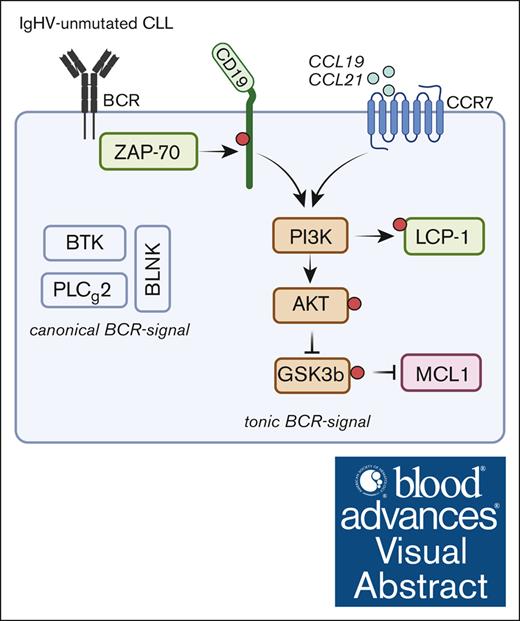
ZAP-70 enhances a tonic BCR-signal exclusively found in IGHV-unmutated CLL.
ZAP-70 augments CCR7-signaling and cell migration of unmutated CLL through activation of LCP-1.
Visual Abstract
Expression of ZAP-70 in a subset of patients with chronic lymphocytic leukemia (CLL) positively correlates with the absence of immunoglobulin heavy-chain gene (IGHV) mutations and is indicative of a more active disease and shorter treatment-free survival. We recently demonstrated that ZAP-70 regulates the constitutive expression of CCL3 and CCL4, activation of AKT, and expression of MYC in the absence of an overt B-cell receptor (BCR) signal, bona fide functions of BCR activation. We, here, provide evidence that these features relate to the presence of a constitutive tonic BCR signal, exclusively found in IGHV-unmutated CLL and dependent on the ZAP-70–mediated activation of AKT and its downstream target GSK-3β. These findings are associated with increased steady-state activation of CD19 and SRC. Notably this tonic BCR signal is not present in IGHV-mutated CLL cells, discordantly expressing ZAP-70. Results of quantitative mass spectrometry and phosphoprotein analyses indicate that this ZAP-70–dependent, tonic BCR signal regulates CLL cell migration through phosphorylation of LCP1 on serine-5. Indeed, we show that CCL19- and CCL21-induced chemotaxis is regulated by and dependent on the expression of ZAP-70 through its function to enhance CCR7 signaling to LCP1. Thus, our data demonstrate that ZAP-70 converges a tonic BCR signal, exclusively present in IGHV-unmutated CLL and CCR7-mediated chemotaxis.
Introduction
Two decades ago, the expression of the tyrosine kinase ZAP-70 was identified in a subset of malignant B cells from patients with chronic lymphocytic leukemia (CLL) and is highly associated with unmutated IGHV genes.1 Similar to patients carrying unmutated IGHV genes, those with ZAP-70 positive CLL have a more active disease and require treatment earlier than patients with ZAP-70 negative CLL.2,3 Notably, the association between unmutated IGHV genes and ZAP-70 expression is not perfect, but ∼20% of mutated CLL cases also express ZAP-70 at detectable levels above the arbitrary defined flow-cytometry threshold of 20%.1,3 The spare clinical data available for those “discordant” cases suggest that ZAP-70 status outperforms mutational status as a prognostic biomarker and that those patients despite carrying mutated IGHV genes have an unusual aggressive clinical course.3 These observations also suggested that ZAP-70 could have a biological role in CLL rather than only being a surrogate marker for genetically defined subgroups. This hypothesis was further corroborated by several groups describing an association of ZAP-70 expression with B-cell receptor (BCR)–signal strength, cytokine secretion, and cell migration.4-7 However, few data clarified whether those functions were related to protein functions or a mere correlation with ZAP-70 expression.
To address this question, we have recently established a method to downregulate ZAP-70 expression in primary CLL cells using small interfering RNA (siRNA). Our data indicated that ZAP-70 is required for the activation of AKT, cytokine expression, and MYC-expression.8 All these effects, previously attributed to BCR-activation, did not require activation of the BCR but were constitutively regulated by ZAP-70, suggesting that ZAP-70 could enhance a constitutive, tonic BCR-signal. Whether these functions of ZAP-70 are restricted to patients carrying unmutated IGHV or are also present in “ZAP-70 discordant” cases remained an open question from our work.
We, here, provide evidence that ZAP-70 augments CD19, SRC, and AKT-activation only in IGHV-unmutated, but not mutated, CLL indicating existence of a tonic BCR signal modulated by ZAP-70. Furthermore, we describe that ZAP-70 directly increases the migration potential of malignant B cells, associated with an enhanced signaling response to CCL19 and CCL21 stimulation.
Material and methods
Cell culture
After obtaining informed consent from patients and in accordance with the Declaration of Helsinki, peripheral blood was obtained from patients with CLL. Studies were approved by the Cambridgeshire Research Ethics Committee (07/MRE05/44). Mononuclear cells were isolated from heparinized blood by centrifuging over a ficoll-hypaque layer (PAN-Biotech). Cells were harvested and cultured in RPMI 1640 (Gibco), supplemented with 10% fetal bovine serum, penicillin/streptomycin 50 U/mL, sodium pyruvate 1 mM, L-glutamine 2 mM, L-asparagine 20 mg/mL, 50 μM 2-mercaptoethanol, 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), and minimum essential medium nonessential amino acids (Gibco). ZAP-70 status was assessed by flow cytometry using intracellular staining with an anti–ZAP-70 antibody (Becton Dickinson Biosciences). Samples with >20% positive cells compared with isotype control were considered positive for ZAP-70.
Nucleofection of primary CLL cells
Nucleofector technology (Lonza) was used to deliver siRNA to primary cells. A total of 5 × 106 CLL cells were resuspended in 100 μL solution-V with 7.5 μL of nonsilencing control siRNA (Thermofisher Scientific 12935300) or 2.5 μL of each ZAP-70 siRNA (Life technologies HSS187732, HSS187733, and HSS187734). The cell-siRNA suspension was nucleofected using the Nucleofector 2b device (Lonza) (program cell type-4, X-001). Cell pellets were collected and resuspended in 3 mL fresh media. siRNA-transfected CLL cells were cocultured with 5 × 104 stromal cells (plated 24 hours before coculturing CLL cells) per well in a 6-well plate for 7 days.
