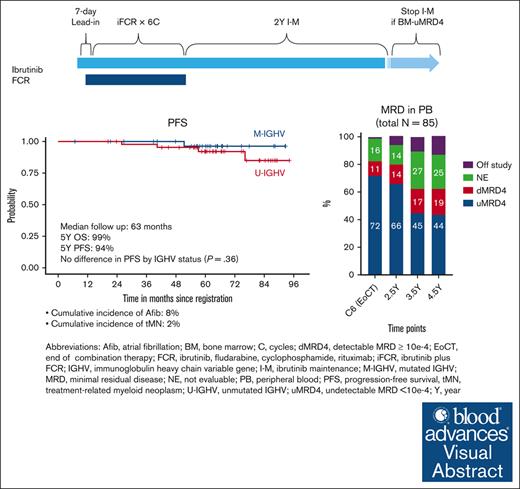
iFCR followed by 2 years of ibrutinib maintenance led to sustained deep remission in CLL irrespective of immunoglobulin heavy-chain variable region gene status.
Recurrent CLL lacked BTK mutations and retained sensitivity to ibrutinib upon retreatment.
Visual Abstract
We previously reported high rates of undetectable minimal residual disease <10−4 (uMRD4) with ibrutinib plus fludarabine, cyclophosphamide, and rituximab (iFCR) followed by 2-year ibrutinib maintenance (I-M) in treatment-naïve chronic lymphocytic leukemia (CLL). Here, we report updated data from this phase 2 study with a median follow-up of 63 months. Of 85 patients enrolled, including 5 (6%) with deletion 17p or TP53 mutation, 91% completed iFCR and 2-year I-M. Five-year progression-free survival (PFS) and overall survival were 94% (95% confidence interval [CI], 89%-100%) and 99% (95% CI, 96%-100%), respectively. No additional deaths have occurred with this extended follow-up. No difference in PFS was observed by immunoglobulin heavy-chain variable region gene status or duration of I-M. High rates of peripheral blood (PB) uMRD4 were maintained (72% at the end of iFCR, 66% at the end of 2-year I-M, and 44% at 4.5 years from treatment initiation). Thirteen patients developed MRD conversion without clinical progression, mostly (77%) after stopping ibrutinib. None had Bruton tyrosine kinase (BTK) mutations. One patient had PLCG2 mutation. Six of these patients underwent ibrutinib retreatment per protocol. Median time on ibrutinib retreatment was 34 months. The cumulative incidence of atrial fibrillation was 8%. Second malignancy or nonmalignant hematologic disease occurred in 13%, mostly nonmelanoma skin cancer. Overall, iFCR with 2-year I-M achieved durably deep responses in patients with diverse CLL genetic markers. Re-emergent clones lacked BTK mutation and retained sensitivity to ibrutinib upon retreatment. This trial is registered at www.clinicaltrials.gov as #NCT02251548.
Introduction
Attainment of durable disease control remains a particular challenge for patients with chronic lymphocytic leukemia (CLL) with unmutated immunoglobulin heavy-chain variable gene (U-IGHV). Treatment with venetoclax plus obinutuzumab (VO) for 1 year is a standard-of-care, fixed-duration treatment regimen that provides high rates (76%-87%) of undetectable minimal residual disease (uMRD) in the peripheral blood (PB).1,2 However, the CLL14 study revealed that patients with U-IGHV lost uMRD twice as fast as those with mutated IGHV (M-IGHV)3 and had significantly shorter progression-free survival (PFS).4 Although used less commonly in recent years, chemoimmunotherapy with fludarabine, cyclophosphamide, and rituximab (FCR) remains a relevant treatment approach in CLL for its potential for functional cure in patients with M-IGHV and its wide availability around the world. However, long-term follow-up from studies testing FCR demonstrated a markedly shorter duration of remission in patients with U-IGHV (median PFS of ∼4 years) compared with those with M-IGHV (12-year PFS of 51%).5-7 Attainment of durable disease control with time-limited therapy remains a particular challenge in patients with CLL U-IGHV, highlighting an unmet need for improved therapies for this subgroup. Long-term remission after FCR is limited to approximately half of the patients with M-IGHV, leaving room for optimization of outcomes for the M-IGHV subgroup.
Small molecules targeting Bruton tyrosine kinase (BTK) provide excellent disease control in CLL irrespective of IGHV status.8 However, treatment with BTK inhibitors alone rarely achieves uMRD, even after several years of continuous therapy.9 Simultaneous inhibition of BTK and BCL2 has recently emerged as a new time-limited treatment strategy with the potential to provide durable remission. Based on preclinical studies suggesting strong synergy between BTK and BCL2 inhibitors,10,11 several clinical trials have tested ibrutinib plus venetoclax as initial therapy and demonstrated high rates of PB-uMRD (55%-75%) that were comparable with those observed with VO.12-14 However, extended follow-up from these studies reported time-dependent attrition of the uMRD state in the U-IGHV subgroup at a rate of 10% per year after stopping treatment.15 These findings are especially relevant for young and fit patients with CLL for whom existing treatment regimens have limited roles in addressing their need for long-lasting, treatment-free remission.
To leverage the curative potential of FCR and the ability of ibrutinib to overcome the poor prognostic impact of U-IGHV, we designed this frontline study to investigate whether time-limited iFCR (ibrutinib plus FCR) followed by 2-year ibrutinib maintenance (I-M) could provide sustained deep remission for young and fit patients with CLL irrespective of IGHV status. We previously reported initial results from this phase 2 study at a median follow-up of 17 months.16 Here, we report updated data at a median follow-up of 63 months.
