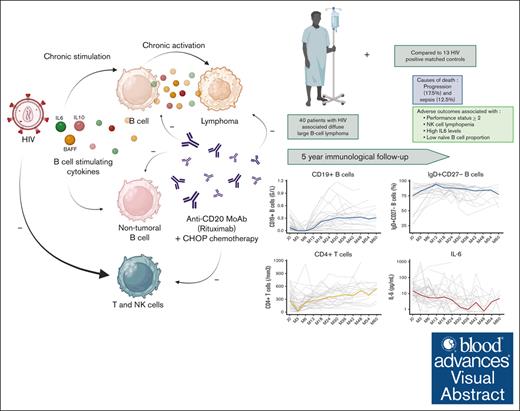
Key Points
Lymphopenia is transient with a persistent lack of effector B cells causing no excess late infectious mortality after R-CHOP for HIV-DLBCL.
R-IPI, NK cell lymphopenia, lower proportion of naïve B cells among the B-cell pool and higher IL-6 levels were linked to poorer outcomes.
Visual Abstract
HIV infection is associated with an increased risk of diffuse large B-cell lymphoma (DLBCL). In this prospective study, we analyzed the evolution of B-cell activating cytokines (interleukin-6 [IL-6], IL-10, and B-cell activating factor [BAFF]) and main functional subsets of circulating B and T cells in 51 patients with HIV-associated DLBCL treated with R-CHOP (rituximab, cyclophosphamide, doxorubicin, Oncovin [vincristine], and prednisone). R-CHOP therapy was associated with a decrease of IL-10, whereas IL-6 levels fluctuated, and BAFF levels increased during the first 3 months and decreased thereafter. We observed a rapid rise in CD19+ B cells composed mostly of naïve B cells whereas marginal zone–like B cells and memory B cells recovered gradually. With a median follow-up of 41 months, progression-free survival and overall survival at 5 years were 61.8% (95% confidence interval [CI], 47.6-80.4) and 67.4% (95% CI, 53.4-85.0), respectively. Progression (17.5%) and sepsis (12.5%) were the main causes of death. Baseline risk factors for death and progression were poor revised International Prognostic Index (P = .049), natural killer cell lymphopenia (P = .001), lower proportion of naïve B cells (P = .017), and higher IL-6 serum levels (P = .001). Our data suggest that patients treated with R-CHOP for HIV-associated DLBCL have a disturbed peripheral B-cell compartment and that the low pool size of circulating naïve B cells negatively affects their clinical outcome. In an era of development of B-cell–depleting therapies including B-cell–targeting chimeric antigen receptor T cells, assessment of perturbations within nontumoral B-cell counterparts are warranted for risk profiling in HIV-associated DLBCL. This trial was registered at www.ClinicalTrials.gov as #NCT01164436.
Introduction
HIV infection is associated with an increased risk of cancer, including non-Hodgkin lymphoma (NHL).1 The widespread use of combined antiretroviral therapy (cART) has significantly decreased the occurrence of NHL,2 improved the prognosis in patients with HIV presenting with diffuse large B-cell lymphoma (DLBCL),3-8 and promotes immune restoration after treatment.9 However, their risk of lymphoma remains higher than that of the general population, still representing >50% of all AIDS-related cancers. The underlying mechanisms could involve chronic B-cell activation, chronic stimulation of the immune system, and immunosuppression.
Indeed, the diagnosis of DLBCL is often preceded by an increase in cytokine levels associated with B-lymphocyte activation.10,11 Elevation of key B-cell stimulating and B-cell survival supporting factors, such as interleukin-6 (IL-6), IL-10, and B-cell activating factor (BAFF) of the tumor necrosis factor family, have also been found to be associated with a poor prognosis in DLBCL, but their relevance in the prognosis of HIV-associated DLBCL remains unknown.11-15 Several studies evaluated the modifications in the lymphocyte subsets in the context of chemotherapy for DLBCL16-19 and demonstrated the efficacy of rituximab-containing immunochemotherapy in this population,20-22 but few studies have specifically addressed the impact of anti-CD20 monoclonal antibody–based therapies on these cytokines and on the reconstitution of the circulating pool of mature B-cell subpopulations in patients with HIV-associated DLBCL.23
In this study, we present a prospective analysis of serum levels of B-cell activating cytokines IL-6, IL-10, and BAFF, and coevolution of main functional subsets of circulating B cells, CD4+ and CD8+ T-cell subsets, and natural killer (NK) cells in patients with HIV-DLBCL treated with R-CHOP (rituximab, cyclophosphamide, doxorubicin, Oncovin [vincristine], and prednisone) from lymphoma diagnosis to a 2/3-year follow-up. We describe the immune reconstitution characteristics of patients treated with R-CHOP for DLBCL in the modern cART era and their role in outcome probabilities in this cohort.
Methods
Patients and study design
The French prospective multicenter cohort study of HIV-related lymphomas (French National Agency for Research on AIDS and Viral Hepatitis CO16 Lymphovir cohort) enrolled consecutive individuals infected with HIV with newly diagnosed lymphoma (NHL or Hodgkin lymphoma) in 22 centers, between 2008 and 2015. Data collection included the medical history, clinical, biological and histological characteristics, and the treatment and outcome of both lymphoma and HIV infection. Histological data were systematically recorded and reviewed. The diagnosis was established by histological analysis, using the 2008 World Health Organization classification. All pathological diagnoses were confirmed within the French hematopathology network (Lymphopath). DLBCL was subclassified with the Hans algorithm when enough material was available.24 Patient evaluation was made at least every 6 months, for a planned follow-up period of 5 years.
Blood samples were collected at DLBCL diagnosis, then every 6 months for up to 60 months in all patients in the longitudinal study. A subpopulation of the Lymphovir cohort with available matched controls was selected for a nested case/control analysis. Patients were matched according to age, sex, viral load, and CD4+ T-cell counts to HIV-1–positive controls without lymphoma recruited in 2 hospitals (Kremlin Bicêtre and Antoine Béclère University Hospitals, Paris-Saclay University). This group was assigned for further case-control analysis of B-cell subsets and serum markers related to B-cell activation.
The study was conducted according to the guidelines of the Declaration of Helsinki. The protocol was approved on the 2 August 2007 by the Ile-de-France VII ethics committee (Comité de Protection des Personnes) and by the competent national authority. Written informed consent was obtained from each patient.
Serum markers analysis
Blood samples were collected from the enrolled patients and controls in dry tubes. Sera were aliquoted and stored at −80°C until use. IL-10, IL-6, and BAFF serum levels were determined using commercially available enzyme-linked immunosorbent assay kits (Quantikine colorimetric sandwich enzyme-linked immunosorbent assay kits, all from R&D Systems, Minneapolis, MS). Serum immunoglobulin G (IgG), IgA, and IgM concentrations (g/L) were determined by nephelometry (BN ProSpec, Siemens Healthcare Diagnostics, Erlangen, Germany).
