Visual Abstract
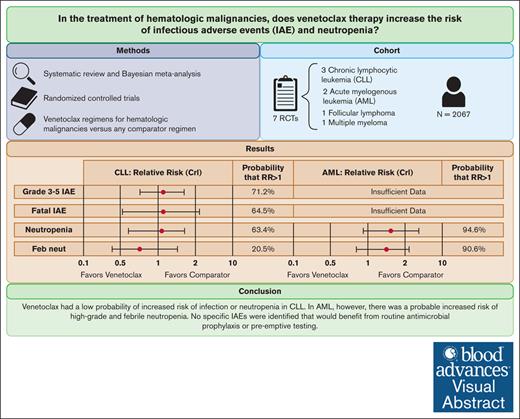
Venetoclax is a small molecule inhibitor of BCL-2 used in the treatment of acute myelogenous leukemia (AML) and chronic lymphocytic leukemia (CLL). Recent postmarketing studies of ibrutinib, another small molecule inhibitor, suggested that these agents may predispose to opportunistic infections. We sought to systematically review the randomized controlled trial (RCT) evidence of venetoclax to assess whether it predisposes patients to infectious adverse events (IAEs) and neutropenia. We systematically reviewed RCTs comparing venetoclax therapy with active or placebo controls for patients with hematologic malignancies. Data on IAEs and neutropenia were pooled by Bayesian meta-analysis, and we computed the probability of any increased risk (P[risk ratio (RR) > 1]) of IAEs or neutropenic complications. Seven RCTs were included, comprising 2067 patients. In CLL (n = 1032), there was a low probability of increased risk of high-grade (P[RR > 1] = 71.2%) and fatal IAEs (P[RR > 1] = 64.5%) and high-grade neutropenia (P[RR > 1] = 63.4%). There were insufficient data to perform a meta-analysis of IAEs in AML; however, 1 trial suggested an increased risk of IAEs with venetoclax. Furthermore, in AML (n = 642), venetoclax was associated with a high probability of increased risk of high-grade neutropenia (P[RR > 1] = 94.6%) and febrile neutropenia (P[RR > 1] = 90.6%). Our results suggest that venetoclax has a low probability of increased risk of IAEs or neutropenia in CLL. By contrast, there is likely increased risk of high-grade neutropenia and febrile neutropenia in AML. Importantly, our analyses did not identify any specific IAEs that would benefit from routine antimicrobial prophylaxis or pre-emptive testing.
Introduction
Infections remain a significant cause of morbidity and mortality among patients with hematologic malignancies.1 Various mechanisms contribute to the increased risk of infectious complications in this population including underlying disease activity, immune dysregulation, and iatrogenic immunosuppression.2 Certain drugs used in the treatment of hematologic malignancies carry a disproportionate risk of opportunistic infections (OIs), such as rituximab and corticosteroids, which are associated with an increased risk of hepatitis B reactivation and Pneumocystis jirovecii pneumonia (PJP), respectively.3,4 Awareness of the specific infectious complications associated with anticancer drugs is critical to guide clinical vigilance, screening (eg, for tuberculosis and hepatitis B), and prophylactic (eg, antifungal and antiviral) strategies.5,6
Although newer generation small molecule inhibitors have improved progression-free survival in patients with hematologic malignancies,7,8 the benefits of these treatments must be weighed against the risk of infectious adverse events (IAEs). Such complications can go unnoticed in initial randomized controlled trials (RCTs) because the trials are not adequately powered to detect rare events and may have shorter durations of follow-up than postmarketing studies. For example, ibrutinib, a Bruton tyrosine kinase inhibitor, was found to be associated with invasive fungal disease (IFD)9-11 in postmarketing studies. Alternatively, the increased risk of IAEs may only manifest in certain populations or in the context of disease-specific interactions. For example, ibrutinib treatment confers a higher risk of PJP in patients with chronic lymphocytic leukemia (CLL) than in those with Waldenström macroglobulinemia.12
Venetoclax is a small molecule inhibitor that targets the antiapoptotic protein BCL-2.13 Venetoclax is approved in the United States alone or in combination with other agents for CLL and acute myelogenous leukemia (AML).14,15 The impact of venetoclax on infectious complications may be complex. On one hand, BCL-2 inhibition may increase the risk of invasive pyogenic and fungal infections through neutropenia.16,17 On the other hand, data from patients with CLL suggest that venetoclax may mitigate some immunosuppressive effects of CLL,18 potentially reducing the risk of IAEs.
Previous 2020 reviews on venetoclax did not include an in-depth analysis of infectious complications and were biased by the inclusion of single-arm studies.19,20 To address this knowledge gap and inform patient management strategies from an infectious disease perspective, we conducted a systematic review and meta-analysis of RCTs to comprehensively evaluate the IAEs associated with venetoclax treatment for hematologic malignancies.
Methods
Protocol
This systematic review and meta-analysis adhered to a protocol registered a priori on the International Prospective Register of Systematic Reviews (PROSPERO) (CRD42021259416) and followed the guidelines from the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA),21 and the Cochrane Handbook for Systematic Reviews of Interventions.22
Search strategy
A comprehensive search strategy was developed in collaboration with a research librarian. MedLine and Embase via Ovid were searched from inception to 22 October 2022 for RCTs of adult populations on venetoclax regimens (supplemental Tables 1 and 2). There were no language restrictions; languages other than English and French were translated to English using Google Translate. Additionally, clinicaltrials.gov was searched for completed interventional studies involving venetoclax.