Methods
Study design
The individual institutional review board of each participating center approved this investigator-initiated, multicenter, open-label, single-arm phase 2 study (NCT02251548). Detailed eligibility and exclusion criteria of the study have been published previously.16 Briefly, eligible patients had treatment-naïve CLL or small lymphocytic lymphoma requiring therapy based on 2008 International Workshop on CLL (iwCLL) treatment criteria,17 were aged between 18 and 65 years, and had Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 1, absolute neutrophil count ≥ 0.75 × 109/L, hemoglobin ≥ 8 g/dL, and platelet count ≥ 50 × 109/L. We excluded patients with known bleeding disorders and those unable to receive primary prophylaxis for Pneumocystis jirovecii. The study originally included patients regardless of TP53 aberration status, which was defined by the detection of deletion 17p by fluorescence in situ hybridization (FISH) and/or TP53 mutation based on sequencing. FISH and TP53 mutations were assessed locally at Clinical Laboratory Improvement Amendments (CLIA)–certified laboratories. The protocol was amended on 21 March 2017 to open an expansion cohort that excluded patients with deletion 17p. All patients provided written informed consent. The study was conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki.
Procedures
Patients received ibrutinib 420 mg by mouth daily during a 7-day lead-in period, followed by the addition of up to 6 cycles of FCR administered with the continuous daily doses of ibrutinib. FCR was administered IV in 28-day cycles using fludarabine 25 mg/m2 on days 1 to 3 of each cycle, cyclophosphamide 250 mg/m2 on days 1 to 3 of each cycle, and rituximab 375 mg/m2 on day 1 of cycle 1 then 500 mg/m2 on day 1 of cycles 2 to 6. Patients in complete or partial response after at least 3 cycles of iFCR continued ibrutinib 420 mg by mouth daily for up to 2 years as maintenance (I-M). For patients in the original cohort, the continuation of ibrutinib beyond 2 years of I-M was allowed regardless of the bone marrow (BM) minimal residual disease (MRD) status. For the expansion cohort, the amended protocol required patients with BM-uMRD4 to stop ibrutinib after 2 years. Retreatment with ibrutinib was allowed when patients developed detectable MRD or clinical progression, at the discretion of the patient and investigators. The study mandated granulocyte colony-stimulating factor support throughout the combination cycles and antimicrobial prophylaxis for Pneumocystis jirovecii, herpes simplex virus, and varicella-zoster virus infections throughout the active treatment period.
Outcomes
The study had 2 coprimary end points. The first primary end point was the rate of BM-uMRD4 with complete response (CR), including CR with or without count recovery after iFCR; these results were previously reported.16 The second primary end point was the rate of sustained BM-uMRD after 2-year I-M in patients who achieved BM-uMRD4 after iFCR; these data are being newly reported in this study. Secondary end points included clinical response, PFS, overall survival (OS), and safety and tolerability of the study regimen. Exploratory end points included pharmacodynamics and correlation of clinical responses and the MRD status with genetic markers of CLL. Assessments of clinical response and the BM MRD status were performed after the last cycle of iFCR and after 1- and 2-year I-M. PB MRD was assessed every 6 months thereafter. Clinical responses were defined by the 2008 iwCLL criteria.17 uMRD4 was defined as <10−4 CLL cells per leukocytes based on multicolor flow cytometry, which was centrally assessed at Mayo Clinic, Rochester, MN or Integrated Oncology, New York, NY following methods recommended by the European Research Initiative in CLL.18 Sanger sequencing of TP53 and pyrosequencing of NOTCH1 was performed before treatment. Clinical targeted next-generation sequencing covering 90 genes was applied to PB samples at MRD conversion in a CLIA-certified laboratory.
Statistical analysis
The original cohort was designed to enroll 35 patients to estimate the primary end point, with a predefined efficacy goal of 11 or more patients achieving CR with BM-uMRD4 after iFCR. After meeting this goal, the study was amended to allow the enrollment of an additional 50 patients without deletion 17p. Eighty-five patients were enrolled in total with an expectation to have 60 patients evaluable for the second coprimary end point (after 2-year I-M). We used the Kaplan-Meier method to estimate PFS and OS. MRD conversion without clinical progression was not considered an event for the PFS analysis. Conversely, clinical progressions, including patients who experienced disease progression and received retreatment with ibrutinib per protocol, were considered events for the PFS analysis. Response and uMRD4 rates and their 95% confidence intervals (CIs) were computed and compared among subgroups by Fisher exact test. Statistical analyses were conducted using R version 4.2.0 (R Foundation for Statistical Computing).
Results
Patient characteristics
Baseline patient characteristics are summarized in Table 1. Eighty-five patients with a median age of 55 years (range, 38-65) were treated, with 52.9% of the patients having U-IGHV, 47.1% having advanced Rai stage (3 or 4), and 5.9% having TP53 aberration (5 patients, including 2 with concurrent deletion 17p and TP53 mutation, 2 with deletion 17p and wild-type TP53, and 1 with TP53 mutation without deletion 17p). NOTCH1 mutation was found in 8.6% of evaluated patients (5 of 58 patients).
