|---|---|---|---|---|
| n events . | n events . | n events . | ||||||||
| Univariate | ||||||||||
| ATG exposure quadrant | ||||||||||
| Q1 (before >50; after <12) | 52 | 3 | 1.0 | .1 | 3 | 1.0 | <.001 | 6 | 1.0 | <.001 |
| Nonoptimal (Q2-4) | 111 | 15 | 2.82 (0.82-9.78) | 28 | 5.32 (1.62-17.53) | 43 | 4.06 (1.72-9.54) | |||
| Bivariate controlling for age (<10 y vs ≥10 y) | ||||||||||
| ATG exposure quadrant | ||||||||||
| Q1 (before >50; after <12) | 52 | 3 | 1.0 | .07 | — | — | — | — | — | — |
| Nonoptimal (Q2-4) | 111 | 15 | 3.1 (0.91-10.95) | |||||||
| Bivariate controlling for MRD status (negative vs positive) | ||||||||||
| ATG exposure quadrant | ||||||||||
| Q1 (before >50; after <12) | 52 | — | — | — | 3 | 1.0 | .003 | 6 | 1.0 | .001 |
| Nonoptimal (Q2-Q4) | 111 | 28 | 6.33 (1.91-20.97) | 43 | 4.24 (1.79-10.03) | |||||
| Bivariate controlling for donor type (parent vs sibling) | ||||||||||
| ATG exposure quadrant | ||||||||||
| Q1 (before >50; after <12) | 52 | 3 | 1.0 | .11 | — | — | — | — | — | — |
| Nonoptimal (Q2-Q4) | 111 | 15 | 2.76 (0.8-9.6) | |||||||
| Bivariate controlling for CMV serostatus (all others vs R+/D−) | ||||||||||
| ATG exposure Q3 | ||||||||||
| Q1 (before >50; after <12) | 52 | 3 | 1.0 | .14 | 3 | 1.0 | .007 | 6 | 1.0 | .002 |
| Nonoptimal (Q2-Q4) | 111 | 15 | 2.59 (0.74-9.05) | 28 | 5.11 (1.55-16.86) | 43 | 3.84 (1.63-9.05) | |||
| Bivariate controlling for CD34 dose (<15 × 106/kg vs ≥15 × 106/kg) | ||||||||||
| ATG exposure quadrant | ||||||||||
| Q1 (before >50; after <12) | 52 | — | — | — | — | — | — | 6 | 1.0 | .002 |
| Nonoptimal (Q2-Q4) | 111 | 43 | 3.99 (1.7-9.4) |
| Variable and categories . | n . | NRM . | SHR estimate∗ (95% CI) . | P value . | Relapse . | SHR estimate∗ (95% CI) . | P value . | 1-DFS . | HR estimate† (95% CI) . | P value . |
|---|---|---|---|---|---|---|---|---|---|---|
| n events . | n events . | n events . | ||||||||
| Univariate | ||||||||||
| ATG exposure quadrant | ||||||||||
| Q1 (before >50; after <12) | 52 | 3 | 1.0 | .1 | 3 | 1.0 | <.001 | 6 | 1.0 | <.001 |
| Nonoptimal (Q2-4) | 111 | 15 | 2.82 (0.82-9.78) | 28 | 5.32 (1.62-17.53) | 43 | 4.06 (1.72-9.54) | |||
| Bivariate controlling for age (<10 y vs ≥10 y) | ||||||||||
| ATG exposure quadrant | ||||||||||
| Q1 (before >50; after <12) | 52 | 3 | 1.0 | .07 | — | — | — | — | — | — |
| Nonoptimal (Q2-4) | 111 | 15 | 3.1 (0.91-10.95) | |||||||
| Bivariate controlling for MRD status (negative vs positive) | ||||||||||
| ATG exposure quadrant | ||||||||||
| Q1 (before >50; after <12) | 52 | — | — | — | 3 | 1.0 | .003 | 6 | 1.0 | .001 |
| Nonoptimal (Q2-Q4) | 111 | 28 | 6.33 (1.91-20.97) | 43 | 4.24 (1.79-10.03) | |||||
| Bivariate controlling for donor type (parent vs sibling) | ||||||||||
| ATG exposure quadrant | ||||||||||
| Q1 (before >50; after <12) | 52 | 3 | 1.0 | .11 | — | — | — | — | — | — |
| Nonoptimal (Q2-Q4) | 111 | 15 | 2.76 (0.8-9.6) | |||||||
| Bivariate controlling for CMV serostatus (all others vs R+/D−) | ||||||||||
| ATG exposure Q3 | ||||||||||
| Q1 (before >50; after <12) | 52 | 3 | 1.0 | .14 | 3 | 1.0 | .007 | 6 | 1.0 | .002 |
| Nonoptimal (Q2-Q4) | 111 | 15 | 2.59 (0.74-9.05) | 28 | 5.11 (1.55-16.86) | 43 | 3.84 (1.63-9.05) | |||
| Bivariate controlling for CD34 dose (<15 × 106/kg vs ≥15 × 106/kg) | ||||||||||
| ATG exposure quadrant | ||||||||||
| Q1 (before >50; after <12) | 52 | — | — | — | — | — | — | 6 | 1.0 | .002 |
| Nonoptimal (Q2-Q4) | 111 | 43 | 3.99 (1.7-9.4) |
Boldface values are considered to be statistically significant.