Study inclusion criteria
RCTs of any phase involving venetoclax for the treatment of hematologic malignancies were eligible for inclusion. This included those comparing venetoclax monotherapy or a venetoclax-containing regimen with another regimen regardless of the line of therapy, underlying hematologic malignancy, or use of antimicrobial prophylaxis. Studies were required to report on the incidence of IAEs and/or neutropenia. Studies with <30 patients in a treatment arm, studies limited to pediatric patients, conference abstracts, or gray literature were excluded.
Study selection
Search results were imported into Covidence and deduplicated.23 Unique articles were then screened for relevance by title and abstract by 2 independent reviewers (C.P. and K.K.). Selected full-text articles were evaluated by 2 independent reviewers (C.P. and K.K.) to confirm they met inclusion criteria. Afterward, the reference lists of the included articles were manually reviewed for additional pertinent studies. When disagreements on the inclusion of an article occurred, they were resolved by consensus or with a third author when necessary.
Quality assessment
Data extraction
Data were extracted by 2 reviewers (C.P. and K.K.) using a standardized form. Discrepancies were resolved by consensus or arbitrated by a third author when necessary. The following data were extracted: study authors, year of publication, study phase, blinding, malignancy type, eligibility criteria, study arms, median follow-up time (according to the study’s definition), antimicrobial prophylaxis, granulocyte colony stimulating factor (G-CSF) use, and timing of IAE data collection. Data on the incidence of neutropenia and IAEs were extracted according to the following groupings: grade 1 to 2, grade 3 to 5, fatal, total, and serious adverse events (SAEs). In addition, details were extracted on OIs and fatal IAEs.
Data analysis
A Bayesian analysis was used because it was thought to provide a more nuanced approach to the assessment of venetoclax’s risk of harm than conventional frequentist analyses, which are dichotomized. When outcome data were reported by at least 2 studies, the data were pooled by Bayesian random effects meta-analysis. Bayesian meta-analyses were conducted using the bayesmeta package in R26 using a weakly informative prior, with a population mean of 0 and standard deviation of 0.71 on the log risk ratio (RR) scale,27 which corresponds to an assumption that the effect size of most interventions in medicine falls between 0.25 and 4. The prior for heterogeneity (Tau) used those proposed by Turner et al28 for the outcome of interest. The probability of any increased risk (P(RR>1)) in the clinical outcomes of interest was computed using the posterior function.26 The primary analyses were conducted separately for the 2 approved indications for venetoclax, AML, and CLL. Additional analyses were performed for other potential indications for venetoclax. Additionally, we pooled the incidence rates of fatal OIs by inverse variance meta-analysis29,30 and compared them between treatment groups by Bayesian meta-analysis. Person-time was estimated using the median treatment exposure time; if this was not reported, then the median follow-up time was used. When only a single data point was available for an outcome, the data were instead presented with descriptive statistics.
Results
Search results
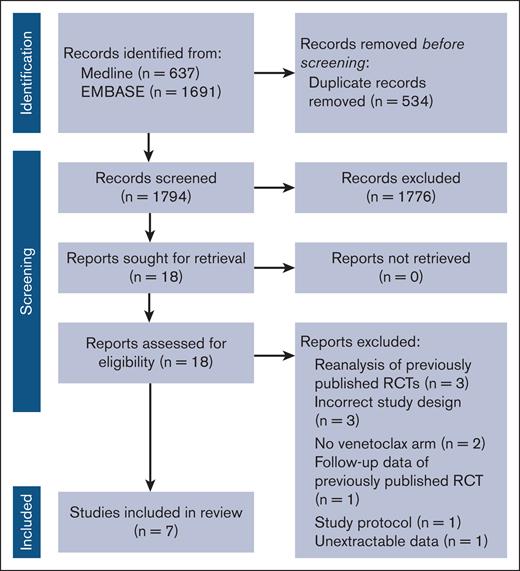
Our search strategy returned a total of 2328 results, including 1777 unique articles. A total of 1759 articles were excluded during screening, and the remaining 18 articles were read in full. Among these 18 articles, 7 were included in the systematic review and meta-analysis, and the remaining were excluded (Figure 1). Hand searching of relevant references and a review of clinicaltrials.gov did not yield additional results.
Study and population characteristics
The 7 RCTs meeting inclusion criteria31-37 were composed of 1190 and 877 patients randomized to a venetoclax-containing and a comparator regimen, respectively. The hematologic malignancies studied included CLL (n = 3),31,32,34 AML (n = 2),33,35 multiple myeloma (MM; n = 1),37 and follicular lymphoma (FL; n = 1).36 Four studies only enrolled patients with previously untreated disease,32-35 whereas the remaining studies only enrolled patients with relapsed or refractory disease.31,36,37 Median follow-up time ranged from 12.0 to 28.1 months. Treatment arms were variable with no 2 studies comparing identical regimens. The majority of patients (62.5%) included were male, and the median age ranged from 61 to 76 years. Only 1 study used different strategies for antimicrobial prophylaxis between the 2 study arms. Although prophylaxis was not included at the initiation of the study, Kumar et al later amended their protocol to administer PJP and antibacterial prophylaxis to all patients assigned to venetoclax.37 G-CSF use was reported in 3 studies32,33,36 and ranged from 32.0% to 43.5% and 34.0% to 45.8% among patients assigned to venetoclax and comparators, respectively. None of the studies elaborated on the duration of G-CSF use. A detailed description of the included studies is outlined in Table 1.
