Maintenance treatment was safe, with low rates of infections and chronic GVHD, and minimal impact on T-cell immune reconstitution.
With 2-year follow-up, Ven/Aza maintenance showed a manageable safety profile and encouraging responses in high-risk MDS/AML.
Visual Abstract
We conducted a phase 1 trial assessing safety and efficacy of prophylactic maintenance therapy with venetoclax and azacitidine (Ven/Aza) for patients with high-risk myelodysplastic syndromes (MDS)/acute myeloid leukemia (AML) undergoing reduced intensity allogeneic stem cell transplantation (allo-SCT) after Ven and fludarabine/busulfan conditioning (Ven/FluBu2 allo-SCT) with tacrolimus and methotrexate as graft-versus-host disease (GVHD) prophylaxis. Among 27 patients who underwent Ven/FluBu2 allo-SCT (55.6% with prior Ven exposure, and 96% with positive molecular measurable residual disease), 22 received maintenance therapy with Aza 36 mg/m2 intravenously on days 1 to 5, and Ven 400 mg by mouth on days 1 to 14 per assigned dose schedule/level (42-day cycles × 8, or 28-day cycles × 12). During maintenance, the most common grade 3-4 adverse events were leukopenia, neutropenia, and thrombocytopenia, which were transient and manageable. Infections were uncommon (n = 4, all grade 1-2). The 1-year and 2-year moderate/severe chronic GVHD rates were 4% (95% confidence interval [CI], 0.3%-18%) and 22% (95% CI, 9%-40%), respectively. After a median follow-up of 25 months among survivors, the median overall survival (OS) was not reached. Among the 22 patients who received Ven/Aza maintenance, the 2-year OS, progression-free survival, nonrelapse mortality, and cumulative incidence of relapse rates were 67% (95% CI, 43%-83%), 59% (95% CI, 36%-76%), 0%, and 41% (95% CI, 20%-61%), respectively. Immune monitoring demonstrated no significant impact on T-cell expansion but identified reduced B-cell expansion compared with controls. This study demonstrates prophylactic Ven/Aza maintenance can be safely administered for patients with high-risk MDS/AML, but a randomized study is required to properly assess any potential benefit. This trial was registered at www.clinicaltrials.gov as #NCT03613532.
Introduction
Relapse is the leading cause of death in patients with myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML) who undergo allogeneic stem cell transplantation (allo-SCT). Modifying the conditioning strategy and therapy in the early posttransplant period represent opportunities to improve outcomes in the majority of patients with MDS/AML, comprising mainly of older patients with higher risk disease features.1-3 Adverse risk disease features and persistent measurable residual disease (MRD) before allo-SCT increase the risk of relapse and are consistently associated with shortened survival.1,2,4-8 Broadly intensifying conditioning regimens with additional chemotherapy and administration of prophylactic maintenance hypomethylating agent therapy after transplant have been met with mixed results.9-11 The ideal MRD- eradicating regimen must improve the chance of curative consolidation with allo-SCT without substantially increasing toxicity or nonrelapse mortality.7
We recently published the results of adding venetoclax to fludarabine/busulfan (Ven/FluBu2) reduced intensity conditioning (RIC) allo-SCT for patients with MDS/AML at high-risk for relapse, defined as those with adverse European LeukemiaNet risk; persistent flow MRD; and mutations in TP53, RAS-pathway, and mutations associated with secondary AML ontogeny.1,12 The regimen was safe without increased infectious complications or impact on donor engraftment. The overall survival (OS) and progression-free survival (PFS) at 1 year were 67% and 53%, respectively.12 Deep sequencing detected molecular MRD before transplant in 18 of 22 patients (82%), which persisted in 9 of 18 patients (50%) by day +100 after transplant, suggesting that, although safe, the addition of Ven to the conditioning regimen still did not achieve the goal of early eradication of MRD in half the patients. Prior studies of preemptive azacitidine (Aza) maintenance therapy after transplant for patients with MDS/AML upon detection of MRD or mixed chimerism demonstrated delay in hematologic relapse, but Aza maintenance itself has not shown a survival benefit in a randomized setting.9,13 Compared with Aza alone, combination Ven and Aza (Ven/Aza) increases rates of remission, survival time, and MRD conversion in patients with AML with overt morphologic disease.14,15 We hypothesized that reduced doses of Ven/Aza maintenance could be safely administered after Ven/FluBu2 allo-SCT without impairing immune reconstitution or increasing risk of infection and graft-versus-host disease (GVHD). Here, we report the safety, tolerability, and efficacy from the phase 1 dose-schedule finding trial of prophylactic Ven/Aza maintenance for patients with high-risk MDS/AML after Ven/FluBu2 allo-SCT.
Methods
Study design
We previously reported the safety and efficacy of Ven/FluBu2 without planned maintenance (Cohort 1; N = 22).12 This is a nonrandomized, open-label phase 1 study of prophylactic posttransplant maintenance therapy with combination Ven/Aza for patients with high-risk MDS/AML (Cohort 2) conducted at the Dana-Farber Cancer Institute (www.clinicaltrials.gov identifier: #NCT03613532). Here, we present the analysis of a separate group of patients enrolled sequentially into Cohort 2 who received Ven/FluBu2 at the recommended phase 2 dose (RP2D) followed by planned study maintenance therapy. The primary objectives were to determine safety, tolerability, and an RP2D of prophylactic maintenance Ven/Aza. Secondary objectives were to measure the clinical outcomes. Exploratory objectives were to evaluate for molecular and flow MRD, assess for immune reconstitution, and measure quality of life (QOL). The study was approved by our institutional review board, and participants provided informed written consent. All authors had access to the primary clinical trial data.
Study patients and conditioning regimen
All patients received RIC chemotherapy according to previously published RP2D of Ven/FluBu2, and GVHD prophylaxis with methotrexate and tacrolimus.12
Eligible population
Eligible patients were aged ≥18 years with high-risk AML/MDS/MDS-myeloproliferative neoplasm (MPN) overlap syndromes who were not eligible for a myeloablative conditioning strategy. For detailed eligibility, please refer to the supplemental Methods. Prior Ven therapy was allowed. Between days +42 and +90, patients were allowed to initiate maintenance therapy if the following criteria were met: absence of morphologic disease (defined as ≥5% bone marrow blasts on maintenance screening bone marrow biopsy), no overall grade 2-4 acute GVHD necessitating prednisone of ≥0.5 mg/kg daily, total bilirubin <2 × upper limit of normal, aspartate aminotransferase and alanine aminotransferase of <3 × upper limit of normal, and CrCl of >30 mL/min. Patients were required to have an absolute neutrophil count (ANC) of ≥1.0 x 103/μL without growth factor support and a platelet count of ≥50 x 103/μL without transfusion within 7 days of starting maintenance therapy unless there was evidence of molecular or cytogenetic disease.
Protocol maintenance treatment
Maintenance treatment included Aza 36 mg/m2 given intravenously on days 1 to 5 plus Ven 400 mg daily given orally on days 1 to 14 regardless of dose cycle length. Patients either received maintenance every 42 days for 8 planned cycles in dose level (DL) 1, or every 28 days for 12 planned cycles in DL2. Antibacterial prophylaxis was required in the first cycle and recommended later at ANC of <0.5 x 103/μL. Antifungal prophylaxis was not required but allowed.16 Dose reductions were allowed for new onset or recurrent grade 4 thrombocytopenia or neutropenia lasting >7 days, including Aza reduction to 24 mg/m2 per day on days 1 to 5, followed by 50% Ven dose reduction, and lastly reduction in Ven duration from 14 to 7 days. Patients were taken off study treatment if donor lymphocyte infusion was administered for any reason. Tacrolimus taper was initiated based on clinician discretion with the goal to be off immune suppression by 6 to 9 months in the absence of GVHD.