Calcium flux
A total of 5 × 106 cells were harvested and resuspended in 500 μL serum-free media. Two microliter Fluo-4 (Invitrogen) was added and incubated for 15 minutes at room temperature with protection from light. Cells were then washed and resuspended in 100 μL Hanks’ balanced salt solution (Ca2+ free). Cells were then labeled with 20 μg biotin-SP AffiniPure Fab fragment goat anti-human immunoglobulin M (IgM) for 20 minutes on ice. Unlabeled anti-IgM was washed off by pelleting cells and resuspending in 500 μL Hanks’ balanced salt solution, followed by 20-minutes incubation at 37°C. 4′,6-diamidino-2-phenylindole was added to identify dead cells. Samples were analyzed on flow cytometry. Measurement was lasting for 20 seconds to record background Ca2+ signal, then 20 μL streptavidin (1 mg/mL) was added to stimulate the Ca2+ flow. Measurement was resumed for up to 180 seconds. Ionomycin was then added to indicate the specific of Ca2+ signal.
Immunoblotting
After the specified treatments, cells were harvested and lysed in RIPA buffer and a total of 10 to 20 μg protein was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, followed by blotting to polyvinylidene difluoride membranes (Millipore), and probing with indicated primary antibodies. Protein signal intensity was measured using Fiji (Image J) for quantification and statistical analysis.
Migration assay
Cells after treatment were resuspended in media (CLL cells in RPMI1640; MEC1 cells in Iscove minimal essential medium) with 5% fetal bovine serum. From this 5 × 105 to 1 × 106 cells were added to the top chamber of a 6.5 mm diameter transwell culture insert with a pore size of 5 μM. Filters with cells were transferred into wells containing medium with the chemokines CCL19 (1 μg/mL) and CCL21 (1 μg/mL) (PeproTech) or media alone. The chambers were incubated for 3 hours at 37°C in 5% CO2. After incubation cells in the lower chamber were thoroughly resuspended, stained with CD5 and CD19 antibody and counted using CountBright Absolute counting beads according to the manufacturers protocol (ThermoFisher). The migration index was calculated as the number of cells transmigrating with chemokine divided by the number of cells loading onto the transwells.
Establishment of MEC1 tetracycline-regulated inducible ZAP-70 expression system
To create an inducible ZAP-70 expression system, Life Technologies’ T-RExTM system was used according to manufacturer’s instructions. The ZAP-70 gene was cloned into the pcDNA4/TO vector backbone. MEC1 cells were transfected by Amaxa nucleofection according to manufacturer’s instructions with pcDNA6/TR vector containing the tet repressor gene. Clones were selected by single-cell dilution using 5 μg/mL blasticidin. A positive clone containing the pcDNA6/TR vector was subsequently transfected with the pcDNA4/TO_ZAP-70 construct (MEC1/ZAP-70) or the unmodified pcDNA4/TO mock control vector (MEC1/parental) as described above. After single-cell dilution under double selection with 5 μg/mL blasticidin and 200 μg/mL zeocin clones were selected by flow cytometry analysis of ZAP-70 expression after treatment with 500 ng/mL tetracycline for 24 hours.
Statistical analyses
All experiments were repeated at least 3 times, and the means ± standard error of the mean were calculated. The exact sample size for each experiment, biological or technical repeats are provided in the figure legends. Statistical analyses of results were performed using 2-tailed Student paired t tests. For experiments in which >2 groups are compared, statistical analyses were performed using 1-way analysis of variance followed by 2-tailed Student paired t tests. Statistical annotations as previously noted were denoted with asterisks according to the following, ∗∗P < .01, ∗P < .05, and ns P > .05.
Results
Constitutive AKT-activation is regulated by ZAP-70 exclusively in UM-CLL
Our previously established protocol to deplete the expression of ZAP-70 in primary CLL cells allowed us to study the functions of ZAP-70 in primary CLL cells without a simultaneous inhibition of the closely related SYK-kinase.8 Our data indicated that ZAP-70 constitutively regulates AKT-activation, cytokine production, and protein synthesis. The effects of ZAP-70 on these BCR-signaling functions were heterogenous, ranging from moderate to profound changes. We hypothesized that the observed differences could be related to differences in the IGHV mutational status of the cells. In particular, we were interested in understanding whether mutated IGHV cases, discordantly expressing ZAP-70, were also reliant on ZAP-70 for a constitutive activation of AKT, similar to IGHV-unmutated (UM-CLL) (ZAP-70-positive, concordant) cases. In keeping with our recent data, downregulation of ZAP-70 did not affect anti-IgM–induced BCR-signaling measured by Ca2+ influx. Notably, M-CLL cases responded poorly to BCR-ligation compared with UM-CLL, irrespective of ZAP-70 expression levels (Figure 1A-B). We previously demonstrated a significant decrease in the baseline phosphorylation of AKT on threonine 308 but less so on serine 473 upon ZAP-70 downregulation in UM-CLL (Figure 1C, left panel, quantification can be found in Chen et al8). In contrast, in M-CLL, the baseline activation of AKT activation, assessed by phosphorylation on serine 473 and threonine 308, was unaffected by knockdown of ZAP-70 (Figure 1C-D). To corroborate the finding that ZAP-70 concordant and discordant cases responded differently to deletion of endogenous ZAP-70, we analyzed the activation of GSK-3β, a bona fide target of AKT. AKT can inhibit constitutively activated GSK-3β through phosphorylation on serine 9.9 Indeed, we observed a strong induction of phospho-GSK-3β (serine 9) after anti-IgM–mediated activation of the BCR in UM-CLL, but not in M-CLL (Figure 1E), in keeping with the known attenuated BCR-signaling capacity of anergic M-CLL.10 Notably, depletion of ZAP-70 decreased the baseline phosphorylation of GSK-3β only in UM-CLL, but not in M-CLL, indicating that the constitutive inhibition of GSK-3β was enhanced by ZAP-70 in the former subgroup (Figure 1E-F). These ZAP-70–dependent effects on AKT and GSK-3β were associated with improved cell survival, predominantly and significantly only in IGHV-unmutated CLL (Figure 1G). Notably, reduced survival of UM-CLL cells depleted of ZAP-70 expressed significantly lower levels of the anti-apoptotic protein MCL1, which is a downstream target of and destabilized by active GSK-3β (Figure 1H-I).