Study characteristics
| Author, y . | Phase . | Design . | Malignancy type . | Eligibility criteria . | Venetoclax regimen, N . | Comparator regimen, N . | Timing of reporting of AEs . | Antimicrobial prophylaxis . | G-CSF use . | Median follow-up time (mo) . |
|---|---|---|---|---|---|---|---|---|---|---|
| DiNardo, 2020 | 3 | Double blind | AML | Untreated disease and ineligible for standard induction therapy | Venetoclax + azacitidine, N = 286 | Placebo + azacitidine, N = 145 | 30 d after discontinuation of the study drug | Antimicrobial prophylaxis was received by 83% and 81% of patients assigned to venetoclax and comparator, respectively | Venetoclax arm: 32% Comparator arm: NR | 20.5 |
| Fischer, 2019 | 3 | Open-label | CLL | Untreated and comorbidities | Venetoclax + Obinutuzumab, N = 216 | Chlorambucil + Obinutuzumab, N = 216 | 28 d after the final dose of venetoclax or 90 d after the final dose of obinutuzumab, whichever is longer. Grade 3 and 4 IAEs were collected until 2 y after the final study drug dose | When clinically indicated, antimicrobial prophylaxis was permitted | Venetoclax arm: 43.5% Comparator arm: 45.8% | 28.1 |
| Kater, 2022 | 3 | Open-label | CLL | Untreated and either older (>65 y) or comorbidities | Venetoclax + ibrutinib, N = 106 | Chlorambucil + obinutuzumab, N = 105 | Unclear | NR | Venetoclax arm: NR Comparator arm: NR | 27.7 |
| Kumar, 2020 | 3 | Doubleblind | MM | Relapsed or refractory disease | Venetoclax + bortezomib + dexamethasone N = 194 | Placebo + bortezomib + dexamethasone, N = 97 | 30 d after the final dose of the study drug | All patients received varicella zoster virus prophylaxis. Initially, antibacterial and PJP prophylaxis were given at the discretion of the study investigator; however, later they were required for all patients receiving venetoclax | Venetoclax arm: NR Comparator arm: NR | 18.7 |
| Seymour, 2018 | 3 | Open-label | CLL | Relapsed or refractory disease | Venetoclax + rituximab, N = 194 | Bendamustine + rituximab, N = 195 | 28 d after the final dose of the study drug, up to a maximum of 2 y for venetoclax, or 90 d after the final dose of rituximab | Antimicrobial prophylaxis was permitted when clinically indicated | Venetoclax arm: NR Comparator arm: NR | 23.8 |
| Wei, 2020 | 3 | Double blind | AML | Previously untreated disease and ineligible for intensive chemotherapy | Venetoclax + cytarabine, N = 143 | Placebo + cytarabine, N = 68 | 30 d after the final dose of the study drug | All subjects with absolute neutrophil counts below 500 were admistered antimicrobial prophylaxis | Venetoclax arm: NR Comparator arm: NR | 12.0 |
| Zinzani, 2020 | 2 | Open-label | FL | Relapsed or refractory disease | Venetoclax + bendamustine + rituximab, N = 51 | Bendamustine + rituximab, N = 51 | 30 d after the final dose of the study drug or 90 d after the final dose of rituximab | NR | Venetoclax arm: 42.9% Comparator arm: 34.0% | 18.2 and 18.4 in the venetoclax and control arms, respectively |
| Author, y . | Phase . | Design . | Malignancy type . | Eligibility criteria . | Venetoclax regimen, N . | Comparator regimen, N . | Timing of reporting of AEs . | Antimicrobial prophylaxis . | G-CSF use . | Median follow-up time (mo) . |
|---|---|---|---|---|---|---|---|---|---|---|
| DiNardo, 2020 | 3 | Double blind | AML | Untreated disease and ineligible for standard induction therapy | Venetoclax + azacitidine, N = 286 | Placebo + azacitidine, N = 145 | 30 d after discontinuation of the study drug | Antimicrobial prophylaxis was received by 83% and 81% of patients assigned to venetoclax and comparator, respectively | Venetoclax arm: 32% Comparator arm: NR | 20.5 |
| Fischer, 2019 | 3 | Open-label | CLL | Untreated and comorbidities | Venetoclax + Obinutuzumab, N = 216 | Chlorambucil + Obinutuzumab, N = 216 | 28 d after the final dose of venetoclax or 90 d after the final dose of obinutuzumab, whichever is longer. Grade 3 and 4 IAEs were collected until 2 y after the final study drug dose | When clinically indicated, antimicrobial prophylaxis was permitted | Venetoclax arm: 43.5% Comparator arm: 45.8% | 28.1 |
| Kater, 2022 | 3 | Open-label | CLL | Untreated and either older (>65 y) or comorbidities | Venetoclax + ibrutinib, N = 106 | Chlorambucil + obinutuzumab, N = 105 | Unclear | NR | Venetoclax arm: NR Comparator arm: NR | 27.7 |
| Kumar, 2020 | 3 | Doubleblind | MM | Relapsed or refractory disease | Venetoclax + bortezomib + dexamethasone N = 194 | Placebo + bortezomib + dexamethasone, N = 97 | 30 d after the final dose of the study drug | All patients received varicella zoster virus prophylaxis. Initially, antibacterial and PJP prophylaxis were given at the discretion of the study investigator; however, later they were required for all patients receiving venetoclax | Venetoclax arm: NR Comparator arm: NR | 18.7 |
| Seymour, 2018 | 3 | Open-label | CLL | Relapsed or refractory disease | Venetoclax + rituximab, N = 194 | Bendamustine + rituximab, N = 195 | 28 d after the final dose of the study drug, up to a maximum of 2 y for venetoclax, or 90 d after the final dose of rituximab | Antimicrobial prophylaxis was permitted when clinically indicated | Venetoclax arm: NR Comparator arm: NR | 23.8 |
| Wei, 2020 | 3 | Double blind | AML | Previously untreated disease and ineligible for intensive chemotherapy | Venetoclax + cytarabine, N = 143 | Placebo + cytarabine, N = 68 | 30 d after the final dose of the study drug | All subjects with absolute neutrophil counts below 500 were admistered antimicrobial prophylaxis | Venetoclax arm: NR Comparator arm: NR | 12.0 |
| Zinzani, 2020 | 2 | Open-label | FL | Relapsed or refractory disease | Venetoclax + bendamustine + rituximab, N = 51 | Bendamustine + rituximab, N = 51 | 30 d after the final dose of the study drug or 90 d after the final dose of rituximab | NR | Venetoclax arm: 42.9% Comparator arm: 34.0% | 18.2 and 18.4 in the venetoclax and control arms, respectively |
NR, not reported.
