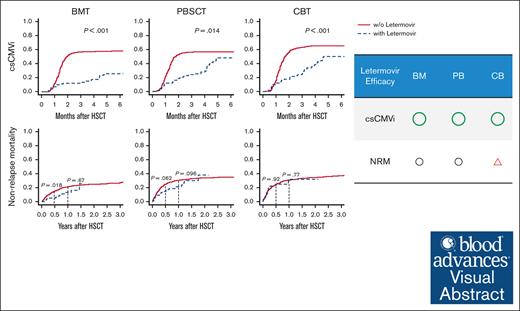
Letermovir prophylaxis after HSCT suppressed clinically significant CMV infection regardless of graft sources.
Impact of letermovir prophylaxis on nonrelapse mortality was remarkable in BM/PBSC transplantation rather than in CB transplantation.
Visual Abstract
Clinically significant cytomegalovirus infection (csCMVi) is frequently observed after allogeneic hematopoietic stem cell transplantation (HSCT) and prophylaxis with letermovir is commonly adopted. However, the clinical benefit of letermovir prophylaxis according to graft sources has not been sufficiently elucidated. We retrospectively analyzed 2194 recipients of HSCT who were CMV-seropositive (236 with letermovir prophylaxis and 1958 without prophylaxis against CMV). csCMVi was significantly less frequent in patients with letermovir prophylaxis than in those without (23.7% vs 58.7% at 100 days after HSCT, P < .001) and the same trend was seen when recipients of bone marrow (BM), peripheral blood stem cell (PBSC), or cord blood (CB) transplantation were separately analyzed. In recipients of BM, nonrelapse mortality (NRM) was significantly lower in the letermovir group at 6 months after HSCT (5.0% vs 14.9%, P = .018), and the same trend was observed in recipients of PBSCs (14.7% vs 24.8%, P = .062); however, there was no statistical significance at 1 year (BM, 21.1% vs 30.4%, P = .67; PBSCs, 21.2% vs 30.4%, P = .096). In contrast, NRM was comparable between recipients of CB with and without letermovir prophylaxis throughout the clinical course (6 months, 23.6% vs 24.3%, P =.92; 1 year, 29.3% vs 31.0%, P = .77), which was confirmed by multivariate analyses. In conclusion, the impact of letermovir prophylaxis on NRM and csCMVi should be separately considered according to graft sources.
Introduction
Cytomegalovirus (CMV) infection is frequently observed after allogeneic hematopoietic stem cell transplantation (HSCT), and is directly/indirectly associated with increased nonrelapse mortality (NRM) and morbidity.1-4 Preemptive therapy with ganciclovir or foscarnet can effectively prevent CMV end-organ diseases, but this strategy cannot reduce CMV DNAemia/antigenemia and indirect effects on NRM.5,6 In 2017, the CMV terminase inhibitor letermovir was reported to be useful for CMV primary prophylaxis,7 and this agent has since been widely adopted.8-10 Nonetheless, the optimal application of letermovir is still unclear. Although many studies reported that clinically significant CMV infection (csCMVi) was reduced by letermovir administration, the beneficial effect on NRM, which was originally the main motive for primary prophylaxis against CMV reactivation, is controversial.11-18 In this multicenter retrospective study, we analyzed the large-cohort transplantation data set of the Kanto Study Group for Cell Therapy (KSGCT) to elucidate the specific patient group most likely to benefit from letermovir.
Methods
Patients
Patients who were CMV seropositive who underwent HSCT from January 2014 to December 2019 at participating institutions in KSGCT were included in this study. Patients who received prophylactic administration of an anti-CMV drug other than letermovir, such as ganciclovir or foscarnet, were excluded. The decision to use letermovir or other prophylaxis was made at the discretion of the individual physician. Letermovir was generally started soon after HSCT and continued until 100 days after HSCT, and the dose of letermovir was 480 mg per day (240 mg per day in patients who received concomitant cyclosporin). Some of the patients in a previous report,13 that is, the patients who underwent HSCT at Keio University Hospital, were also included in this study. Disease risk was classified as previously published.19 HLA was considered as mismatched when at least 1 disparity was present in the HLA-A, -B, -C, or -DRB1 allele. Performance status was evaluated according to Eastern Cooperative Oncology Group criteria. CMV monitoring with CMV antigenemia testing was generally started after neutrophil engraftment, and csCMVi was defined as initiation of preemptive therapy or diagnosis of CMV end-organ disease. Preemptive therapy was generally initiated when ≥3 antigenemia-positive cells were detected every 2 slides2,20 and antiviral therapy was administered as recommended.10 Causes of death were classified according to a previous report.21 This study was performed in accordance with the Declaration of Helsinki and was approved by the ethics committee of Tokyo Metropolitan Komagome Hospital (approval number 2474) and participating institutions.
