Key Points
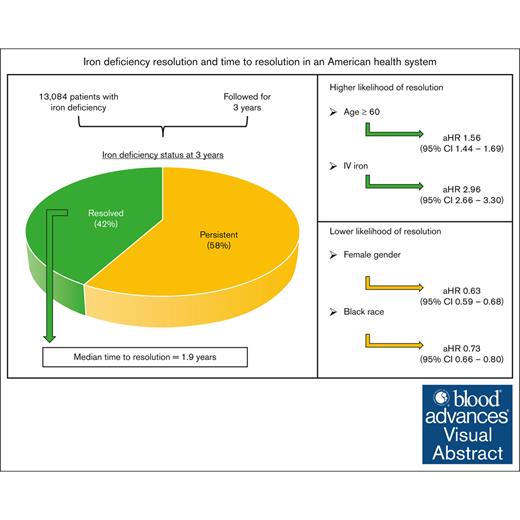
In a statewide cohort, time to resolution of iron deficiency was 1.9 years, and 58% of patients did not achieve resolution within 3 years.
Greater awareness of nonanemic iron deficiency and diagnostic ferritin levels is needed, particularly in females and Black individuals.
Visual Abstract
Iron deficiency (ID) is a global health problem with consequences independent of anemia, including impaired cognition and exercise tolerance. The time from laboratory diagnosis to resolution of ID has not been defined. In a retrospective review of electronic medical record data from a Minnesota statewide health system, we identified patients with ID (ferritin level ≤25 ng/mL). Patients with at least 1 follow-up ferritin level within 3 years were included. Patients with a subsequent ferritin of ≥50 ng/mL were classified as having resolved ID. Descriptive statistics and time-to-event analyses were used to determine proportion of ID resolution and time to resolution, and to evaluate characteristics predictive of resolution. We identified 13 084 patients with ID between 2010 to 2020. We found that 5485 (41.9%) had resolution within 3 years of diagnosis, whereas 7599 (58.1%) had no documented resolution. The median time to resolution was 1.9 years (interquartile range, 0.8-3.9). Factors associated with greater likelihood of resolution included age of ≥60 years (adjusted hazard ratio [aHR], 1.56; 95% confidence interval [CI], 1.44-1.69]), male sex (aHR, 1.58; 95% CI, 1.48-1.70]) and treatment with intravenous iron (aHR, 2.96; 95% CI, 2.66-3.30). Black race was associated with a lower likelihood of resolution (aHR, 0.73; 95% CI, 0.66-0.80). We observed a high proportion of persistent ID and prolonged time to resolution overall, with greater risk of lack of resolution among females and Black individuals. Targeted knowledge translation interventions are required to facilitate prompt diagnosis and definitive treatment of this prevalent and correctable condition.
Introduction
Iron deficiency (ID) is more prevalent and clinically important than currently appreciated. The global anemia burden was 33% in 2010, with half of cases driven by ID.1 In high-risk patients such as adolescent and young adult women, the prevalence of ID in the United States is ∼39%, comprised primarily of nonanemic ID (NAID).2 NAID is associated with fatigue and impaired cognitive functioning.3-7 Yet despite this anemia-independent morbidity, up to 70% of ID goes undiagnosed in high-risk populations.8
Underdiagnosis of ID has several possible driving factors.8 First, the clinical consequences of NAID are nonspecific, not well recognized, and thought to only be of clinical consequence when ID causes anemia. Second, ID can be challenging to diagnose. Failure to obtain and properly interpret all necessary laboratory parameters (for example, iron, transferrin saturation [TSAT], ferritin, etc) can lead to missed diagnoses. Furthermore, clinically actionable laboratory parameter values may fall within existing laboratory reference intervals and be falsely interpreted as normal.8,9 Third, oral iron is often poorly absorbed and limited by side effects,10 whereas the cost of IV iron and misconceptions over its safety limit its use.
These structural challenges likely contribute to a high prevalence of clinically significant ID that is either undiagnosed or incompletely treated. In this study, we sought to define both the proportion of resolution and time to resolution of ID in an American statewide health system.
Methods
Data source
The M Health Fairview system (MHFV), a partnership between the University of Minnesota and Fairview Pharmacy Services, is 1 of the largest health providers in the state of Minnesota. MHFV comprises 12 hospitals and medical centers, with >100 000 admissions in the system annually. The Clinical and Translational Science Institute of the University of Minnesota maintains a clinical data repository comprising data from >4.5 million patients seen at 8 hospitals and >40 clinics in the MHFV system. The institutional review board determined that this study meets the criteria for exemption from institutional review board review.