Flow cytometry analysis
All flow cytometry analysis were performed at the laboratory of Immunology of Bicêtre Hospital (H.H.-C., R.K., and Y.T.). Blood samples collected in EDTA tubes were analyzed within 24 hours after shipment. Absolute counts of CD3 T cells, CD4 and CD8 T cells, B, and NK cells were determined by using the BD Multitest 4-color TBNK reagent, with BD Trucount tubes (BD Biosciences, San Jose, CA). The following antibodies were used to assess B-cell subsets: anti-CD19 (phycoerythrin), anti-IgD (fluoroscein isothiocyanate) and anti-CD27 (allophycocyanin; all from BD Biosciences). Flow cytometry analysis was performed by using a FACSCalibur cytometer (BD Biosciences).
Statistical analysis
Categorical variables were expressed as numbers (percentages) and quantitative variables as medians (interquartile range [IQR]). Comparisons between groups were made using the Mann-Whitney U test for continuous variables, and χ2 or Fisher exact tests for categorical variables, as appropriate. We used paired statistical tests when comparing patients with matched controls and when comparing the evolution of lymphocyte counts and cytokines at 24 to 36 months. For the lymphocyte counts and cytokines at 24 to 36 months, we used the mean when multiple values were available. We calculated Kaplan-Meier estimates for all-cause death and progression-free survival (PFS). We used log-rank tests to assess characteristics associated with PFS. Because of the small sample size, we did not perform multivariate analysis. For survival analyses, continuous variables were dichotomized. The significance level was 5% on both sides. All analyses were performed using R software (version 4.1.1).
Results
Among 179 consecutive patients with newly diagnosed HIV-associated lymphoma enrolled in the cohort between 2008 and 2015, 110 patients (62%) had NHL. Of these patients, 51 had DLBCL, of whom 40 had available clinical and immunological data with longitudinal follow-up, and these patients were included in this analysis. Among them, 13 cases had a matched control at baseline.
Clinical, immunological and pathological findings at diagnosis are shown in Table 1. Thirty-five patients (87.5%) were male, with a median age of 50 years (IQR, 44.0-56.2). HIV infection had been diagnosed for a median of 16.0 years (IQR, 5.7-23.2) and 35 patients (87.5%) were receiving cART at lymphoma diagnosis. Thirty-six (90.0%) had a disseminated DLBCL (Ann-Harbor stage ≥3) and the revised International Prognostic Index (R-IPI) score was poor in 18 (45.0%). DLBCL had a germinal center phenotype in 13 of 37 (35.1%). Bcl-2 and Bcl-6 were overexpressed in 18 of 30 (60.0%) and 20 of 29 (69.0%) of patients, respectively. Median CD4+ T-cell count was 0.272 (IQR, 0.029 x 109/L to 0.423 × 109/L) × 109/L and CD19+ B-cell count was 0.073 (IQR, 0.029 × 109/L to 0.0135 × 109/L) x 109/L.
Patient characteristics at HIV-DLBCL diagnosis
| . | Patients . | Missing . |
|---|---|---|
| N | 40 | |
| Demographics | ||
| Age (y) | 50.0 (44.0-56.2) | 0 |
| Sex (male) | 35 (87.5) | 0 |
| HIV infection | ||
| Time since HIV diagnosis (y) | 16.0 (5.7-23.2) | 1 |
| AIDS stage | 14 (35.0) | 0 |
| Active HIV replication (>200 copies per mL) | 14 (35.0) | 0 |
| Ongoing cART | 35 (87.5) | 0 |
| Protease inhibitor | 20 (57.1) | 5 |
| Nucleoside inhibitor of reverse transcriptase | 9 (25.7) | 5 |
| Nonnucleoside inhibitor of reverse transcriptase | 8 (22.9) | 5 |
| Positive HCV serological test | 13 (34.2) | 2 |
| Replicative HBV (positive HBV DNA) | 1 (2.5) | 0 |
| Lymphoma | ||
| Performance status | 0 | |
| 0-1 | 25 (62.5) | |
| 2 | 11 (27.5) | |
| 3-4 | 4 (10.0) | |
| Elevated LDH | 20 (51.3) | 1 |
| Lymphoma stage | 0 | |
| I-II | 1 (10.0) | |
| III-IV | 36 (90.0) | |
| >1 extranodal site | 22 (56.4) | 1 |
| R-IPI score | 0 | |
| Very good | 2 (5.0) | |
| Good | 20 (50.0) | |
| Poor | 18 (45.0) | |
| Immunological phenotypes | ||
| Immunoglobulin | 1 | |
| IgG (g/L) | 14.9 (9.9-18.8) | |
| IgA (g/L) | 2.6 (1.7-3.3) | |
| IgM (g/L) | 1.0 (0.6-1.5) | |
| CD45+ leucocytes (× 109/L) | 1.12 (0.74-1.55) | 1 |
| T lymphocytes | 1 | |
| CD3+ T lymphocytes (× 109/L) | 0.89 (0.58-1.28) | |
| CD3+CD4+ T lymphocytes (× 109/L) | 0.27 (0.13-0.42) | |
| CD3+CD8+ T lymphocytes (× 109/L) | 0.55 (0.36-0.76) | |
| CD4+:CD8+ ratio | 0.4 (0.3-0.7) | |
| NK lymphocytes | 1 | |
| NK lymphocytes (× 109/L) | 0.09 (0.05-0.14) | |
| B lymphocytes | ||
| CD19+ B lymphocytes (× 109/L) | 0.07 (0.03-0.14) | 1 |
| CD19+CD27−IgD+ among B lymphocytes (%) | 74.0 (47.0-87.0) | 8 |
| CD19+CD27+IgD+ among B lymphocytes (%) | 5.5 (2.0-10.2) | 8 |
| CD19+CD27+IgD− among B lymphocytes (%) | 11.0 (4.8-21.5) | 8 |
| Cytokines | ||
| IL-6 blood level (pg/mL) | 14.7 (6.9-24.8) | 1 |
| IL-10 blood level (pg/mL) | 26.4 (13.6-54.7) | 0 |
| BAFF blood level (pg/mL) | 1012.8 (677.4-1690.1) | 2 |
| . | Patients . | Missing . |
|---|---|---|
| N | 40 | |
| Demographics | ||
| Age (y) | 50.0 (44.0-56.2) | 0 |
| Sex (male) | 35 (87.5) | 0 |
| HIV infection | ||
| Time since HIV diagnosis (y) | 16.0 (5.7-23.2) | 1 |
| AIDS stage | 14 (35.0) | 0 |
| Active HIV replication (>200 copies per mL) | 14 (35.0) | 0 |
| Ongoing cART | 35 (87.5) | 0 |
| Protease inhibitor | 20 (57.1) | 5 |
| Nucleoside inhibitor of reverse transcriptase | 9 (25.7) | 5 |
