Key Points
With DSMB oversight, we introduce a novel stepwise eligibility strategy for pediatric SCD curative trials based on 4 stages.
A committee assessed eligibility based on pain incidence rate, undertreated asthma, chronic stress, and adherence to maximum medical therapy.
Visual Abstract
The Blood and Marrow Transplant Clinical Trials Network (BMT CTN) 1507 leadership and the data safety monitoring board (DSMB) established incremental entry criteria for children aged 5 to 14.99 years with sickle cell disease (SCD) enrolling in a phase 2 trial of HLA-haploidentical hematopoietic stem cell transplantation. First, the enrollment was limited to overt stroke in the first 10 participants (stage 4). Subsequently, the DSMB reviewed the interim results and expanded the eligibility to include children with silent cerebral infarcts or abnormal transcranial Doppler velocities with magnetic resonance angiography–defined cerebral vasculopathy (stage 3). A third cohort was enrolled after the DSMB reviewed the clinical outcomes in these cumulative initial enrollments (n = 18) and additions were made to the entry criteria that included nonneurologic morbidities (stage 2). Added eligibility criteria included the following: (1) life-threatening acute chest syndrome requiring exchange transfusion; (2) right heart catheterization confirmed pulmonary hypertension; (3) persistent systemic hypertension despite maximum medical therapy; (4) acute pain despite maximum medical therapy in the absence of psychosocial factors and unmanaged asthma after adjudication; and (5) 2 major priapism episodes in 12 months or 3 in 24 months. Children with SCD who did not meet the criteria for stages 4, 3, and 2 were not eligible. To our knowledge, for the first time, we introduce a staged strategy for eligibility in a curative therapy trial for children with SCD concordant with 45 Code of Federal Regulations § 46.405(b). The research governance–mandated eligibility strategy used within the BMT CTN 1507 phase 2 study may apply to future pediatric SCD curative therapy trials. This trial was registered at www.ClinicalTrials.gov as #NCT032635590.
Introduction
Recent advancements in therapies for sickle cell disease (SCD) have been met with great enthusiasm from the parents of children with SCD; however, the rationale for determining who would optimally benefit from these novel therapies is unclear. The eligibility criteria for potentially curative therapy for children with SCD have varied between clinical trials, with marked differences in entry criteria between children and adults across industry-sponsored and investigator-initiated trials.
For children with SCD, evidence supports the inclusion of overt stroke as an indication for investigative therapy with curative intent because of the palliative nature of regular blood transfusion therapy for secondary stroke prevention.1-9 More recently, investigators have included children with abnormal transcranial Doppler (TCD) measurements and silent cerebral infarct (SCI) because of the risk of overt stroke or cerebral infarct recurrence (overt and SCI) despite receiving life-long monthly blood transfusion therapy.10-14 Still, others advocate considering allogeneic hematopoietic stem cell transplant (HCT) for children with little or no evidence of SCD severity based on the possibility of cure before becoming an adult, in part because no substantial change in the last 2 decades has been seen in the median survival of ∼48 years for individuals with SCD,15,16 and these therapies have the potential to halt progressive organ injury and disease symptoms.17 Parents of children with SCD and medical providers are currently facing inconsistent eligibility criteria for investigative curative clinical trials, and experts in hematology and hematopoietic stem cell transplantation may have contrasting views on eligibility.18,19
We describe a novel strategy of incremental entry criteria for children with SCD used within the Blood and Marrow Transplant Clinical Trials Network (BMT CTN) 1507 study, a phase 2 curative trial for the treatment of SCD. This strategy accounts for the Code of Federal Regulations (CFR) in the United States, protecting children as a vulnerable population. The strategy reflects a consensus among the trial’s data safety and monitoring board (DSMB) and hematology and stem cell transplant physicians.
Methods
To maximize the benefits and minimize toxicity for children aged 5 to 14.99 years participating in a phase 2 trial, an independent National Heart, Lung, and Blood Institute–appointed protocol review committee and DSMB for the BMT CTN elected to limit the initial enrollments to children with overt stroke. After conferring with participating site investigators and with the approval of the DSMB, eligibility was broadened.