Statistics
Differences in numerical and categorical variables were compared using a t test and Fisher exact test, respectively. Survival time was calculated with the Kaplan-Meier method, and the log-rank test was used to evaluate the differences. The factors with P < .05 in the univariate analyses were included in the multivariate analyses. In the multivariate analyses, overall survival (OS) was evaluated using a Cox proportional hazard model, and the cumulative incidence of relapse and NRM were evaluated using the Gray method by considering each risk as a competing risk. A logistic regression model was used to identify the clinical factors significantly associated with letermovir use. The cumulative incidence of csCMVi was evaluated using the Gray method by considering relapse and NRM as competing risks. The association of NRM and letermovir use was also evaluated using a Cox proportional hazard model in multivariate analysis. The level of statistical significance was set at P < .05. Statistical analyses were performed using R version 4.0.5.
Results
Patient characteristics
A total of 2194 patients who underwent HSCT during the study period were enrolled in this study. Among them, 236 patients (10.8%) received letermovir prophylaxis, and 1958 patients received no prophylactic medication against CMV. The median age at transplantation was 52 years (range, 16-71). Graft sources were bone marrow (BM) in 948, peripheral blood stem cells (PBSCs) in 657, and cord blood (CB) in 589 patients. The cumulative incidence of relapse was 24.3% at 3 years after HSCT, and the 3-year NRM was 31.5%. Letermovir was started a median of 1 day after HSCT (interquartile range, 0-7) and the median duration of letermovir administration was 90 days (interquartile range, 41.3-96.0). In total, 99 patients discontinued letermovir prophylaxis before 70 days after HSCT, and the main reasons were difficulty in oral administration in 28 patients, CMV reactivation in 26, declined systemic condition and/or death in 11, and human herpes virus-6 (HHV-6) reactivation in 10 patients (supplemental Table 1). In addition, 11 patients temporarily discontinued letermovir and restarted thereafter, and the causes of discontinuation were difficulty in oral administration in 5 patients, HHV-6 reactivation in 3, decline of serum voriconazole concentration in 1, CMV reactivation in 1, and declined systemic condition in 1 patients.
Details of the patient characteristics are shown in Table 1. There were several significant differences between patients receiving letermovir prophylaxis and those who were not. Patients with letermovir administration were significantly older (median age, 54.0 vs 51.0 years, P < .001), received BM transplantation less frequently than PBSCs or umbilical CB (33.9% vs 44.3%, P = .004), received HLA-matched HSCT less frequently (32.2% vs 41.1%, P = .009), less frequently received HSCT from CMV–immunoglobulin G (IgG)-negative donors (26.7% vs 39.2%, P = .006), and were more frequently administered antithymocyte globulin (ATG) as a conditioning regimen (28.0% vs 16.5%, P < .001). Patient characteristics varied based on graft sources, and the prevalence of patients who used ATG was significantly lower in recipients of CB (supplemental Table 2). Logistic regression analysis revealed that recipients of CB (odds ratio [OR], 2.30; 95% confidence interval [CI], 1.47-3.59; P < .001), donor CMV IgG positivity (OR, 1.61; 95% CI, 1.10-2.36; P = .014), and the use of ATG (OR, 2.37; 95% CI, 1.61-3.50; P < .001) were significantly associated with letermovir usage.
The association between patient characteristics and letermovir use
| Characteristics . | Patients with letermovir prophylaxis (n = 236) . | Patients without CMV prophylaxis (n = 1958) . | P value . |
|---|---|---|---|
| Patient age at HSCT, y, median | 54.0 | 51.0 | <.001 |
| Sex mismatch | .74 | ||
| Female to male | 48 (20.3%) | 419 (21.4%) | |
| Other | 188 (79.7%) | 1539 (78.6%) | |
| Underlying disease | .24 | ||
| Acute myeloid leukemia | 104 (44.1%) | 867 (44.3%) | |