Data on the extracted IAEs are presented in supplemental Table 3, and details on the fatal IAEs are presented in supplemental Table 4. Importantly, there was heterogeneity in the reporting of rare and nonfatal IAEs, especially OIs, among the included studies. Only Kumar et al37 reported all adverse events occurring during the study. The other studies applied a minimum threshold prevalence to report the adverse events ranging from >2% to 20% for grade 3/4 adverse events, >1% to 5% for SAEs, and >10% to 20% for the remainder of adverse events.
Quality assessments
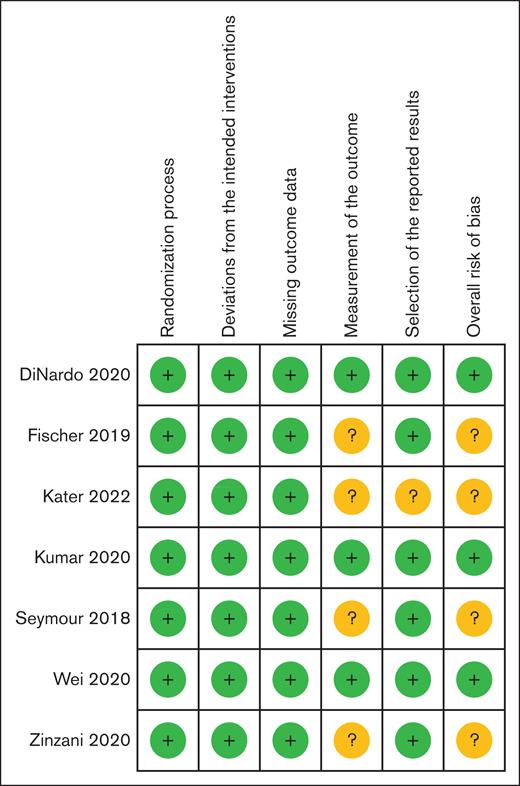
Three studies were judged at a low overall risk of bias,33,35,37 and 4 had some concerns31,32,34,36 (Figure 2). The predominant concern was related to the open-label study design and resultant potential for ascertainment bias in the reporting and/or grading of IAEs or in the differential use of antimicrobial prophylaxis. All 3 CLL trials were at some risk of bias, and both AML trials had a low risk of bias.
Cochrane RoB 2 summary. RoB 2, Cochrane risk-of-bias tool for randomized trials version 2.
Cochrane RoB 2 summary. RoB 2, Cochrane risk-of-bias tool for randomized trials version 2.
CLL
The 3 CLL RCTs were open-label and head-to-head comparisons, comprising a total of 1032 patients.31,32,34 Two studies involved patients receiving firstline therapy, and the other involved subsequent-line therapy.
The pooled RR of grade 3 to 5 and fatal IAEs were 1.11 (95% credible interval [CrI], 0.74-1.68; P[RR > 1] = 71.2%) and 1.16 (95% CrI, 0.53-2.57; P[RR > 1] = 64.5%), respectively (Table 2). The risk of high-grade neutropenia (RR = 1.07; 95% CrI, 0.64-1.74; P[RR > 1] = 63.4%) and febrile neutropenia (RR = 0.76; 95% CrI, 0.40-1.49; P[RR > 1] = 20.5%) were also similar between the 2 groups. Similarly, we did not identify a highly probable increased risk of sepsis, pneumonia, upper respiratory tract infections, or cellulitis.