Cohort
Within the Clinical and Translational Science Institute clinical data repository, patients aged ≥18 years who had a laboratory diagnosis of ID (with or without anemia) within the MHFV system since 2010 were eligible for inclusion. We sought to construct a cohort of patients with (1) laboratory evidence of ID, (2) regular care in the MHFV system, and (3) enough follow-up time to allow for documented resolution of ID with treatment. The assumptions guiding each of these components are described as follows:
Laboratory evidence of ID: we defined ID as a ferritin value of ≤25 ng/mL. A ferritin value in this range is highly predictive of ID.11,12 Although there is disagreement about the ferritin level that can be considered diagnostic of ID,13 we chose 25 ng/mL as a conservative threshold with a physiologic basis11,14 and currently accepted in clinical practice15-19;
Regular care in the MHFV system: we included patients with at least 2 ferritin values (1 initial value of ≤25 ng/mL, and at least 1 subsequent value) measured in the MHFV system. The second value was required to occur at least 2 months after the initial value, and no later than 3 years after the initial value. These criteria are intended to signify both ongoing engagement with the MHFV system (suggesting opportunities for diagnosis, treatment, and follow-up on treatment efficacy), and that providers caring for a given patient considered the diagnosis of ID multiple times in a 3-year period.
Sufficient follow-up time for documented resolution: patients were required to have 3 years of follow-up data available after diagnosis. Resolution of ID could occur at any time point in the 3-year window. The 3-year time frame was chosen as an amount of time in which any recognized case of ID should be able to be sufficiently treated. A common approach to the management of ID is to start with oral iron and, if there is no improvement, to transition to IV iron. Oral iron is generally trialed for 3 months before its efficacy can be adequately assessed. IV iron, once approved by insurance, can often replete iron stores within 2 months of treatment. We felt that a 3-year study period would be more than sufficient for the initial diagnosis, at least 1 trial of oral iron, time for insurance approval of IV iron, and time after IV iron treatment to test for resolution.
Based on these principles, patients with a ferritin value of ≤25 ng/mL who had at least 1 subsequent ferritin value measured in the MHFV system within 3 years were further included in the study cohort (Figure 1). Resolution of ID was defined as a subsequent ferritin value of ≥50 ng/mL. Although the optimal laboratory value threshold for when ID can be considered resolved is not defined, several studies suggest that a ferritin of ≥50 ng/mL is the level at which gastrointestinal absorption of iron returns to baseline and symptoms of ID are likely to resolve.5,20,21 Patients with an initial ferritin value of ≤25 ng/mL who had a subsequent ferritin of ≥50 ng/mL were classified as having resolved ID. Those with an initial ferritin value of ≤25 ng/mL who had at least 1 subsequent ferritin measured, all of which were <50 ng/mL, were classified as having persistent ID.
Study schema. Patients included in the final study cohort had an initial ferritin value of ≤25 ng/mL and had at least 1 subsequent ferritin measured in the MHFV system between 2 months and 3 years after the initial value.
Study schema. Patients included in the final study cohort had an initial ferritin value of ≤25 ng/mL and had at least 1 subsequent ferritin measured in the MHFV system between 2 months and 3 years after the initial value.
We further determined demographic, insurance, and medication (oral and IV iron) data for the entire study cohort (all patients with resolved and persistent ID). Patients in the study cohort were classified as having received treatment with oral iron only, IV iron only, both, or neither within the 3 years of data (eg, from the initial low ferritin value to 3 years after).
Statistical analysis
Patient demographic and clinical characteristics were summarized as medians and interquartile ranges (IQRs) for continuous variables, and frequencies and percentages for categorical variables. Proportions of resolved and persistent ID at 3 years after diagnosis were determined, and Wilcoxon rank sum and χ2 tests were used for comparison of continuous and categorical variables between these 2 groups, respectively. To determine characteristics associated with achieving resolution, multivariable Cox proportional hazard regression modeling was performed, including patient age at ID diagnosis, sex, race/ethnicity, insurance, and treatment. For the cohort with resolution, time to resolution of ID was estimated. The remaining patients without resolution were censored at 3 years. The regression results were reported as adjusted hazard ratios (aHRs) and their corresponding 95% confidence intervals (CIs). All hypothesis tests were 2-sided and conducted at the .05 significance level using the R environment (version 4.2.3) and through R Studio (version 2023.06.0+421).