| Nonnucleoside inhibitor of reverse transcriptase | 8 (22.9) | 5 |
| Positive HCV serological test | 13 (34.2) | 2 |
| Replicative HBV (positive HBV DNA) | 1 (2.5) | 0 |
| Lymphoma | ||
| Performance status | 0 | |
| 0-1 | 25 (62.5) | |
| 2 | 11 (27.5) | |
| 3-4 | 4 (10.0) | |
| Elevated LDH | 20 (51.3) | 1 |
| Lymphoma stage | 0 | |
| I-II | 1 (10.0) | |
| III-IV | 36 (90.0) | |
| >1 extranodal site | 22 (56.4) | 1 |
| R-IPI score | 0 | |
| Very good | 2 (5.0) | |
| Good | 20 (50.0) | |
| Poor | 18 (45.0) | |
| Immunological phenotypes | ||
| Immunoglobulin | 1 | |
| IgG (g/L) | 14.9 (9.9-18.8) | |
| IgA (g/L) | 2.6 (1.7-3.3) | |
| IgM (g/L) | 1.0 (0.6-1.5) | |
| CD45+ leucocytes (× 109/L) | 1.12 (0.74-1.55) | 1 |
| T lymphocytes | 1 | |
| CD3+ T lymphocytes (× 109/L) | 0.89 (0.58-1.28) | |
| CD3+CD4+ T lymphocytes (× 109/L) | 0.27 (0.13-0.42) | |
| CD3+CD8+ T lymphocytes (× 109/L) | 0.55 (0.36-0.76) | |
| CD4+:CD8+ ratio | 0.4 (0.3-0.7) | |
| NK lymphocytes | 1 | |
| NK lymphocytes (× 109/L) | 0.09 (0.05-0.14) | |
| B lymphocytes | ||
| CD19+ B lymphocytes (× 109/L) | 0.07 (0.03-0.14) | 1 |
| CD19+CD27−IgD+ among B lymphocytes (%) | 74.0 (47.0-87.0) | 8 |
| CD19+CD27+IgD+ among B lymphocytes (%) | 5.5 (2.0-10.2) | 8 |
| CD19+CD27+IgD− among B lymphocytes (%) | 11.0 (4.8-21.5) | 8 |
| Cytokines | ||
| IL-6 blood level (pg/mL) | 14.7 (6.9-24.8) | 1 |
| IL-10 blood level (pg/mL) | 26.4 (13.6-54.7) | 0 |
| BAFF blood level (pg/mL) | 1012.8 (677.4-1690.1) | 2 |
The results are presented as n (%) for categorical variables and as median (IQR) for quantitative variables.
HBV, hepatitis B virus; HCV, hepatitis C virus; LDH, lactate dehydrogenase.
Immune characteristics of HIV-associated DLBCL at diagnosis
Nested case control study
Among the 13 pairs at baseline, cases differed from their HIV+ nonlymphoma controls by having a lower IgG level (12.6 g/L IQR [10.2-15.1] vs 14.7 IQR [11.9-18.5] g/L; P = .033) with no difference in IgA or IgM levels. Lymphocyte subpopulation distribution did not show any statistical difference between patients and matched controls. In contrast, HIV-associated DLBCL cases had significantly higher levels of circulating IL-10 and BAFF as compared with controls (23.7 pg/mL IQR [11.6-36.9] vs 4.4 IQR [3.9-12.0] pg/mL; P = .001 and 1114.0 IQR [850.2-1880.8] vs 426.5 IQR [362.0-581.0] pg/mL; P = .016, respectively; Table 2) whereas IL-6 level did not differ between groups (P = .78).
Immunological characteristics of patients with HIV-DLBCL and their matched controls at DLBCL diagnosis
| . | Patients . | Matched controls . | P value . |
|---|---|---|---|
| n | 13 | 13 | |
| Immunoglobulin | |||
| IgG (g/L) | 12.6 (10.2- 15.1) | 14.7 (11.9- 18.5) | .03 |
| IgA (g/L) | 1.7 (1.2-2.3) | 2.3 (1.9-2.7) | .22 |
| IgM (g/L) | 0.7 (0.4-0.8) | 0.9 (0.6-1.2) | .33 |
| CD45+ leucocytes (× 109/L) | 1.52 (1.22- 2.03) | 1.67 (1.31- 1.96) | .84 |
| T lymphocytes | |||
| CD3+ T lymphocytes (× 109/L) | 1.23 (0.9- 1.44) | 1.15 (0.91- 1.37) | .79 |
| CD3+CD4+ T lymphocytes (× 109/L) | 0.43 (0.28- 0.59) | 0.42 (0.33- 0.50) | .74 |
| CD3+CD8+ T lymphocytes (× 109/L) | 0.66 (0.40- 1.1) | 0.62 (0.51- 0.81) | .31 |
| CD4+:CD8+ ratio | 0.7 (0.4-0.9) | 0.7 (0.5-1.1) | .73 |
| NK lymphocytes | |||
| NK lymphocytes (× 109/L) | 0.14 (0.10- 0.22) | 0.16 (0.13- 0.30) | .64 |
| B lymphocytes | |||
| CD19+ B lymphocytes (× 109/L) | 0.17 (0.05- 0.4) | 0.23 (0.12- 0.29) | .86 |
| CD19+IgD+CD27− among B lymphocytes (%) | 84.5 (75.0- 90.0) | 70.0 (61.0- 79.0) | .17 |
| CD19+IgD+CD27+ among B lymphocytes (%) | 4.5 (2.0-9.8) | 7.0 (3.0-19.0) | .29 |
| CD19+IgD−CD27+ among B lymphocytes (%) | 6.0 (3.8- 10.8) | 15.0 (13.0- 20.0) | .37 |
| Cytokines | |||
| IL-6 blood level (pg/mL) | 9.2 (0.0- 38.6) | 5.4 (1.0-35.0) | .78 |
| IL-10 blood level (pg/mL) | 23.7 (11.6- 36.9) | 4.4 (3.9-12.0) | .001 |
| BAFF blood level (pg/mL) | 1114.0 (850.2- 1880.8) | 426.5 (362.0- 581.0) | .016 |
| . | Patients . | Matched controls . | P value . |
|---|---|---|---|
| n | 13 | 13 | |
| Immunoglobulin | |||
| IgG (g/L) | 12.6 (10.2- 15.1) | 14.7 (11.9- 18.5) | .03 |
| IgA (g/L) | 1.7 (1.2-2.3) | 2.3 (1.9-2.7) | .22 |
| IgM (g/L) | 0.7 (0.4-0.8) | 0.9 (0.6-1.2) | .33 |
| CD45+ leucocytes (× 109/L) | 1.52 (1.22- 2.03) | 1.67 (1.31- 1.96) | .84 |
| T lymphocytes | |||
| CD3+ T lymphocytes (× 109/L) | 1.23 (0.9- 1.44) | 1.15 (0.91- 1.37) | .79 |
| CD3+CD4+ T lymphocytes (× 109/L) | 0.43 (0.28- 0.59) | 0.42 (0.33- 0.50) | .74 |
| CD3+CD8+ T lymphocytes (× 109/L) | 0.66 (0.40- 1.1) | 0.62 (0.51- 0.81) | .31 |
| CD4+:CD8+ ratio | 0.7 (0.4-0.9) | 0.7 (0.5-1.1) | .73 |
| NK lymphocytes | |||
| NK lymphocytes (× 109/L) | 0.14 (0.10- 0.22) | 0.16 (0.13- 0.30) | .64 |
| B lymphocytes | |||
| CD19+ B lymphocytes (× 109/L) | 0.17 (0.05- 0.4) | 0.23 (0.12- 0.29) | .86 |
| CD19+IgD+CD27− among B lymphocytes (%) | 84.5 (75.0- 90.0) | 70.0 (61.0- 79.0) | .17 |
| CD19+IgD+CD27+ among B lymphocytes (%) | 4.5 (2.0-9.8) | 7.0 (3.0-19.0) | .29 |
| CD19+IgD−CD27+ among B lymphocytes (%) | 6.0 (3.8- 10.8) | 15.0 (13.0- 20.0) | .37 |
| Cytokines | |||
| IL-6 blood level (pg/mL) | 9.2 (0.0- 38.6) | 5.4 (1.0-35.0) | .78 |
| IL-10 blood level (pg/mL) | 23.7 (11.6- 36.9) | 4.4 (3.9-12.0) | .001 |
| BAFF blood level (pg/mL) | 1114.0 (850.2- 1880.8) | 426.5 (362.0- 581.0) | .016 |
The results are presented as median [IQR].