Pooled CLL overall outcomes
| Outcome . | Venetoclax, n/N (%) . | Comparator, n/N (%) . | Number of studies included . | RR (95% CrI) . | P(RR > 1), % . |
|---|---|---|---|---|---|
| Total IAE | — | — | — | — | — |
| Grade 3-5 IAEs | 101/516 (19.6%) | 92/516 (17.8%) | 3 | 1.11 (0.74-1.68) | 71.2 |
| Fatal IAEs | 10/516 (1.9%) | 8/516 (1.6%) | 3 | 1.16 (0.53-2.57) | 64.5 |
| Fatal OI∗ | 1/516 (0.2%) | 2/516 (0.4%) | 3 | 0.83 (0.27-2.58) | 37.6 |
| Grade 1-2 neutropenia | 23/516 (4.5%) | 38/516 (7.4%) | 3 | 0.66 (0.37-1.21) | 8.6 |
| Grade 3-5 neutropenia | 261/516 (50.6%) | 228/516 (44.2%) | 3 | 1.07 (0.64-1.74) | 63.4 |
| Total neutropenia | 284/516 (55.0%) | 266/516 (51.6%) | 3 | 1.02 (0.63-1.63) | 54.1 |
| SAE neutropenia | — | — | — | — | — |
| Grade 1-2 febrile neutropenia | — | — | — | — | — |
| Grade 3-5 febrile neutropenia | 20/516 (3.9%) | 29/516 (5.6%) | 3 | 0.76 (0.40-1.49) | 20.5 |
| Total febrile neutropenia | — | — | — | — | — |
| SAE febrile neutropenia | 19/516 (3.7%) | 27/516 (5.2%) | 3 | 0.76 (0.39-1.50) | 20.9 |
| SAE sepsis | 7/516 (1.4%) | 7/516 (1.4%) | 3 | 1.00 (0.39-2.58) | 50.0 |
| Grade 1-2 pneumonia | 9/300 (3%) | 11/300 (3.7%) | 2 | 0.88 (0.39-1.96) | 37.2 |
| Grade 3-5 pneumonia | 30/516 (5.8%) | 30/516 (5.8%) | 3 | 1.01 (0.58-1.74) | 50.9 |
| Total pneumonia | 29/300 (9.7%) | 29/300 (9.7%) | 2 | 0.93 (0.52-1.67) | 39.7 |
| SAE pneumonia | 32/516 (6.2%) | 30/516 (5.8%) | 3 | 1.06 (0.62-1.81) | 58.2 |
| Total upper respiratory tract infections | 56/300 (18.7%) | 43/300 (14.3%) | 2 | 1.23 (0.69-2.08) | 78.5 |
| Total nasopharyngitis | — | — | — | — | — |
| Total bronchitis | — | — | — | — | — |
| Total urinary tract infections | — | — | — | — | — |
| SAE cellulitis | 4/322 (1.2%) | 0/321 (0.0%) | 2 | 1.54 (0.47-5.03) | 76.3 |
| Outcome . | Venetoclax, n/N (%) . | Comparator, n/N (%) . | Number of studies included . | RR (95% CrI) . | P(RR > 1), % . |
|---|---|---|---|---|---|
| Total IAE | — | — | — | — | — |
| Grade 3-5 IAEs | 101/516 (19.6%) | 92/516 (17.8%) | 3 | 1.11 (0.74-1.68) | 71.2 |
| Fatal IAEs | 10/516 (1.9%) | 8/516 (1.6%) | 3 | 1.16 (0.53-2.57) | 64.5 |
| Fatal OI∗ | 1/516 (0.2%) | 2/516 (0.4%) | 3 | 0.83 (0.27-2.58) | 37.6 |
| Grade 1-2 neutropenia | 23/516 (4.5%) | 38/516 (7.4%) | 3 | 0.66 (0.37-1.21) | 8.6 |
| Grade 3-5 neutropenia | 261/516 (50.6%) | 228/516 (44.2%) | 3 | 1.07 (0.64-1.74) | 63.4 |
| Total neutropenia | 284/516 (55.0%) | 266/516 (51.6%) | 3 | 1.02 (0.63-1.63) | 54.1 |
| SAE neutropenia | — | — | — | — | — |
| Grade 1-2 febrile neutropenia | — | — | — | — | — |
| Grade 3-5 febrile neutropenia | 20/516 (3.9%) | 29/516 (5.6%) | 3 | 0.76 (0.40-1.49) | 20.5 |
| Total febrile neutropenia | — | — | — | — | — |
| SAE febrile neutropenia | 19/516 (3.7%) | 27/516 (5.2%) | 3 | 0.76 (0.39-1.50) | 20.9 |
| SAE sepsis | 7/516 (1.4%) | 7/516 (1.4%) | 3 | 1.00 (0.39-2.58) | 50.0 |
| Grade 1-2 pneumonia | 9/300 (3%) | 11/300 (3.7%) | 2 | 0.88 (0.39-1.96) | 37.2 |
| Grade 3-5 pneumonia | 30/516 (5.8%) | 30/516 (5.8%) | 3 | 1.01 (0.58-1.74) | 50.9 |
| Total pneumonia | 29/300 (9.7%) | 29/300 (9.7%) | 2 | 0.93 (0.52-1.67) | 39.7 |
| SAE pneumonia | 32/516 (6.2%) | 30/516 (5.8%) | 3 | 1.06 (0.62-1.81) | 58.2 |
| Total upper respiratory tract infections | 56/300 (18.7%) | 43/300 (14.3%) | 2 | 1.23 (0.69-2.08) | 78.5 |
| Total nasopharyngitis | — | — | — | — | — |
| Total bronchitis | — | — | — | — | — |
| Total urinary tract infections | — | — | — | — | — |
| SAE cellulitis | 4/322 (1.2%) | 0/321 (0.0%) | 2 | 1.54 (0.47-5.03) | 76.3 |
Missing data (—) indicate <2 studies reporting the outcome.
All 3 CLL studies had some risk of bias on the Cochrane risk-of-bias tool for randomized trials version 2 (RoB 2) grading.