Results
We identified 13 084 patients with ID detected between 2010 to 2020. These patients could be further divided into those who had resolution of ID and those who did not have resolution of ID within 3 years of diagnosis (Table 1). In both cohorts, the majority of patients were female (80.9% resolved; 90.7% persistent), identified their race/ethnicity as White (84.1%; 75.9%), had private insurance (56.9%; 58.9%), and received some form of treatment (84.7%; 72.5%).
Baseline patient characteristics of patients with ID
| Characteristics . | Resolved, n (%) . | Persistent, n (%) . |
|---|---|---|
| 5485 (100) | 7599 (100) | |
| Sex | ||
| Female | 4436 (80.9) | 6889 (90.7) |
| Male | 1049 (19.1) | 710 (9.3) |
| Age, y | ||
| 18-39 | 1540 (28.1) | 3511 (46.2) |
| 40-59 | 2291 (41.8) | 2891 (38.0) |
| ≥60 | 1654 (30.1) | 1297 (15.8) |
| Race/ethnicity | ||
| White | 4611 (84.1) | 5765 (75.9) |
| Black | 553 (10.0) | 1218 (16.0) |
| Asian | 218 (4.0) | 455 (6.0) |
| Other/not specified | 103 (1.9) | 161 (2.1) |
| Insurance | ||
| Medicare | 1275 (23.2) | 949 (12.5) |
| Private insurance | 3123 (56.9) | 4473 (58.9) |
| Medicaid/other public | 789 (14.4) | 1362 (17.9) |
| Uninsured | 298 (5.5) | 815 (10.7) |
| Medication type | ||
| Oral only | 2488 (45.4) | 4625 (60.8) |
| IV only | 390 (7.1) | 133 (1.8) |
| Both | 1766 (32.2) | 751 (9.9) |
| Neither | 841 (15.3) | 2090 (27.5) |
| Characteristics . | Resolved, n (%) . | Persistent, n (%) . |
|---|---|---|
| 5485 (100) | 7599 (100) | |
| Sex | ||
| Female | 4436 (80.9) | 6889 (90.7) |
| Male | 1049 (19.1) | 710 (9.3) |
| Age, y | ||
| 18-39 | 1540 (28.1) | 3511 (46.2) |
| 40-59 | 2291 (41.8) | 2891 (38.0) |
| ≥60 | 1654 (30.1) | 1297 (15.8) |
| Race/ethnicity | ||
| White | 4611 (84.1) | 5765 (75.9) |
| Black | 553 (10.0) | 1218 (16.0) |
| Asian | 218 (4.0) | 455 (6.0) |
| Other/not specified | 103 (1.9) | 161 (2.1) |
| Insurance | ||
| Medicare | 1275 (23.2) | 949 (12.5) |
| Private insurance | 3123 (56.9) | 4473 (58.9) |
| Medicaid/other public | 789 (14.4) | 1362 (17.9) |
| Uninsured | 298 (5.5) | 815 (10.7) |
| Medication type | ||
| Oral only | 2488 (45.4) | 4625 (60.8) |
| IV only | 390 (7.1) | 133 (1.8) |
| Both | 1766 (32.2) | 751 (9.9) |
| Neither | 841 (15.3) | 2090 (27.5) |
ID resolution
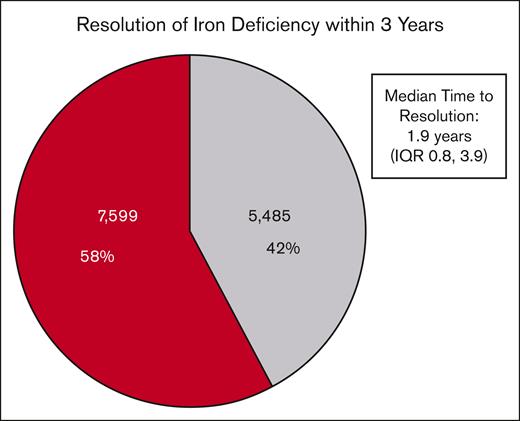
The majority of patients in the cohort (7599, 58.1%) did not have documented resolution of ID within 3 years of diagnosis despite multiple ferritin measurements (Figure 2). Among the remaining patients who had documented ID resolution (5485, 41.9%), the median time to resolution was 1.9 years (IQR, 0.8-3.9).