Safety and response assessments
All patients were included in safety and efficacy analyses. Adverse events (AEs) were assessed per US National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0. Dose-limiting toxicities were captured during first cycle and defined as any treatment-related death, failure of ANC to recover to ≥0.5 × 103/μL, sustained ANC level of <0.5 x 103/μL or platelet level of <25 x103/μL for >2 weeks with hypocellular marrow, grade 4 neutropenia complicated by infection, and any nonhematologic grade 4 toxicities or tumor lysis syndrome event. Responses after transplant were assessed at approximately day +28, day +100, 6 months, and 12 months, and then per standard of care or at disease progression using modified International Working Group criteria for MDS, or European LeukemiaNet criteria for AML.3,17 MRD by multiparametric flow cytometry (estimated lower level of detection of <0.02%) was performed by Hematologics, Inc.
Statistical analysis
Clinical characteristics and safety and laboratory data were summarized descriptively.
OS and PFS were estimated by Kaplan-Meier method. Cumulative incidence of relapse (CIR) and nonrelapse mortality were estimated in the competing risks framework treating each other as a competing event. Cumulative incidence of acute and chronic GVHD were also estimated in the competing risks framework, treating relapse or death without developing GVHD as a competing event. To identify prognostic factors, univariable Cox regression analysis was performed. All analyses were based on data cutoff of 31 July 2023. QOL surveys, including FACT-BMT, were conducted before transplant and on cycle 1, day 1; cycle 2, day 1; and cycle 4, day 1 of maintenance treatment for exploratory descriptive analysis. Pairwise comparisons were made using Wilcoxon signed rank test. All P values were 2-sided at the significance level of .05. Multiplicity was not considered. All analyses were performed using SAS 9.4 (SAS Institute Inc, Cary, NC), and R version 3.6.1 (the CRAN project, www.cran.r-project.org).
Additional details for methods for trial eligibility, trial design, exploratory, and correlative studies including clinical next-generation sequencing, BH3 profiling, immune monitoring, and research-level duplex genetic sequencing on paired samples are provided in supplemental Methods.
Results
Patients and disease characteristics
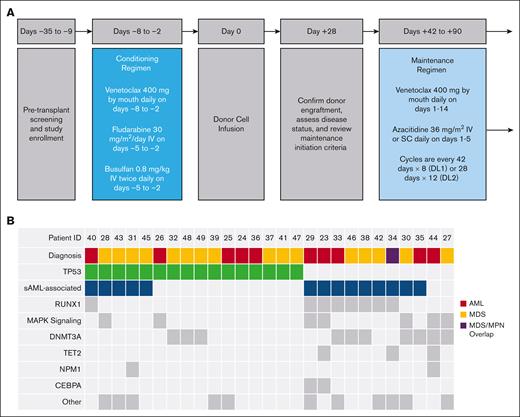
Between May 2020 and February 2022, 27 patients (17 males and 10 females) were enrolled with the intent to administer Ven/FluBu2 allo-SCT followed by Ven/Aza maintenance therapy and included in the primary analysis (Table 1; Figure 1). Patients had a median age of 67 years (range, 47-78 years). Pretransplant disease status included 10 patients (37%) with AML (9 in complete remission [CR] and 1 in morphologic leukemia free state), 16 patients (59.3%) with MDS (1 in CR, 7 in marrow CR with hematologic improvement, 4 in marrow CR without hematologic improvement, and 4 with 5%-10% excess blasts), and 1 patient (3.7%) with MDS/MPN in CR. Six cases (22.2%) were considered therapy-related myeloid neoplasms. Flow MRD positivity was detected in 15 of 27 (55.6%) immediately before transplant. Previous Ven exposure was also common (15 of 27; 55.6%), including 2 of 15 patients who were refractory to prior hypomethylating agent/Ven treatment. TP53 mutations were detected at diagnosis in 16 of 27 patients (59.3%), including 8 with monoallelic and 8 with biallelic status. Complex karyotype was present at baseline in 10 of 27 patients (37%).
Patient baseline demographics and clinical/disease characteristics
| Characteristic . | Total, intent-to-treat cohort (N = 27) . |
|---|---|
| Median age (range), y | 67 (47-78) |
| ≥65, n (%) | 18 (66.7) |
| Median donor age (range), y | 28 (20-64) |
| Patient sex, n (%) | |
| Female | 10 (37) |
| Male | 17 (63) |
| Donor sex, n (%) | |
| Female | 11 (40.7) |
| Male | 16 (59.3) |
| Male patient, female donor, n (%) | 4 (14.8) |
| ECOG performance status, n (%) | |
| 0 | 6 (22.2) |
| 1 | 12 (44.4) |
| 2 | 9 (33.3) |
| HCT-CI, n (%) | |
| 1 | 8 (29.6) |
| 2-3 | 4 (14.8) |
| ≥4 | 15 (55.6) |
| Median (range) | 4 (1-10) |
| Histology, n (%) | |
| MDS | 16 (59.3) |
| AML | 10 (37) |
| MDS/MPN | 1 (3.7) |
| Disease risk index | |
| Intermediate | 9 (33.3) |
| High | 17 (63) |
| Very high | 1 (3.7) |
| Number of prior therapies, median (range) | 1 (0-3) |
| Prior Ven exposure, n (%) | 15 (55.6) |
| Flow MRD positivity, n (%) | 15 (55.6) |
| TP53 mutation, n (%) | |
| No | 11 (40.7) |
| Yes | 16 (59.3) |
| HLA molecular typing (A, B, C, and DRB1), n (%) | |
| Matched related | 3 (11.1) |
| Matched unrelated | 24 (88.9) |
| Patient and donor CMV serostatus | |
| Negative/negative | 12 (44.4) |
| Negative/positive | 6 (22.2) |
| Positive/negative | 5 (18.5) |
| Positive/positive | 4 (14.8) |
| Characteristic . | Total, intent-to-treat cohort (N = 27) . |
|---|---|
| Median age (range), y | 67 (47-78) |
| ≥65, n (%) | 18 (66.7) |
| Median donor age (range), y | 28 (20-64) |
| Patient sex, n (%) | |
| Female | 10 (37) |
| Male | 17 (63) |
| Donor sex, n (%) | |
| Female | 11 (40.7) |
| Male | 16 (59.3) |
| Male patient, female donor, n (%) | 4 (14.8) |
| ECOG performance status, n (%) | |
| 0 | 6 (22.2) |
| 1 | 12 (44.4) |
| 2 | 9 (33.3) |
| HCT-CI, n (%) | |
| 1 | 8 (29.6) |
| 2-3 | 4 (14.8) |
| ≥4 | 15 (55.6) |
| Median (range) | 4 (1-10) |
| Histology, n (%) | |
| MDS | 16 (59.3) |
| AML | 10 (37) |
| MDS/MPN | 1 (3.7) |
| Disease risk index | |
| Intermediate | 9 (33.3) |
| High | 17 (63) |
| Very high | 1 (3.7) |
| Number of prior therapies, median (range) | 1 (0-3) |
| Prior Ven exposure, n (%) | 15 (55.6) |
| Flow MRD positivity, n (%) | 15 (55.6) |
| TP53 mutation, n (%) | |
| No | 11 (40.7) |
| Yes | 16 (59.3) |
| HLA molecular typing (A, B, C, and DRB1), n (%) | |
| Matched related | 3 (11.1) |
| Matched unrelated | 24 (88.9) |
| Patient and donor CMV serostatus | |
| Negative/negative | 12 (44.4) |
| Negative/positive | 6 (22.2) |
| Positive/negative | 5 (18.5) |
| Positive/positive | 4 (14.8) |
CMV, cytomegalovirus; ECOG, Eastern Cooperative Oncology Group; HCT-CI, hematopoietic cell transplantation–specific comorbidity index.