| Acute lymphoblastic leukemia | 47 (19.9%) | 341 (17.4%) | |
| Aplastic anemia | 9 (3.8%) | 50 (2.6%) | |
| Chronic myeloid leukemia | 3 (1.3%) | 75 (3.8%) | |
| Myelodysplastic syndromes | 36 (15.3%) | 327 (16.7%) | |
| Other | 11 (4.7%) | 93 (4.7%) | |
| Disease risk | .84 | ||
| Low | 109 (46.2%) | 909 (46.4%) | |
| High | 127 (53.8%) | 1027 (52.5%) | |
| NA | 0 | 22 (1.1%) | |
| Number of HSCTs | .93 | ||
| 1 | 193 (81.8%) | 1590 (81.2%) | |
| 2 | 43 (18.2%) | 368 (18.8%) | |
| Graft source | .004 | ||
| BM | 80 (33.9%) | 868 (44.3%) | |
| PBSCs | 75 (31.8%) | 582 (29.7%) | |
| CB | 81 (34.3%) | 508 (25.9%) | |
| HLA | .009 | ||
| Matched | 76 (32.2%) | 804 (41.1%) | |
| Mismatched | 160 (67.8%) | 1154 (58.9%) | |
| Relationship | .77 | ||
| Related | 75 (31.8%) | 644 (32.9%) | |
| Unrelated | 161 (68.2%) | 1314 (67.1%) | |
| Performance status | .51 | ||
| 0-1 | 214 (90.7%) | 1741 (88.9%) | |
| 2-4 | 22 (9.3%) | 217 (11.1%) | |
| HCT-CI | .36 | ||
| 0-2 | 178 (75.4%) | 1531 (78.2%) | |
| ≥3 | 58 (24.6%) | 427 (21.8%) | |
| Donor CMV IgG | .006 | ||
| Positive | 98 (41.5%) | 750 (38.3%) | |
| Negative | 63 (26.7%) | 767 (39.2%) | |
| Unknown | 75 (31.8%) | 441 (22.5%) | |
| Conditioning intensity | .098 | ||
| Myeloablative | 109 (46.2%) | 1020 (52.1%) | |
| Reduced intensity | 127 (53.8%) | 938 (47.9%) | |
| ATG use before HSCT | <.001 | ||
| Yes | 66 (28.0%) | 323 (16.5%) | |
| No | 170 (72.0%) | 1635 (83.5%) | |
| Acute GVHD, grade | 1.0 | ||
| 0-1 | 148 (62.7%) | 1197 (61.1%) | |
| 2-4 | 86 (36.4%) | 699 (35.7%) | |
| NA | 2 (0.8%) | 62 (3.2%) |
| Characteristics . | Patients with letermovir prophylaxis (n = 236) . | Patients without CMV prophylaxis (n = 1958) . | P value . |
|---|---|---|---|
| Patient age at HSCT, y, median | 54.0 | 51.0 | <.001 |
| Sex mismatch | .74 | ||
| Female to male | 48 (20.3%) | 419 (21.4%) | |
| Other | 188 (79.7%) | 1539 (78.6%) | |
| Underlying disease | .24 | ||
| Acute myeloid leukemia | 104 (44.1%) | 867 (44.3%) | |
| Acute lymphoblastic leukemia | 47 (19.9%) | 341 (17.4%) | |
| Aplastic anemia | 9 (3.8%) | 50 (2.6%) | |
| Chronic myeloid leukemia | 3 (1.3%) | 75 (3.8%) | |
| Myelodysplastic syndromes | 36 (15.3%) | 327 (16.7%) | |
| Other | 11 (4.7%) | 93 (4.7%) | |
| Disease risk | .84 | ||
| Low | 109 (46.2%) | 909 (46.4%) | |
| High | 127 (53.8%) | 1027 (52.5%) | |
| NA | 0 | 22 (1.1%) | |
| Number of HSCTs | .93 | ||
| 1 | 193 (81.8%) | 1590 (81.2%) | |
| 2 | 43 (18.2%) | 368 (18.8%) | |
| Graft source | .004 | ||
| BM | 80 (33.9%) | 868 (44.3%) | |
| PBSCs | 75 (31.8%) | 582 (29.7%) | |
| CB | 81 (34.3%) | 508 (25.9%) | |
| HLA | .009 | ||
| Matched | 76 (32.2%) | 804 (41.1%) | |
| Mismatched | 160 (67.8%) | 1154 (58.9%) | |
| Relationship | .77 | ||
| Related | 75 (31.8%) | 644 (32.9%) | |
| Unrelated | 161 (68.2%) | 1314 (67.1%) | |
| Performance status | .51 | ||
| 0-1 | 214 (90.7%) | 1741 (88.9%) | |
| 2-4 | 22 (9.3%) | 217 (11.1%) | |
| HCT-CI | .36 | ||
| 0-2 | 178 (75.4%) | 1531 (78.2%) | |
| ≥3 | 58 (24.6%) | 427 (21.8%) | |
| Donor CMV IgG | .006 | ||
| Positive | 98 (41.5%) | 750 (38.3%) | |
| Negative | 63 (26.7%) | 767 (39.2%) | |
| Unknown | 75 (31.8%) | 441 (22.5%) | |
| Conditioning intensity | .098 | ||
| Myeloablative | 109 (46.2%) | 1020 (52.1%) | |
| Reduced intensity | 127 (53.8%) | 938 (47.9%) | |
| ATG use before HSCT | <.001 | ||
| Yes | 66 (28.0%) | 323 (16.5%) | |
| No | 170 (72.0%) | 1635 (83.5%) | |
| Acute GVHD, grade | 1.0 | ||
| 0-1 | 148 (62.7%) | 1197 (61.1%) | |
| 2-4 | 86 (36.4%) | 699 (35.7%) | |
| NA | 2 (0.8%) | 62 (3.2%) |
HCT-CI, hematopoietic cell transplantation-specific comorbidity index; NA, not assessed.
csCMVi
The cumulative incidence of csCMVi at 100 days after HSCT among the total cohort was 55.0% (95% CI, 52.9-57.1), and it was significantly lower in patients receiving letermovir than in those not receiving prophylaxis (23.7% vs 58.7% at 100 days after HSCT, P < .001; Figure 1A). Multivariate analysis revealed that letermovir use was significantly associated with csCMVi (hazard ratio [HR], 0.488; 95% CI, 0.405-0.589; P < .001; Table 2).