Rate of resolution of ID and time to resolution. Of 13 084 patients in the final study cohort, 5485 (42%) had documented ID resolution within 3 years of their initial diagnostic ferritin level. The median time to resolution among these patients was 1.9 years (IQR, 0.8-3.9). There were 7599 patients (58%) who did not have documented resolution within 3 years (eg, at least 1 additional ferritin level of <50 ng/mL).
Rate of resolution of ID and time to resolution. Of 13 084 patients in the final study cohort, 5485 (42%) had documented ID resolution within 3 years of their initial diagnostic ferritin level. The median time to resolution among these patients was 1.9 years (IQR, 0.8-3.9). There were 7599 patients (58%) who did not have documented resolution within 3 years (eg, at least 1 additional ferritin level of <50 ng/mL).
Patients with resolution had a median of 4 ferritin values measured during the 3-year study period (IQR, 3-6). The median ferritin level at diagnosis was 12 ng/mL (IQR, 7-19) and at resolution was 78 ng/mL (IQR, 60-131).
Patients without resolution had a median of 2 ferritin values measured (IQR, 2-4). The median ferritin level at diagnosis in these patients was 10 ng/mL (IQR, 6-16).
ID resolution at 1 year
We conducted a sensitivity analysis in which we evaluated resolution of ID within 1 year of diagnosis. We found that 882 patients had documented resolution of ID by this time point. This equates to 6.8% of all patients in the study cohort (N = 13 084), and 16.1% of patients with resolution within 3 years of diagnosis (n = 5485).
Factors associated with resolution of ID
Several factors were associated with a higher likelihood of resolution of ID (Table 2). First, with increasing age, the likelihood of resolution of ID increased as well (aHR, 1.26; 95% CI, 1.18-1.35; P < .001 for age 40-59 years vs age 18-39 years; and aHR, 1.56; 95% CI, 1.44-1.69; P < .001, for age ≥60 years). Second, male sex was associated with increased likelihood of resolution relative to female sex (aHR, 1.58; 95% CI, 1.48-1.70; P < .001). Third, patients with Medicare insurance had an increased likelihood of resolution of ID compared with private insurance (aHR, 1.13; 95% CI, 1.05-1.21; P = .001). Finally, treatment with IV iron alone (aHR, 2.96; 95% CI, 2.66-3.30; P < .001) and treatment with both oral and IV iron (aHR, 1.93; 95% CI, 1.81-2.06; P < .001) were associated with increased likelihood of resolution compared with those treated with oral iron alone.
Association of patient characteristics and resolution of ID
| Characteristic . | aHR . | 95% CI . | P . |
|---|---|---|---|
| Age, y | |||
| 40-59 vs 18-39 | 1.26 | (1.18-1.35) | <.001 |
| ≥60 vs 18-39 | 1.56 | (1.44-1.69) | <.001 |
| Sex | |||
| Male vs female | 1.58 | (1.48-1.70) | <.001 |
| Race | |||
| Asian vs White | 0.89 | (0.78-1.02) | .1 |
| Black vs White | 0.73 | (0.66-0.80) | <.001 |
| Multiracial vs White | 0.97 | (0.79-1.18) | 1.0 |
| Insurance | |||
| Medicaid/other public vs private | 1.03 | (0.95-1.12) | .4 |
| Medicare vs private | 1.13 | (1.05-1.21) | .001 |
| Uninsured vs private | 0.97 | (0.86-1.10) | 1.0 |
| Medication type | |||
| IV only vs oral only | 2.96 | (2.66-3.30) | <.001 |
| Both vs oral only | 1.93 | (1.81-2.06) | <.001 |
| Neither vs oral only | 0.86 | (0.79-0.93) | <.001 |
| Characteristic . | aHR . | 95% CI . | P . |
|---|---|---|---|
| Age, y | |||
| 40-59 vs 18-39 | 1.26 | (1.18-1.35) | <.001 |
| ≥60 vs 18-39 | 1.56 | (1.44-1.69) | <.001 |
| Sex | |||
| Male vs female | 1.58 | (1.48-1.70) | <.001 |
| Race | |||
| Asian vs White | 0.89 | (0.78-1.02) | .1 |
| Black vs White | 0.73 | (0.66-0.80) | <.001 |
| Multiracial vs White | 0.97 | (0.79-1.18) | 1.0 |
| Insurance | |||
| Medicaid/other public vs private | 1.03 | (0.95-1.12) | .4 |
| Medicare vs private | 1.13 | (1.05-1.21) | .001 |
| Uninsured vs private | 0.97 | (0.86-1.10) | 1.0 |
| Medication type | |||
| IV only vs oral only | 2.96 | (2.66-3.30) | <.001 |
| Both vs oral only | 1.93 | (1.81-2.06) | <.001 |
| Neither vs oral only | 0.86 | (0.79-0.93) | <.001 |
There were also several variables associated with a lower likelihood of ID resolution. Black patients were less likely to achieve resolution relative to White patients (aHR, 0.73; 95% CI, 0.66-0.80; P < .001). Additionally, those who were not treated with iron supplementation of any kind were associated with a lower likelihood of resolved ID than those treated with oral iron (aHR, 0.86; 95% CI, 0.79-0.93; P < .001).