The RR was computed using the incidence.
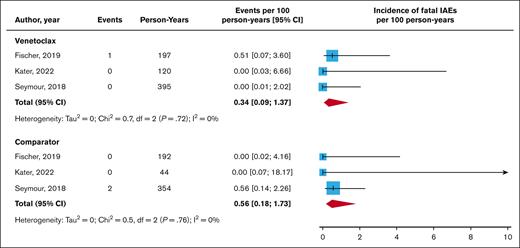
Three fatal OIs were reported in the CLL trial: 2 in the comparator group involving a case of invasive Scedosporium infection and sepsis from Listeria monocytogenes, and the third fatal OI was a case of fungal pneumonia in a patient treated with venetoclax. The incidences of fatal OIs per 100 person-years were 0.34 (95% confidence interval [CI], 0.09-1.37) and 0.56 (95% CI, 0.18-1.73) for venetoclax and comparator regimens (Figure 3), respectively (RR = 0.83; 95% CrI, 0.27-2.58; P[RR > 1] = 37.6%). Only 1 other IFD was reported; Kater et al observed 1 case (0.9%) of nonfatal bronchopulmonary aspergillosis in the venetoclax arm.34 The Kater et al study also reported 1 case each of nonfatal cytomegalovirus pneumonia and PJP among venetoclax recipients but none among controls.34
AML
The 2 included AML RCTs were double-blind and placebo-controlled, using venetoclax as the firstline agent, and comprised 642 patients in total.33,35
There were insufficient data points to compute the risk of total, fatal, and high-grade IAEs as well as fatal OIs in patients with AML. However, in the venetoclax arm of the DiNardo et al33 trial, there was a higher number of total (239 [83.6%] vs 97 [66.9%]) and high-grade (180 [62.9%] vs 74 [51.0%]) IAEs. Venetoclax was associated with a probable increased risk of high-grade neutropenia (RR = 1.71; 95% CrI, 0.87-3.17; P[RR > 1] = 94.6%) and febrile neutropenia (RR = 1.49; 95% CrI, 0.78-2.60; P[RR > 1] = 90.6%; Table 3). However, the rates of sepsis and pneumonia were comparable. No information on fatal IAEs or OIs was reported in the AML studies.
Pooled AML overall outcomes
| Outcome . | Venetoclax, n/N (%) . | Comparator, n/N (%) . | Number of studies included . | RR (95% CrI) . | P(RR>1), % . |
|---|---|---|---|---|---|
| Total IAE | — | — | — | — | — |
| Grade 3-5 IAEs | — | — | — | — | — |
| Fatal IAEs | — | — | — | — | — |
| Fatal OI | — | — | — | — | — |
| Grade 1-2 neutropenia | — | — | — | — | — |
| Grade 3-5 neutropenia | 185/429 (43.1%) | 52/213 (24.4%) | 2 | 1.71 (0.87-3.17) | 94.6 |
| Total neutropenia | — | — | — | — | — |
| SAE neutropenia | 17/429 (4.0%) | 3/213 (1.4%) | 2 | 1.62 (0.60-4.26) | 83.3 |
| Grade 1-2 febrile neutropenia | — | — | — | — | — |
| Grade 3-5 febrile neutropenia | 163/429 (38.0%) | 47/213 (22.1%) | 2 | 1.49 (0.78-2.60) | 90.6 |
| Total febrile neutropenia | — | — | — | — | — |
| SAE febrile neutropenia | 107/429 (24.9%) | 27/213 (12.7%) | 2 | 1.53 (0.71-2.97) | 87.8 |
| SAE sepsis | 24/429 (5.6%) | 16/213 (7.5%) | 2 | 0.80 (0.41-1.59) | 25.5 |
| Grade 1-2 pneumonia | — | — | — | — | — |
| Grade 3-5 pneumonia | — | — | — | — | — |
| Total pneumonia | 94/429 (21.9%) | 50/213 (23.5%) | 2 | 0.96 (0.59-1.63) | 43.3 |
| SAE pneumonia | 65/429 (15.2%) | 39/213 (18.3%) | 2 | 0.87 (0.51-1.56) | 29.8 |
| Total upper respiratory tract infections | — | — | — | — | — |
| Total nasopharyngitis | — | — | — | — | — |
| Total bronchitis | — | — | — | — | — |
| Total urinary tract infections | — | — | — | — | — |
| SAE cellulitis | — | — | — | — | — |
| Outcome . | Venetoclax, n/N (%) . | Comparator, n/N (%) . | Number of studies included . | RR (95% CrI) . | P(RR>1), % . |
|---|---|---|---|---|---|
| Total IAE | — | — | — | — | — |
| Grade 3-5 IAEs | — | — | — | — | — |
| Fatal IAEs | — | — | — | — | — |
| Fatal OI | — | — | — | — | — |
| Grade 1-2 neutropenia | — | — | — | — | — |
| Grade 3-5 neutropenia | 185/429 (43.1%) | 52/213 (24.4%) | 2 | 1.71 (0.87-3.17) | 94.6 |
| Total neutropenia | — | — | — | — | — |
| SAE neutropenia | 17/429 (4.0%) | 3/213 (1.4%) | 2 | 1.62 (0.60-4.26) | 83.3 |
| Grade 1-2 febrile neutropenia | — | — | — | — | — |
| Grade 3-5 febrile neutropenia | 163/429 (38.0%) | 47/213 (22.1%) | 2 | 1.49 (0.78-2.60) | 90.6 |
| Total febrile neutropenia | — | — | — | — | — |
| SAE febrile neutropenia | 107/429 (24.9%) | 27/213 (12.7%) | 2 | 1.53 (0.71-2.97) | 87.8 |
| SAE sepsis | 24/429 (5.6%) | 16/213 (7.5%) | 2 | 0.80 (0.41-1.59) | 25.5 |
| Grade 1-2 pneumonia | — | — | — | — | — |
| Grade 3-5 pneumonia | — | — | — | — | — |
| Total pneumonia | 94/429 (21.9%) | 50/213 (23.5%) | 2 | 0.96 (0.59-1.63) | 43.3 |
| SAE pneumonia | 65/429 (15.2%) | 39/213 (18.3%) | 2 | 0.87 (0.51-1.56) | 29.8 |
| Total upper respiratory tract infections | — | — | — | — | — |
| Total nasopharyngitis | — | — | — | — | — |
| Total bronchitis | — | — | — | — | — |
| Total urinary tract infections | — | — | — | — | — |
| SAE cellulitis | — | — | — | — | — |
Missing data (—) indicate <2 studies reporting the outcome.