Patient characteristics
| Total, N . | 85 . |
|---|---|
| Age, median (range), y | 55 (38-65) |
| Sex, N (%) | |
| Female | 29 (34.1) |
| Male | 56 (65.9) |
| Race, N (%) | |
| Caucasian | 83 (97.6) |
| Asian | 2 (2.4) |
| Ethnicity, N (%) | |
| Hispanic or Latino | 3 (3.5) |
| Non-Hispanic or Latino | 81 (95.3) |
| Unknown | 1 (1.2) |
| ECOG PS | |
| 0 | 50 (58.8) |
| 1 | 35 (41.2) |
| Rai stage, N (%) | |
| 0-2 | 42 (49.4) |
| 3-4 | 40 (47.1) |
| Missing | 3 (3.5) |
| Bulky disease (any target lesion > 5 cm), N (%) | 27 (31.8) |
| IGHV status, N (%) | |
| Mutated | 34 (40.0) |
| Unmutated | 45 (52.9) |
| Indeterminate | 6 (7.1) |
| Hierarchical FISH at enrollment, N (%) | |
| Deletion 13q | 37 (43.5) |
| Normal | 15 (17.6) |
| Trisomy 12 | 12 (14.1) |
| Deletion 11q | 16 (18.8) |
| Deletion 17p | 4 (4.7) |
| Missing | 1 (1.2) |
| Complex karyotype (3 or more aberrations), N (%) | 14 (16.9) |
| Driver gene mutation at enrollment, N mutated/N tested (%) | |
| TP53 mutation | 3/81 (3.7) |
| NOTCH1 mutation | 5/58 (8.6) |
| MYD88 mutation | 2/19 (10.5) |
| Laboratory values at enrollment, median (range) | |
| White blood cell count (109/L) | 109 (3-776) |
| Hemoglobin (g/dL) | 12 (7.8-15.9) |
| Platelet count (109/L) | 127 (43-366) |
| Beta-2 microglobulin (mg/dL) | 3.4 (0.3-12.6) |
| Total, N . | 85 . |
|---|---|
| Age, median (range), y | 55 (38-65) |
| Sex, N (%) | |
| Female | 29 (34.1) |
| Male | 56 (65.9) |
| Race, N (%) | |
| Caucasian | 83 (97.6) |
| Asian | 2 (2.4) |
| Ethnicity, N (%) | |
| Hispanic or Latino | 3 (3.5) |
| Non-Hispanic or Latino | 81 (95.3) |
| Unknown | 1 (1.2) |
| ECOG PS | |
| 0 | 50 (58.8) |
| 1 | 35 (41.2) |
| Rai stage, N (%) | |
| 0-2 | 42 (49.4) |
| 3-4 | 40 (47.1) |
| Missing | 3 (3.5) |
| Bulky disease (any target lesion > 5 cm), N (%) | 27 (31.8) |
| IGHV status, N (%) | |
| Mutated | 34 (40.0) |
| Unmutated | 45 (52.9) |
| Indeterminate | 6 (7.1) |
| Hierarchical FISH at enrollment, N (%) | |
| Deletion 13q | 37 (43.5) |
| Normal | 15 (17.6) |
| Trisomy 12 | 12 (14.1) |
| Deletion 11q | 16 (18.8) |
| Deletion 17p | 4 (4.7) |
| Missing | 1 (1.2) |
| Complex karyotype (3 or more aberrations), N (%) | 14 (16.9) |
| Driver gene mutation at enrollment, N mutated/N tested (%) | |
| TP53 mutation | 3/81 (3.7) |
| NOTCH1 mutation | 5/58 (8.6) |
| MYD88 mutation | 2/19 (10.5) |
| Laboratory values at enrollment, median (range) | |
| White blood cell count (109/L) | 109 (3-776) |
| Hemoglobin (g/dL) | 12 (7.8-15.9) |
| Platelet count (109/L) | 127 (43-366) |
| Beta-2 microglobulin (mg/dL) | 3.4 (0.3-12.6) |
ECOG PS, ECOG performance status; N, number of patients.
Treatment course and patient disposition
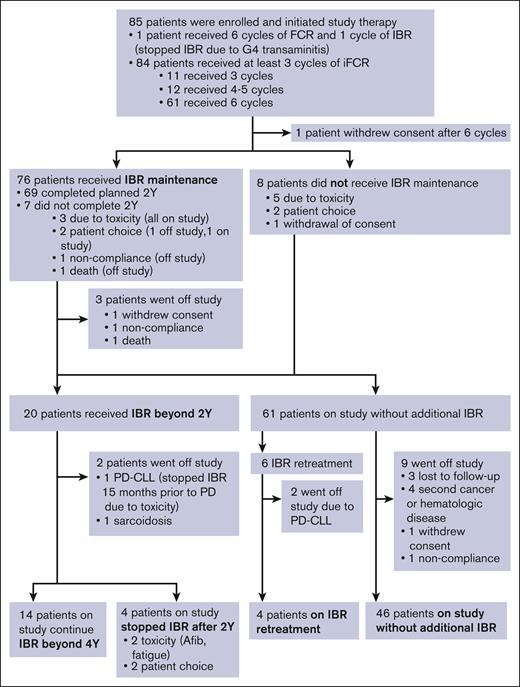
Figure 1 summarizes the treatment course and disposition of the study population. Ibrutinib administration for 1 patient was stopped during cycle 1 because of grade 4 transaminitis; the patient remained on the study and completed 6 cycles of FCR without ibrutinib. The remaining 84 patients received at least 3 cycles of iFCR. Most patients (61 patients, 71.8%) completed the planned 6 cycles of iFCR. Twelve (14.1%) received 4 to 5 cycles, and 11 (12.9%) received 3 cycles of iFCR.
Patient outcome and disposition. Afib, atrial fibrillation; FCR, fludarabine, cyclophosphamide, and rituximab; IBR, ibrutinib, PD-CLL, progression of disease with chronic lymphocytic leukemia; Y, years.
Patient outcome and disposition. Afib, atrial fibrillation; FCR, fludarabine, cyclophosphamide, and rituximab; IBR, ibrutinib, PD-CLL, progression of disease with chronic lymphocytic leukemia; Y, years.