The primary protocol (v1.0) was released on 25 April 2017 and included a pediatric stratum with SCD (hemoglobin [Hb] SS or Sβ° thalassemia) aged 5 to 14.99 years with a singular eligibility criterion of overt stroke history based on conventional clinical and imaging definitions (defined as stage 4). Protocol v2.0, released on 28 January 2018, included amendments to define further documentation requirements for stroke, including overt stroke and transient ischemic attack. The original protocol did not have a staged eligibility strategy. The stopping rule was not based on the incremental enrollment criteria later applied in the pediatric stratum but on event-free survival (EFS). The decision to have the first 12 participants with a stroke enter the trial was based on a clinical impression of what is a reasonable number to make a decision to move forward or to stop the trial. The study was designed as a phase 2 trial to determine the feasibility of achieving a high rate of EFS at 2 years after transplant. EFS was defined as survival without 1 of the following qualifying events: primary or secondary graft failure by day +42, second hematopoietic cell infusion, or death. The BMT CTN set the sample size at 40 participants. Statistical leadership evaluated a range of probabilities and estimated an EFS probability of 80% with a 95% confidence interval of 67.6 to 92.4.
Key safety end points for the study factored in known outcomes for matched sibling HCT for SCD. A protocol modification of the conditioning regimen was planned if 100-day graft failure rates exceeded 15% in the first 12 evaluable participants in a stratum. Monitoring guidelines were established to track graft failure within 100 days, grade 3 to 4 acute graft-versus-host disease (GVHD) within 100 days, overall mortality within 180 days after the start of hydroxyurea treatment (day –70), and severe chronic GVHD within 18 months after HCT. Monitoring was conducted monthly. The set thresholds were to trigger consultation with the DSMB to determine the continuation of participant accrual for the pediatric stratum. The feasibility and safety end points were cumulative in the pediatric stratum and did not change from the original study design due to the small numbers in each subgroup in the pediatric stratum. Only 1 statistical threshold, set at the onset of the trial, mandated a change in the conditioning regimen from thiotepa and 200 cGY to 400 cGY alone as part of the conditioning regimen.
Initially, the DSMB decided to limit enrollment to approximately the first 12 children with strokes and evaluate their outcomes before enrolling any additional children in the pediatric stratum. The DSMB's decision to expand enrollment to include additional eligiblity criteria was base on their judgment. Enrolling additional child participants was not based on any statistical threshold to stop the trial but on the DSMB's expert opinion and impression that the interim results in the pediatric stratum were acceptable and that the subsequent cohort of children with expanded eligibility criteria should be enrolled.
Two subsequent protocol iterations included amendments that expanded pediatric eligibility criteria. Protocol v3.0, released on 13 December 2018, expanded eligibility to include SCI or abnormal TCD velocities with magnetic resonance angiography–defined vasculopathy, requiring life-long blood transfusion therapy (defined as stage 3). Protocol v4.0 was approved on 3 December 2019, after the protocol committee members elected to better define the minimum eligibility criteria interval between HCT and acute stroke, transient ischemic attack, and central nervous system revascular procedures of 180 days. Finally, on 19 May 2021, protocol v5.0 included an amendment to expand eligibility criteria beyond neurologic indications based on evidence of progressive organ disease despite the receipt of maximum medical disease-modifying therapies defined as severe acute chest syndrome (ACS), pulmonary hypertension, essential hypertension, severe recurrent acute vaso-occlusive pain events per 4-member adjudication review, and priapism (defined as stage 2). Potential participants with few or no symptoms were excluded from the trial (stage 1). Table 1 displays the protocol changes.