ZAP-70 contributes to the constitutive phosphorylation of AKT and GSK3β in IGHV–unmutated CLL cells. (A) Representative kinetic blots show the calcium flux of IGHV–unmutated (UM-CLL) or IGHV–mutated (M-CLL) CLL cells with NSC or ZAP-70 siRNA transfection. CLL cells were harvested and labeled with Fluo-4 (fluorescein isothiocyanate). BCR-signaling was activated with anti-IgM beads, triggered 20 to 30 seconds after flow cytometric measurement started. (B) Quantification of calcium flux response of CLL samples (n = 4 for both UM-CLL and M-CLL) transfected with NSC or ZAP-70 siRNA. The Ca2+ ratios were calculated by using kinetic blots and divided peak Fluo-4 (FITC-A) mean intensity upon anti-IgM activation by baseline. (C) Phospho-AKT (T308), phospho-AKT (S473), total AKT, ZAP-70, and β-actin immunoblots of primary M-CLL cells monocultured for 24 hours after nonspecific control siRNA or ZAP-70 siRNA transfection. For anti-IgM stimulated samples, CLL cells were treated with beads-bound anti-IgM for 20 minutes. (D) Phosphorylated protein levels of AKT (relative to total AKT levels) 24 hours after siRNA transfection in M-CLL samples. For anti-IgM stimulated samples, monocultured M-CLL cells were treated with beads-bound anti-IgM for 20 minutes (n = 4 for pi-AKT [T-308] and n = 3 for pi-AKT [S473]). (E) Phospho-GSK-3β (S9), total GSK-3β, ZAP-70, and β-actin immunoblots of primary UM-CLL or M-CLL cells monocultured for 24 hours after nonspecific control siRNA or ZAP-70 siRNA transfection. For anti-IgM stimulated samples, monocultured CLL cells were treated with beads-bound anti-IgM for 20 minutes. (F) Phosphorylated GSK-3β levels (relative to total GSK-3β levels) 24 hours after siRNA transfection in UM-CLL and M-CLL samples. For anti-IgM stimulated samples, monocultured CLL cells were treated with beads-bound anti-IgM for 20 minutes (n = 3 for both UM-CLL and M-CLL). (G) Cell viability of primary CLL cells assessed using annexin-V/propidium iodide staining, 9 days after siRNA transfection. Cells were monocultured for 48 hours after nonspecific control siRNA or ZAP-70 siRNA transfection for 7 days on stromal cells. (H) MCL1 and β-actin immunoblots of primary UM-CLL cells cultured under identical conditions as described in panel G. (I) Relative MCL1 protein level (compared to with β-actin levels) 24 hours after siRNA transfection in UM-CLL samples (n = 3). Statistical significance between samples was assessed by paired 2-tailed Student t tests. ∗P < 5 × 10−2, ∗∗∗P < 10−4. LE, long exposure; ns, not significant; NSC, non-specific control; SE, short exposure.
ZAP-70 contributes to the constitutive phosphorylation of AKT and GSK3β in IGHV–unmutated CLL cells. (A) Representative kinetic blots show the calcium flux of IGHV–unmutated (UM-CLL) or IGHV–mutated (M-CLL) CLL cells with NSC or ZAP-70 siRNA transfection. CLL cells were harvested and labeled with Fluo-4 (fluorescein isothiocyanate). BCR-signaling was activated with anti-IgM beads, triggered 20 to 30 seconds after flow cytometric measurement started. (B) Quantification of calcium flux response of CLL samples (n = 4 for both UM-CLL and M-CLL) transfected with NSC or ZAP-70 siRNA. The Ca2+ ratios were calculated by using kinetic blots and divided peak Fluo-4 (FITC-A) mean intensity upon anti-IgM activation by baseline. (C) Phospho-AKT (T308), phospho-AKT (S473), total AKT, ZAP-70, and β-actin immunoblots of primary M-CLL cells monocultured for 24 hours after nonspecific control siRNA or ZAP-70 siRNA transfection. For anti-IgM stimulated samples, CLL cells were treated with beads-bound anti-IgM for 20 minutes. (D) Phosphorylated protein levels of AKT (relative to total AKT levels) 24 hours after siRNA transfection in M-CLL samples. For anti-IgM stimulated samples, monocultured M-CLL cells were treated with beads-bound anti-IgM for 20 minutes (n = 4 for pi-AKT [T-308] and n = 3 for pi-AKT [S473]). (E) Phospho-GSK-3β (S9), total GSK-3β, ZAP-70, and β-actin immunoblots of primary UM-CLL or M-CLL cells monocultured for 24 hours after nonspecific control siRNA or ZAP-70 siRNA transfection. For anti-IgM stimulated samples, monocultured CLL cells were treated with beads-bound anti-IgM for 20 minutes. (F) Phosphorylated GSK-3β levels (relative to total GSK-3β levels) 24 hours after siRNA transfection in UM-CLL and M-CLL samples. For anti-IgM stimulated samples, monocultured CLL cells were treated with beads-bound anti-IgM for 20 minutes (n = 3 for both UM-CLL and M-CLL). (G) Cell viability of primary CLL cells assessed using annexin-V/propidium iodide staining, 9 days after siRNA transfection. Cells were monocultured for 48 hours after nonspecific control siRNA or ZAP-70 siRNA transfection for 7 days on stromal cells. (H) MCL1 and β-actin immunoblots of primary UM-CLL cells cultured under identical conditions as described in panel G. (I) Relative MCL1 protein level (compared to with β-actin levels) 24 hours after siRNA transfection in UM-CLL samples (n = 3). Statistical significance between samples was assessed by paired 2-tailed Student t tests. ∗P < 5 × 10−2, ∗∗∗P < 10−4. LE, long exposure; ns, not significant; NSC, non-specific control; SE, short exposure.
In summary, ZAP-70 promotes the constitutive activation of AKT in UM but not M-CLL. This activation is associated with an inhibition of GSK-3β and stabilization of MCL1, promoting cell survival.
ZAP-70 augments a tonic BCR signal in IGVH-UM but not M-CLL
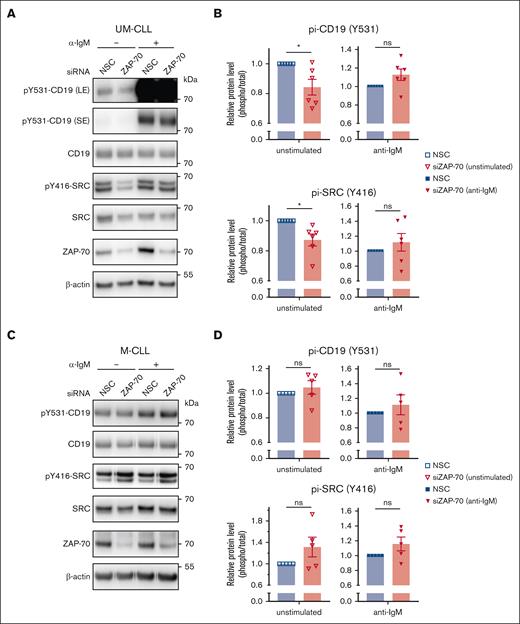
Our data suggested that ZAP-70 augments a tonic BCR signal exclusively in UM-CLL but not in M-CLL discordantly expressing ZAP-70. To provide further evidence for this hypothesis, we analyzed the activation of CD19. Signaling through the BCR activates the coreceptor CD19, which amplifies the signal by recruiting phosphatidylinositol 3-kinase (PI3K) to the plasma membrane.11 Depletion of ZAP-70 slightly, but significantly, reduced the constitutive phosphorylation of CD19, which was not affected under conditions of BCR-stimulation using anti-IgM beads. These effects were only observed in UM-CLL (Figure 2A-B), but not in M-CLL discordantly expressing ZAP-70 (Figure 2C-D). The tyrosine protein kinase LYN is a member of the Src-family kinases and operates upstream of CD19 and is involved in the BCR-mediated phosphorylation of CD79A, CD79B, and SYK. Similar to CD19, but overall more pronounced, we observed that depletion of ZAP-70 inhibited the constitutive phosphorylation of members of Src-family kinases on tyrosine 416, which increases kinase activity by locking the activation loop in a substrate-binding permissive configuration. In contrast, the ZAP-70–dependent constitutive tyrosine phosphorylation of Src was not observed in ZAP-70–positive M-CLL (Figure 2A-D).