Clinical outcomes after allogeneic HSCT according to the presence or absence of letermovir prophylaxis. (A) Cumulative incidence of csCMVi, (B) OS, (C) CIR, and (D) NRM in patients with letermovir prophylaxis (blue, dotted line) or without cytomegalovirus prophylaxis (red, solid line).
Clinical outcomes after allogeneic HSCT according to the presence or absence of letermovir prophylaxis. (A) Cumulative incidence of csCMVi, (B) OS, (C) CIR, and (D) NRM in patients with letermovir prophylaxis (blue, dotted line) or without cytomegalovirus prophylaxis (red, solid line).
Univariate and multivariate analyses for csCMVi
| Characteristics . | Univariate analysis . | Multivariate analysis . | |
|---|---|---|---|
| P value . | HR (95% CI) . | P value . | |
| Letermovir prophylaxis | <.001 | 0.488 (0.405-0.589) | <.001 |
| Patient age at HSCT, >50 y | .26 | ||
| Diagnosis | .82 | ||
| Disease risk high | .021 | 0.884 (0.790-0.990) | .033 |
| PS > 1 | <.001 | 0.617 (0.498-0.764) | <.001 |
| HCT-CI > 2 | .51 | ||
| Donor CMV IgG | .15 | ||
| BMT | .024 | 0.955 (0.827-1.10) | .54 |
| PBSCT | 1.0 | ||
| CBT | .011 | 1.08 (0.902-1.30) | .40 |
| HLA-mismatched donor | <.001 | 1.41 (1.23-1.62) | <.001 |
| Female donor to male recipient | .61 | ||
| Unrelated donor | .070 | ||
| Number of HSCT > 1 | .34 | ||
| MAC | .13 | ||
| ATG use | .001 | 1.29 (1.08-1.54) | .005 |
| Characteristics . | Univariate analysis . | Multivariate analysis . | |
|---|---|---|---|
| P value . | HR (95% CI) . | P value . | |
| Letermovir prophylaxis | <.001 | 0.488 (0.405-0.589) | <.001 |
| Patient age at HSCT, >50 y | .26 | ||
| Diagnosis | .82 | ||
| Disease risk high | .021 | 0.884 (0.790-0.990) | .033 |
| PS > 1 | <.001 | 0.617 (0.498-0.764) | <.001 |
| HCT-CI > 2 | .51 | ||
| Donor CMV IgG | .15 | ||
| BMT | .024 | 0.955 (0.827-1.10) | .54 |
| PBSCT | 1.0 | ||
| CBT | .011 | 1.08 (0.902-1.30) | .40 |
| HLA-mismatched donor | <.001 | 1.41 (1.23-1.62) | <.001 |
| Female donor to male recipient | .61 | ||
| Unrelated donor | .070 | ||
| Number of HSCT > 1 | .34 | ||
| MAC | .13 | ||
| ATG use | .001 | 1.29 (1.08-1.54) | .005 |
BMT, bone marrow transplantation; CBT, cord blood transplantation; HCT-CI, hematopoietic cell transplantation-specific comorbidity index; HR, hazard ratio; MAC, myeloablative conditioning; PBSCT, peripheral blood stem cell transplantation.
Transplantation outcomes
Among the total cohort, the 1-year OS, cumulative incidences of relapse (CIR), and NRM were 62.2% (95% CI, 60.2-64.3), 20.0% (95% CI, 18.3-21.7), and 25.9% (95% CI, 24.0-27.7) and the 3-year OS, CIR, and NRM were 50.0% (95% CI, 47.8-52.3), 24.3% (95% CI, 22.5-26.2), and 31.5% (95% CI, 29.4-33.6). The OS (P = .50) and CIR (P = .93) were not significantly different between patients administered letermovir and those who did not receive letermovir (Figure 1B-C). Multivariate analyses revealed that letermovir prophylaxis was not a significant risk factor for OS (HR, 0.866; 95% CI, 0.687-1.09; P = .22; supplemental Table 3) or for CIR (HR, 0.973; 95% CI, 0.713-1.33; P = .86; supplemental Table 4). NRM (P = .24) was also not significantly different between the 2 treatment groups (Figure 1D). However, multivariate analysis using a competing regression model showed that letermovir use was significantly associated with lower NRM (HR, 0.743; 95% CI, 0.556-0.993; P = .045; Table 3), although the difference was not statistically significant when the Cox proportional hazard model was used to evaluate the impact of letermovir use on NRM more rigorously (HR, 0.800; 95% CI, 0.601-1.06; P = .12; supplemental Table 5).