Staged pediatric eligibility criteria of the phase 2 trial
| Protocol version release date . | Indication . | Definitions of SCD-specific eligibility indications . |
|---|---|---|
| V1.0 25 April 2017 | Stroke | A neurological event resulting in focal neurologic deficits that lasted ≥24 h (classical clinical definition of stroke, not requiring imaging studies of the brain) or a focal neurological event resulting in abnormalities on T2-weighted or FLAIR images using an MRI scan, indicative of an acute infarct, with no other reasonable medical explanation (definition of a stroke supported with MRI imaging scans of the brain) or both. |
| V2.0 12 January 2018 | Updated the definition of stroke to provide additional detail regarding the requirements for documenting a stroke. | |
| V3.0 13 December 2018 | Nonstroke neurologic changes requiring chronic transfusion therapy | Expanded pediatric stroke eligibility criteria to include patients with abnormal TCD or SCI requiring chronic transfusions.
|
| V4.0 3 December 2019 | Clarification to stroke eligibility criteria to exclude patients with overt stroke, transient ischemic attack, or EDAS within 180 d before enrollment. | |
| V5.0 19 May 2021 | Nonneurologic changes | Expanded pediatric eligibility criteria beyond neurological indications
|
| Protocol version release date . | Indication . | Definitions of SCD-specific eligibility indications . |
|---|---|---|
| V1.0 25 April 2017 | Stroke | A neurological event resulting in focal neurologic deficits that lasted ≥24 h (classical clinical definition of stroke, not requiring imaging studies of the brain) or a focal neurological event resulting in abnormalities on T2-weighted or FLAIR images using an MRI scan, indicative of an acute infarct, with no other reasonable medical explanation (definition of a stroke supported with MRI imaging scans of the brain) or both. |
| V2.0 12 January 2018 | Updated the definition of stroke to provide additional detail regarding the requirements for documenting a stroke. | |
| V3.0 13 December 2018 | Nonstroke neurologic changes requiring chronic transfusion therapy | Expanded pediatric stroke eligibility criteria to include patients with abnormal TCD or SCI requiring chronic transfusions.
|
| V4.0 3 December 2019 | Clarification to stroke eligibility criteria to exclude patients with overt stroke, transient ischemic attack, or EDAS within 180 d before enrollment. | |
| V5.0 19 May 2021 | Nonneurologic changes | Expanded pediatric eligibility criteria beyond neurological indications
|
BMT CTN 1507 phase 2 HLA-haploidentical hematopoietic cell trial (NCT03263559) protocol versions describing the staged additions to eligibility.
AAP, American Academy of Pediatrics; aTCD, abnormal TCD; BiPAP, bilevel positive airway pressure; CPAP, continuous positive airway pressure; EDAS, encephaloduroarteriosynangiosis; FLAIR, fluid-attenuated inversion recovery; HFNC, high-flow nasal cannula; MRI, magnetic resonance imaging; SiPAP, synchronized inspiratory positive airway pressure; VOP, vaso-occlusive pain.
Eligibility expansion strategy
As part of the incremental eligibility process, the study chairs and the DSMB used evidence-based rationale to define, in children, progressive features outside neurologic indications, including (1) severe ACS resulting in intensive care admission for nonmechanical or invasive ventilatory support20-22; (2) pulmonary hypertension based on right heart catheterization23-26; (3) essential hypertension27-29; (4) recurrent acute vaso-occlusive pain events despite maximum medical therapy, evidence of medication adherence, and the absence of plausible chronic psychosocial stressors and undertreated or previously undiagnosed asthma30-34; and (5) refractory priapism.35-37Table 1 includes detailed definitions for each eligibility criterion in all stages, and Table 2 highlights the rationale for each stage and indication.
Rationale for the stepwise strategy for eligibility for a pediatric SCD curative trial
| Pediatric SCD staging system for curative therapies . | ||
|---|---|---|
| Stage . | Symptoms . | Rationale . |
| 4 | Neurologic event with imminent risk for progression
|
|
| 3 | Nonstroke neurologic event requiring chronic blood transfusion therapy and imminent risk for progression
| |
| 2 | Nonneurologic event with rationale indicating imminent risk of progressive vital organ disease or impaired QoL
|
|
| 1 | Symptoms with low imminent risk of progressive organ disease or impaired QoL |
|
| Pediatric SCD staging system for curative therapies . | ||
|---|---|---|
| Stage . | Symptoms . | Rationale . |
| 4 | Neurologic event with imminent risk for progression
|
|
| 3 | Nonstroke neurologic event requiring chronic blood transfusion therapy and imminent risk for progression
| |
| 2 | Nonneurologic event with rationale indicating imminent risk of progressive vital organ disease or impaired QoL
|
|
| 1 | Symptoms with low imminent risk of progressive organ disease or impaired QoL |
|
The incremental expansion strategy was developed using rationale based on the time-sensitive nature of brain, heart, lung, and genitourinary disease while receiving optimal medical care.