Study design and baseline disease and mutational profiles. (A) Pretransplant screening and enrollment of patients were conducted in the 28-day period before day −8. Schematic and timeline of events for the phase 1 trial of Ven plus Ven/FluBu2 conditioning chemotherapy, including reviewing criteria to initiate maintenance (after day +28), and starting maintenance therapy with Ven and Aza between days +42 to +90. SC, subcutaneously; DL1, 42-day cycles; DL2, 28-day cycles. (B) Comutation plot of diagnostic mutations amenable to MRD tracking. Columns represent individual patients by study identifier (ID) and rows represent clinical variables or the presence of mutation(s) identified at diagnosis or mutations at screening with VAF of ≥1%. This VAF cutoff is suggestive of a diagnostic mutation, which was not confirmed at diagnosis because of lack of diagnostic sample or technical assay differences. Secondary AML (sAML)-associated genes include the following SRSF2, SF3B1, U2AF1, ZRSR2, ASXL1, EZH2, BCOR/L1, and STAG2. MAPK signaling genes included were NRAS, KRAS, FLT3, and PTPN11. "Other" includes mutations in the following genes: JAK2, SETBP1, WT1, MYC, EP300, PRPF8, PPM1D, BRAF, CSF3R, PHF6, and GATA2.
Study design and baseline disease and mutational profiles. (A) Pretransplant screening and enrollment of patients were conducted in the 28-day period before day −8. Schematic and timeline of events for the phase 1 trial of Ven plus Ven/FluBu2 conditioning chemotherapy, including reviewing criteria to initiate maintenance (after day +28), and starting maintenance therapy with Ven and Aza between days +42 to +90. SC, subcutaneously; DL1, 42-day cycles; DL2, 28-day cycles. (B) Comutation plot of diagnostic mutations amenable to MRD tracking. Columns represent individual patients by study identifier (ID) and rows represent clinical variables or the presence of mutation(s) identified at diagnosis or mutations at screening with VAF of ≥1%. This VAF cutoff is suggestive of a diagnostic mutation, which was not confirmed at diagnosis because of lack of diagnostic sample or technical assay differences. Secondary AML (sAML)-associated genes include the following SRSF2, SF3B1, U2AF1, ZRSR2, ASXL1, EZH2, BCOR/L1, and STAG2. MAPK signaling genes included were NRAS, KRAS, FLT3, and PTPN11. "Other" includes mutations in the following genes: JAK2, SETBP1, WT1, MYC, EP300, PRPF8, PPM1D, BRAF, CSF3R, PHF6, and GATA2.
The median time to maintenance treatment start was 57 days (range, 42-103 days). Among the 27 patients that received Ven/FluBu2 allo-SCT, 5 of 27 (18.5%) did not initiate maintenance because of early relapse (n = 3, all occurring within 68 days after transplant), patient preference (n = 1), and provider preference (n = 1). Ultimately, 22 of 27 patients (81.5%) received Ven/Aza maintenance therapy. Study disposition is provided in supplemental Table 1.
Safety and toxicity
Ven/Aza maintenance was well tolerated and the toxicity profile is shown in Table 2 (toxicity profile for the intent-to-treat population shown in supplemental Table 2). No unexpected AEs were observed. The most common grade 3-4 treatment-emergent AEs were leukopenia (95.5%, n = 21), neutropenia (81.8%, n = 18), thrombocytopenia (77.3%, n = 17), and anemia (45.5%, n = 10). Cytopenias were generally transient and reversible in each dose schedule (Figure 2; supplemental Tables 3-4). After 1 cycle of Ven/Aza and before dosing on day 1 of cycle 2, 20 (100%) and 16 (80%) patients had ANC recovery to >1.0 x 103/μL and platelet count of 100 x 103/μL. The median neutrophil counts were >1.0 × 103/μL and platelet counts were >100 × 103/μL at day 1 and day 15 of each subsequent cycle regardless of cycle length (Figure 2). No growth factor support was used during maintenance. There was 1 febrile neutropenia event (grade 3). Infections were uncommon (n = 4 total), including grade 2 COVID-19 infections requiring treatment delay and nirmatrelvir/ritonavir (n = 2), grade 1 parainfluenza infection (n = 1), and grade 2 BK polyomavirus associated–hemorrhagic cystitis (n = 1). No bleeding events were noted except for the patient with symptomatic BK viruria who had no further hematuria upon resuming maintenance. No grade 3-4 gastrointestinal events were observed. Grade 1-2 diarrhea and nausea were observed in 77.3% and 72.7% of patients, respectively; most were grade 1 and manageable.
Treatment-emergent AEs regardless of attribution in patients on maintenance
| Toxicity grade CTCAE version 5.0 . | Dose level . | All N = 22 . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DL1 42-day cycles n = 11 . | DL2 28-day cycles n = 11 . | |||||||||||
| 1 . | 2 . | 3 . | 4 . | 1 . | 2 . | 3 . | 4 . | 1 . | 2 . | 3 . | 4 . | |
| N . | N . | N . | N . | N . | N . | N . | N . | N . | N . | N . | N . | |
| Hematologic | ||||||||||||
| Anemia | 4 | - | 4 | - | 1 | 4 | 6 | - | 5 | 4 | 10 | - |
| Neutrophil count decreased | - | 2 | 1 | 7 | 1 | - | 1 | 9 | 1 | 2 | 2 | 16 |
| Platelet count decreased | 1 | 2 | 4 | 3 | - | 1 | 2 | 8 | 1 | 3 | 6 | 11 |
| WBC count decreased | - | - | 4 | 6 | - | - | - | 11 | - | - | 4 | 17 |
| Nonhematologic | ||||||||||||
| Abdominal pain | 1 | - | - | - | - | - | - | - | 1 | - | - | - |
| ALT increased | 5 | 1 | - | - | 4 | 1 | - | - | 9 | 2 | - | - |
| Alk Phos increased | 2 | - | - | - | 2 | - | - | - | 4 | - | - | - |
| Anorexia | 1 | 1 | - | - | 3 | - | - | - | 4 | 1 | - | - |
| Arthralgia | - | - | - | - | 1 | - | - | - | 1 | - | - | - |
| AST increased | 2 | - | - | - | 4 | - | - | - | 6 | - | - | - |
| Bloating | - | - | - | - | 1 | - | - | - | 1 | - | - | - |
| Blood bilirubin increased | 1 | 1 | - | - | - | - | - | - | 1 | 1 | - | - |
| Bone pain | - | - | - | - | 1 | - | - | - | 1 | - | - | - |
| Constipation | 1 | - | - | - | 4 | - | - | - | 5 | - | - | - |
| Cough | - | - | - | - | 1 | - | - | - | 1 | - | - | - |
| Creatinine increased | 3 | - | - | - | 5 | 1 | - | - | 8 | 1 | - | - |
| Diarrhea | 7 | 2 | - | - | 7 | 1 | - | - | 14 | 3 | - | - |
| Dizziness | 1 | - | - | - | 2 | - | - | - | 3 | - | - | - |
| Dysgeusia | 3 | - | - | - | 2 | - | - | - | 5 | - | - | - |