Univariate and multivariate analyses for NRM
| Characteristics . | Univariate analysis . | Multivariate analysis . | |
|---|---|---|---|
| P value . | HR (95% CI) . | P value . | |
| Letermovir prophylaxis | .24 | 0.743 (0.556-0.993) | .045 |
| Patient age at HSCT, >50 y | <.001 | 1.56 (1.30-1.87) | <.001 |
| AML | .88 | ||
| ALL | <.001 | 0.996 (0.786-1.26) | .97 |
| AA | .002 | 0.613 (0.286-1.32) | .21 |
| CML | .072 | ||
| MDS | .087 | ||
| Disease risk high | <.001 | 1.93 (1.61-2.32) | <.001 |
| PS > 1 | <.001 | 1.84 (1.46-2.32) | <.001 |
| HCT-CI > 2 | <.001 | 1.28 (1.06-1.53) | <.001 |
| Donor CMV IgG | .58 | ||
| HLA-matched BMT | <.001 | 0.768 (0.592-0.998) | .048 |
| HLA-matched PBSCT | .008 | 0.776 (0.576-1.05) | .095 |
| HLA-mismatched BMT | .24 | ||
| HLA-mismatched PBSCT | <.001 | 1.11 (0.856-1.45) | .42 |
| CBT | <.001 | 1.19 (0.942-1.49) | .15 |
| Female donor to male recipient | .053 | ||
| Unrelated donor | .15 | ||
| Number of HSCT > 1 | <.001 | 1.84 (1.51-2.26) | <.001 |
| MAC | <.001 | 1.16 (0.970-1.40) | .10 |
| ATG use | .003 | 1.14 (0.902-1.45) | .27 |
| Characteristics . | Univariate analysis . | Multivariate analysis . | |
|---|---|---|---|
| P value . | HR (95% CI) . | P value . | |
| Letermovir prophylaxis | .24 | 0.743 (0.556-0.993) | .045 |
| Patient age at HSCT, >50 y | <.001 | 1.56 (1.30-1.87) | <.001 |
| AML | .88 | ||
| ALL | <.001 | 0.996 (0.786-1.26) | .97 |
| AA | .002 | 0.613 (0.286-1.32) | .21 |
| CML | .072 | ||
| MDS | .087 | ||
| Disease risk high | <.001 | 1.93 (1.61-2.32) | <.001 |
| PS > 1 | <.001 | 1.84 (1.46-2.32) | <.001 |
| HCT-CI > 2 | <.001 | 1.28 (1.06-1.53) | <.001 |
| Donor CMV IgG | .58 | ||
| HLA-matched BMT | <.001 | 0.768 (0.592-0.998) | .048 |
| HLA-matched PBSCT | .008 | 0.776 (0.576-1.05) | .095 |
| HLA-mismatched BMT | .24 | ||
| HLA-mismatched PBSCT | <.001 | 1.11 (0.856-1.45) | .42 |
| CBT | <.001 | 1.19 (0.942-1.49) | .15 |
| Female donor to male recipient | .053 | ||
| Unrelated donor | .15 | ||
| Number of HSCT > 1 | <.001 | 1.84 (1.51-2.26) | <.001 |
| MAC | <.001 | 1.16 (0.970-1.40) | .10 |
| ATG use | .003 | 1.14 (0.902-1.45) | .27 |
AA, aplastic anemia; AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; BMT, bone marrow transplantation; CBT, cord blood transplantation; CML, chronic myeloid leukemia; HCT-CI, hematopoietic cell transplantation-specific comorbidity index; MAC, myeloablative conditioning; MDS, myelodysplastic syndromes; PBSCT, peripheral blood stem cell transplantation.
csCMVi and NRM according to graft sources and the presence or absence of letermovir use
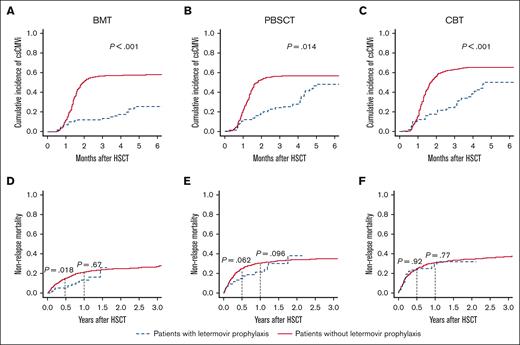
Next, we evaluated the clinical impact of letermovir on csCMVi and NRM according to graft sources. The cumulative incidence of csCMVi tended to be lower in patients with letermovir prophylaxis regardless of the graft source (Figure 2A-C).