One patient withdrew consent immediately after 6 cycles of iFCR because of no longer being able to travel to the study center. Eight patients elected not to receive I-M because of toxicity (n = 5; 1 patient each had gastrointestinal perforation, recurrent syncope, recurrent cytopenia, atrial fibrillation, and transaminitis during iFCR), patient choice (n = 2), or withdrawal of consent (n = 1). Seventy-six patients (89.4%) proceeded to I-M, 90.7% of whom (or 69 patients) completed the planned 2-year I-M. I-M was stopped for 7 patients (8.2%) before reaching 2 years because of toxicity (n = 3, including 2 patients with arthralgia and 1 with rash), patient choice (n = 2), noncompliance (n = 1), and death (n = 1, sudden cardiac death). Twenty patients continued ibrutinib beyond 2 years, including 13 patients with detectable MRD in BM or PB after 2-year I-M and 7 patients from the initial cohort who elected to remain on I-M despite having achieved BM-uMRD4. Six of these 20 patients eventually discontinued ibrutinib after 2-year I-M because of toxicity (n = 2; atrial fibrillation and fatigue in 1 patient each), patient choice (n = 2), CLL progression (n = 1), or another medical condition (n = 1; sarcoidosis requiring systemic therapy); median time on I-M was 46 months (range 28-69) for the 6 patients. In total, 61 patients discontinued ibrutinib and underwent observation, which included 8 patients who chose not to receive any I-M after iFCR, 4 who stopped I-M within 2 years because of toxicity or patient choice, and 49 patients who completed 2-years of I-M and stopped as per protocol. Six patients received ibrutinib retreatment because of clinical progression (n = 2) or detectable MRD (n = 4).
Dose reduction of fludarabine and cyclophosphamide occurred in 19 patients (22.4%), most commonly because of grade 3 or 4 thrombocytopenia. Febrile neutropenia leading to dose reduction was rare (1.2% or 1 patient). Prolonged cytopenia was the most common reason for dose reduction and discontinuation of FCR. Dose reduction of ibrutinib occurred in 10 patients (11.8%). Arthralgia was the most common adverse event associated with ibrutinib dose reduction (5.9% [or 5 patients]). One patient permanently discontinued ibrutinib because of recurrent arthralgias despite dose reduction. Three patients resumed full-dose ibrutinib after temporary dose reduction (1 patient each with transaminitis, P shigelloides infection, and need for treatment with isavuconazole owing to histoplasmosis).
Safety
Safety was similar to that presented in our previous report (supplemental Table 1).16 The most common hematologic toxicity was thrombocytopenia (84.7%, 31.8% grade ≥3 [G ≥ 3]), followed by neutropenia (67.1%; 40.0% G ≥ 3). Febrile neutropenia occurred in 11.8% (10 patients; all G ≥ 3). The most common nonhematologic toxicity was nausea (74.1%; 1.2% G ≥ 3), followed by hyperglycemia (69.4%; 8.2% G ≥ 3) and fatigue (65.9%; 0% G ≥ 3). The cumulative incidence of atrial fibrillation and hypertension was 8.2% (7 patients; 2.4% G ≥ 3) and 27.1% (23 patients; 7.1% G ≥ 3), respectively (supplemental Table 2). Atrial fibrillation occurred early in the treatment course (median 1.4 months after treatment initiation [range, 0.4-51.3]), whereas hypertension occurred later (median 40.8 months after treatment initiation [range, 1.2-80.1]). All atrial fibrillation events and 70% of hypertension events occurred while patients were on active treatment with ibrutinib. Second malignancies or nonmalignant hematologic diseases were reported in 11 patients (12.9%), mostly nonmelanoma skin cancer (n = 7). The remaining 4 patients developed myelodysplastic syndrome, clonal cytopenia of undetermined significance (CCUS), aplastic anemia, and hemophagocytic lymphohistiocytosis (n = 1 each). The myelodysplastic syndrome was considered treatment-related because the patient had new monosomy of chromosome 7 and 5%-10% BM blasts at 70 months on I-M. The patient withdrew consent to pursue allogeneic stem cell transplant. Another patient who was diagnosed with CCUS at 25 months on I-M remained on study. No clonal evolution was detected on FISH and karyotype of the patient with CCUS. Given the limited data from these 2 patients, we could not identify high-risk clonal aberrations associated with the risk of treatment-related myeloid neoplasm (tMN). No new deaths have occurred since the 1 death reported in the prior publication (sudden cardiac-related death after 6 cycles of iFCR followed by 20 months of I-M).16
Survival, clinical response, and the MRD status
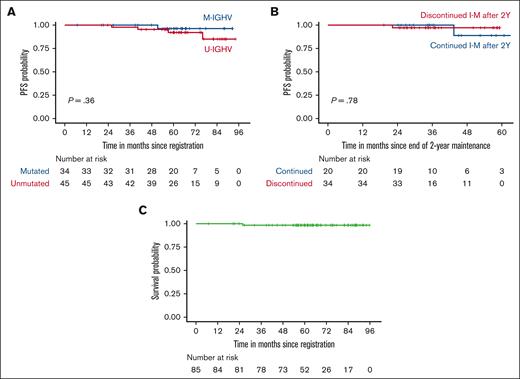
At a median follow-up of 63 months (range, 6.8-95.8), 5 patients developed CLL progression along with 1 sudden death. Survival analyses were performed without censoring patients who received retreatment with ibrutinib. Five-year PFS was 94% (95% CI, 89%-100%). Five-year OS was 99% (95% CI, 96%-100%). There was no statistically significant difference in PFS based on IGHV status or duration of I-M beyond 2 years, although the comparison was limited by the relatively low number of patients in each IGHV subgroup (Figure 2). Five-year PFS was found to be 97% when patients who received retreatment with ibrutinib were censored (supplemental Figure 1).
PFS and OS. (A) PFS of subgroups divided by IGHV mutation status. (B) PFS of patients who discontinued or continued I-M after 2 years. The x-axis indicates months since the end of 2-year I-M. (C) OS of all study participants. Patients who received ibrutinib retreatment were included in the analysis of PFS and OS.
PFS and OS. (A) PFS of subgroups divided by IGHV mutation status. (B) PFS of patients who discontinued or continued I-M after 2 years. The x-axis indicates months since the end of 2-year I-M. (C) OS of all study participants. Patients who received ibrutinib retreatment were included in the analysis of PFS and OS.