| Dyspepsia | - | - | - | - | 2 | - | - | - | 2 | - | - | - |
| Dyspnea | 1 | 1 | - | - | - | - | - | - | 1 | 1 | - | - |
| Dysuria | - | - | - | - | 1 | - | - | - | 1 | - | - | - |
| Fatigue | 2 | 3 | - | - | 9 | - | - | - | 11 | 3 | - | - |
| Febrile neutropenia | - | - | - | - | - | - | 1 | - | - | - | 1 | - |
| Headache | 1 | - | - | - | - | - | - | - | 1 | - | - | - |
| Hyperkalemia | 1 | - | - | - | 1 | 1 | - | - | 2 | 1 | - | - |
| Hyperuricemia | 1 | - | - | - | 1 | - | - | - | 2 | - | - | - |
| Hypocalcemia | - | - | - | - | 2 | 1 | - | - | 2 | 1 | - | - |
| Hypomagnesemia | 1 | 1 | - | - | 3 | - | - | - | 4 | 1 | - | - |
| Lip pain | 1 | - | - | - | - | - | - | - | 1 | - | - | - |
| Mucositis oral | - | - | - | - | 3 | - | - | - | 3 | - | - | - |
| Nail discoloration | - | - | - | - | 1 | - | - | - | 1 | - | - | - |
| Nausea | 3 | 3 | - | - | 7 | 3 | - | - | 10 | 6 | - | - |
| Parainfluenza infection | - | - | - | - | 1 | - | - | - | 1 | - | - | - |
| Peripheral sensory neuropathy | - | - | - | - | 1 | - | - | - | 1 | - | - | - |
| Pruritus | 1 | - | - | - | 1 | - | - | - | 2 | - | - | - |
| Rash maculopapular | 1 | 1 | - | - | - | - | - | - | 1 | 1 | - | - |
| Urinary frequency | - | - | - | - | 1 | - | - | - | 1 | - | - | - |
| Vomiting | - | 1 | - | - | 1 | - | - | - | 1 | 1 | - | - |
| Weight loss | - | - | - | - | 1 | - | - | - | 1 | - | - | - |
| Toxicity grade CTCAE version 5.0 . | Dose level . | All N = 22 . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DL1 42-day cycles n = 11 . | DL2 28-day cycles n = 11 . | |||||||||||
| 1 . | 2 . | 3 . | 4 . | 1 . | 2 . | 3 . | 4 . | 1 . | 2 . | 3 . | 4 . | |
| N . | N . | N . | N . | N . | N . | N . | N . | N . | N . | N . | N . | |
| Hematologic | ||||||||||||
| Anemia | 4 | - | 4 | - | 1 | 4 | 6 | - | 5 | 4 | 10 | - |
| Neutrophil count decreased | - | 2 | 1 | 7 | 1 | - | 1 | 9 | 1 | 2 | 2 | 16 |
| Platelet count decreased | 1 | 2 | 4 | 3 | - | 1 | 2 | 8 | 1 | 3 | 6 | 11 |
| WBC count decreased | - | - | 4 | 6 | - | - | - | 11 | - | - | 4 | 17 |
| Nonhematologic | ||||||||||||
| Abdominal pain | 1 | - | - | - | - | - | - | - | 1 | - | - | - |
| ALT increased | 5 | 1 | - | - | 4 | 1 | - | - | 9 | 2 | - | - |
| Alk Phos increased | 2 | - | - | - | 2 | - | - | - | 4 | - | - | - |
| Anorexia | 1 | 1 | - | - | 3 | - | - | - | 4 | 1 | - | - |
| Arthralgia | - | - | - | - | 1 | - | - | - | 1 | - | - | - |
| AST increased | 2 | - | - | - | 4 | - | - | - | 6 | - | - | - |
| Bloating | - | - | - | - | 1 | - | - | - | 1 | - | - | - |
| Blood bilirubin increased | 1 | 1 | - | - | - | - | - | - | 1 | 1 | - | - |
| Bone pain | - | - | - | - | 1 | - | - | - | 1 | - | - | - |
| Constipation | 1 | - | - | - | 4 | - | - | - | 5 | - | - | - |
| Cough | - | - | - | - | 1 | - | - | - | 1 | - | - | - |
| Creatinine increased | 3 | - | - | - | 5 | 1 | - | - | 8 | 1 | - | - |
| Diarrhea | 7 | 2 | - | - | 7 | 1 | - | - | 14 | 3 | - | - |
| Dizziness | 1 | - | - | - | 2 | - | - | - | 3 | - | - | - |
| Dysgeusia | 3 | - | - | - | 2 | - | - | - | 5 | - | - | - |
| Dyspepsia | - | - | - | - | 2 | - | - | - | 2 | - | - | - |
| Dyspnea | 1 | 1 | - | - | - | - | - | - | 1 | 1 | - | - |
| Dysuria | - | - | - | - | 1 | - | - | - | 1 | - | - | - |
| Fatigue | 2 | 3 | - | - | 9 | - | - | - | 11 | 3 | - | - |
| Febrile neutropenia | - | - | - | - | - | - | 1 | - | - | - | 1 | - |
| Headache | 1 | - | - | - | - | - | - | - | 1 | - | - | - |
| Hyperkalemia | 1 | - | - | - | 1 | 1 | - | - | 2 | 1 | - | - |
| Hyperuricemia | 1 | - | - | - | 1 | - | - | - | 2 | - | - | - |
| Hypocalcemia | - | - | - | - | 2 | 1 | - | - | 2 | 1 | - | - |
| Hypomagnesemia | 1 | 1 | - | - | 3 | - | - | - | 4 | 1 | - | - |
| Lip pain | 1 | - | - | - | - | - | - | - | 1 | - | - | - |
| Mucositis oral | - | - | - | - | 3 | - | - | - | 3 | - | - | - |
| Nail discoloration | - | - | - | - | 1 | - | - | - | 1 | - | - | - |
| Nausea | 3 | 3 | - | - | 7 | 3 | - | - | 10 | 6 | - | - |
| Parainfluenza infection | - | - | - | - | 1 | - | - | - | 1 | - | - | - |
| Peripheral sensory neuropathy | - | - | - | - | 1 | - | - | - | 1 | - | - | - |
| Pruritus | 1 | - | - | - | 1 | - | - | - | 2 | - | - | - |
| Rash maculopapular | 1 | 1 | - | - | - | - | - | - | 1 | 1 | - | - |
| Urinary frequency | - | - | - | - | 1 | - | - | - | 1 | - | - | - |
| Vomiting | - | 1 | - | - | 1 | - | - | - | 1 | 1 | - | - |
| Weight loss | - | - | - | - | 1 | - | - | - | 1 | - | - | - |
ALT, alanine aminotransferase; Alk Phos, alkaline phosphatase; AST, aspartate aminotransferase; CTCAE, Common Terminology Criteria for Adverse Events; WBC, white blood cell.
Neutrophil and platelet counts on venetoclax and azacitidine maintenance. Peripheral blood counts for patients on DL1 (42-day cycles with 8 planned cycles) and DL2 (28-day cycles with 12 planned cycles) and maintenance Ven/Aza on days 1, 8, 15, and 22 of cycle 1, and then days 1 and 15 each of each subsequent cycle. (A) Median ANC (K/μL) (± standard deviation [SD]) with dashed line at grade 4 neutrophil level (<0.5 x 103/μL). (B) Median platelet counts (K/μL) (± SD) with dashed line representing grade 3 platelet level (<50 x103/μL). The bars in all plots represent median values with error bars representing the interquartile range between the 25% and 75% quartiles. Details for each time point are shown in supplemental Tables 3-4.
Neutrophil and platelet counts on venetoclax and azacitidine maintenance. Peripheral blood counts for patients on DL1 (42-day cycles with 8 planned cycles) and DL2 (28-day cycles with 12 planned cycles) and maintenance Ven/Aza on days 1, 8, 15, and 22 of cycle 1, and then days 1 and 15 each of each subsequent cycle. (A) Median ANC (K/μL) (± standard deviation [SD]) with dashed line at grade 4 neutrophil level (<0.5 x 103/μL). (B) Median platelet counts (K/μL) (± SD) with dashed line representing grade 3 platelet level (<50 x103/μL). The bars in all plots represent median values with error bars representing the interquartile range between the 25% and 75% quartiles. Details for each time point are shown in supplemental Tables 3-4.