csCMVi and NRM according to graft sources. Cumulative incidence of csCMVi in recipients of (A) BM, (B) PBSC, and (C) CB transplantation, and NRM in recipients of (D) BM, (E) PBSC, and (F) CB transplantation. Data in patients with letermovir prophylaxis are indicated by dotted blue lines and those in patients without CMV prophylaxis are shown with solid red lines. Black dotted lines indicate 6 months or 1 year after transplantation.
csCMVi and NRM according to graft sources. Cumulative incidence of csCMVi in recipients of (A) BM, (B) PBSC, and (C) CB transplantation, and NRM in recipients of (D) BM, (E) PBSC, and (F) CB transplantation. Data in patients with letermovir prophylaxis are indicated by dotted blue lines and those in patients without CMV prophylaxis are shown with solid red lines. Black dotted lines indicate 6 months or 1 year after transplantation.
In recipients of BMs, NRM was significantly lower in the letermovir group than in the nonletermovir group at 6 months after HSCT (5.0% vs 14.9%, P = .018), but the differences were not significant 1 year after HSCT (BM: 21.1% vs 30.4%, P = .67; Figure 2D). The same trend was seen in recipients of PBSC, although the difference at 6 months after HSCT was not statistically significant (6 months: 14.7% vs 24.8%, P = .062; 1 year: 21.2% vs 30.4%, P = .096; Figure 2E). However, in recipients of CB, NRM was not significantly different between the letermovir and nonletermovir group throughout the posttransplant clinical course (6 months: 23.6% vs 24.3%, P = .92; 1 year: 29.3% vs 31.0%, P = .77; Figure 2F). The OS and CIR were not significantly different among the graft sources or between patients with and without letermovir prophylaxis, but we noted a nonsignificant trend toward improved OS in patients with letermovir prophylaxis who underwent PBSC transplantation (P = .050; supplemental Figure 1). Multivariate analysis also revealed that letermovir prophylaxis was significantly associated with lower NRM in recipients of BM/PBSC (HR, 0.623; 95% CI, 0.427-0.908; P = .014; supplemental Table 6) but not in recipients of CB (HR, 0.968; 95% CI, 0.624-1.50; P = .88; supplemental Table 7). Multivariate analysis using a Cox proportional hazard model also showed that letermovir use was significantly associated with lower NRM in recipients of BM/PBSC (HR, 0.631; 95% CI, 0.433-0.921; P = .017; supplemental Table 8) but not in recipients of CB (HR, 1.10; 95% CI, 0.707-1.71; P = .67; supplemental Table 9).
Causes of death at 6 months after HSCT according to the presence or absence of letermovir prophylaxis
At 6 months after HSCT, 43 of 236 patients with letermovir prophylaxis and 494 of 1958 patients without prophylaxis against CMV died, and the causes in 32 and 344 cases were other than primary disease. When comparing the proportion of causes of deaths other than primary disease at 6 months after HSCT according to letermovir prophylaxis and graft sources, the proportion of bacterial infection seemed to be larger in CB transplantation than in BM/PBSC transplantation, and the proportion of graft-versus-host disease (GVHD) seemed to be larger in recipients of CB with letermovir prophylaxis than in patients without prophylaxis against CMV, but statistical analysis was not performed because the numbers of deaths in the letermovir groups with the different graft sources were quite small (Figure 3 and supplemental Table 10).
Stacked area chart of causes of early death after transplantation according to letermovir prophylaxis. Proportion of causes of death other than primary disease at 6 months after transplantation are shown for patients receiving (A) BM, (B) PBSC, and (C) CB transplantation.
Stacked area chart of causes of early death after transplantation according to letermovir prophylaxis. Proportion of causes of death other than primary disease at 6 months after transplantation are shown for patients receiving (A) BM, (B) PBSC, and (C) CB transplantation.
Clinical outcomes in patients with letermovir prophylaxis
Among the 236 patients with letermovir prophylaxis, the cumulative incidence of csCMVi was 23.7% at 100 days after HSCT and 43.5% at 1 year after HSCT. Multivariate analysis for csCMVi showed that a performance status score of 2 to 4 (HR, 0.328; 95% CI, 0.114-0.943; P = .039) and HLA-matched BM transplantation (HR, 0.346; 95% CI, 0.165-0.724; P = .005) were less associated with csCMVi in recipients of HSCT with letermovir prophylaxis (supplemental Table 11). To assess the impact of early letermovir discontinuation, we conducted a landmark analysis involving 162 patients who received letermovir prophylaxis and remained free from both relapse and csCMVi for 70 days after HSCT. The analysis revealed that discontinuing letermovir before the 70-day mark after HSCT was significantly associated with a subsequent cumulative incidence of csCMVi (6 months, 58.8% vs 25.0%; P < .0001). However, it was not correlated with the risk of relapse (1 year, 21.3% vs 16.0%; P = .75) or NRM (1 year, 18.5% vs 12.1%, P = .37; supplemental Figure 2A). In addition, we also conducted landmark analysis for 137 patients who survived without relapse or csCMVi for 100 days after HSCT, indicating that there was no significant difference in cumulative incidence of csCMVi among patients with or without grade II to IV acute GVHD (6 months, 33.8% vs 25.5%, P = .32; supplemental Figure 2B).