Both AML studies had low risk of bias on the Cochrane risk-of-bias tool for randomized trials version 2 (RoB 2) grading of indication for venetoclax.
MM
Only 1 study on MM was included,37 which precluded meta-analysis. Progression-free survival was improved in the venetoclax arm of the Kumar et al study.37 However, overall survival was poorer in this arm, predominantly driven by an increase in fatal IAEs (8 [4.1%] vs 0 [0.0%]) despite a protocol amendment implementing antibacterial, α herpesvirus, and PJP prophylaxis to the venetoclax arm. Of the 8 fatal IAEs in the venetoclax arm, 5 (2.6%) were unspecified sepsis and 3 (1.5%) were pneumonia. A total of 9 cases (4.6%) of varicella were reported among venetoclax recipients vs 1 (1.0%) among controls. No other OIs were reported. Both high-grade neutropenia (35 [18.0%] vs 7 [7.2%]) and febrile neutropenia (5 [2.6%] vs 0 [0.0%]) were numerically higher in the venetoclax arm.
FL
Only 1 trial on FL was identified, preventing meta-analysis.36 There was 1 episode (2.0%) of fatal pneumonia in the venetoclax arm and no fatal IAEs in the comparator arm. High-grade neutropenia (29 [56.9%] vs 14 [27.5%]) and febrile neutropenia (6 [11.8%] vs 3 [5.9%]) were numerically higher in the venetoclax arm. For OIs, Zinzani et al documented 3 cases (5.9%) of PJP in the venetoclax arm but none in the comparator arm.36
Overall analyses
Pooled analyses irrespective of indication for venetoclax are presented in supplemental Table 5. The pooled RR of total and high-grade IAEs were 1.14 (95% CrI, 0.76-1.66; P[RR > 1] = 81.1%) and 1.16 (95% CrI, 0.86-1.55; P[RR > 1] = 86.6%), respectively. There was a probable increased risk of high-grade neutropenia (RR = 1.44; 95% CrI, 1.01-2.10; P[RR > 1] = 97.8%).
Discussion
This extensive review of the venetoclax RCT data suggests that venetoclax has a low probability of increasing the risk of infection or neutropenia in CLL. There was insufficient evidence to determine whether venetoclax increases the risk of IAEs in AML; however, there was a probable increase in high-grade neutropenia (94.6%) and febrile neutropenia (90.6%).
As demonstrated in early trials of venetoclax,16,17 we found a high prevalence of high-grade and febrile neutropenia. In CLL, there was only a 63.4% probability of increased risk of high-grade neutropenia and only a 71.2% probability of association with an increased risk of IAEs. Our findings may be explained by the immunomodulatory effects of venetoclax in CLL. Although venetoclax treatment results in an absolute reduction in T, B, and natural killer cells, it also restores natural killer cell function.18 Therefore, the immunosuppressive effects of venetoclax-induced neutropenia may be attenuated by a partial recovery of cellular immunity in CLL. In AML, however, a probable increased risk of neutropenia was noted, which may have driven the numerically increased risk of infection observed in the DiNardo et al study.33
Initial trials of ibrutinib failed to recognize an increased risk of rare but severe IFDs in patients with hematologic malignancies,8,38,39 and the first report suggesting the association was published 3 years after its licensure.40 Concern over ibrutinib conferring an increased risk of IFDs41,42 underscores the importance of scrutinizing the IAEs of novel small molecule inhibitors. Recent observational studies have reported a 4% to 13% incidence of probable or proven IFDs on venetoclax; however, this risk was not modified by antifungal prophylaxis.43-45 A large study pooling the safety data from 3 venetoclax CLL phase 1/2 trials found a lower prevalence of OIs of 3.1%, with a median time to OI of 4.5 months.46 Our results confirm a low incidence of fatal OIs in CLL. Furthermore, we did not identify specific infectious complications that would benefit from specific antimicrobial prophylaxis or screening measures beyond the current standard of care.