Acute and chronic GVHD
The cumulative incidence of grade 2-4 acute GVHD at 6 months was 22% (95% confidence interval [CI], 9%-29%) for the entire cohort. There were 2 cases of grade 3 acute GVHD, and no cases of grade 4 GVHD.
At 1 year and 2 years, the cumulative incidences of chronic GVHD were 23% (95% CI, 8%-42%) and 30% (95% CI, 14%-48%), respectively. The 1-year and 2-year moderate/severe chronic GVHD rates were 4% (95% CI, 0.3%-18%) and 22% (95% CI, 9%-40%), respectively. Only 2 cases of severe GVHD occurred. The overall frequency of acute and chronic GVHD events were similar to that in our previously published study with Ven/FluBu2 without any planned Ven/Aza maintenance.12
Maintenance tolerability
The median duration of Ven/Aza maintenance therapy was 33.9 weeks (range, 4-56.2 weeks). The median number of cycles received in the 42-day and 28-day cycles were 3 of 8 (range, 1-8) and 5.5 of 12 (range, 1-12), respectively. Nine of 22 patients (40.9%) completed all planned cycles, including 4 of 11 (36.4%) in the 42-day (DL1) and 5 of 11 (45.5%) in the 28-day (DL2) cohorts. The median length of the first cycle was 42 days (range, 40-91 days) for DL1 and 28 days (range, 27-29 days) for DL2. Reasons for treatment discontinuation included relapse (n = 8; 36.3%), GVHD (n = 3; 13.6%), and intolerability (n = 1 [4.5%]; discontinued after receiving 9 of 12 planned 28-day maintenance treatment cycles). Dose reductions for toxicity or tolerability were performed in only 2 of 22 (9.1%) patients (both in the 28-day cohort). One patient underwent second matched unrelated donor allo-SCT upon detection and confirmation of donor-derived TP53 clonal hematopoiesis, which confers exceedingly high risk for evolution of donor cell leukemia.18 No significant differences were detected between the 2 dose schedules. Serious AEs included 1 case of grade 2 maculopapular rash and 1 case of overlap chronic (moderate) GVHD in setting of rapid tacrolimus taper. There were no study treatment-related deaths. No dose-limiting toxicities were observed. In the absence of an MTD, we determined the RP2D to be Aza 36 mg/m2 days 1 to 5, plus Ven 400 mg daily on days 1 to 14, on a 28-day cycle.
Chimerism
The day +28 and day +100 median donor-derived chimerism values for leukocytes were 99% (range, 13%-100%) and 99% (range, 5%-100%), CD33+ granulocytes were 100% (range, 2%-100%) and 100% (range, 1%-100%), and CD3+ T cells were 69% (range, 15%-100%) and 83% (range, 31%-100%), respectively. The median marrow chimerism at day +28 was 98% (range, 4%-100%), day +100 was 98% (range, 2%-100%), 6 months was 100% (range, 22%-100%), and 12 months was 100% (range, 99%-100%; supplemental Table 5). No patients experienced primary or secondary graft failure.
Treatment efficacy
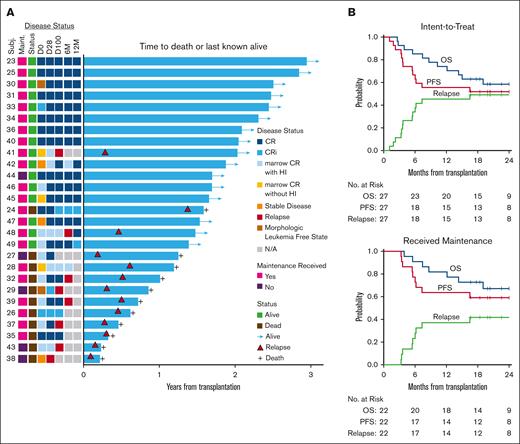
With a median follow-up of 24 months (range, 17-35 months) among survivors and regardless of maintenance receipt, the 2-year OS was 58% (95% CI, 35%-75%) and PFS was 52% (95% CI, 32%-69%; Figure 3). The 2-year CIR was 48% (95% CI, 28%-66%) and nonrelapse mortality was 0%. For those that received Ven/Aza maintenance, with a median follow-up time of 25 months (range, 17-35 months), the median OS has not been reached. After Ven/Aza maintenance, the 2-year OS was 67% (95% CI, 43%-83%), and PFS was 59% (95% CI, 36%-76%). The 2-year CIR was 41% (95% CI, 20%-61%).
Outcomes after Ven plus FluBu2 allo-SCT followed by Ven/Aza maintenance. (A) Swimmer plot for the intent-to-treat cohort (N = 27) and time to death or last known alive. Shown with accompanying heat map for disease status over time (D0, D28, D100, 6M, and 12M), maintenance receipt, and survival status. D0, day 0; D28, day +28 after transplant; D100, day +100 after transplant; 6M, 6 months after transplant; and 12M, 12 months after transplant. Relapse indicated by red-filled triangle. (B) Kaplan-Meier estimate and log-rank test of OS (blue line), PFS (red line), and relapse (green line) in the intent-to-treat cohort (top, N = 27) and in the cohort that ultimately received maintenance Ven/Aza (bottom, N = 22). 95% CIs are reported for each outcome.
Outcomes after Ven plus FluBu2 allo-SCT followed by Ven/Aza maintenance. (A) Swimmer plot for the intent-to-treat cohort (N = 27) and time to death or last known alive. Shown with accompanying heat map for disease status over time (D0, D28, D100, 6M, and 12M), maintenance receipt, and survival status. D0, day 0; D28, day +28 after transplant; D100, day +100 after transplant; 6M, 6 months after transplant; and 12M, 12 months after transplant. Relapse indicated by red-filled triangle. (B) Kaplan-Meier estimate and log-rank test of OS (blue line), PFS (red line), and relapse (green line) in the intent-to-treat cohort (top, N = 27) and in the cohort that ultimately received maintenance Ven/Aza (bottom, N = 22). 95% CIs are reported for each outcome.
At time of data cutoff, 13 of 27 patients (48%) experienced morphologic relapse overall, including 9 of 22 (40.9%) after Ven/Aza maintenance. All but 1 relapse occurred within the first year of transplant (12/13 [92.3%]), and 4 of 13 relapses occurred by day +100. Among the 13 relapsed cases, 4 were AML and 9 were MDS. Nine of 13 (69.2%) had previously received Ven, which was expected, given the older patient population in the current treatment era.
The median time to relapse for those that received maintenance therapy was 112 days (range, 101-502 days) with 6 of 9 relapses occurring within the first 6 months of transplant. Among the relapses that occurred on maintenance, 8 of 9 cases (88.9%) had a baseline TP53 mutation, including 5 of 8 (62.5%) with biallelic TP53 status. Notably, 2 of 9 relapses (22.2%) with TP53-mutated disease later regained remission after immune suppression tapering with subsequent GVHD induction without needing additional chemotherapy or donor lymphocyte infusion. Thirteen of 22 patients who received maintenance are alive and remain in remission, including 7 with a TP53 mutation.
Univariable analysis was performed to identify risk factors for OS, PFS, and likelihood of relapse among patients that received maintenance, including Molecular International Prognostic Scoring System status among those with MDS, older age, donor recipient sex mismatch, cytomegalovirus serostatus, MDS vs AML, marrow blast percentage, hematopoietic cell transplantation–specific comorbidity index, Eastern Cooperative Oncology Group performance status score (2 vs 0-1), disease risk index, baseline TP53 mutation status, baseline flow MRD status, and prior Ven use. These risk factors did not impact OS or PFS. Flow MRD positivity at day +28 was the only factor that was associated with relapse or PFS (hazard ratio, 9.43; 95% CI, 1.56-56.92; P = .015). Four patients were flow MRD positive at day +28 and relapsed early after transplant (between day +34 and day +169), which precluded maintenance initiation in 2 of them (supplemental Figure 1).