Comparison of PB and BM-uMRD results revealed high rates of concordance between the 2 compartments (95.5% concordance after completing iFCR, 94.6% after 2-year I-M). The timing of achievement of PB-uMRD4 during the course of therapy for individual patients is depicted in supplemental Figure 2. The rate of BM-uMRD4 with CR was 32.9% when assessed 2 months after completing iFCR combination therapy and 34.1% after 2-year I-M (Table 2). Subgroup analyses revealed no significant difference in CR or BM-uMRD4 rates based on IGHV mutation status (P > .05 for all time points). The rate of CR with BM-uMRD4 at best response was 55.9% for the M-IGHV group and 46.7% for the U-IGHV subgroup (odds ratio [OR], 2.4; 95% CI, 0.6-11.5; P = .25 Fisher exact test). We observed CR with BM-uMRD4 in 40.0% of patients with NOTCH1 mutation (2 of 5 patients) and 28.6% of those with complex karyotype with ≥3 abnormalities (4 of 14 patients) as best response. None of the 5 patients with TP53 aberration achieved CR with BM-uMRD4.
Clinical response and bone marrow MRD status
| Number of patients (%) . | During iFCR (after 3 cycles) . | After iFCR . | After 2-y I-M . |
|---|---|---|---|
| CR with BM-uMRD4 | 9 (10.6) | 28 (32.9) | 29 (34.1) |
| CR with BM-dMRD4 | 6 (7.1) | 2 (2.4) | 10 (11.8) |
| PR with BM-uMRD4 | 31 (36.5) | 40 (47.1) | 10 (11.8) |
| PR with BM-dMRD4 | 28 (32.9) | 11 (12.9) | 1 (1.2) |
| Off study | 1 (1.2) | 3 (3.5) | 11 (12.9) |
| Not assessed | 2 (2.4) | 1 (1.2) | 24 (28.2) |
| Other | 8 (9.4)∗ | 0 | 0 |
| Number of patients (%) . | During iFCR (after 3 cycles) . | After iFCR . | After 2-y I-M . |
|---|---|---|---|
| CR with BM-uMRD4 | 9 (10.6) | 28 (32.9) | 29 (34.1) |
| CR with BM-dMRD4 | 6 (7.1) | 2 (2.4) | 10 (11.8) |
| PR with BM-uMRD4 | 31 (36.5) | 40 (47.1) | 10 (11.8) |
| PR with BM-dMRD4 | 28 (32.9) | 11 (12.9) | 1 (1.2) |
| Off study | 1 (1.2) | 3 (3.5) | 11 (12.9) |
| Not assessed | 2 (2.4) | 1 (1.2) | 24 (28.2) |
| Other | 8 (9.4)∗ | 0 | 0 |
BM-dMRD4, bone marrow detectable MRD; BM-uMRD4, bone marrow undetectable MRD; PR, partial response.
Eight patients discontinued iFCR after 3 cycles and proceeded to ibrutinib maintenance thereafter. Clinical and MRD responses captured after 3 cycles of iFCR were considered as post-iFCR responses.
When the analysis was restricted to 49 evaluable patients with clinical response and BM MRD data available after iFCR and 2-year I-M, I-M doubled the CR rate over time (34.7% [17/49], 2 months after iFCR to 79.6% [39/49], after 2-year I-M). A total of 89.8% (44 of the 49 patients) had BM-uMRD4, two months after iFCR including 32.7% (16 patients) who achieved CR with or without count recovery with BM-uMRD4. The high rate of BM-uMRD4 was durable (77.6% [38/49] after 2 years of I-M).
In 1 of the 5 patients with TP53 aberration, detectable MRD converted to undetectable MRD in the BM after 2-year I-M; however, the patient continued to be in partial response based on the iwCLL response criteria.
PB MRD kinetics
We longitudinally tracked the PB MRD of 69 patients who were treated with at least 2 years of I-M after iFCR and had PB MRD assessed at least twice during I-M. The rate of missing MRD assessment was 1.4% at the 2 months after iFCR time point, 1.4% at the 2-year I-M time point, and <25% for all other time points. Patients, who went off study or those who did not reach the time point, were included in the intent-to-treat analysis. In the analysis including all 85 participants in the study, we observed high rates of PB-uMRD4 after iFCR (71.8%), which was sustained after 2 years of I-M (65.9%) and, for many patients, even 4.5 years after treatment initiation (43.5%; Figure 3A). Among evaluable patients, rates of PB-uMRD4 were 75.4% after iFCR and 77.9% after 2 years of I-M (supplemental Figure 3). At 4.5 years since initiation of iFCR, 64.2% of evaluable patients maintained PB-uMRD4.
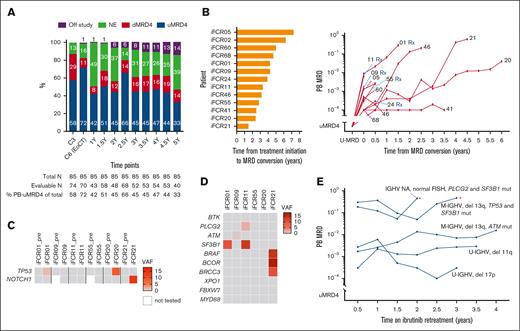
Kinetics and genomic characteristics of MRD. (A) Serial PB MRD assessments of all participants. (B) Time from initiation of iFCR until MRD conversion (left), and MRD growth kinetics without intervention in 13 patients with MRD conversion (right). Each patient’s study identification number is shown at the end of each line. Serial PB MRD data during retreatment are shown separately in panel E. (C) Status of TP53 and NOTCH1 mutations at pretreatment and at the time of MRD conversion. (D) Status of BTK, PLCG2, and CLL driver gene mutations at MRD conversion. (E) MRD kinetics during ibrutinib retreatment. Asterisks indicate clinical CLL progression. C3, cycle 3 of iFCR; del, deletion; dMRD, detectable MRD (≥10-4); EoCT, end of combination therapy with iFCR; mut, mutated; NA, not assessed; NE, not evaluable; pre, before initiation of iFCR; Rx, patient who received retreatment with ibrutinib; uMRD4, undetectable MRD (<10−4); VAF, variant allele frequency; Y, years after iFCR.