Longitudinal study
At diagnosis, median T-cell count was 0.89 × 109/L (IQR, 0.58-1.28) with median CD4+ T-cell count of 0.27 × 109/L (IQR, 0.13-0.42) and median CD8+ T-cell count of 0.55 × 109/L (IQR, 0.36-0.76). Median NK cell count was 0.08 × 109/L (IQR, 0.05-0.14). Median CD19+ B-cell count was 0.07 × 109/L (IQR, 0.03-0.14). The B-cell pool was made up of 74% naïve B cells (CD19+IgD+CD27−), 5.5% marginal zone–like B cells (CD19+IgD+CD27+), and 11% memory B cells (CD19+IgD−CD27+). Median IL-6, IL-10, and BAFF levels were 14.7 pg/mL (IQR, 6.9-24.8), 26.4 pg/mL (IQR, 13.6-54.7), and 1012.8 pg/mL (IQR, 677-1690), respectively.
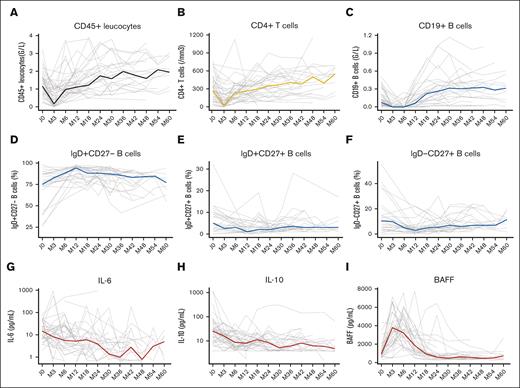
During R-CHOP therapy, the overall lymphocyte count declined, with a decrease in T cells, NK cells, and B cells. Within the B-cell pool, CD27-expressing effector populations of CD19+IgD−CD27+ memory and CD19+IgD+CD27+ marginal zone–like B cells decreased steadily, with a nadir observed at month 12 after treatment initiation for both B-cell subsets. An increase in the proportion of circulating CD19+IgD+CD27− naïve B-cell pool was observed in parallel. Interestingly, although IL-10 decreased steadily in most patients during the 12-month period after treatment initiation and serum levels of IL-6 fluctuated, a strong increase in serum levels of BAFF was observed, reaching peak values between month 3 and month 6 in most patients (Figure 1; supplemental Figure 1).
B-cell reconstitution and cytokine evolution after R-CHOP therapy. Evaluation of absolute lymphocyte counts of CD45+ leucocytes (A), CD4+ T cells (B) and CD19+ B cells (C) during a 5-year follow-up. Proportions of naïve B cells (D), marginal zone-like B cells (E) and memory B cells (F) among the CD19 B-cell pool during a 5-year follow-up. Evolution of IL-6 (G), IL-10 (H), and BAFF (I) serum levels during a 5-year follow-up. Grey lines represend individual values and colored lines represent median values.
B-cell reconstitution and cytokine evolution after R-CHOP therapy. Evaluation of absolute lymphocyte counts of CD45+ leucocytes (A), CD4+ T cells (B) and CD19+ B cells (C) during a 5-year follow-up. Proportions of naïve B cells (D), marginal zone-like B cells (E) and memory B cells (F) among the CD19 B-cell pool during a 5-year follow-up. Evolution of IL-6 (G), IL-10 (H), and BAFF (I) serum levels during a 5-year follow-up. Grey lines represend individual values and colored lines represent median values.
Posttreatment immune reconstitution in HIV-associated DLBCL
After treatment initiation, a sustained depletion of total CD19+ B-cell pool was observed recovering pretreatment levels after 18 months (Figure 1). The proportion of naïve CD19+IgD+CD27− B cells increased initially and tended to decrease after 12 months, whereas marginal zone–like CD19+IgD+CD27+ and memory CD19+IgD−CD27+ B cells increased progressively in proportion after 12 months (Figure 1). CD4+ T-cell counts decreased during treatment, reaching their lowest level at 3 months. Pretreatment levels were obtained after 18 months in most patients. Similarly, CD8+ T-cell and NK cell counts decreased during the 3 first months before increasing and reaching pretreatment levels after 6 months after treatment initiation (supplemental Figure 1). B-cell activating cytokines, IL-6 and IL-10, decreased steadily over time reaching the lowest concentration after 36 months of follow-up. BAFF levels decreased after termination of therapy, reaching their lowest level after 30 months of follow-up.