Flow MRD
Flow MRD was detected in 15 of 27 (55.6%) patients before transplant, which converted to negative in 11 of 14 patients (78.6%) at day +28 (supplemental Figure 1). However, in 5 of these 11 pretransplant flow MRD-positive and day +28 MRD-negative cases that demonstrated early conversion, flow MRD–positive disease was later redetected at day +100. Among the 12 of 22 patients with flow MRD–positive disease at study entry that received maintenance, 6 of 12 (50%) patients achieved long-term flow MRD–negative remission after transplant. On study maintenance therapy, the 2-year PFS for patients who were flow MRD positive at day +100 was 36% compared with 78% for those who were flow MRD negative at day +100 (P = .0078; supplemental Figure 2).
Molecular MRD
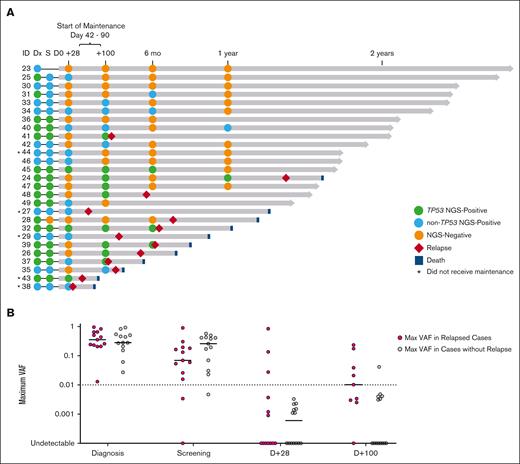
Targeted duplex sequencing was used to quantify tumor mutational burden (molecular MRD) at serial time points before and after transplant (Figure 4A). Before transplantation, 25 of 26 (96%) patients with available bone marrow sample were MRD positive, including 13 of 25 (52%) with TP53 mutations. We found no correlation between pretransplant molecular MRD variant allele frequency (VAF) and probability of relapse. Pretransplant molecular MRD was assessed in 21 of 22 patients that received maintenance, and all but 1 had detectable disease. While on study, 11 of 20 (55%) patients with pretransplant molecular MRD underwent MRD clearance. Among patients who were molecular MRD negative at day +28, 10 of 14 (71%) became molecular MRD positive at later time points, and 6 of 14 (42.9%) ultimately relapsed, including 5 with a TP53 mutation and 1 without a TP53 mutation who developed new subclonal KRAS mutations (supplemental Figures 3-4). Among patients who were molecular MRD negative at day +100, 1 of 9 (11.1%) became molecular MRD positive at a later time point but remains in remission, and 1 of 9 (11.1%) ultimately relapsed. In contrast, in the early posttransplant period, patients who relapsed more commonly had day +100 persistent molecular MRD positivity with maximum VAF of >1% (5 of 9; 55.6%) than those patients who did not relapse (1 of 14; 7.1%; Figure 4B). Among the remaining 4 of 9 patients that relapsed, all had TP53-mutated disease and day +100 MRD at low VAF (<1%).
Serial molecular MRD surveillance before and after transplant. (A) Molecular MRD swimmer plot. Each bar represents a patient, with the length of gray bars indicating follow-up time. Patients arranged by follow-up time and labeled by patient/study ID number. Recommended window for maintenance initiation indicated (day +42 to day +90). Symbols indicate molecular status (defined as detection of pre-allo-SCT mutation) at serial time points, relapse, and death; asterisk indicates those that did not receive maintenance (n = 5), and blue- or green-filled circles indicate TP53 mutation status. Notably, ID 40 had molecular MRD positive clone detected at 1 year (RUNX1 p.L175fs at VAF of 0.19%) but did not relapse at time of data cutoff. (B) Plot of mutational burden by VAF over time early in the transplant course. Line presents median. VAF > 0.01 (1%) at day +100 identifies cases that are most likely to relapse. One exception to this is a 4.8% VAF TP53-mutated clone identified at day +100 that was not detected at subsequent time points.
Serial molecular MRD surveillance before and after transplant. (A) Molecular MRD swimmer plot. Each bar represents a patient, with the length of gray bars indicating follow-up time. Patients arranged by follow-up time and labeled by patient/study ID number. Recommended window for maintenance initiation indicated (day +42 to day +90). Symbols indicate molecular status (defined as detection of pre-allo-SCT mutation) at serial time points, relapse, and death; asterisk indicates those that did not receive maintenance (n = 5), and blue- or green-filled circles indicate TP53 mutation status. Notably, ID 40 had molecular MRD positive clone detected at 1 year (RUNX1 p.L175fs at VAF of 0.19%) but did not relapse at time of data cutoff. (B) Plot of mutational burden by VAF over time early in the transplant course. Line presents median. VAF > 0.01 (1%) at day +100 identifies cases that are most likely to relapse. One exception to this is a 4.8% VAF TP53-mutated clone identified at day +100 that was not detected at subsequent time points.
Next, we evaluated whether maintenance had any impact on molecular MRD detected in the early posttransplant period. After transplantation, 13 of 27 (48.1%) were MRD positive at day +28, and 14 of 23 patients (60.9%) were MRD positive at day +100. Several patients had molecular MRD conversion on study and remain in remission. Molecular MRD at day +100 was positive in 14 of 22 patients (63.6%) who received maintenance. Six of 14 patients (42.9%) underwent MRD conversion, including 4 patients by +6 months, and 2 more patients by +12 months. These 6 patients remain in remission at a median follow-up of 24 months (range, 20.2-30.2). Notably, in these “MRD-conversion” cases, all or most of planned maintenance therapy was administered, 3 of 6 have a TP53 mutation at baseline, and 4 of 6 developed GVHD. Similar to what has been observed in patients with AML receiving combination Ven and Aza for frontline treatment on the VIALE-A trial, time to MRD conversion on maintenance Ven/Aza varied.15 Patient-level responses, including molecular and flow MRD status, are detailed in supplemental Table 6. On study maintenance therapy, the 2-year PFS for patients who were molecular MRD positive at day +100 was 50% compared with 89% for those who were molecular MRD negative at day +100 (P = .028).
Historical control cohort
Although this study was not randomized, we compared the outcome of patients who received a similar conditioning backbone (Ven/FluBu2) before transplant who were not intended to receive any maintenance therapy (Cohort 1, which was previously published,12 served as a “control cohort”) with the outcome of patients in the intent-to-treat Cohort 2 (N = 27) to reduce selection bias.12 Although limited in sample size, a comparison of Cohort 1 (N = 22) and Cohort 2 (including 22 who received maintenance and 5 patients who did not receive maintenance) did not detect a difference in 2-year OS (P = .96) or PFS (P = .88). In Cohort 1, 6 of 18 patients (33%) with pretransplant molecular MRD underwent clearance on study (without planned maintenance). Notably, the cohorts had markedly different follow-up time because they were sequentially enrolled, and prior Ven exposure determined new standard-of-care treatment options. In Cohort 1, very few patients had seen prior Ven (only 3 of 22; 13.6%), whereas 55.6% of patients in Cohort 2 have previously been treated with Ven.
Patient-reported outcomes
To explore whether maintenance therapy incurred additional toxicities or burden, we assessed for QOL using the Functional Assessment of Cancer Therapy–Bone Marrow Transplant (FACT-BMT) QOL assessment among patients before and after transplant (n = 10). BMT subscale scores did not worsen during the first 4 cycles of maintenance than the pretransplant status. FACT-General, social well-being, and functional well-being scores initially declined as expected immediately after transplant (P = .02), and soon after returned to pretransplant levels (supplemental Table 7).