ZAP-70 enhances the constitutive activation of CD19 and SRC. (A,C) Phospho-CD19 (Y531), phospho-SRC (Y416), total CD19, total SRC, ZAP-70, and β-actin immunoblots of primary UM-CLL (in panel A) or M-CLL cells (in panel C), monocultured for 24 hours after nonspecific siRNA or ZAP-70 siRNA transfection. For anti-IgM stimulated samples, CLL cells were treated with bead-bound anti-IgM for 20 minutes. (B,D) Phosphoprotein levels of CD19 and SRC (relative to total CD19 and SRC-protein levels, respectively) 24 hours after siRNA transfection in UM-CLL (in panel B) or M-CLL samples (in panel D). For anti-IgM stimulated samples, CLL cells were treated with bead-bound anti-IgM for 20 minutes. (n = 6 for UM-CLL, n = 5 for M-CLL). Statistical significance between samples was assessed by paired 2-tailed Student t tests. ∗P < 5 × 10−2. LE, long exposure; ns, not significant; SE, short exposure.
ZAP-70 enhances the constitutive activation of CD19 and SRC. (A,C) Phospho-CD19 (Y531), phospho-SRC (Y416), total CD19, total SRC, ZAP-70, and β-actin immunoblots of primary UM-CLL (in panel A) or M-CLL cells (in panel C), monocultured for 24 hours after nonspecific siRNA or ZAP-70 siRNA transfection. For anti-IgM stimulated samples, CLL cells were treated with bead-bound anti-IgM for 20 minutes. (B,D) Phosphoprotein levels of CD19 and SRC (relative to total CD19 and SRC-protein levels, respectively) 24 hours after siRNA transfection in UM-CLL (in panel B) or M-CLL samples (in panel D). For anti-IgM stimulated samples, CLL cells were treated with bead-bound anti-IgM for 20 minutes. (n = 6 for UM-CLL, n = 5 for M-CLL). Statistical significance between samples was assessed by paired 2-tailed Student t tests. ∗P < 5 × 10−2. LE, long exposure; ns, not significant; SE, short exposure.
In conclusion, these data provide further evidence that ZAP-70 augments a constitutive, tonic BCR signal exclusively in UM-CLL.
ZAP-70 binds to cytoskeletal proteins and regulates LCP1-activation
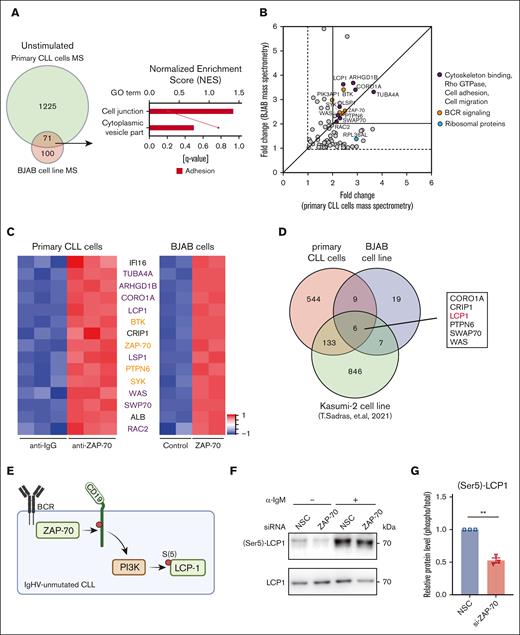
We previously demonstrated an enhanced binding of ZAP-70 to ribosomal proteins after activation of the BCR.8 For this, we had generated the human Burkitt-like lymphoma cell line (BJAB) (BJABcontrol) overexpressing a BirA-tagged ZAP-70 (BJABZAP-70), allowing for the in vivo biotinylation of ZAP-70 and immunoprecipitation without anti–ZAP-70 antibodies. In addition, we isolated ZAP-70 immunocomplexes from double-crossed linked primary CLL cells.8 To identify binding partners to ZAP-70, constitutively bound in the absence of an induced BCR-signal, we reanalyzed the data to identify ZAP-70–binding proteins in unstimulated primary CLL and BJAB cells, and we excluded all proteins that were not present in both experiments to reduce false-positive nonspecific hits (Figure 3A). With this approach we identified 71 unique proteins bound to ZAP-70 in the absence of anti-IgM treatment (P < .01; log2 fold-change >1). Gene ontology enrichment analysis identified only 1 cluster, named “cell junction” (Figure 3A). By further restricting our analysis to greater than twofold changes (compared with BJABcontrol or IgG, respectively) we identified a cluster of proteins important for cell adhesion and migration (Figure 3B-C). These included the actin binding proteins Coronin A1 (CORO1A1), lymphocyte-specific protein 1, Wiskott-Aldrich syndrome protein (WASP), switch-associated protein 70, and lymphocyte cytosolic protein 1 (LCP1). Next, we overlayed our data to recently published ZAP-70 interactome-data obtained from a human B-cell acute lymphoblastic leukemia cell line.12 This analysis further revealed conserved binding of ZAP-70 to protein tyrosine phosphatase (PTPN6) and several cytoskeletal proteins (Figure 3D) and thus suggested that ZAP-70 is functionally important for actin-remodeling in B cells with different maturation status.
ZAP-70 constitutively binds to cytoskeleton proteins. (A) Venn diagram compares the ZAP-70-binding proteins identified from mass spectrometry (MS) in unactivated primary CLL cells and BJAB cell lines. Gene set enrichment analysis showing the gene ontology terms extracted from the unique 71 proteins (primary CLL cells MS and BJAB cell line MS, log2 fold-change (log2FC >1, adj P < .01). (B) The 71 unique proteins from panel A were plotted by the log2FC. x-axis, identified in primary CLL cells MS; y-axis, identified in BJAB cell line MS. Colors indicate the subgrouped functions of the proteins. Dots in gray are the proteins involved in other cellular functions. (C) Heat map of proteins presented in panel B (primary CLL cells MS and BJAB cell line MS, log2FC >2, adj P < .01). Purple and orange indicate relative high and low protein abundance, respectively. Each condition analyzed depicts 3 (primary CLL cells) or 2 (BJAB cell line) technical replicates. Proteins were ranked from top to bottom by average log2FC combine primary CLL cells MS and BJAB cell line MS. (D) Venn diagram comparing ZAP-70–binding proteins identified from MS in unactivated primary CLL cells using ZAP-70–specific antibody pull down, in unactivated BJAB cell lines using biotinylated–ZAP-70 streptavidin pull down and in unactivated Kasumi-2 cells using a ZAP-70 proximity dependent biotinylation strategy (Sadras et al12) (log2FC >2, adj P < .01). (E) Proposed model for tonic BCR signal regulating LCP1 serine(5) phosphorylation in UM-CLL. Created with BioRender.com. (F) Phospho-LCP1 (S5) and total LCP1 immunoblots of primary UM-CLL cells monocultured for 24 hours after nonspecific control siRNA or ZAP-70 siRNA transfection. For anti-IgM–stimulated samples, monocultured CLL cells were treated with bead-bound anti-IgM for 20 minutes. (G) Phosphorylated LCP1 levels (relative to total LCP1 levels) 24 hours after siRNA transfection in UM-CLL samples (n = 3). Statistical significance between samples was assessed by paired 2-tailed Student t tests. ∗∗P < 10−3. ns, not significant.