Long-term immune reconstitution was assessed in 27 patients with at least 1 analyzable sample in-between 24 and 36 months of follow-up: compared with pretreatment level, B-cell counts (median, 0.33 vs 0.11 × 109/L; P = .001) were significantly increased and the phenomenon reflected mostly the increase in the proportion of naïve CD19+IgD+CD27− B-cell pool (median, 85.2% vs 77.5%; P = .009). CD4+ and CD8+ T-cell counts did not statistically differ from baseline levels (P = .437 and P = .178, paired Wilcoxon test), whereas NK cell counts increased (median, 0.15 vs 0.09 × 109/L; P = .001; Table 3). When stratifying our analysis on initial cART treatment or on the absolute CD4 count at baseline, we did not observe any significant difference in the reconstitution profile (supplemental Figures 2 and 3). At 24 to 36 months, serum IL-10 levels decreased significantly (median, 7.2 pg/mL vs 24.8 pg/mL; P < .001) whereas the decrease did not reach statistical significance for IL-6 and BAFF (Table 3).
T, NK, and B-cell reconstitution and cytokine evolution 24 to 36 months after R-CHOP therapy
| . | At diagnosis . | 24-36 months∗ . | P value . |
|---|---|---|---|
| n | 27 | 27 | |
| Immunoglobulin levels | |||
| IgG (g/L) | 14.9 (10.8- 17.2) | 11.8 (9.6-14.2) | <.001 |
| IgA (g/L) | 2.5 (2.0-3.2) | 2.1 (1.5-2.6) | .002 |
| IgM (g/L) | 0.9 (0.5-1.7) | 0.7 (0.4-1.0) | .012 |
| Leucocytes CD45+ (× 109/L) | 1.40 (0.85- 1.70) | 1.88 (1.27- 2.33) | .014 |
| T lymphocytes | |||
| CD3+ T lymphocytes (× 109/L) | 1.07 (0.64-1.46) | 1.30 (0.96-1.71) | .11 |
| CD3+CD4+ T lymphocytes (× 109/L) | 0.29 (0.2-0.48) | 0.4 (0.25-0.54) | .44 |
| CD3+CD8+ T lymphocytes (× 109/L) | 0.62 (0.42-1.01) | 0.87 (0.58-1.21) | .13 |
| B lymphocytes | |||
| CD19+ B lymphocytes (× 109/L) | 0.10 (0.04-0.23) | 0.33 (0.22-0.41) | .001 |
| CD19+IgD+CD27− among B lymphocytes (%) | 77.5 (65.0- 87.0) | 85.2 (77.5- 90.4) | .009 |
| CD19+IgD+CD27+ among B lymphocytes (%) | 5.0 (2.0-10.2) | 3.0 (1.9-4.9) | .036 |
| CD19+IgD−CD27+ among B lymphocytes (%) | 10.5 (4.8-16.5) | 6.5 (4.2-9.7) | .031 |
| NK lymphocytes | |||
| NK lymphocytes (× 109/L) | 0.09 (0.06- 0.14) | 0.15 (0.12-0.2) | .001 |
| Cytokines | |||
| IL-6 blood level (pg/mL) | 9.8 (6.0-21.7) | 4.1 (1.4-8.7) | .14 |
| IL-10 blood level (pg/mL) | 24.8 (9.4-35.0) | 7.2 (5.3-12.7) | <.001 |
| BAFF blood level (pg/mL) | 847.0 (656.2- 1241.0) | 604.8 (494.2- 920.0) | .200 |
| . | At diagnosis . | 24-36 months∗ . | P value . |
|---|---|---|---|
| n | 27 | 27 | |
| Immunoglobulin levels | |||
| IgG (g/L) | 14.9 (10.8- 17.2) | 11.8 (9.6-14.2) | <.001 |
| IgA (g/L) | 2.5 (2.0-3.2) | 2.1 (1.5-2.6) | .002 |
| IgM (g/L) | 0.9 (0.5-1.7) | 0.7 (0.4-1.0) | .012 |
| Leucocytes CD45+ (× 109/L) | 1.40 (0.85- 1.70) | 1.88 (1.27- 2.33) | .014 |
| T lymphocytes | |||
| CD3+ T lymphocytes (× 109/L) | 1.07 (0.64-1.46) | 1.30 (0.96-1.71) | .11 |
| CD3+CD4+ T lymphocytes (× 109/L) | 0.29 (0.2-0.48) | 0.4 (0.25-0.54) | .44 |
| CD3+CD8+ T lymphocytes (× 109/L) | 0.62 (0.42-1.01) | 0.87 (0.58-1.21) | .13 |
| B lymphocytes | |||
| CD19+ B lymphocytes (× 109/L) | 0.10 (0.04-0.23) | 0.33 (0.22-0.41) | .001 |
| CD19+IgD+CD27− among B lymphocytes (%) | 77.5 (65.0- 87.0) | 85.2 (77.5- 90.4) | .009 |
| CD19+IgD+CD27+ among B lymphocytes (%) | 5.0 (2.0-10.2) | 3.0 (1.9-4.9) | .036 |
| CD19+IgD−CD27+ among B lymphocytes (%) | 10.5 (4.8-16.5) | 6.5 (4.2-9.7) | .031 |
| NK lymphocytes | |||
| NK lymphocytes (× 109/L) | 0.09 (0.06- 0.14) | 0.15 (0.12-0.2) | .001 |
| Cytokines | |||
| IL-6 blood level (pg/mL) | 9.8 (6.0-21.7) | 4.1 (1.4-8.7) | .14 |
| IL-10 blood level (pg/mL) | 24.8 (9.4-35.0) | 7.2 (5.3-12.7) | <.001 |
| BAFF blood level (pg/mL) | 847.0 (656.2- 1241.0) | 604.8 (494.2- 920.0) | .200 |
The results are presented as median [IQR]. Statistical analyses were conducted using paired Wilcoxon tests.
When multiple values were available for a patient, the mean value was used.
Risk factors for death or progression
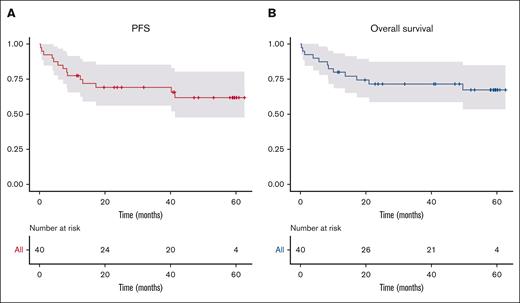
With a median follow-up time of 41 months (IQR, 11.8-51.0), estimated PFS and overall survival at 5 years were 61.9% (95% confidence interval [CI], 47.6-80.4) and 67.4% (95% CI, 53.4-85.0), respectively (Figure 2). The main causes of deaths were progression (17.5%, n = 7) and sepsis (12.5%, n = 5). Of 5 patients who have died of sepsis, 4 patients died within the first 6 months after the start of lymphoma treatment (2 patients did not experience progressive disease, and 2 patients died after receiving the first cycle of R-CHOP therapy). The fifth death from an infectious cause occurred in a patient undergoing autologous stem cell transplantation (ASCT) after a relapse of his lymphoma 2 years after diagnosis.
Progression-free and overall survival of the study population during a 5-year follow-up. Curves represent progression-free (A) and overall (B) survival.