CI, confidence interval, MRA, magnetic resonance angiography; pHTN, pulmonary hypertension; QoL, quality of life; SBP, systolic blood pressure.
Adjudication procedure
Acute vaso-occlusive pain episodes are common but are a subjective complication of SCD. An adjudication procedure was adopted to establish objectivity via an independent assessment of whether the participant received maximized medical therapy and continued to have ongoing acute vaso-occlusive pain episodes. For participants referred for acute pain as the indication for enrollment, a clinical survey was completed by site investigators for adjudication by the committee. The adjudication survey is shown in Table 3. Detailed definitions of comorbidities and maximum or optimal medical therapy were deliberately not included in the survey to account for variability and allow for collegial exchange because no finite definition exists. The local definition of maximum tolerated hydroxyurea therapy was based on the local hematologist. The fixed 4-member adjudication committee, including the protocol cochairs, consisted of a pediatric hematologist with expertise in pain management of SCD, a second pediatric hematologist with SCD HCT expertise, and 2 adult hematologists with SCD HCT expertise. The expected interval between receiving the information for pain adjudication and decision-making was 5 business days. However, protocol chairs were permitted to request additional information from the enrolling team about documentation of the episodes and evidence that maximal medical therapy had been attempted. Discrepancies among the 4 adjudication members were resolved via video conference, requests for additional data from the primary site via email, or both. A dialogue continued until the majority decided among the 4 as to the eligibility.
Adjudication survey
| Pain . |
|---|
|
|
|
|
|
|
| Priapism (Only report episodes happening at least 4 h) |
|
| Pain . |
|---|
|
|
|
|
|
|
| Priapism (Only report episodes happening at least 4 h) |
|
The survey was completed by enrolling institutions and reviewed by the adjudication committee.
AVN, avascular necrosis; Hb F, hemoglobin F; MCV, mean corpuscular volume; NA, not applicable; VOE, vaso occlusive event.
The trial was approved by the institutional review board at each participating institution.
Results
The trial opened for enrollment in February 2018, and during the subsequent 39 months, 25 participants who met the combined criteria for neurological morbidity (stages 4 and 3) were enrolled. In September 2021, over 13 months, 16 participants were enrolled, 12 within stage 2 (with nonneurologic indications), for a total of 41 participants for the pediatric stratum. Overall enrollment included 17 (42%) with overt stroke, 12 (29%) with nonstroke neurologic indications, and 12 (29%) with nonneurologic indications, including 3 with severe ACS, 5 with severe vaso-occlusive pain per adjudication, and 4 meeting both ACS and pain criteria. A total of 6 participants met multiple eligibility criteria and were counted once at the first stage met within our system: stroke and abnormal TCD velocities (n = 3); stroke and SCI (n = 1); stroke, SCI, and abnormal TCD velocity (n = 1); and abnormal TCD velocities, severe ACS, and severe vaso-occlusive pain per adjudication (n = 1; Table 4).