Treatment by sex and race
Regarding treatment by sex, there was no difference (P = .26) between the proportion of women with ID who received treatment of any kind (8807 of 11 325 women, 77.8%) compared with the proportion of men who received treatment (1346 of 1759 men, 76.5%). Regarding race, a higher proportion (P < .001) of Black patients (1570 of 1771, 88.7%) received treatment compared with White patients (7819 of 10 376, 75.4%).
Discussion
In this retrospective study of electronic medical record data, we sought to estimate the proportion of ID resolution and time to resolution in a statewide health system. We identified 13 084 patients over a 10-year period with a laboratory diagnosis of ID and at least 1 subsequent laboratory assessment of iron stores within 3 years of diagnosis. The majority of patients (58.1%) did not achieve resolution in that 3-year period. Those that did have documented resolution (41.9%) had a median time to resolution of 1.9 years. Several factors were associated with a higher likelihood of achieving resolution, including older age, male sex, Medicare insurance, and IV iron treatment. Black race was associated with a lower likelihood of resolution.
The high proportion of persistent ID in this cohort suggests that providers may be failing to recognize laboratory evidence of ID. This condition can present across the life span: bleeding can cause ID in both young women with heavy menses and older men with colon cancer; malnutrition can occur in children with suboptimal intake and older individuals with malabsorptive conditions. Thus, a wide variety of providers, from primary care providers to general surgeons, must be able to suspect and diagnose ID. However, up to 1 in 4 providers misinterpret laboratory assessments of iron stores,22 and lack of awareness of the symptoms of NAID may lead providers to not offer treatment in the absence of anemia. This may result in large numbers of untreated patients with symptomatic NAID, reducing quality of life and leading to increased health care use.
Furthermore, the estimated time to resolution of ID in our study, almost 2 years from diagnosis in the subset of patients with documented resolution, suggests inefficiencies in treatment delivery. There are likely many factors driving this prolonged time to resolution. Intolerance of oral iron is common, and there are often challenges in obtaining insurance coverage for IV iron. Providers may not immediately recognize laboratory evidence of ID, or another provider may recognize it later on, leading to delayed treatment initiation. There may also be socioeconomic factors precluding access to efficient medical care. Regardless of the exact explanation, there appears to be a substantial gap between the most efficient treatment of ID (∼2 months with IV iron treatment) and what is occurring in real-world clinical practice.
Several variables associated with the likelihood of ID resolution merit further discussion. We found that increasing age, Medicare insurance, and male sex were associated with a greater probability of ID resolution. Unexplained ID anemia (IDA) is known to be a sign of possible gastrointestinal cancer,23 which may translate to more urgency around the workup and management of this diagnosis in older individuals. Mortality related to colorectal cancer is 33% higher in men relative to women,24 again potentially driving increased attention to this condition in males. Additionally, in some older individuals, cessation of menstrual bleeding may have led to resolution of ID. Finally, sex-based inequities in care may also drive this finding, explained further in the subsequent paragraph.