Kinetics and genomic characteristics of MRD. (A) Serial PB MRD assessments of all participants. (B) Time from initiation of iFCR until MRD conversion (left), and MRD growth kinetics without intervention in 13 patients with MRD conversion (right). Each patient’s study identification number is shown at the end of each line. Serial PB MRD data during retreatment are shown separately in panel E. (C) Status of TP53 and NOTCH1 mutations at pretreatment and at the time of MRD conversion. (D) Status of BTK, PLCG2, and CLL driver gene mutations at MRD conversion. (E) MRD kinetics during ibrutinib retreatment. Asterisks indicate clinical CLL progression. C3, cycle 3 of iFCR; del, deletion; dMRD, detectable MRD (≥10-4); EoCT, end of combination therapy with iFCR; mut, mutated; NA, not assessed; NE, not evaluable; pre, before initiation of iFCR; Rx, patient who received retreatment with ibrutinib; uMRD4, undetectable MRD (<10−4); VAF, variant allele frequency; Y, years after iFCR.
Of 69 patients included in the MRD kinetics analysis, 36 patients had sustained PB-uMRD4 at the last follow-up (sustained uMRD4), 13 had detectable MRD converted from undetectable (MRD conversion), 6 had persistently detectable MRD without meeting the iwCLL criteria for clinical progression of CLL (persistent dMRD4), and 14 had missing long-term MRD data. Because of the limited sample size per group, we could not detect any statistically significant differences in baseline characteristics of patients with or without sustained uMRD4 (supplemental Table 3). All 6 patients in the persistent dMRD4 group had U-IGHV, and 2 of the 6 patients had TP53 aberration.
Thirteen (18.8%) patients developed MRD conversion without clinical progression, mostly (76.9% [10/13]) after stopping ibrutinib (supplemental Table 4). Six of these patients had U-IGHV (46.2%). Three had deletion 11q (23.1%) and 1 had deletion 17p (7.7%). None had TP53 mutation. The median time from the initiation of iFCR to MRD conversion was 3.9 years (range, 1.5-7.3 years; Figure 3B). Most (76.9%) patients had >1 log-value increase in MRD levels, whereas 3 patients had relatively stable MRD over time.
To investigate the genetic characteristics of re-emergent CLL, we applied 90-gene targeted next-generation sequencing to PB samples collected from 7 patients at the time of MRD conversion. Two patients had newly detectable TP53 mutation at the time of MRD conversion, whereas 1 patient had new NOTCH1 mutation (Figure 3C). Only 1 patient had PLCG2 D1140G mutation at a low variant allele frequency (2.2%; Figure 3D). None had BTK mutations. Driver gene mutations were commonly identified (71.4% [5/7]), including mutations of TP53 (n = 2), SF3B1 (n = 2), ATM (n = 1), NOTCH1 (n = 1), and BRAF (n = 1). Frequent detection of driver gene mutations suggested selective expansion of mutated clones as a possible mechanism contributing to MRD conversion. BTK and PLCG2 mutations were rare, indicating that recurrent MRD should remain sensitive to BTK inhibition.
Consistent with our expectations from these genomic data, 6 patients who were retreated with ibrutinib responded to retreatment. All 6 patients had confirmed MRD conversion, including 5 who were included in the MRD kinetics analysis. Two patients also met criteria for clinical progression of CLL at the time of retreatment. During retreatment with ibrutinib, 2 patients had temporary stabilization of CLL followed by clinical progression at 32 and 23 months, respectively. Four patients responded to retreatment with ibrutinib: 3 achieved partial response and 1 had unconfirmed CR at best response (CR was unconfirmed owing to lack of BM biopsy). Serial PB MRD data from 5 patients on ibrutinib retreatment revealed that MRD growth stabilized but was not eradicated during retreatment (Figure 3D).
We conducted similar analyses for patients with persistent dMRD after iFCR and 2-year I-M (supplemental Figure 4). All 9 patients with dMRD after iFCR received I-M. One of these patients temporarily achieved PB-uMRD4 during I-M; the patient developed MRD conversion during I-M (18 months on ibrutinib), stopped I-M after 3 years owing to frequent infections, subsequently progressed with CLL after 2.5 years of off-therapy observation, and pursued treatment with acalabrutinib with good response. All other patients had dMRD throughout, including 6 patients who continued ibrutinib beyond 2 years. Further continuation of I-M reduced circulating disease burden by at least >1 log in 5 patients. One patient had >1 log increase of MRD over time. None of the 6 patients on long-term I-M developed clinical disease progression at the time of data analyses.