Enrollment in the pediatric stratum of the BMT CTN 1507 trial of HLA-haploidentical HCT for SCD
| Stage . | Enrollment per stage and symptom . | Total . |
|---|---|---|
| 4 | Stoke only (n = 12) Stroke and aTCD (n = 3) Stroke and SCI (n = 1) Stroke and SCI and aTCD (n = 1) | 17 |
| 3 | SCI only (n = 7) aTCD only (n = 3) SCI and aTCD (n = 1) aTCD and ACS and severe vaso-occlusive pain per adjudication (n = 1) | 12 |
| 2 | ACS (n = 3) Right heart catheter defined pHTN (n = 0) Essential HTN despite therapy (n = 0) Severe vaso-occlusive pain by adjudication (n = 5) Priapism per adjudication (n = 0) ACS and severe vaso-occlusive pain by adjudication (n = 4) | 12 |
| 1 | Not open for enrollment |
| Stage . | Enrollment per stage and symptom . | Total . |
|---|---|---|
| 4 | Stoke only (n = 12) Stroke and aTCD (n = 3) Stroke and SCI (n = 1) Stroke and SCI and aTCD (n = 1) | 17 |
| 3 | SCI only (n = 7) aTCD only (n = 3) SCI and aTCD (n = 1) aTCD and ACS and severe vaso-occlusive pain per adjudication (n = 1) | 12 |
| 2 | ACS (n = 3) Right heart catheter defined pHTN (n = 0) Essential HTN despite therapy (n = 0) Severe vaso-occlusive pain by adjudication (n = 5) Priapism per adjudication (n = 0) ACS and severe vaso-occlusive pain by adjudication (n = 4) | 12 |
| 1 | Not open for enrollment |
Eligibility was stepwise after National Heart, Lung, and Blood Institute-appointmentd protocol review committee and DSMB panel insight.
Adjudication procedure
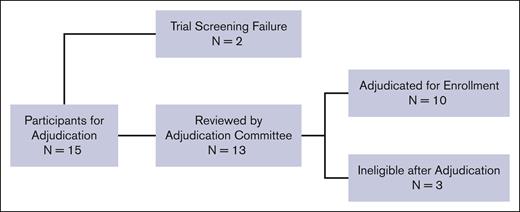
Fifteen potential participants were evaluated for eligibility based on having severe vaso-occlusive pain. Figure 1. Two participants were ineligible due to screen failure (site investigator decision and presence of donor-specific HLA antibodies, respectively) and were not reviewed by the adjudication team. Ultimately, the adjudication committee reviewed 13 participants, of whom 10 (77%) were deemed to have met the criteria outlined in the trial. Three potential participants were deemed ineligible because there was insufficient documentation that maximal medical therapies had been provided before referral to HCT. The first potential participant was withdrawn after the protocol chairs asked for additional information to document maximal medical therapy. The second potential participant was disqualified because they did not receive maximal medical therapy with hydroxyurea, initially 15 mg/kg per day, with no evidence of myelosuppression. The third potential participant had no home treatment for severe vaso-occlusive pain episodes and was not offered L-glutamine, the US Food and Drug Administration–approved therapy for decreasing vaso-occlusive pain events while receiving hydroxyurea therapy.38 The average review interval was 11 calendar days (range: 1-39), from when the coordinating center sent the adjudication request to the committee to when the site was notified of a decision. All information was reviewed and discussed via email or videoconference call until 3 of the 4 protocol chairs agreed on eligibility.
CONSORT diagram of participant enrollments that underwent adjudication for vaso-occlusive pain. A total of 15 patients were enrolled under the indication of severe vaso-occlusive pain. Two patients failed screening due to investigator decision and the presence of donor-specific antibodies, respectively, and were not reviewed by the adjudication committee. Thirteen were reviewed by the adjudication committee, and 10 were successfully adjudicated and enrolled. A total of 3 patients were deemed ineligible by adjudication.
CONSORT diagram of participant enrollments that underwent adjudication for vaso-occlusive pain. A total of 15 patients were enrolled under the indication of severe vaso-occlusive pain. Two patients failed screening due to investigator decision and the presence of donor-specific antibodies, respectively, and were not reviewed by the adjudication committee. Thirteen were reviewed by the adjudication committee, and 10 were successfully adjudicated and enrolled. A total of 3 patients were deemed ineligible by adjudication.