R+/D−, recipient seropositive and donor seronegative.
Determined using Fine-Gray competing risk regression.
Determined using Cox regression proportional hazard models.
In the bivariate analysis that controlled for ATG exposure quadrant (optimal vs nonoptimal), being MRD positive (P = .003) and having CMV R+/D− serostatus (P < .001) were associated with an increased HR for 1-DFS (Table 2). When controlling for MRD status, CMV serostatus, and CD34 dose, ATG exposure quadrant was significantly associated with an increased HR for relapse (P = .001; P = .002; and P = .002, respectively; Table 3). Patients who were ≥10 years of age with optimal rATG exposure (Q1) had a 3-year DFS of 86.2% (95% CI, 73.5-98.9; n = 35) as opposed to 48.2% (95% CI, 34.3-62.1; n = 69) for those with suboptimal rATG exposure (Q2-4) (P < .001). The difference among those <10 years of age was less profound. The 3-year DFS was 87.4% (95% CI, 70.9-99.9; n = 17) for those in Q1 vs 64.6% (95% CI, 48.7-80.5; n = 42) for those in Q2 to 3 (P = .19; Figure 2C).
For patients who were MRD negative, those with optimal rATG exposure had a 3-year relapse incidence of 0% (95% CI, 0-11.8; n = 35) as opposed to 24.1% (95% CI, 12.9-35.3; n = 84) among those with suboptimal exposure (P = .008) (Figure 4A) and a 3-year DFS of 90.6% (95% CI, 80.4-99.9) as opposed to 61.5% (95% CI, 49.5-73.5) among those with suboptimal exposure (P = .01; Figure 4B). For patients who were MRD positive, those with optimal rATG exposure had a 3-year relapse incidence of 25.5% (95% CI, 0.1-51.2; n = 14) as opposed to 59.2% (95% CI, 38.2-80.2; n = 25) among those with suboptimal exposure (P = .02; Figure 4A) and a 3-year DFS of 74.5% (95% CI, 48.8-99.9) as opposed to 36.7% (95% CI, 17.1-56.3) among those with suboptimal exposure (P = .01; Figure 4B).
Predicted rATG optimal exposure and MRD status effects on cumulative incidence of relapse (A) and DFS (B).P values were determined using the 2-sided log-rank test.
Predicted rATG optimal exposure and MRD status effects on cumulative incidence of relapse (A) and DFS (B).P values were determined using the 2-sided log-rank test.