Discussion
iFCR followed by 2 years of I-M led to durably deep responses in young, fit patients with previously untreated CLL with relatively favorable genetic risk profiles (only 6% with TP53 aberration). We observed high rates of cytopenias, particularly thrombocytopenia, leading to FCR dose reduction in 22% of the patients. Nevertheless, the treatment was largely deliverable, with the majority of patients receiving 6 cycles of FCR and a low rate of ibrutinib discontinuation owing to toxicity during 2-year maintenance (8%). Most hematologic adverse events were limited to the combination phase and serious complications of cytopenia were relatively uncommon (febrile neutropenia in 12%, tMN in 2.4%, and hematoma in 1%). These toxicities are similar to what has previously been reported with FCR alone. Irrespective of IGHV mutation status, patients achieved high rates of PB-uMRD4 after iFCR (75%), which was sustained in most patients 4 years after completion of iFCR (64% of 53 evaluable patients and 44% of 85 enrolled patients). Two years of I-M improved the rate of CR (35% [17/49] after iFCR and 80% [39/49] after 2-year I-M in the evaluable population), indicating that extended duration of BTK inhibition can deepen lymph node response, which may be an important factor in the long PFS observed with the regimen. With limitations of cross-trial comparison, the 5-year PFS rate of 94% in this study was substantially higher than those observed in the FCR arms of other studies conducted in young and fit patients with CLL including the original MD Anderson FCR300 trial (median PFS, 6.4 years), the German CLL8 study (5-year PFS, 47%),5,6 the ECOG E1912 study (5-year PFS, 51%),19 and the UK FLAIR study (5-year PFS, 64%).20 Furthermore, the OS rate of iFCR is favorable in light of those reported among young, fit patients on continuous BTK inhibition, such as ibrutinib plus rituximab in the ECOG E1912 study (5-year PFS 89%),19 despite the fact that the study excluded patients with deletion 17p.20
Two recent studies have also reported excellent outcomes after first-line treatment with ibrutinib with chemoimmunotherapy in CLL. One study at MD Anderson combined ibrutinib with 3 cycles of fludarabine, cyclophosphamide, and obinutuzumab followed by up to 9 cycles of ibrutinib plus obinutuzumab for patients with CLL with M-IGHV.21 The other study, reported by the French Innovative Leukemia Organization (FILO) group, used 9 months of ibrutinib plus obinutuzumab, followed by 4 cycles of fludarabine, cyclophosphamide, and obinutuzumab in CLL regardless of IGHV status.22 Both studies demonstrated durable PFS (96%-98% at 3 or 4 years) and high rates of CR (60% in the MD Anderson study) or CR with BM-uMRD (62% in the FILO study). The FILO study also demonstrated that deep response was sustained, an 81% rate of PB-uMRD4 at 5 years. These data suggest the possibility that the number of chemoimmunotherapy cycles could be reduced to 4, potentially lowering the long-term risk of treatment-related myeloid neoplasms without compromising the durability of response to ibrutinib-based combinations. Targeted triplet combination regimens are alternative time-limited treatment approaches associated with high rates of BM-uMRD.2,23-25 However, follow-up with such combinations remains short, making it difficult to compare directly with our FCR-based regimen. Potential research designs that could begin to help us understand the relative efficacy and safety of iFCR would need to adapt to current standards of care in which the studies are being performed. For example, a study could compare iFCR with VO in countries where the latter regimen is widely available. In contrast, in some countries with limited availability of frontline targeted therapies, a straightforward study comparing iFCR with FCR could help clarify the added benefit of ibrutinib and, given the time-limited nature of ibrutinib in the regimen, could potentially represent an economically feasible way to deliver a time-limited therapy that incorporates a targeted therapy with the potential of durable benefit.
Although <6 cycles of chemoimmunotherapy may be as effective as 6 cycles, our data argue against the association between cumulative doses of chemoimmunotherapy and tMN. To date, the rates of tMN are similar in our iFCR study (2.4% [2/85]; 5-year median follow-up), the study by Jain et al (2% [1/45]; 3-year median follow-up),21 and the FILO study (2% [2/115]; 5-year median follow-up).22 Likewise, a retrospective analysis of 3200 patients with CLL treated with FCR or fludarabine plus cyclophosphamide demonstrated no difference in the incidence of tMN in subgroups divided by ≤6 cycles of FCR.26 Thus, the number of chemoimmunotherapy cycles added to ibrutinib may not be the most important factor contributing to tMN. Genomic (ie, mutations linked to clonal hematopoiesis) and host-related factors (ie, age) have been identified as reliable predictors of myeloid neoplasms in large-scale sequencing of healthy individuals.27 Further research is necessary to identify predictors of tMN in CLL and understand the role of CLL-directed therapy as a modifiable risk factor for tMN.
Although most patients had sustained uMRD4 after treatment cessation, 19% (13/69) developed MRD conversion during the follow-up. We could not identify enrichment of high-risk prognostic markers at baseline among patients with MRD conversion. None had TP53 aberration at baseline. Forty-six percent of the patients had U-IGHV, a rate similar to the overall study population (53%). Sequencing of re-emergent CLL demonstrated evidence of clonal evolution with newly detectable driver gene mutations of TP53 in 2 patients and NOTCH1 in 1 patient. Enrichment of CLL driver gene mutations has been commonly observed after chemoimmunotherapy alone, and we did not see any evidence that such mutations were more likely with our regimen.28,29 It is possible that these mutations may have been present at low variant allele frequencies below the reporting cutoff of 5% used for clinical-grade sequencing. Although limited data exist on genetic characteristics of re-emergent MRD after frontline CLL therapy, TP53 and NOTCH1 mutations at pretreatment were associated with lower rates of uMRD and shorter PFS in patients with CLL who received VO as first-line therapy.30 The median time from treatment initiation to MRD conversion was ∼48 months in our iFCR study, comparable with 33 months reported with VO.4 CLL reemerging after iFCR lacked BTK mutations and retained sensitivity to ibrutinib at retreatment, a finding analogous to the lack of BCL2 mutations30 and responsiveness to venetoclax retreatment in patients treated with time-limited venetoclax-based regimens.31
In conclusion, these 5-year results suggest that in patients with previously untreated CLL, treatment with iFCR followed by 2 years of I-M continues to provide sustained high rates of PB-uMRD4 and durable PFS. Acquired BTK mutations associated with ibrutinib resistance were not observed at MRD conversion, with early data suggesting that ibrutinib retreatment is a viable option for these patients to prolong time until the next class of therapy is required. Given the long time horizon for younger patients with CLL in terms of life expectancy, the significant expense associated with continuous BTK inhibition, and the logistical challenges of initiation with time-limited venetoclax-based combinations, time-limited combinations of a BTK inhibitor plus FCR represent a promising treatment strategy for this select group of patients with CLL and should be explored further in larger comparative studies, although, at this time, we are not aware of any definite plans for such a study.