Progression-free and overall survival of the study population during a 5-year follow-up. Curves represent progression-free (A) and overall (B) survival.
Patient baseline characteristics were assessed to identify risk factors for death or progression in survival analysis (Table 4). Compared with patients with HIV-DLBCL who survived, those who died or progressed had poorer R-IPI scores (poor R-IPI, 71.4% vs 30.8%; P = .049), lower absolute number of NK cells (NK cells <0.05 × 109/L, 57.1% vs 8.0%; P = .001), and a lower proportion of naïve B cells at baseline (CD19+IgD+CD27− cells among circulating CD19+ B cells >50%, 45.5% vs 85.7%; P = .017). In addition, we observed an increased risk of death or progression in patients with higher serum IL-6 level at baseline (IL-6 > 20 pg/mL, 57.1% vs 20.0%; P = .001).
Univariate analysis of PFS or overall survival in patients with HIV-DLBCL
| . | Overall survival . | PFS . | Death or progression . | P value (log-rank) . | Missing . |
|---|---|---|---|---|---|
| N | 40 | 26 | 14 | ||
| Demographics | |||||
| Age >50 y | 20 (50.0) | 14 (53.8) | 6 (42.9) | 0.60 | 0 |
| Sex (male) | 35 (87.5) | 23 (88.5) | 12 (85.7) | 0.87 | 0 |
| HIV infection | |||||
| Ongoing cART | 35 (87.5) | 23 (88.5) | 12 (85.7) | 0.99 | 0 |
| New HIV diagnosis | 4 (10.3) | 4 (15.4) | 0 (0.0) | 0.15 | 1 |
| AIDS stage | 14 (35.0) | 11 (42.3) | 3 (21.4) | 0.23 | 0 |
| Active HIV replication (>200/mL) | 14 (35.0) | 8 (30.8) | 6 (42.9) | 0.45 | 0 |
| Positive HCV serological test | 13 (34.2) | 7 (29.2) | 6 (42.9) | 0.32 | 2 |
| Lymphoma | |||||
| PS ≥ 2 | 15 (37.5) | 6 (23.1) | 9 (64.3) | < 0.01 | 0 |
| Elevated LDH | 20 (51.3) | 11 (44.0) | 9 (64.3) | 0.32 | 1 |
| Lymphoma stage | 0.20 | 0 | |||
| I-II | 4 (10.0) | 4 (15.4) | 0 (0) | ||
| III-IV | 36 (90.0) | 22 (84.6) | 14 (100.0) | ||
| R-IPI score | 0.049 | 0 | |||
| Very good | 2 (5.0) | 2 (7.7) | 0 (0.0) | ||
| Good | 20 (50.0) | 16 (61.5) | 4 (28.6) | ||
| Poor | 18 (45.0) | 8 (30.8) | 10 (71.4) | ||
| Germinal center phenotype | 13 (35.1) | 9 (39.1) | 4 (28.6) | 0.70 | 3 |
| Bcl2 expression | 18 (60.0) | 8 (44.4) | 10 (83.3) | 0.07 | 10 |
| Bcl6 expression | 20 (69.0) | 12 (70.6) | 8 (66.7) | 0.77 | 11 |
| LMP expression | 2 (15.4) | 1 (12.5) | 1 (20.0) | 0.65 | 27 |
| EBER | 6 (18.2) | 5 (22.7) | 1 (9.1) | 0.38 | 7 |
| Immunoglobulin | |||||
| IgG <10 g/L | 10 (25.6) | 4 (16.0) | 6 (42.9) | 0.095 | 1 |
| IgA <1 g/L | 3 (7.7) | 1 (4.0) | 2 (14.3) | 0.28 | 1 |
| IgM <1 g/L | 19 (48.7) | 11 (44.0) | 8 (57.1) | 0.58 | 1 |
| T lymphocytes | |||||
| CD3+CD4+ T lymphocytes <0.2 × 109/L | 16 (41.0) | 10 (40.0) | 6 (42.9) | 0.66 | 1 |
| CD3+CD8+ T lymphocytes <0.5 × 109/L | 17 (43.6) | 10 (40.0) | 7 (50.0) | 0.65 | 1 |
| B lymphocytes | |||||
| CD19+ B lymphocytes <0.050 × 109/L | 16 (41.0) | 9 (36.0) | 7 (50.0) | 0.35 | 1 |
| CD19+CD27−IgD+ among B cells >50% | 23 (71.9) | 18 (85.7) | 5 (45.5) | 0.017 | 8 |
| CD19+CD27+IgD+ among B cells >5% | 16 (50.0) | 9 (42.9) | 7 (63.6) | 0.29 | 8 |
| CD19+CD27+IgD− among B cells >10% | 17 (53.1) | 10 (47.6) | 7 (63.6) | 0.38 | 8 |
| NK lymphocytes | |||||
| NK lymphocytes <0.05 × 109/L | 10 (25.6) | 2 (8.0) | 8 (57.1) | 0.001 | 1 |
| Cytokines | |||||
| IL-6 > 20 pg/mL | 13 (33.3) | 5 (20.0) | 8 (57.1) | 0.011 | 1 |
| IL-10 > 20 pg/mL | 24 (60.0) | 16 (61.5) | 8 (57.1) | 0.77 | 0 |
| BAFF > 20 pg/mL | 19 (50.0) | 10 (41.7) | 9 (64.3) | 0.17 | 2 |
| . | Overall survival . | PFS . | Death or progression . | P value (log-rank) . | Missing . |
|---|---|---|---|---|---|
| N | 40 | 26 | 14 | ||
| Demographics | |||||
| Age >50 y | 20 (50.0) | 14 (53.8) | 6 (42.9) | 0.60 | 0 |
| Sex (male) | 35 (87.5) | 23 (88.5) | 12 (85.7) | 0.87 | 0 |
| HIV infection | |||||
| Ongoing cART | 35 (87.5) | 23 (88.5) | 12 (85.7) | 0.99 | 0 |
| New HIV diagnosis | 4 (10.3) | 4 (15.4) | 0 (0.0) | 0.15 | 1 |
| AIDS stage | 14 (35.0) | 11 (42.3) | 3 (21.4) | 0.23 | 0 |
| Active HIV replication (>200/mL) | 14 (35.0) | 8 (30.8) | 6 (42.9) | 0.45 | 0 |
| Positive HCV serological test | 13 (34.2) | 7 (29.2) | 6 (42.9) | 0.32 | 2 |
| Lymphoma | |||||
| PS ≥ 2 | 15 (37.5) | 6 (23.1) | 9 (64.3) | < 0.01 | 0 |
| Elevated LDH | 20 (51.3) | 11 (44.0) | 9 (64.3) | 0.32 | 1 |
| Lymphoma stage | 0.20 | 0 | |||
| I-II | 4 (10.0) | 4 (15.4) | 0 (0) | ||
| III-IV | 36 (90.0) | 22 (84.6) | 14 (100.0) | ||