Immune reconstitution
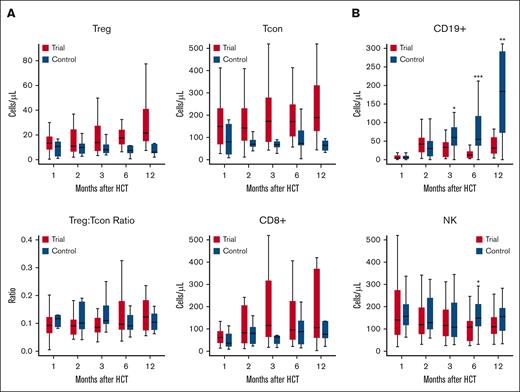
We examined the dynamics of immune reconstitution on Ven/Aza maintenance therapy at 1, 2, 3, 6, and 12 months after transplant. Myelosuppression is a well-recognized side effect of Ven/Aza, but at reduced doses in the posttransplant setting its impact on immunosuppression has not been described. We compared this to a matched contemporaneous cohort of 25 adult patients with paired blood samples who underwent standard FluBu2 RIC transplant followed by tacrolimus/methotrexate GVHD prophylaxis without maintenance therapy at our center. Serial analysis of peripheral blood samples by flow cytometry after transplant revealed that Ven/Aza had limited impact on T-cell immune reconstitution of regulatory CD4+ T, conventional CD4+ T, and CD8+ T cells (Figure 5; supplemental Figure 5). Levels of peripheral conventional CD4+ T and CD8+ T cells were lower at 6 and 12 months after transplant than in controls, but these differences were not statistically significant. At 6 months, peripheral natural killer cells were significantly lower in patients on maintenance compared with in controls (median and interquartile range [IQR]: 108.6 cells per μL [IQR, 45.6-136] vs 148.5 cells per μL [IQR, 111.9-211.8]; P = .03). Patients on Ven/Aza also had significantly delayed CD19+ B-cell reconstitution compared with controls at 6 months (median and IQR: 12.6 cells per μL [IQR, 4.5-22.5] vs 55 cells per μL [IQR, 37.8-117.8]; P = .002) and at 12 months (median and IQR: 31 cells per μL [IQR, 16.6-60.4] vs 184.2 cells per μL [IQR, 72.4-291.9]; P = .01). However, no significant differences were observed in immunoglobulin G levels among 19 patients with available laboratory tests at 6 months, and among 11 patients at 12 months.
Assessment of peripheral blood for immune reconstitution. Flow cytometry immune monitoring was performed on peripheral blood samples collected from patients on Ven/Aza maintenance and matched controls at 1 (n = 51, including 26 study patients and 25 controls), 2 (n = 41, including 24 study patients and 17 controls), 3 (n = 47, including 24 study patients and 23 controls), 6 (n = 38, including 18 study patients and 20 controls), and 12 (n = 34, including 13 study patients and 21 controls) months after transplant. (A) T-cell populations were measured at serial time points after transplant to evaluate for impaired immune cell expansion. Three major T-cell populations, regulatory CD4+ T (CD4Treg), conventional CD4+ T (CD4Tcon), and CD8+ T cells, were defined as CD3+CD4+CD8−CD25+CD127− cells, CD3+CD4+CD8−CD25−/lowCD127+/− cells, and CD3+CD4−CD8+, respectively. (B) Natural killer (NK) cells were defined as CD3−CD56+ lymphocytes. NK T cells were defined as CD3+CD56+ lymphocytes. B cells were defined as CD45+CD19+ lymphocytes. Details of gating strategy are shown in supplemental Figure 5. Data are median (thick black line), and box plots indicate the interquartile range.
Assessment of peripheral blood for immune reconstitution. Flow cytometry immune monitoring was performed on peripheral blood samples collected from patients on Ven/Aza maintenance and matched controls at 1 (n = 51, including 26 study patients and 25 controls), 2 (n = 41, including 24 study patients and 17 controls), 3 (n = 47, including 24 study patients and 23 controls), 6 (n = 38, including 18 study patients and 20 controls), and 12 (n = 34, including 13 study patients and 21 controls) months after transplant. (A) T-cell populations were measured at serial time points after transplant to evaluate for impaired immune cell expansion. Three major T-cell populations, regulatory CD4+ T (CD4Treg), conventional CD4+ T (CD4Tcon), and CD8+ T cells, were defined as CD3+CD4+CD8−CD25+CD127− cells, CD3+CD4+CD8−CD25−/lowCD127+/− cells, and CD3+CD4−CD8+, respectively. (B) Natural killer (NK) cells were defined as CD3−CD56+ lymphocytes. NK T cells were defined as CD3+CD56+ lymphocytes. B cells were defined as CD45+CD19+ lymphocytes. Details of gating strategy are shown in supplemental Figure 5. Data are median (thick black line), and box plots indicate the interquartile range.
BH3 profiling of immune cells
We assessed the impact of Ven on apoptotic priming in immune cells using available paired peripheral blood samples. BH3 profiling of immune cells revealed no significant change in apoptotic priming in T cells (CD3+, CD3+CD4+, CD3+CD8+), regulatory T cells (CD3+CD4+ CD25+ CD127−), or B cells (CD19+) during the first 3 cycles of Ven/Aza maintenance (supplemental Figure 6).
Discussion
This trial is, to our knowledge, the first study to assess Ven/FluBu2 allo-SCT followed by prophylactic maintenance therapy with combination Aza/Ven for patients with high-risk MDS/AML. Overall, Ven/Aza maintenance was safe and well tolerated. Cytopenias were transient, front loaded, and manageable. Infections were uncommon. A dose of 400 mg of Ven for a duration of 14 days was chosen given the excellent safety and tolerability data, suggesting that this is the appropriate RP2D for higher risk MDS. However, as myelosuppression is a known toxicity of Ven/Aza treatment, we tested this regimen at 2 different cycle lengths (42 and 28 days) and found no difference in toxicity or tolerability. Notably, the cumulative incidence of acute and chronic GVHD were lower than standard RIC regimens and similar to our prior experience with Ven/FluBu2 allo-SCT without planned maintenance.12,19 Altogether, these data are encouraging in a high-risk MDS/AML cohort not eligible or recommended for a myeloablative conditioning strategy but raises the question of whether maintenance added benefit to transplant and in which patients it should even be considered.
Limiting prophylactic maintenance to those most vulnerable to relapse is a priority to avoid unnecessary toxicity. All except 1 patient who relapsed had detectable molecular MRD at study entry before transplant (12 of 13; 92.3%). Although early posttransplant MRD persistence is a clear relapse risk factor, serial time points are required to confirm clearance. For instance, relapses occurred despite MRD negativity at day +28 in 6 of 20 patients (30%) by flow, and in 7 of 14 patients (50%) by molecular testing, suggesting that MRD negativity at a single time point especially in the very early phase of engraftment may provide premature reassurance. We propose dynamic MRD monitoring to facilitate the optimal selection of candidates at high risk who would benefit from preemptive maintenance therapy or tapering of immunosuppressive therapy in the early posttransplant period. Although this needs to be confirmed in a randomized setting, we recommend consideration of prophylactic maintenance for patients at exceedingly high risk for relapse including those with pretransplant molecular MRD and particularly for those with MRD persistence at day +28 or day +100. Because there is evidence for rapid disease reduction with Ven/Aza, monitoring for posttransplant disease kinetics using molecular MRD can identify patients who may benefit most from the addition of maintenance therapy to control resistant or emerging clones. Among the 16 patients with TP53-mutated disease, 9 have relapsed (1 patient did not initiate maintenance) and 7 remain in remission. In these 7 remission cases, molecular MRD negativity was achieved by day +100 (n = 4) or underwent conversion after day +100 (n = 3), raising the possibility that Ven-augmented conditioning chemotherapy may have been sufficient to suppress disease clone expansion after transplant in some patients and that maintenance therapy provided continued disease eradication after transplant in others.