Discussion
We identified that low pre-HCT exposures and high post-HCT exposures of rATG were associated with worse NRM, relapse, and survival among recipients who underwent AB-TCD haploidentical HCT. The other patient-specific factors found to be independent predictors of poor outcomes were positive MRD status before HCT and the use of a CMV seronegative donor for a seropositive patient. A pre-HCT exposure of >50 AU per day per milliliter in conjunction with a post-HCT exposure of <12 AU per day per milliliter was determined to represent the optimal exposure to support DFS, primarily by minimizing relapse, although some other rATG exposure cutoff points also performed well, suggesting that there may be a range of optimal doses to target in an effort to mitigate poor outcomes for this transplant approach. A pre-HCT exposure of >50 AU per day per milliliter was also associated with lower rates of cGVHD, potentially via improved elimination of host antigen-presenting cells, a finding that has been reported previously.11,18 Optimal exposure of rATG was therefore also associated with higher cGRFS. Previous studies on rATG exposure suggested that higher pre-HCT exposures led to improved clearance of host T cells, potentially facilitating improved post-HCT expansion of infused donor T cells and GVL.19 Conversely, higher post-HCT exposures may lead to excess in vivo depletion of infused donor lymphocytes cells, thereby slowing immune reconstitution and abrogating GVL.
Previous PK- pharmacodynamics analyses demonstrated that weight-based dosing of rATG led to highly variable exposures before and after HCT, especially in patients >10 years of age.18 Exposure to rATG was associated with survival in several studies of adult and pediatric patients who underwent T-replete HCT7,18,20 (optimal exposure was identified as a pre-HCT AUC of >40 AU per day per milliliter and a post-HCT AUC of <50 AU per day per milliliter), children who underwent umbilical cord blood HCT (optimal post-HCT exposure was originally identified as <16 AU per day per milliliter and more recently as <10 AU per day per milliliter),8,10 and in adults and children who underwent CD34-selected matched donor HCT without pharmacologic GVHD prophylaxis (optimal post-HCT exposure was identified as <30 AU per day per milliliter, but immune reconstitution was even better with lower exposures).9 Because in AB-TCD haploidentical HCT, a low dose of donor T cells (primarily TCR-G/D CD3+ cells with a median of 7.7 × 106 cells per kg)6 is administered in a range comparable with that of umbicial cord blood transplant (median dose of 3.3 × 106 to 4.8 × 106 cells per kg),21,22 our identified optimal exposure is similar to these findings. A prospective, single-arm phase 2 trial of 58 pediatric patients who underwent T-replete HCT used model-based dosing of rATG to achieve the goal exposure and to validate the original reports. This demonstrated improved CD4 reconstitution when compared with historic controls and improved OS in the group that received bone marrow grafts.11
Identification of an optimal rATG exposure for patients who are undergoing AB-TCD HCT for hematologic malignant disease has similar potential to improve outcomes. A report of 149 pediatric patients who underwent AB-TCD HCT (mainly with haploidentical donors) with complete omission of rATG and replacement with abatacept and tocilizumab demonstrated similar rates of rejection and GVHD with lower risks for NRM in the rATG-free group, although there were some potentially important differences between the study cohort and the historic control.23 In general, our study suggests that the dosing strategy employed in the PTCTC ONC1401 study of 3 mg/kg per day on days -12 to -10 for all patients should be modified to account for slower clearance in adolescents and young adults with low ALCs and faster clearance in younger children with high ALCs. Our data also support the continued use of a day -12 start date for rATG with this platform, because more proximal timing generally leads to high post-HCT levels and significantly higher relapse rates and suggests that optimization of that dose based on model prediction may lead to better outcomes. Of note, given the differential elimination of the 2 formulations of rATG, Thymoglobulin and Grafalon, these findings are confined to approaches using Thymoglobulin.24
We demonstrated that rATG exposure interacts with MRD status to influence patient outcomes. Among patients who were MRD negative before HCT, no relapses occurred in the optimal rATG exposure group (Q1), and the only deaths were the consequence of NRM. This suggests that this is a patient group in which safety of the HCT approach could be prioritized by using less intensive conditioning regimens. Among patients who were MRD positive before HCT, excellent 3-year DFS rates of 74.5% could be achieved in the optimal rATG exposure group, demonstrating the preservation of GVL in this high-risk population. The sample size of patients with targetable lesions who received post-HCT maintenance with tyrosine-kinase or FLT3 inhibitors was small (n = 19); however, it was noteworthy that none of these patients relapsed. This suggests that the use of targeted post-HCT maintenance may allow sufficient time for adequate GVL to develop, but this need to be validated prospectively.