| R-IPI score | 0.049 | 0 | |||
| Very good | 2 (5.0) | 2 (7.7) | 0 (0.0) | ||
| Good | 20 (50.0) | 16 (61.5) | 4 (28.6) | ||
| Poor | 18 (45.0) | 8 (30.8) | 10 (71.4) | ||
| Germinal center phenotype | 13 (35.1) | 9 (39.1) | 4 (28.6) | 0.70 | 3 |
| Bcl2 expression | 18 (60.0) | 8 (44.4) | 10 (83.3) | 0.07 | 10 |
| Bcl6 expression | 20 (69.0) | 12 (70.6) | 8 (66.7) | 0.77 | 11 |
| LMP expression | 2 (15.4) | 1 (12.5) | 1 (20.0) | 0.65 | 27 |
| EBER | 6 (18.2) | 5 (22.7) | 1 (9.1) | 0.38 | 7 |
| Immunoglobulin | |||||
| IgG <10 g/L | 10 (25.6) | 4 (16.0) | 6 (42.9) | 0.095 | 1 |
| IgA <1 g/L | 3 (7.7) | 1 (4.0) | 2 (14.3) | 0.28 | 1 |
| IgM <1 g/L | 19 (48.7) | 11 (44.0) | 8 (57.1) | 0.58 | 1 |
| T lymphocytes | |||||
| CD3+CD4+ T lymphocytes <0.2 × 109/L | 16 (41.0) | 10 (40.0) | 6 (42.9) | 0.66 | 1 |
| CD3+CD8+ T lymphocytes <0.5 × 109/L | 17 (43.6) | 10 (40.0) | 7 (50.0) | 0.65 | 1 |
| B lymphocytes | |||||
| CD19+ B lymphocytes <0.050 × 109/L | 16 (41.0) | 9 (36.0) | 7 (50.0) | 0.35 | 1 |
| CD19+CD27−IgD+ among B cells >50% | 23 (71.9) | 18 (85.7) | 5 (45.5) | 0.017 | 8 |
| CD19+CD27+IgD+ among B cells >5% | 16 (50.0) | 9 (42.9) | 7 (63.6) | 0.29 | 8 |
| CD19+CD27+IgD− among B cells >10% | 17 (53.1) | 10 (47.6) | 7 (63.6) | 0.38 | 8 |
| NK lymphocytes | |||||
| NK lymphocytes <0.05 × 109/L | 10 (25.6) | 2 (8.0) | 8 (57.1) | 0.001 | 1 |
| Cytokines | |||||
| IL-6 > 20 pg/mL | 13 (33.3) | 5 (20.0) | 8 (57.1) | 0.011 | 1 |
| IL-10 > 20 pg/mL | 24 (60.0) | 16 (61.5) | 8 (57.1) | 0.77 | 0 |
| BAFF > 20 pg/mL | 19 (50.0) | 10 (41.7) | 9 (64.3) | 0.17 | 2 |
The results are presented as n (%). Statistical analyses were conducted using survival analysis (Kaplan Meier estimate and log-rank test).
EBER, Ebstein-Barr virus encoded small RNAs; HCV, hepatitis C virus; LDH, lactate dehydrogenase; LMP, latent membrane protein.
Discussion
This study is the first to focus specifically on the role of the B-cell compartment at baseline and the posttreatment reconstitution pattern of main B-cell subpopulations and B-cell activating and survival factors IL-6, IL-10, and BAFF during R-CHOP therapy in patients with HIV-DLBCL.
We found no difference in main T-cell subsets (CD4+ and CD8+ T cells), and B and NK lymphocyte profiles between patients with DLBCL at lymphoma diagnosis and control patients with HIV who did not develop lymphoma. However, we did find a significant increase in 2 B-cell activating cytokines, that is, IL-10 and BAFF, in patients with HIV-DLBCL, with no significant increase in IL-6. Of note, in our previous work on Hodgkin lymphoma, CD19+IgD+CD27− naïve B-cell subpopulation, as well as IL-6, IL-10, and BAFF, were significantly higher at lymphoma diagnosis in patients infected with HIV compared with their matched controls.25
We observed a rapid increase in CD19+ B-cell counts after rituximab discontinuation, with an initial reconstitution dominated by naïve B-cell pool whereas innate and adaptive effector B-cell pools, such as marginal zone–like B cells and memory B cells, recovered gradually over months after completion of treatment. The early and preferential recovery of the mature naïve B-cell pool in our cohort mimics the B-cell pool reconstitution pattern in patients infected with HIV treated with ASCT.18 In comparison with patients who are not infected with HIV, B-cell compartment immune recovery in those infected with HIV was characterized by a significant expansion of naïve CD19+IgD+CD27− B cells over a period between 6 to 12 months after ASCT.18 Notably, in those infected with HIV treated with rituximab and ASCT, the reconstitution of the B-cell compartment is mainly characterized by the restoration of the naïve B-cell pool with a maturation defect within the memory B-cell pool associated with B-cell dysfunction and hypogammaglobulinemia.19,23 Moreover, in addition to impaired B-cell function, a persistent defect in the process of somatic hypermutation of immunoglobulin variable region genes is observed up to 6 years after rituximab treatment.26 In contrast, we found a rapid increase in the CD4+ and CD8+ circulating T-cell pool after R-CHOP treatment, with a stable CD4+:CD8+ ratio over time, with return to basal levels after 12 months, as previously described.27 The peripheral pool of NK cells showed a similar reconstitution profile to CD4+ T cells and CD8+ T cells.