Discussion
In this study, we retrospectively analyzed 2194 recipients of HSCT, including 236 cases who received letermovir prophylaxis, and showed that letermovir prophylaxis suppressed csCMVi in patients with any of the graft sources. However, the difference in csCMVi incidence was reduced after discontinuation of letermovir, as in previous reports.7,12-17,22 Late CMV infection after letermovir discontinuation, which might be because of delayed reconstitution of anti-CMV immunity,23 is the Achilles heel of letermovir prophylaxis.16,24,25 Thus, letermovir continuation >100 days after HSCT is an expected option to reduce late-onset CMV infection.26,27 Another promising strategy is a risk-based letermovir use, which could reduce csCMVi while allowing some patients at low risk to avoid letermovir use.14,28 However, although both strategies could efficiently suppress csCMVi, their effects on survival are unclear.
In this study, prophylaxis with letermovir was significantly associated with lower NRM at 6 months after BMT and the same tendency was seen after PBSC transplantation, although the difference was not statistically significant. The superiority diminished thereafter, as shown in a phase 3 trial in which most participants were recipients of BM/PBSC transplantation,7 and this decline in effectiveness was attributed to frequent delayed-onset csCMVi, which directly or indirectly increased CMV-related mortality after letermovir discontinuation.16,25 Therefore, recipients of BM/PBSC transplantation might be promising candidates for extended-duration letermovir prophylaxis, which has mainly drawn attention in the CB transplantation setting.26 In contrast, in CB transplantation, NRM was comparable between patients with and without letermovir use throughout the clinical course, possibly because the higher mortality rate early after CB transplantation29-31 interfered with the survival benefit from prophylaxis against CMV. Therefore, although letermovir prophylaxis could decrease csCMVi, rehospitalization, and medical costs,32,33 our data indicate that letermovir prophylaxis alone cannot reduce early NRM, and additional intervention is necessary to improve the early outcomes after CB transplantation.
Causes of death in this cohort are described in Figure 3 and supplemental Table 11. Bacterial infection was the major cause of early death in CB transplantation, as in earlier reports.21,34 Although the small number of deaths of each cause in the letermovir group precluded statistical analysis, the proportion of deaths due to bacterial infection in the letermovir group seemed to be lower than that in the nonletermovir group in BM/PBSC transplantation but not in the CB transplantation setting. In addition, although no patient died of hemorrhage in the letermovir group of BM/PBSC transplantation recipients, in the CB transplantation setting, hemorrhagic deaths seemed to be rather frequent in the letermovir group. These possible declines in various causes of deaths in letermovir group in BM/PBSC transplantation might inversely reflect the indirect effect of csCMVi.5 The exact reason why the decreased NRM was not seen in CB transplantation was unclear, but delayed hematopoietic recovery after CB transplantation might be the key, which can be associated with frequent deaths due to bacterial infections and hemorrhages.21 Perhaps various attempts to improve the hematologic recovery in CB transplantation such as ex vivo expansion35 and administration of recombinant human thrombopoietin36 might reinforce the positive impact of letermovir prophylaxis on NRM, but further studies with much larger cohorts are warranted. In contrast, the underlying mechanism of the apparent increase in deaths due to GVHD in recipients of CB with letermovir prophylaxis is unclear (Figure 3), although the number is relatively small. The nonsignificant higher prevalence of grade II to IV acute GVHD in recipients of CB with letermovir prophylaxis might be associated with the discrepancy in GVHD-related deaths (supplemental Table 2). However, previous studies that primarily included recipients of BM/PBSC transplantation did not suggest a significant increase in GVHD-related mortality with letermovir prophylaxis.17,22 Future studies on CB transplantation are warranted.