ZAP-70 constitutively binds to cytoskeleton proteins. (A) Venn diagram compares the ZAP-70-binding proteins identified from mass spectrometry (MS) in unactivated primary CLL cells and BJAB cell lines. Gene set enrichment analysis showing the gene ontology terms extracted from the unique 71 proteins (primary CLL cells MS and BJAB cell line MS, log2 fold-change (log2FC >1, adj P < .01). (B) The 71 unique proteins from panel A were plotted by the log2FC. x-axis, identified in primary CLL cells MS; y-axis, identified in BJAB cell line MS. Colors indicate the subgrouped functions of the proteins. Dots in gray are the proteins involved in other cellular functions. (C) Heat map of proteins presented in panel B (primary CLL cells MS and BJAB cell line MS, log2FC >2, adj P < .01). Purple and orange indicate relative high and low protein abundance, respectively. Each condition analyzed depicts 3 (primary CLL cells) or 2 (BJAB cell line) technical replicates. Proteins were ranked from top to bottom by average log2FC combine primary CLL cells MS and BJAB cell line MS. (D) Venn diagram comparing ZAP-70–binding proteins identified from MS in unactivated primary CLL cells using ZAP-70–specific antibody pull down, in unactivated BJAB cell lines using biotinylated–ZAP-70 streptavidin pull down and in unactivated Kasumi-2 cells using a ZAP-70 proximity dependent biotinylation strategy (Sadras et al12) (log2FC >2, adj P < .01). (E) Proposed model for tonic BCR signal regulating LCP1 serine(5) phosphorylation in UM-CLL. Created with BioRender.com. (F) Phospho-LCP1 (S5) and total LCP1 immunoblots of primary UM-CLL cells monocultured for 24 hours after nonspecific control siRNA or ZAP-70 siRNA transfection. For anti-IgM–stimulated samples, monocultured CLL cells were treated with bead-bound anti-IgM for 20 minutes. (G) Phosphorylated LCP1 levels (relative to total LCP1 levels) 24 hours after siRNA transfection in UM-CLL samples (n = 3). Statistical significance between samples was assessed by paired 2-tailed Student t tests. ∗∗P < 10−3. ns, not significant.
Among these interacting proteins, LCP1 was demonstrated to function as a regulatory switch for assembling actin-rich structures required for cell migration. By cross linking actin filaments into tight bundles LCP1 can regulate T- and B-cell chemotaxis.13,14 Because these functions also require calcium binding, we hypothesized that BCR-activation may further increase binding of ZAP-70 to LCP1. Contrary to this hypothesis, we did not observe increased binding of the 2 proteins in an immunoprecipitation assay (supplemental Figure 1A). Notably, knockdown of ZAP-70 did not decrease the total abundancy of LCP1 in CLL cells (supplemental Figure 1B), indicating that expression levels of total LCP1 are not regulated by ZAP-70 and its stimulatory effects on protein synthesis.8
Importantly, the avidity of LCP1 for intracellular actin is also regulated by posttranslational protein modifications and serine(5)-phosphorylation was shown to positively regulate migration and invasion of epithelial cancer cells.15 Notably, PI3K is a known upstream regulator of this phosphorylation site.16 We, therefore, hypothesized that serine(5) on LCP1 may also be regulated by a tonic BCR-signal in malignant B cells (Figure 3E). Indeed, in the absence of an induced BCR-signal, ZAP-70 levels correlated with phospho-Ser(5)-LCP1 levels (Figure 3F-G), indicating that a ZAP-70–dependent, constitutive signal contributes to the baseline activation of LCP1 in UM-CLL cells. Crosslinking of IgM induced a strong phosphorylation of LCP1 on Ser(5), which was not further modulated by ZAP-70.
In summary, our data indicate that a tonic BCR-signaling is enhanced by ZAP-70, which physically associates with cytoskeletal proteins and promotes the phosphorylation and activation of LCP1 in UM-CLL cells.
ZAP-70 promotes CCR7-signaling and chemokine-induced activation of LCP1
Our data suggested that ZAP-70 can directly and positively regulate CLL-cell migration. To test this hypothesis, we performed migration assays on IGHV–unmutated CLL cells, using transwells against a gradient of CCL19 and CCL21. Although spontaneous migration of cells in the absence of chemokines was minimal, the siRNA-mediated decrease in ZAP-70 expression significantly reduced the migration capacity of primary CLL cells toward both chemokines. Migration was enhanced toward CCL19 compared with CCL21 (Figure 4A). Both, CCL19 and CCL21, signal through CCR7 expressed on CLL cells. To assess whether the impaired migration toward these chemokines was related to reduced receptor expression after siRNA-mediated knock down of ZAP-70, we analyzed CCR7 expression on ZAP-70 modulated CLL cells. Importantly, knockdown of ZAP-70 did not alter CCR7 surface expression, which was overall readily detectable (Figure 4B). To corroborate our findings and to exclude off-target effects from the siRNAs, possibly contributing to these results, we engineered MEC1 cells with an inducible, tetracycline-regulated ZAP-70 expression vector. Although endogenous ZAP-70 levels in MEC1 cells were low, ectopic expression can be induced in these cells simply by adding doxycycline. Notably, we observed a leakiness of the system, which caused higher expression of baseline ZAP-70 compared with the parental MEC1 clone in the absence of doxycycline (Figure 4C). Migration assays of genetically modified MEC1 cells showed a strong correlation between ZAP-70 expression and cell mobility induced by CCL19. The minimal migration of the parental MEC1 cells was significantly enhanced by a moderate expression of ZAP-70 in the leaky clone, which was further increased by doxycycline-induced expression of high levels of ZAP-70 (Figure 4D). Contrary to primary CLL cells, genetically modified MEC1 cells responded poorly to stimulation with CCL21 (not shown). These data confirm that ZAP-70 is not only associated with higher migration potential of CLL cells but showed that it is directly involved in chemotaxis.