The proposed pathophysiology of lymphomagenesis in patients with HIV involves Tat-induced abnormal production of B-cell growth–stimulating cytokines such as IL-6 and IL-10, and Nef-induced (a viral accessory negative factor that has a key role in HIV replication) production of BAFF.28 Indeed, IL-6, IL-10, and BAFF are known to be increased in patients infected with HIV29-31 as well as in the context of B-cell lymphoma.14,32 In addition, the role of HIV-1 matrix protein p17 variants, which accumulate in the lymph nodes of patients infected with HIV-1 even under cART and have enhanced B-cell clonogenic and lymphomagenic potential, is also highlighted.33,34 Also, high levels of IL-6 and IL-10 are correlated with B-cell tumor mass,14 and their rapid decline may reflect the decrease in tumor burden. In contrast, BAFF levels inversely correlate with the peripheral B-cell pool, rising rapidly during rituximab-induced B-cell depletion, and decreasing during the B-cell pool reconstitution process, emphasizing the important role of BAFF in homeostatic regulation of the peripheral B-cell compartment.35
Evaluating the impact of baseline immune characteristics on the outcome of HIV-associated DLBCL cases, a univariate analysis showed that NK cell lymphopenia at diagnosis was associated with poorer outcomes, as previously described in non-HIV–associated DLBCL.36 Consistent with a previous study, we found no evidence of an increased risk of relapse or death associated with profound CD4+ T-cell lymphopenia.37 Additionally, baseline IL-6 was associated with a poorer outcome in accordance with the literature in non-HIV–associated DLBCL cases.12,14,38 IL-6 levels do not only reflect tumor mass but is also involved in a proinflammatory and tumor growth–promoting tumor microenvironment,39 representing an independent risk factor associated with poor prognosis.11 Baseline level of IL-10 and BAFF, which were higher in patients with HIV-associated DLBCL as compared with matched controls, had no prognostic impact in our cohort in contrast to patients with non-HIV–associated lymphoma in other studies.13-15
A significant aspect of our study is related to the assessment of B-cell reconstitution after anti-CD20 monoclonal antibody–based therapy in patients infected with HIV. Indeed, a significantly increased risk of infectious adverse events related to B-cell–depleting therapies were initially reported in this context.40 In our study, 12.5% of patients died after infection in the absence of disease progression, which is comparable with that observed in elderly patients (11.1%) and higher than in the selected population from the POLARIX trial control group (2.3%).41,42 These results are similar to those observed in a phase 3 trial investigating the safety and efficacy of rituximab in combination with conventional CHOP therapy in patients who are HIV positive, in which 14% of patients died of infectious causes in the rituximab group vs 2% in the CHOP group, although this trial was conducted in the early time period of cART.40 In a meta-analysis demonstrating the efficacy of rituximab in HIV-associated DLBCL in the era of cART, median CD4 count at inclusion was similar to that observed in this study (0.248 × 109/L) but only 52% of patients were under cART (vs 87.5% in our cohort). Patients had a similar AIDS stage rate to our cohort (38% vs 35%, respectively). In this meta-analysis, rituximab was neither associated with increased nonrelapsed mortality nor increased HIV-associated mortality. More recently, in the AMC-075 trial evaluating rituximab, etoposide, prednisone, oncovin (vincristine), cyclophosphamide and doxorubicin with/without vorinostat in HIV-associated DLBCL, patients treated with rituximab did not experience significant deaths from infectious causes, despite having a lower CD4 count than those in our cohort (0.185 vs 0.272 × 109/L, respectively).43 However, in the Lymphovir cohort, all patients were included without the exclusion criteria imposed by prospective therapeutic studies (Eastern Cooperative Oncology Group performance status score of ≥2, organ failure, and CD4 count of <0.05 × 109/L at inclusion). Together, our results, and data from the literature, suggest that performance status and comorbidity burden have a stronger impact on clinical outcomes than immunosuppression at diagnosis.
The study of immune reconstitution is important in the context of anti-CD19 chimeric antigen receptor (CAR) T-cell therapy because infections represent the first cause of nonrelapse mortality in CAR T-cell therapy.44-46 However, patients who are infected with HIV are still excluded from CAR T-cell–based protocols, although preliminary efficacy results seem promising.47-49 The CAR T-cell approach in the context of HIV infection will require close monitoring of potential alterations in immune reconstitution due to CAR T-cell lymphodepletion and related toxicities. In our study, we observed satisfactory effector B-cell reconstitution after the end of treatment, and deaths of infectious origin were mainly observed in the early stages. Our study supports reevaluation of the contraindication of CAR T cells in patients with HIV, and paves the way for a larger use of CAR T cells in HIV-associated lymphoma. A study is currently underway to evaluate the efficacy and safety of CAR T cells and immune reconstitution in patients with HIV-associated DLBCL (ClinicalTrials.gov identifier: #NCT05077527).50
Our study has several limitations. Because of limited sample size, we did not perform a multivariate analysis to assess the prognostic interaction between disturbances within functional B-cell subsets and serum levels of B-cell–activating cytokines. With only 13 included patients per group, the nested case control analysis also lacked statistical power. In addition, blood samples were not available for all time points for patients still alive at study completion. Another limitation is the absence of a dedicated cohort of non-HIV–related DLBCL as a control group.
Conclusion
In this study, we present a set of characteristics regarding reconstitution of the main functional B-cell subsets as well as serum levels of B-cell–activating cytokines (IL-6, IL-10, and BAFF) during R-CHOP therapy in patients with HIV infection and DLBCL. After completion of treatment, a rapid rise in peripheral CD19+ B-cell counts was observed. Although substantial heterogeneity patterns for key B-cell subsets analyzed was observed, a possible defect in CD27+ effector B-cell reconstitution was revealed. Also, higher IL-6 serum level and lower NK cell count at DLBCL diagnosis were associated with a poorer prognosis. In conclusion, in an era of rapid development of B-cell–depleting therapies including B-cell–targeting CAR T cells, the findings of this study support the further evaluation of the nontumoral B-cell counterpart perturbations as a monitoring tool in the HIV-associated DLBCL setting. The reconstitution of a B-cell–effector pool and the absence of infectious deaths after the end of treatment prompt us to support the CAR T-cell approach for patients with HIV-associated DLBCL.
Acknowledgments
This research was funded by French National Agency for Research on AIDS and Viral Hepatitis grant Lymphovir-CO16. The publication charges were supported by the Centre Hospitalier de Versailles.
Authorship
Contribution: R. Liévin, A.M., H.H.-C., R.K., D.C., Y.T., and C.B. conceptualized the study; A.M., R. Lancar, and M.A.G. were responsible for data curation; R. Liévin, A.M., R. Lancar, and L.A. were responsible for formal analysis; M.A.G. acquired funding; M.A.G., D.C., and C.B. were responsible for project administration; R.K., D.C., Y.T., and C.B. supervised the study; H.H.-C. was responsible for study/data validation; R. Liévin, A.M., H.H.-C., L.A., Y.T., and C.B. were responsible for data visualization; and R. Liévin, A.M., R.K., and C.B. wrote the original draft of the manuscript.
Conflict-of-interest disclosure: D.C. reports personal fees from Pfizer (2022) for lectures outside the submitted work. The remaining authors declare no competing financial interests.
Correspondence: Caroline Besson, Centre Hospitalier de Versailles, 177 Rue de Versailles, Le Chesnay-Rocquencourt 78150; France; email: cbesson@ght78sud.fr.
References
Author notes
R.L. and A.M. contributed equally to this work.
The data set is owned by the French National Agency for Research on AIDS and Viral Hepatitis (France REcherche Nord & Sud Sida-hiv Hépatites), an autonomous agency within INSERM. Data requests may be submitted to the data monitoring and analysis center of the study and must be approved by the French computer watchdog authority, la Commission Nationale de l’Informatique et des Libertés. Data requests may be sent to coauthor Lambert Assoumou (lambert.assoumou@iplesp.upmc.fr).
The full-text version of this article contains a data supplement.