Requests from the committee for additional information occurred in 8 of 13 cases. Common requests for further information included the following: (1) confirmation from the local hematologists that the child with SCD received maximum medical therapy, including maximum tolerated hydroxyurea therapy, and still had multiple acute pain episodes; (2) clarification that the patient had a full psychosocial evaluation to decrease the likelihood of modifiable risk factors for acute vaso-occlusive pain episodes; (3) exclusion of undertreated or unrecognized asthma as a contributing factor to the increased acute vaso-occlusive pain incidence rate; and (4) confirmation of a laboratory response to receiving the maximum tolerated dose of hydroxyurea (increased total hemoblogin and Hb F percent and decreased absolute neutrophil count, white blood cell count, and platelet count).
Discussion
A lack of a global consensus regarding indications for curative trials for children with SCD presents a significant and unique challenge for families and referring physicians. In addition, children participating in clinical trials in the United States are considered vulnerable as potential research participants. Following the US CFR, the fundamental mandate for considering a trial with greater than minimum risk in children is whether the therapy is at least as beneficial to the child as standard care (research involving greater than minimal risk but presenting the prospect of direct benefit to the individual patients; 45 CFR § 46.405[b]).39 Four cohort studies in children with SCD have demonstrated that the expected survival of children aged ≤18 years in high-income countries is at least 98%.40-43 Thus, enrollment eligibility criteria for children could not be based on preventing death before age 18 years, but on attenuating progressive brain disease (stages 4 and 3), heart, lung, or genitourinary disease (stage 2), or improving the quality of life because of unremitting vaso-occlusive pain events after maximal medical therapy (stage 2).
Children with neurological morbidities (stages 4 and 3) have a well-defined near-term risk of progressive and permanent neurologic damage despite receiving monthly blood transfusion therapy.1 Thus, the decision to include participants with neurological morbidities in our phase 2 trial was readily accepted. However, for children with heart, lung, and kidney disease, as well as recurrent acute vaso-occlusive pain events and priapism, the entry criteria for curative trials are less defined, given there is limited evidence of childhood, progressive, life-long morbidity in these organs that precedes early mortality in adulthood. The lack of consensus is in part based on the poorly defined incidence of progressive heart, lung, and kidney disease in children, coupled with the relatively low mortality rate before age 18 years. In contrast, in adults, continued decline in the function of these vital organs (heart,44 lungs,45 and kidneys46-48) does lead to premature death, and recurrent vaso-occlusive pain49,50 and major priapism events37,51,52 lead to significant morbidity. Neither the protocol chairs nor the DSMB believed that asymptomatic or mildly symptomatic children with SCD (stage 1) should be offered an opportunity to participate in a phase 2 curative trial with an unknown risk of mortality and poorly defined transplant-related late health effects.
A strength of our trial entry criteria is the selection of incremental eligibility based on the time-sensitive nature of brain, heart, lung, and genitourinary disease. However, as anticipated in a rare disease, our strategy has limitations due to the lack of clinical history data to facilitate risk stratification when selecting comorbidities in stage 2. Therefore, when deciding on the medical conditions for stage 2, the group elected to identify a central theme, namely progressive organ disease despite maximum medical interventions. The protocol chairs targeted medical indications with evidence of imminent risk of morbidity that may be modifiable with HCT and not mortality, because the rate of death is low in children with SCD.40-43,53 The sequelae associated with a single episode of life-threatening ACS are not well documented. Although risk factors for recurrent ACS, including asthma20,21,54 and asthma risk factors,22 are well defined in children, scant data exist regarding whether an initial life-threatening ACS event is temporally associated with a second life-threatening event. Nevertheless, the protocol chairs believed that a single event of life-threatening ACS was sufficient to consider a curative trial. Another limitation was defining frequent acute vaso-occlusive pain events despite maximized medical therapy. There are generally 2 different starting doses of hydroxyurea for adults and children with SCD: 15 mg/kg per day and 20 mg/kg per day, respectively.55-57 Furthermore, there are multiple cytopenia thresholds for holding hydroxyurea therapy based on reaching the maximum tolerated hydroxyurea therapy dose55-57 (supplemental Table 1). Because there was no standard for maximum tolerated dose, we set a threshold of at least 20 mg/kg per day of hydroxyurea minimum level in the evaluated children, unless there was evidence of locally defined cytopenia. To address the heterogeneity in defining repetitive severe pain episodes, trial leadership elected to include pain events that occurred at home that were documented in the electronic record and developed an adjudication process imperative for the standardization of entry.