ZAP-70 enhances CCR7 signaling and chemotaxis. (A) Migration capacity in response to chemokine CCL19 or CCL21 (1 μg/mL, 3 hours) were quantified in primary CLL cells (n = 11) transfected with nonspecific siRNA or ZAP-70 siRNA. Percentage of migrated CLL cells were quantified by using counting beads and divided numbers of migrated CD5+ CD19+ cells through transwells by total cell numbers that were loaded onto transwells. Nontreated controls were the samples that were cultured in media without chemokines. (B) Representative fluorescence-activated cell sorter histograms (left) and quantification (right, n = 6) of surface CCR7 levels on CD19+ cells in primary CLL cells transfected with nonspecific siRNA or ZAP-70 siRNA. (C) ZAP-70 immunoblots of whole-cell lysates from MEC1/parental or MEC1/ZAP-70 cells. Cells were treated with doxycyline (1 μg/mL) for 72 hours as indicated. (D) Migration capacity in response to chemokine CCL19 (1 μg/mL, 3 hours) was quantified in MEC1/parental cells or MEC1/ZAP-70 cells treated with 1 μg/mL doxycycline. Percentage of migrated MEC1 cells were quantified using counting beads and numbers of migrated CD19+ cells through transwells were divided by total input cells numbers. (E) Phospho-AKT (T308), phospho-AKT (S473), total AKT, and ZAP-70 immunoblots of primary CLL cells after nonspecific control siRNA or ZAP-70 siRNA transfection. For cytokines stimulated samples, cultured CLL cells were treated with CCL19 or CCL21 at 1 μg/mL for 5 minutes. (F) Graphs of relative phosphorylated AKT level (relative to total AKT levels) in CLL samples transfected with control siRNA or ZAP-70 siRNA and treated with CCL19 or CCL21 at 1 μg/mL for 5 minutes. (G) Phospho-LCP1 (S5), total LCP1, ZAP-70, and β-actin immunoblots of primary CLL cells after nonspecific control siRNA or ZAP-70 siRNA transfection. For cytokines stimulated samples, cultured CLL cells were treated with CCL19 or CCL21 at 1 μg/mL for 5 minutes (n = 3). Statistical significance between samples was assessed by paired 2-tailed Student t tests. ∗P < 5 × 10−2; ∗∗P < 10−3. ns, not significant.
ZAP-70 enhances CCR7 signaling and chemotaxis. (A) Migration capacity in response to chemokine CCL19 or CCL21 (1 μg/mL, 3 hours) were quantified in primary CLL cells (n = 11) transfected with nonspecific siRNA or ZAP-70 siRNA. Percentage of migrated CLL cells were quantified by using counting beads and divided numbers of migrated CD5+ CD19+ cells through transwells by total cell numbers that were loaded onto transwells. Nontreated controls were the samples that were cultured in media without chemokines. (B) Representative fluorescence-activated cell sorter histograms (left) and quantification (right, n = 6) of surface CCR7 levels on CD19+ cells in primary CLL cells transfected with nonspecific siRNA or ZAP-70 siRNA. (C) ZAP-70 immunoblots of whole-cell lysates from MEC1/parental or MEC1/ZAP-70 cells. Cells were treated with doxycyline (1 μg/mL) for 72 hours as indicated. (D) Migration capacity in response to chemokine CCL19 (1 μg/mL, 3 hours) was quantified in MEC1/parental cells or MEC1/ZAP-70 cells treated with 1 μg/mL doxycycline. Percentage of migrated MEC1 cells were quantified using counting beads and numbers of migrated CD19+ cells through transwells were divided by total input cells numbers. (E) Phospho-AKT (T308), phospho-AKT (S473), total AKT, and ZAP-70 immunoblots of primary CLL cells after nonspecific control siRNA or ZAP-70 siRNA transfection. For cytokines stimulated samples, cultured CLL cells were treated with CCL19 or CCL21 at 1 μg/mL for 5 minutes. (F) Graphs of relative phosphorylated AKT level (relative to total AKT levels) in CLL samples transfected with control siRNA or ZAP-70 siRNA and treated with CCL19 or CCL21 at 1 μg/mL for 5 minutes. (G) Phospho-LCP1 (S5), total LCP1, ZAP-70, and β-actin immunoblots of primary CLL cells after nonspecific control siRNA or ZAP-70 siRNA transfection. For cytokines stimulated samples, cultured CLL cells were treated with CCL19 or CCL21 at 1 μg/mL for 5 minutes (n = 3). Statistical significance between samples was assessed by paired 2-tailed Student t tests. ∗P < 5 × 10−2; ∗∗P < 10−3. ns, not significant.
To further investigate the mechanisms underlying the hyperresponsiveness of ZAP-70–positive CLL cells toward CCL19- and CCL21-induced migration, we investigated whether ZAP-70 had a direct effect on the activation of AKT, which is a bona fide target downstream of PI3K and CCR7.17 To define a timepoint for the optimal assessment of CCL-induced AKT-activation, we first analyzed the kinetics of AKT-activation after stimulation with CCL21. Our data indicated that CCR7-activation causes a rapid and readily detectable phosphorylation of AKT which peaked after 5 minutes, followed by a rapid dephosphorylation within 1 hour (supplemental Figure 1C). Notably, the signal change amplitude on AKT was higher for serine 473 compared with threonine 308. We then investigated whether modification of ZAP-70 expression in primary CLL cells affected CCL19 and CCL21-induced signal-transduction. Unexpectedly, reduced expression of ZAP-70 strongly mitigated CCL19-dependent phosphorylation of serine 473 on AKT. Similar, though less pronounced effects were observed for CCL21–induced AKT activation. Here, phosphorylation of AKT at threonine 308 was also reduced by downregulation of AKT, but these effects were more subtle and only seen for CCL19 but not CCL21 (Figure 4E-F).
Lastly, to test whether these signal-modulations also affected the activation of LCP1, we assessed the expression of Ser(5)-LCP1 in chemokine-activated cells, which were either wild-type (nonsilencing) or expressed low levels of ZAP-70 (siZAP-70). Downregulation of ZAP-70 significantly decreased phospho-Ser(5)-LCP1 levels induced by CCL19 and CCL21 (Figure 4G).
Conclusively, our data provide evidence that ZAP-70 augments CCR7 signaling and activation of the PI3K pathway, which promotes AKT- and LCP1-activation and enhances chemotaxis of CLL cells.