We also found that female sex and Black race were associated with a lower likelihood of resolution of ID. Linkages between sex and suboptimal ID care are well documented.25 In 1 study of adolescent women presenting to the emergency department with heavy menstrual bleeding, only 8.9% had iron studies with serum ferritin performed; of these, 78.3% had laboratory results consistent with ID, but only 12.4% were discharged on iron therapy.26 Our finding that there was no difference in the proportion of men and women who received iron treatment implies that women may have ongoing risk factors (eg, menorrhagia) for ID that can affect treatment efficacy. Regarding race, anemia and ID are common in pregnant individuals, with Black patients having the highest rates of both.8,27 We found that, despite receiving more treatment overall, Black patients were less likely than White patients to achieve ID resolution. It is possible that Black patients receive IV iron at lower rates, have less management of ongoing comorbidities such as menorrhagia, or suffer from other limitations in care delivery quality. Our study both adds to the prior literature in reinforcing the disparities that exist in ID care and adds granularity, in that ID is both less likely to be diagnosed and treated to resolution in these populations.
The intention of this study is to highlight that ID has consequences independent of anemia, that this condition is prevalent even in developed nations, and that there are numerous challenges in the accurate diagnosis of ID. The global morbidity of ID is undoubtedly linked primarily to IDA, and the nutritional, economic, and public health drivers of this end-stage manifestation of ID. Although IDA is not nearly as prevalent in the United States, there are likely many individuals who suffer the consequences of undiagnosed NAID and IDA. The proper diagnosis of ID involves an accurate laboratory evaluation including ferritin and TSAT values, with the requisite dietary considerations for the latter. Although a focus on ferritin allowed for the best estimation of ID resolution in this study, a ferritin value alone is not sufficient for a thorough evaluation of ID.
Limitations of this study include its retrospective nature, precluding our ability to gather important data such as reasons for ferritin testing, etiology of ID in each patient, etc. It is possible that patients may have received care at other institutions outside the MHFV system, limiting completeness of data. We attempted to mitigate this issue by requiring patients to have at least 2 ferritin values measured within 3 years, indicating ongoing engagement with the MHFV system. Ideally, multiple iron parameters, particularly TSAT, would have been included in the laboratory assessment. However, given the requirement that iron and TSAT values be drawn while fasting, the inability to determine this retrospectively, and the lack of impact of fasting on ferritin validity, we opted to use ferritin alone for this study. We were unable to determine whether patients were symptomatic from ID and cannot determine whether providers decided not to offer treatment in the absence of clear symptoms and/or anemia. We were also unable to determine when concomitant inflammation may have falsely elevated ferritin values, leading to some patients with ID being classified as iron replete. Finally, our time-to-resolution estimate is limited by the lack of standardized posttreatment ferritin testing and, as such, the true time to resolution in this cohort may be shorter or longer than 1.9 years.
In conclusion, we found that in an American statewide health system, there was a high proportion of persistent ID (58% of patients within 3 years of diagnosis), as well as prolonged times to resolution of ID (1.9 years). These findings suggest substantial gaps in both the appropriate recognition and efficient treatment of this disease, particularly in younger individuals, females, and those identifying as Black. Potential interventions to improve care delivery could include provider education about the symptoms of NAID, laboratory flags for ferritin levels suggestive of ID despite falling within test reference ranges, and increased use of IV iron.
Acknowledgments
This research was supported by the National Institutes of Health National Center for Advancing Translational Sciences, grant UL1TR002494∗. This project was also supported by grant number 1UL1RR033183 from the National Center for Research Resources and by grant Number 8 UL1 TR000114-02 from the National Center for Advancing Translational Sciences of the National Institutes of Health to the Clinical and Translational Science Institute (CTSI).
The contents of this report are solely the responsibility of the authors and do not necessarily represent the official views of the CTSI or the National Institutes of Health. The University of Minnesota CTSI is part of a national Clinical and Translational Science award consortium created to accelerate laboratory discoveries into treatments for patients.
Authorship
Contribution: J.C.C. designed the research and analyzed the data; M.S. assisted with research design; J.C.C. and J.M. performed the literature search, performed the research, and wrote the manuscript; Z.J. performed statistical analysis; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: M.S. reports research funding from Octapharma, CSL Behring, and Pfizer; and reports honoraria for advisory boards from Octapharma and Pfizer. The remaining authors declare no competing financial interests.
Correspondence: Jacob C. Cogan, Division of Hematology, Oncology and Transplantation, University of Minnesota, 516 Delaware St SE, PWB 14-100, Minneapolis, MN 55455; email: cogan029@umn.edu.
References
Author notes
Data are available on request from the corresponding author, Jacob C. Cogan (cogan029@umn.edu).