Although small molecule inhibitors significantly improve various oncologic outcomes (eg, progression- and event-free survival), it is important to weigh these benefits against the risk of infectious complications and overall survival. Although venetoclax improved progression-free survival in the MM study by Kumar et al,37 it increased the risk of all-cause mortality, powered predominantly by an increase in fatal IAEs. Therefore, in MM, the risk-benefit profile of venetoclax appears unfavorable. In contrast, in CLL, venetoclax appears more beneficial given that our results found a low probability of increased risk of IAEs or neutropenia, and the 3 included trials demonstrated improved progression-free survival with venetoclax as well as an overall mortality benefit in the Seymour et al study.31,32,34 In AML, our results suggested a probable increased risk of neutropenia, and 1 study suggested an increased risk of infection, but both AML trials demonstrated improved oncologic outcomes with 1 finding an overall mortality benefit with venetoclax.33,35
To our knowledge, this systematic review is the first investigating the impact of venetoclax on IAEs using RCT data. We used a comprehensive search strategy, and no additional articles were identified through hand searching. The inclusion of only RCTs limited the risk of selection, detection, and attrition bias that confound observational studies. Moreover, none of the included RCTs were considered at a high risk of bias. Despite these strengths, our results must be interpreted within the included studies’ contexts. First, our study may be underpowered to detect differences in rare IAEs, and the included studies were inconsistent about reporting infrequent IAEs. Second, because there are few RCTs published on venetoclax, RCTs were included regardless of underlying malignancy, line of therapy, and concomitant chemotherapy regimen, which introduces some interstudy heterogeneity. Third, studies were pooled regardless of the dose or duration of venetoclax, which may obscure dose- or duration-dependent toxicities. Fourth, we were unable to perform time-dependent analyses because these data were unavailable, which could introduce bias from a competing risk of malignancy-specific mortality. Fifth, an inherent limitation of including open-label studies is the possibility of ascertainment bias in the reporting/grading of IAEs and the differential use of antimicrobial prophylaxis or G-CSF. Sixth, there was significant heterogeneity in the reporting and definitions of IAEs, and many studies did not provide sufficient detail for more granular analyses. Lastly, multiple hypothesis testing increases the risk of type 1 error, and these results must be interpreted with this consideration. Nevertheless, our study is the most current and comprehensive assessment of IAEs of venetoclax to date, and our probabilistic analysis provides some insight beyond the traditional P value–driven dichotomous analysis.
Conclusions
Venetoclax had a low probability of increased risk of infection or neutropenia in CLL. In AML, however, a probable increased risk of high-grade and febrile neutropenia was noted, with 1 study suggesting an increased risk of IAEs. The risk of OIs was overall low. Our analyses did not identify any specific IAEs that would necessitate additional antimicrobial prophylaxis or pre-emptive testing. Nevertheless, we recommend that postmarketing studies continue surveillance of the IAEs associated with venetoclax treatment.
Acknowledgment
The authors thank Ibtisam Mahmoud, research librarian, for her assistance in designing the search strategy.
This work had no sources of funding.
Authorship
Contribution: M.P.C., K.D., V.C., and C.P. conceptualized the study and investigated the data; M.P.C., K.D., V.C., C.P., and T.C.L. designed the methodology; C.P., K.D., and V.C. validated the data; C.P. and T.C.L. provided formal analysis; C.P. and M.P.C. provided resources; C.P., K.D., V.C., A.N., O.D.L., M. Sorin, and K.K. curated the data; C.P., M.P.C., E.G.M., T.C.L., M. Sebag, and K.D. wrote the original draft; C.P. provided visualization; M.P.C. supervised the project; C.P., M.P.C., and K.D. administered the project; and all authors reviewed and edited the manuscript.
Conflict-of-interest disclosure: M.P.C. reports operating grants from the Canadian Institutes of Health Research during the conduct of the study and salary support from the Fonds de Recherche du Québec – Santé, both unrelated to this work; personal fees from GEn1E Lifesciences and Nomic Bio as a member of the scientific advisory board; personal fees from AstraZeneca as a scientific consultant; research support from Cidara Therapeutics, Scynexis, Inc, and Amplyx Pharmaceutics during the conduct of the study but outside the submitted work; is the cofounder of Kanvas Biosciences Inc and owns equity in the company; and has pending patents, including: (1) methods for detecting tissue damage, graft-versus-host disease, and infections using cell-free DNA profiling, (2) methods for assessing the severity and progression of severe acute respiratory syndrome coronavirus 2 infections using cell-free DNA pending, and (3) rapid identification of antimicrobial resistance and other microbial phenotypes using highly multiplexed fluorescence in situ hybridization. M. Sebag is on the advisory boards of Janssen, Pfizer, Amgen, Novartis, Gilead, Bristol Myers Squibb, Focus Therapeutics, and Sanofi, and reports funding from the Canadian Institutes of Health Research and Janssen Pharmaceuticals. E.G.M. and T.C.L. have operating grants from the Canadian Institutes of Health Research and receive salary support from the Fonds de Recherche du Québec – Santé outside of this work. The remaning authors declare no competing financial interests.
Correspondence: Connor Prosty, Infectious Diseases, McGill University, 1001 Decarie Blvd E5-1820, Montréal, QC H4A 3J1, Canada; email: connor.prosty@mail.mcgill.ca; and Matthew P. Cheng, Infectious Diseases, McGill University, 1001 Decarie Blvd EM3.3218, Montréal, QC H4A 3J1, Canada; email: matthew.cheng@mcgill.ca.
References
Author notes
Data are available on request from the corresponding author, Connor Prosty (connor.prosty@mail.mcgill.ca).
The full-text version of this article contains a data supplement.