Discussion
The oncogenic addiction of CLL cells on BCR-signals has been recognized for >2 decades and instigated the clinical development of kinase inhibitors, which have impressively transformed treatment of patients with CLL and some other indolent B-cell lymphoma.18 Prosurvival signals can be transmitted through the BCR through distinct mechanisms, differing in their developmental requirement and signal strength. In CLL, 3 types of BCR signals have been identified, namely ligand-dependent, tonic, and cell-autonomous BCR signaling. Although the cell-autonomous BCR signaling is a peculiar finding in CLL and relies on the interaction between HCDR3-region and an internal epitope within the BCR,19 tonic, and ligand-dependent BCR-signals are essential for the survival of normal and malignant B cells. The existence of a tonic BCR signal in CLL cells was proposed and supported by the presence of constitutively active BCR-signaling molecules, including PI3K,20 ERK,21 and LYN22 in the absence of an overt antigen-stimulus. More recently, the concept of tonic BCR-signaling in CLL has been further corroborated by experiments using single-cell proteomic analyses and super-resolution microscopy, demonstrating that CLL-derived BCR forms dimers and oligomers in the absence of an antigen.23,24 Notably, to our knowledge, no differences in the signal strength of a tonic BCR signal have been reported between IGHV-mutated and -unmutated CLL or other common prognostic markers. Our data provide more granularity on the molecular mechanism driving tonic BCR signaling by identifying that ZAP-70 acts as a signal enhancer for a constitutive, tonic BCR signal. These data corroborate recently published data from the Müschen group. In a set of elegant experiments, they demonstrated that ZAP-70 diverted BCR-signals toward a PI3K-dependent survival signal to the expense of nuclear factor of activated T cells activation and impaired negative-selection.12 Importantly in this context, in our previous work, we have not detected ZAP-70 dependent effects on the phosphorylation of Bruton's tyrosine kinase (BTK) or B-cell linker, which are signal-mediators of an autonomous BCR-signal.19,25 Therefore, and in keeping with the original data that autonomous BCR-signaling is not restricted to either M- or UM-CLL cells,19 we conclude that ZAP-70 regulates a tonic BCR signal, which is different from an autonomous BCR-signal that is not modulated by the constitutive expression of ZAP-70 in subsets of CLL cells.
The ZAP-70–mediated effects, cumulating in improved cell survival, were largely absent in IGHV-mutated CLL, raising the question what other factors determine these opposing effects. We considered that the abundancy of surface IgM and the known decreased expression of membrane bound IgM in mutated CLL10 may be a contributing factor. However, although the average surface expression of IgM is lower in mutated than in unmutated CLL,10 this is not the case for mutated CLL cells discordantly expressing ZAP-70 (supplemental Figure 1D; Rassenti et al3) and hence largely exclude this as an underlying mechanism. Alternatively, posttranslational modifications on IgM also affect the signal capacity and may contribute to a tonic BCR-signal.26 Other factors to be considered relate to differences in the protein abundancies of other BCR-signaling molecules, which do not necessarily correlate with genetic markers.27 It, therefore, is important to assess in future work whether binding of ZAP-70 to BCR signaling molecules is quantitatively and/or qualitatively different between UM- and M-CLL.
By experimentally decreasing the expression of ZAP-70 in primary CLL cells, we identified that it predominantly regulates steady-state functions of CLL, including chemokine secretion, protein synthesis, and chemotaxis. These ZAP-70–dependent functions were overwritten by a strong BCR-signal, mimicking binding of a putative antigen. This suggests that ZAP-70 promotes disease progression only in the absence of a coexisting autonomous or ligand-dependent BCR signal. Although the latter is likely to be dependent on cell migration and influenced by the presence of antigen in different microenvironments in vivo, cell-autonomous signaling seems unlikely to be affected by this, questioning why we have not observed ZAP-70–mediated effects in anti-IgM stimulated cells. In fact, the Kipps’ group reported that ectopic expression of ZAP-70 enhanced anti-IgM–induced BCR signaling, notably only IGHV-mutated CLL were investigated.28 To experimentally address this conundrum, a careful downtitration of the anti-IgM signal would be needed to assess whether ZAP-70 also enhances ligand-dependent BCR signaling below a defined threshold. The prognostic impact of ZAP-70 expression on the natural course of the disease certainly suggests that its disease promoting functions extend to different types of BCR stimulations and are not restricted to the enhancement of a tonic BCR signal only.
Applying proximity ligation assays and proteomic analyses, we identified that ZAP-70 is constitutively bound to cytoskeletal proteins, suggesting that its tonic BCR signal-enhancing function may be directly related to and the consequence of actin-remodeling. Notably, the upstream mechanisms of BCR activation involve clustering of BCRs in lipid rafts to assemble a signaling complex in which immunoreceptor tyrosine-based activation motifs are phosphorylated.29 This process is associated and dependent on a transient disassembly and reassembly of cortical actin network, which increases BCR lateral mobility.30,31 These dynamic changes in cortical actin enhance BCR signaling, which is further amplified by the activation of BCR-associated kinases, such as Btk.32 Proposed mechanisms of how BCR-kinase regulate actin-remodeling include activation of GTPases Cdc42 and Rac,33,34 which themselves modulate actin polymerization. This intimate interplay between actin remodeling and BCR signaling is best illustrated by increased levels of autoantibodies derived from B cells lacking the actin-nucleation–promoting factor WASP.35 In T cells, ZAP-70 has been implicated in remodeling the actin network after T-cell receptor-activation, a process involving the RhoGTPase Cdc4236 and Vav137 in addition to activation of the Cdc42-associated WASP.38 Importantly, our mass spectrometry data identified the entire complex associated with ZAP-70, strongly suggesting that ZAP-70 remodels the actin network in leukemic B cells similar to that in activated T cells. In addition, we found a physical association between ZAP-70 and LCP1, an actin-bundling protein required for actin polymerization and the formation of immune-synapses in T cells and recently characterized as an abundantly expressed antigen in CLL and required for niche residency.39 Peculiarly, a recent single-cell sequencing study identified subclonal mutations of LCP1 in CLL and its association with an impaired DNA-damage response.40 Whether binding of wild-type LCP1 to ZAP-70 phenocopies these functions of mutated LCP1 remains a possibility and to be experimentally addressed. In conclusion, it seems very reasonable to speculate that the signal-enhancing effects of ZAP-70 are predominantly driven by its actin-modulating functions, which improve the assembly of BCR signalosomes in the absence of antigen.
In addition to the BCR-related functions of ZAP-70, cell migration remains an additional mechanism how ZAP-70 favors CLL-microenvironment interactions and disease progression.41 Indeed, enhanced migration of ZAP-70–positive CLL cells toward the CCR7 ligands, CCL19 and CCL21 has previously been reported, though it remained unknown from these studies whether this was a direct consequence of ZAP-70 or a mere correlation.5,7,42 Furthermore, published data indicate that differences in CCR7 expression contribute to this phenotype.7 Notably, we have not observed a decrease in CCR7 protein abundance in primary CLL cells depleted of ZAP-70. Unexpectedly, we observed that ZAP-70 not only enhances a tonic BCR-signal, but also CCL19- and CCL21-induced activation and phosphorylation of AKT and LCP1. CCR7-signaling has been extensively investigated in dendritic cells and there it engages MAPK-pathway43 and regulates actin polymerization through RhoA,43 in addition to the aforementioned activation of PI3K/AKT. Indeed, it was previously shown that the latter arm of the CCR7 signaling pathway mediates antiapoptotic signals also in CLL cells,44 in keeping with other’s observations.20 Integrating these and our data, we hypothesize that 2 signaling pathways, BCR and CCR7, converge on PI3K, which in the absence of antigen remains in a poised activation state because of the presence of a tonic BCR-signal. Under this condition, a CCR7-mediated signal is then augmented, leading to enhanced cell migration of ZAP-70–positive UM-CLL cells. Whether complex formation of PI3 subunits, ZAP-70 and LCP1 is required for this, remains still unanswered. This also relates to the open question whether the observed effects are dependent on ZAP-70’s kinase function or not. In support of the latter, the Kipps and Efremov groups identified that the signal-enhancing effects of ZAP-70 were not dependent on its kinase activity and suggest that it can act as a chaperon molecule facilitating assembly of larger signaling complexes.28,45 Addressing these questions remains challenging from a technical point because it would require replacing endogenous ZAP-70 with a kinase-dead protein in primary cells to avoid issues related to nonspecific binding of ZAP-70, expressed at supraphysiological levels, to other proteins. However, our recently developed methods to genetically manipulate primary human malignant B cells46,47 now allow to address these questions in the future.