Our approach for incremental entry criteria for a curative clinical trial in children with SCD is consistent with well-recognized incremental eligibility criteria for oncology phase 1 and 2 clinical trials, in which the individuals with the highest likelihood of disease progression are enrolled first. In contrast, the currently enrolling (ClinicalTrials.gov; March 2024) and completed gene therapy trials for SCD that include children58-60 have excluded participants who meet stages 3 and 4 eligibility, those at high risk of stroke or stroke recurrence. For other children at risk of strokes, those with abnormal TCD velocities and normal magnetic resonance angiography evaluations, after a year of regular blood transfusion therapy, maximum tolerated hydroxyurea has been demonstrated as an effective basis for primary stroke prevention.61 Finally, few trials have reported using an adjudication procedure to assess comorbidities that may be associated with an increase in the pain incidence rate, and no trial has defined ACS as a life-threatening event requiring respiratory support and admission to the intensive care unit. The importance of a clear diagnosis of ACS is based on the absence of uniform diagnostic criteria and the significant overlap between ACS and an asthma exacerbation diagnosis.62 We emphasize that our strategy for incremental clinical trial eligibility is intended to support standardized decision-making within future pediatric SCD curative trials and not therapeutic decision-making outside of a clinical trial.
For the first time, to our knowledge, we introduce a staged strategy for eligibility in children with SCD for a curative therapy clinical trial concordant with 45 CFR § 46.405(b). The research governance–mandated incremental eligibility strategy used within the BMT CTN 1507 phase 2 study may apply to future pediatric SCD curative therapy trials.
Acknowledgments
The author thanks Nicole Ritzau for her organizational processes as associate project leader for the Blood and Marrow Transplant Clinical Trials Network (BMT CTN) 1507 study.
The work was supported by grants U10HL069294 and U24HL138660 to the BMT CTN from the National Heart, Lung, and Blood Institute and the National Cancer Institute.
The content is solely the authors' responsibility and does not necessarily represent the official view of the National Institutes of Health.
Authorship
Contribution: T.D.J. and M.R.D. contributed equally to the first and multiple manuscript drafts, contributed data, and performed the analysis; A.A.K., H.G.R., G.A., C.M.B., M.Q.R., C.M., and M.E. contributed data and reviewed the manuscript; and M.C.W. and M.R.D. conceived and designed the analysis.
Conflict-of-interest disclosure: T.D.J. has participated as an advisory board member and received consulting fees from bluebird bio, Vertex Pharmaceuticals, and BioLineRx; is a medical monitor for the BMT CTN 2001 (GRASP) trial, for which she receives compensation; and is the Stanford site principal investigator of a clinical trial for genome editing with Beam Therapeutics (NCT04443907). H.G.R. participated as an advisory board member and received consultancy fees from Medexus; is a medical monitor for the National Marrow Donor Program CD33 Chimeric Antigen Receptor-T Cell trial but does not receive compensation; and is the Nationwide Children’s Hospital site principal investigator of a clinical trial for gene editing with Autologous Clustered Regularly Interspaced Short Palindromic Repeats Gene-edited Cluster of Differentiation 34 (CD34+) Human Hematopoietic Stem and Progenitor Cells (EDIT-301) (RUBY trial; NCT04853576). The remaining authors declare no competing financial interests.
Correspondence: Michael R. DeBaun, Vanderbilt-Meharry, Center of Excellence in Sickle Cell Disease Vanderbilt University School of Medicine, 2200 Children’s Way, 11206DOT, Nashville, TN 37232-9000; email: m.debaun@vumc.org.
References
Author notes
The data that support the findings of this study are available on reasonable request from the corresponding author, Michael R. DeBaun (m.debaun@vumc.org).
The full-text version of this article contains a data supplement.