We did not confirm the previous finding from the PTCTC ONC1401 study that patients ≥10 years of age had inferior DFS. The population in this study differed by containing Complete Remission 3+ patients, more MRD-positive patients, and diseases other than acute lymphoblastic leukemia/acute myeloid leukemia. Notably, older patients did have higher predicted pre- and post-HCT rATG exposures, and the improvement in DFS for such patients when in the optimal rATG exposure group was particularly striking (86.2% vs 48.2% in the nonoptimal exposure group). We identified a strong association between R+/D− CMV serostatus and NRM. Because no patients were identified to die directly from CMV, further work will be required to determine the mechanisms underpinning this and to develop methods to abrogate this risk when seronegative donors must be used for a seropositive patient.
This study has several limitations. First, we used predicted exposure of rATG based on a PK model validated in other transplant settings instead of directly measured levels, and some patients may have had actual exposures either slightly higher or lower than predicted. However, the model could be studied prospectively and correlated with actual levels for further refinement in this setting. This finding requires validation in an independent cohort of patients who are undergoing AB-TCD haploidentical HCT using Thymoglobulin. The variability in dosing and timing of rATG, although accounted for by the PK model, introduces potential bias regarding why certain patients were given a regimen other than 3 mg/kg over 3 doses starting on day -12. Although, to our knowledge, this was one of the largest reports of AB-TCD haploidentical HCT that used Thymoglobulin to date, some analyses were limited by small numbers in certain subgroups. Finally, the model employed to predict rATG AUCs does not account for possible early exposure to granulocyte–colony stimulating factor, administration of which was not collected, and this might enhance T-cell clearance by residual ATG exposure.25
In conclusion, we identified that among patients who underwent AB-TCD haploidentical HCT for treatment of a hematologic malignancy, certain rATG exposures (such as pre-HCT AUC >50 AU per day per milliliter and post-HCT AUC <12 AU per day per milliliter) led to lower rates of NRM, relapse, and cGVHD and to improved DFS and cGRFS. Validation with prospective drug levels and an independent cohort would strengthen these conclusions. Our data indicate that model-based dosing of rATG to target optimal pre- and post-HCT exposure levels may be superior to weight-based dosing in patients who are undergoing AB-TCD HCT.
Acknowledgments
This work was supported by the National Institutes of Health (NIH) through National Cancer Institute (NCI) grant R01 CA181050. Additional funding for PTCTC activities was provided by the NIH through National Heart, Lung, and Blood Institute grant UG1HL069254 and a grant from the St. Baldrick’s Consortium. M.A.P. received additional support from St. Baldrick’s Johnny Christopher grant P30CA040214.
The funder of the study had no role in study design, data collection, analysis, interpretation, or writing of the report.
Authorship
Contribution: C.C.D. and M.A.P. conceived and designed the study; C.C.D., W.C., and M.A.P. analyzed the data and prepared the manuscript; all authors were responsible for patient inclusion and data collection; and all authors reviewed the manuscript, vouched for the accuracy and completeness of the data and analyses, had access to the statistical reports, and had final responsibility for the decision to submit for publication.
Conflict-of-interest disclosure: C.C.D. reports being a consultant for and serving on advisory boards of Jazz Pharmaceuticals, Alexion Inc, and AlloVir. J-A.T. reports receiving study support from Miltenyi. H.A.-A. reports serving on the advisory boards for Adaptive, Vertex, and Johnson & Johnson, and receiving study support from Adaptive. M.A.P. reports serving on advisory boards for Novartis, Equillium, bluebird bio, Medexus, Pfizer, GentiBio, and Vertex; engaging in educational activities for Novartis; and receiving study support from Miltenyi (not for this trial) and Adaptive. The remaining authors declare no competing financial interests.
Correspondence: Christopher C. Dvorak, Division of Pediatric Allergy, Immunology, and Bone Marrow Transplantation, University of California San Francisco, Benioff Children’s Hospitals, 550 16th St, 4th Floor, San Francisco, CA 94143; email: christopher.dvorak@ucsf.edu.
References
Author notes
Deidentified participant data and a data dictionary are available on request from the corresponding author, Christopher C. Dvorak (christopher.dvorak@ucsf.edu).
The full-text version of this article contains a data supplement.