Our study had several limitations. First, CMV monitoring was generally performed by pp65 antigenemia rather than polymerase chain reaction in this study because polymerase chain reaction testing was not covered by the public insurance system in Japan during the study period. This could have affected the results because CMV viremia before engraftment might have been overlooked.37 Second, the retrospective nature of this study is a limitation, because the decision to use letermovir was at the discretion of each physician. In our cohort, CB transplantation, donor CMV IgG positivity, and the use of ATG were independently associated with letermovir use, suggesting the potential presence of unaccounted biases. Discrepancies in patient characteristics related to graft sources, including the indications for letermovir, could influence the results, although such variations in transplantation settings align with previous reports.38,39 In addition, the percentage of patients who discontinued letermovir was notably high in this study. This was partially attributed to the limited supply of intravenous letermovir during a specific period of the study in Japan because of manufacturing process issues. Consequently, some physicians might have had to discontinue letermovir altogether instead of switching to intravenous letermovir when patients were unable to swallow the letermovir tablets. Third, CB transplantation is a major risk factor for HHV-6 reactivation and encephalitis,40-43 and some of the recipients of CB transplantation were not included in this study because they received prophylactic foscarnet to prevent HHV-6 encephalitis,44 which also can suppress CMV,10,45 and this procedure might have affected the results. The indication of prophylactic foscarnet in each patient was based on each physician’s choice and this also might have influenced the results. Fourth, post-HSCT risk factors for CMV reactivation, such as GVHD, were not included in this study because such factors are not useful to determine the indication for letermovir at the time of HSCT. Risk-based letermovir use, that is, initiating letermovir prophylaxis when systemic corticosteroid is started,14 could be a reasonable option, and further studies are needed to evaluate this approach.
In conclusion, our large-scale, multicenter retrospective study showed that the impact of letermovir prophylaxis on NRM was limited in patients undergoing CB transplantation and further strategies for reducing NRM in these patients will be needed, although letermovir prophylaxis tended to prevent csCMVi regardless of the graft source. Application of letermovir could be variable according to the objective and, thus, further investigation of the goal-directed application of letermovir prophylaxis after HSCT is warranted.
Acknowledgments
The authors thank all patients who participated in the study and all members of Kanto Study of Group for Cell Therapy (KSGCT). The authors also thank Toyohiro Kawano and the members of the KSGCT data center for data management.
Authorship
Contribution: T.T. performed statistical analyses, wrote the manuscript, and made the figures; T.T., K.M., M.S., J.K., T.M., N.D., S. Masuda., N.A., S.T., E.S., Y.N., S.F., S. Machida, Y.A., H.Y., K.S., Y.H., K.U., K.K., and Y.K. contributed to data collection and revised the manuscript; N.D. and Y.K. supervised the study; and all authors read and approved the manuscript.
Conflict-of-interest disclosure: M.S. owns stock in Celaid Therapeutics. T.M. has received grants or contracts from each of Mochida Pharmaceutical, Bristol Myers Squibb, Merck Sharp & Dohme (MSD), AbbVie GK, Novartis Pharma, CSL Behring, Eisai, Teijin Pharma, Medical & Biological Laboratorie, KM Biologics, Otsuka Pharmaceutical, Kyowa Kirin, Shionogi, Chugai Pharmaceutical, Takeda Pharmaceutical, and Asahi Kasei Corporation; and has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Pfizer Inc, AbbVie GK, MSD, Meiji Seika Pharma, Janssen Pharma, CSL Behring, Sumitomo Pharma, SymBio Pharmaceuticals, Novartis Pharma, AstraZeneca, Kyowa Kirin Co Ltd, Daiichi Sankyo, Chugai Pharmaceutical, Mochida Pharmaceutical, Asahi Kasei Corporation, Sanofi, Japan Blood Products Organization, Otsuka Pharmaceutical, Takeda Pharmaceutical, Mitsubishi Tanabe Pharma, Ono Pharmaceutical, Eisai Pharmaceuticals, and Astellas Pharma. K.U. has received research funding from Astellas, AbbVie, Bristol Myers Squibb, Janssen, Ono, Otsuka, Chugai, Aperis, Yakult, MSD, Amgen, Alxion, Incyte, Eisai, Kyowa Kirin, Sanfi, SymBio, Celgene, Daiichi Sankyo, Sumitomo-Dainippon, Nippon Shinyaku, Novartis, Mundi, and Takeda; has served on speaker bureaus for Novartis, AbbVie, Alexion, Incyte, Ono, Kyowa-Kirin, Sanfi, Takeda, Nippon-Shinyaku, Pfizer, and Bristol Myers Squibb; and has served on consulting bureaus for Astellas, Amgen, Alnylam Japan, Alexion, Eisai, Otsuka, Ohara, Kyowa-Kirin, Sanofi, Sando, SymBio, Takeda, Chugai, and Nippon-Shinyaku. The remaining authors declare no competing financial interests.
Correspondence: Takashi Toya, Hematology Division, Tokyo Metropolitan Cancer and Infectious Diseases Center, Komagome Hospital, 3-18-22 Hon-komagome, Bunkyo-ku, Tokyo 1138677, Japan; email: tooya-tky@umin.ac.jp.
References
Author notes
The data sets generated and/or analyzed during this study are available on reasonable request from the corresponding author, Takashi Toya (tooya-tky@umin.ac.jp).
The full-text version of this article contains a data supplement.