Acknowledgments
The authors thank the patients and their families who participated in this trial, research coordinators, research nurses, advanced practice providers, and site staff for their support of the trial.
Pharmacyclics, LLC, an AbbVie company, provided research funding and study drug for this study. I.E.A. is supported by an American Society of Hematology Scholar Award and Leukemia and Lymphoma Society Scholar in Clinical Research Award. J.R.B. is supported by National Institutes of Health R01CA25892. M.S.D. is supported by a National Institutes of Health 1R01CA266298-01A1 award and is a Leukemia and Lymphoma Society Scholar in Clinical Research.
The funder (Pharmacyclics, LLC) had no role in study design, data collection, analysis, or interpretation of this investigator-sponsored trial. Investigators analyzed the data and wrote this manuscript independently without medical writing support.
Authorship
Contribution: J.R.B. and M.S.D. conceived and designed the study; D.M.B., H.A.W., R.B., A.A., L.S., M.O., C.A.J., P.A., S.Y.N., J.C., D.C.F., A.S.L., J.A., E.P.H., R.W.T., J.S.A., J.R.B., and M.S.D. acquired the data; I.E.A., Y.R., Y.Z., and S.T. analyzed and interpreted the data; Y.R., Y.Z., and S.T. performed statistical analyses; I.E.A. and M.S.D. drafted the manuscript; all authors edited and approved the manuscript; and M.S.D. was the principal investigator and sponsor-investigator for this trial, had full access to all the data in the study, and had final responsibility for the content of the report and the decision to submit for publication.
Conflict-of-interest disclosure: I.E.A. reports receiving consulting fees from AstraZeneca and BeiGene. D.M.B. received research funding from Ascentage, Juno/Celgene/Bristol Myers Squibb (BMS), AstraZeneca/Acerta, Allucent, Catapult, NeWave, DTRM, Genentech, BeiGene, MEI Pharma, ArQule/Merck, and AbbVie; and consulting fees from Pfizer, Pharmacyclics, Genentech, TG Therapeutics, ArQule/Merck, and AbbVie. J.M. reports receiving consulting fees from Pharmacyclics, AbbVie, and AstraZeneca. A.A. reports receiving consulting fees from Amgen, Kite, Seagen, Epizyme, Janssen, BeiGene, Incyte, TG Therapeutics, Genentech, and Lilly; and research funding from Loxo, BeiGene, and Incyte. C.A.J. reports receiving consulting fees from Kite/Gilead, BMS/Celgene, Novartis, Miltenyi, Abintus Bio, Ipsen, Instil Bio, Daiichi-Sankyo, MorphoSys, Caribou Bio, ImmPACT Bio, and AstraZeneca; and research funding from Kite/Gilead, and Pfizer. P.A. reports receiving consulting fees from Merck, BMS, Pfizer, Affimed, Adaptive, Infinity, ADC Therapeutics, Celgene, MorphoSys, Daiichi-Sankyo, Miltenyi, Tessa, Genmab, C4, Enterome, Regeneron, Epizyme, AstraZeneca, Genentech, and Xencor; research funding from Kite; institutional research funding from Merck, BMS, Affimed, Adaptive, Tensha, Otsuka, Sigma-Tau, Genentech/Roche, and IGM; and honoraria from Merck and BMS. J.C. reports receiving consulting fees from Incyte, Karyopharm, Kite, ADC therapeutics, Genmab; and research funding from Roche, Merck, AbbVie, and Bayer. A.S.L. received fees from Research To Practice; and consulting fees from Seagen, and Kite. J.A reports receiving consulting fees from BMS. E.P.H. holds equity at Leuko. J.S.A. reports receiving consulting fees from AbbVie, AstraZeneca, BeiGene, bluebird bio, BMS, Celgene, Epizyme, Incyte, Kymera, Genmab, Genentech, Ono Pharma, Mustang Bio, MorphoSys, Regeneron, Century, Kite Pharma, Lilly, Janssen, Takeda, Caribou Biosciences, Interius, and Cellectar. J.R.B. reports receiving consulting fees from AbbVie, Acerta/AstraZeneca, BeiGene, Genentech/Roche, Grifols Worldwide Operations, Hutchmed, iOnctura, Janssen, Kite, Loxo/Lilly, MEI Pharma, Numab Therapeutics, Pfizer, and Pharmacyclics; and research funding from BeiGene, Gilead, iOnctura, Loxo/Lilly, MEI Pharma, and TG Therapeutics. M.S.D. received research funding from AbbVie, AstraZeneca, Ascentage Pharma, Genentech, Novartis, Surface Oncology; and consulting fees from AbbVie, Adaptive Biosciences, Ascentage Pharma, AstraZeneca, BeiGene, BMS, Eli Lilly, Genentech, Genmab, Janssen, Merck, Mingsight Pharmaceuticals, Nuvalent, Ono Pharmaceuticals, Secura Bio, TG Therapeutics, and Takeda. The remaining authors declare no competing financial interests.
Correspondence: Matthew S. Davids, Department of Medical Oncology, Dana-Farber Cancer Institute, 450 Brookline Ave, Boston, MA 02215; email: matthew_davids@dfci.harvard.edu.
References
Author notes
Original data, individual deidentified participant data, and study protocol are available after 9 months of publication upon approved request from the corresponding author, Matthew S. Davids (matthew_davids@dfci.harvard.edu).
The full-text version of this article contains a data supplement.