In summary, our data demonstrate that a tonic BCR- and CCR7-signaling converge in a subset of patients with CLL through the expression of ZAP-70 and activation of LCP1. Because both signals share common downstream pathways, the presence of a positive feedback loop between BCR- and CCR7-signaling appears to be a possible mechanism for this. Activation of either pathway improves cellular fitness and cell migration and is likely to improve T-cell support and to drive disease progression. The constitutive activation of these pathways suggests that ZAP-70–positive patients may benefit from early intervention with BCR-inhibitors, a concept that was trialed in the CLL-12 study. Although a subgroup analysis based on ZAP-70 expression was not reported in this trial, patients with IGHV-unmutated CLL had a marked benefit from ibrutinib- vs placebo-treated patients compared with mutated CLL,48 suggesting that interference with a tonic BCR signal can delay disease progression, particularly for ZAP-70–positive patients.
Acknowledgments
The authors express gratitude to all patients from Addenbrooke’s outpatient clinic that provided blood samples for this research. The help of Alison Wray and Joanna Baxter to recruit patients to this study was essential to the work.
This research was supported by Kay Kendell Foundation grant KKL1070 and Cancer Research UK grant C49940/A17480. J.C. receives funding from the Natural Science Foundation of China (82200196).
The visual abstract was created with BioRender.com.
Authorship
Contribution: J.C. performed wet lab experiments, analyzed data, and created figures; V.S. performed proteomic experiments in close collaboration with C.D.; C.S.R.C. and V.N.R.F. analyzed data and provided raw data and figures; C.A.J. generated switchable MEC1 cells; and I.R. planned the study and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ingo Ringshausen, University College London, Cancer Institute, Paul O'Gorman Building, 72 Huntley St, London, WC1E 6BT United Kingdom; email: i.ringshausen@ucl.ac.uk; and Jingyu Chen, Department of Biochemistry and Molecular Biology, West China School of Basic Medical Sciences & Forensic Medicine, Sichuan University, Chengdu, 610041 People’s Republic of China; email: jchen2022@scu.edu.cn.
References
Author notes
The authors will share publication-related data upon reasonable request to the corresponding author, Ingo Ringshausen (i.ringshausen@ucl.ac.uk).
The full-text version of this article contains a data supplement.

![ZAP-70 contributes to the constitutive phosphorylation of AKT and GSK3β in IGHV–unmutated CLL cells. (A) Representative kinetic blots show the calcium flux of IGHV–unmutated (UM-CLL) or IGHV–mutated (M-CLL) CLL cells with NSC or ZAP-70 siRNA transfection. CLL cells were harvested and labeled with Fluo-4 (fluorescein isothiocyanate). BCR-signaling was activated with anti-IgM beads, triggered 20 to 30 seconds after flow cytometric measurement started. (B) Quantification of calcium flux response of CLL samples (n = 4 for both UM-CLL and M-CLL) transfected with NSC or ZAP-70 siRNA. The Ca2+ ratios were calculated by using kinetic blots and divided peak Fluo-4 (FITC-A) mean intensity upon anti-IgM activation by baseline. (C) Phospho-AKT (T308), phospho-AKT (S473), total AKT, ZAP-70, and β-actin immunoblots of primary M-CLL cells monocultured for 24 hours after nonspecific control siRNA or ZAP-70 siRNA transfection. For anti-IgM stimulated samples, CLL cells were treated with beads-bound anti-IgM for 20 minutes. (D) Phosphorylated protein levels of AKT (relative to total AKT levels) 24 hours after siRNA transfection in M-CLL samples. For anti-IgM stimulated samples, monocultured M-CLL cells were treated with beads-bound anti-IgM for 20 minutes (n = 4 for pi-AKT [T-308] and n = 3 for pi-AKT [S473]). (E) Phospho-GSK-3β (S9), total GSK-3β, ZAP-70, and β-actin immunoblots of primary UM-CLL or M-CLL cells monocultured for 24 hours after nonspecific control siRNA or ZAP-70 siRNA transfection. For anti-IgM stimulated samples, monocultured CLL cells were treated with beads-bound anti-IgM for 20 minutes. (F) Phosphorylated GSK-3β levels (relative to total GSK-3β levels) 24 hours after siRNA transfection in UM-CLL and M-CLL samples. For anti-IgM stimulated samples, monocultured CLL cells were treated with beads-bound anti-IgM for 20 minutes (n = 3 for both UM-CLL and M-CLL). (G) Cell viability of primary CLL cells assessed using annexin-V/propidium iodide staining, 9 days after siRNA transfection. Cells were monocultured for 48 hours after nonspecific control siRNA or ZAP-70 siRNA transfection for 7 days on stromal cells. (H) MCL1 and β-actin immunoblots of primary UM-CLL cells cultured under identical conditions as described in panel G. (I) Relative MCL1 protein level (compared to with β-actin levels) 24 hours after siRNA transfection in UM-CLL samples (n = 3). Statistical significance between samples was assessed by paired 2-tailed Student t tests. ∗P < 5 × 10−2, ∗∗∗P < 10−4. LE, long exposure; ns, not significant; NSC, non-specific control; SE, short exposure.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/8/5/10.1182_bloodadvances.2022009557/2/m_blooda_adv-2022-009557-gr1.jpeg?Expires=1769094719&Signature=WUbqjL3zmaJYURhokr0jw2xd139P-o068Lld15bnTfXh4jg4rRT0brKMbziO6QC2SsZI~x09UQQs29AgBPwZYSbu6MHt7ROjKVqj2xYog9Jif5VH5rSC4S71MDmmim75C8safi7rg1o2qgWpEuHwykiiT~7KrfST~NNrRk289OjUa42f-S5mwEwLHwVfqso32mQDq0v69yDcpu1zSeLvVce~eHYy98THnnMbAOeV9Unyal6S64-JUAx23oBY0jlRqVcM7tDZq3HiTcK9TkbwxR6DF4G9RLVS6~2wbh7mSBckEhm0C6SMlmFescPAUyvaXSfKAZotSVLTcXf6pXUsnA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)