The optimal treatment choice for posttransplant maintenance therapy is not clear. Even among prospective and retrospective studies with tyrosine kinase inhibitors targeting BCR-ABL or FLT3 and small molecule inhibitors targeting IDH1/2 the data are not entirely definitive, except when pretransplant MRD was persistent, in which case maintenance therapy improves relapse-free survival.16,20,21 However, there is good rationale for identifying broad-acting agents, including combination Ven and Aza given underlying disease heterogeneity, significant clinical activity in AML/MDS, and evidence for MRD conversion in AML.14,15,22 As a next step for this trial to further improve feasibility, we are separately assessing the safety and efficacy of Ven/FluBu2 allo-SCT followed by an all oral maintenance regimen with decitabine-cedazuridine and Ven.
This study was limited by the small sample size and because it was not randomized we are unable to confirm the benefit of either modified RIC strategy with the addition of Ven or prophylactic maintenance treatment. However, strengths of this study are the long-term follow-up; detailed MRD assessments, which revealed depth of responsiveness (conversion) and uncommonly resistance (emergence of new mutations); and serial immune profiling. Serial molecular MRD surveillance identified the emergence of a previously undetected KRAS mutations in 1 patient during Ven/Aza maintenance who ultimately relapsed. Activation of the RAS/MAPK-signaling pathway is a previously described mechanism of Ven resistance that involves upregulation of the antiapoptotic protein MCL-1.23,24 In an exploratory analysis, although posttransplant Ven/Aza maintenance therapy did not appear to have a negative impact on T-cell immune reconstitution, immune profiling revealed that B cells did not expand as robustly as controls 6 months after transplant and this may have contributed to the relatively lower rate of chronic GVHD during the first year of transplant.
In conclusion, administering Ven/Aza at reduced doses might be a nontoxic strategy after nonmyeloablative allo-SCT to provide further disease reduction, which may prevent relapse until the benefits of transplant fully manifest. A randomized trial is necessary to confirm the true impact of Ven when added to RIC chemotherapy and prophylactic Ven/Aza maintenance after transplant.
Acknowledgments
Research reported in this publication was supported by the Ted and Eileen Pasquarello Tissue Bank in Hematologic Malignancies, the National Cancer Institute (NCI) of the National Institutes of Health (NIH) under award numbers CA066996 and K08CA245209, the American Society of Hematology MRHAP award (H.M.M.), and a scholar award from the Leukemia & Lymphoma Society (R.C.L.). This work was also supported by grants from the Frederick A. DeLuca Foundation and the Jock and Bunny Adams Research and Education Endowment. Genentech provided trial and drug (venetoclax) support.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or NCI.
Authorship
Contribution: J.S.G., H.T.K., R.C.L., R.J.S., and J.H.A. designed the study; J.S.G., C.S.C., J.B., M.G., V.T.H., J.K., S.N., R.R., R.S., R.J.S., and J.H.A. enrolled and treated the patients; M.E.C., M.A., J.R., and H.M.M. processed and performed biomarker assays; J.S.G., H.T.K., H.M.M., R.C.L., R.J.S., and J.H.A. analyzed and interpreted the data and created the figures; J.S.G., H.T.K., H.M.M., R.C.L., R.J.S., and J.H.A. drafted the manuscript; and all authors contributed to data collection, revision of the manuscript, and approval of the final version.
Conflict-of-interest disclosure: J.S.G. reports grants from and serves on advisory boards for AbbVie, Astellas, Bristol Myers Squibb, Genentech, and Servier, and receives grants/research funding from AbbVie, Genentech, New Wave, and Pfizer. C.S.C. serves on the advisory board/consults for Sanofi, InhibRx, Cellarity, Astellas, Rigel, Novartis, Incyte, Cimeio, and Oxford Immune Algorithmics, and serves on the data and safety monitoring board for AlloVir and Angiocrine. J.K. serves on board and consults for Mallinckrodt, EMD Serono, Merck, Cugene, Cue Biotherapeutics, Biolojic Design, Gentibio, Nekonal, Equillium, and Amgen, and receives grants/research support from Bristol Myers Squibb, Miltenyi, Regeneron, Equillium, Amgen and Clinigen. R.R. receives grant support from CRISPR Therapeutics and serves on the advisory board for Glycostem. D.J.D. consults for AbbVie, Amgen, Agios, Autolus, Blueprint, Forty-Seven, Glycomimetics, Incyte, Jazz, Kite, Novartis, Pfizer, Servier, and Takeda. R.M.S. reports grants and personal fees from AbbVie, Agios, and Novartis; personal fees from Actinium, Argenx, Astellas, AstraZeneca, BioLineRx, Celgene, Daiichi Sankyo, Elevate, GEMoaB, Janssen, Jazz, MacroGenics, Otsuka, Pfizer, Hoffmann–La Roche, Stemline, Syndax, Syntrix, Syros, Takeda, and Trovagene; and grants from Arog. J.H.A. serves on the data and safety monitoring board for CSL Behring and Janssen, and serves on the scientific advisory board for Pharmacosmos. J.R. receives research support from Equillium, Kite/Gilead, Novartis, and Oncternal Therapeutics, and serves on advisory boards for Akron Biotech, Clade Therapeutics, Garuda Therapeutics, LifeVault Bio, Novartis, Smart Immune, and TScan Therapeutics. A.L. serves on the scientific advisory board of Flash Therapeutics, Trueline Therapeutics, and Zentalis Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Jacqueline S. Garcia, Department of Medical Oncology, Dana-Farber Cancer Institute, 450 Brookline Ave, Boston, MA 02215; email: jacqueline_garcia@dfci.harvard.edu.
References
Author notes
R.J.S. and J.H.A. are joint senior authors.
Presented in abstract form at the 64th annual meeting of the American Society of Hematology, New Orleans, LA, 10 December 2022.
Trial data will be posted on ClinicalTrials.gov as required upon completion of the study. Deidentified individual participant data that underlie the reported results will be made available for up to 2 years after publication after approved use by the study authors. Proposals for access should be sent to the corresponding author, Jacqueline S. Garcia (jacqueline_garcia@dfci.harvard.edu).
The full-text version of this article contains a data supplement.


![Neutrophil and platelet counts on venetoclax and azacitidine maintenance. Peripheral blood counts for patients on DL1 (42-day cycles with 8 planned cycles) and DL2 (28-day cycles with 12 planned cycles) and maintenance Ven/Aza on days 1, 8, 15, and 22 of cycle 1, and then days 1 and 15 each of each subsequent cycle. (A) Median ANC (K/μL) (± standard deviation [SD]) with dashed line at grade 4 neutrophil level (<0.5 x 103/μL). (B) Median platelet counts (K/μL) (± SD) with dashed line representing grade 3 platelet level (<50 x103/μL). The bars in all plots represent median values with error bars representing the interquartile range between the 25% and 75% quartiles. Details for each time point are shown in supplemental Tables 3-4.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/8/4/10.1182_bloodadvances.2023012120/2/m_blooda_adv-2023-012120-gr2.jpeg?Expires=1769081051&Signature=VaqJkpV1ozl1vZuYIVKapMnId0tl6EYKrSkCU3BKwpoivK4avA2XxTIE5qUWRaeBjRUCTCs~Oy1uojXLObb5-rx0TaKrRMSIHBe35WGL9ZJM7hcl~JW2ogBxE9DyU0XDfawJezqODDs08NFYDS1q~uVEOiXCdXDFuzNspmN5r4gXFRELVvNovGEWmBv-Rx~5DLSnr8YodY8h74Mn897JP3EhX18o-RQN4BvYyhdjJT3dALtjejVR6wqwwxtA8VOip6VzLCrLfH~OxeuYl1J~j8TmdYlWfrm0PAeMOx1WeFrzt6Wv7ZItr4PSJ8pzcWYDPILKu08HUSUgKLi1dfoDHw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)