Because of short term and long term care needs, allo-HCT is costly.
Novel therapies that reduce both length of hospitalization after HCT and complication rates may significantly reduce allo-HCT costs.
Visual Abstract
Patients with hematologic malignancies undergoing allogeneic hematopoietic cell transplant (allo-HCT) require extensive care. Using the Merative MarketScan Commercial Claims and Encounters database (2016 Q1-2020 Q2), we quantified the costs of care and assessed real-world complication rates among commercially insured US patients diagnosed with a hematologic malignancy and aged between 12 and 64 years undergoing inpatient allo-HCT. Health care resource use and costs were assessed from 100 days before HCT to 100 days after HCT. Primary hospitalization was defined as the time from HCT until first discharge date. Incidence of complications was assessed using medical billing codes from HCT date to 100 days after HCT. Among the 1082 patients analyzed, allo-HCT grafts included peripheral blood (79%), bone marrow (11%), and umbilical cord blood (3%). In the 100 days after HCT, 52% of the patients experienced acute graft-versus-host disease; 21% had cytomegalovirus infection. The median primary hospitalization length of stay (LOS) was 28 days; 31% required readmission in first 100 days after HCT. Across the transplant period (14 days pretransplant to 100 days posttransplant), 44% of patients were admitted to the intensive care unit with a median LOS of 29 days. Among those with noncapitated health plans (n = 937), median cost of all-cause health care per patient during the transplant period was $331 827, which was driven by primary hospitalization and readmission. Additionally, the predicted median incremental costs per additional day in an inpatient setting increased with longer LOS (eg, $3381-$4071, 10th-20th day.) Thus, decreasing length of primary hospitalization and avoiding readmissions should significantly reduce the allo-HCT cost of care.
Introduction
Allogeneic hematopoietic cell transplantation (allo-HCT) is a potentially curative therapy for many patients with high-risk hematologic malignancies.1 According to the 2021 data from the World Marrow Donor Association, >24 000 allo-HCTs are performed annually worldwide.2 Additionally, based on 2020 data from the Center for International Blood and Marrow Transplant Research, the annual number of allo-HCT recipients in the United States is slightly >8000.1 In the 2017 activity survey of the European Society of Blood and Marrow Transplantation, conducted in 50 countries (including the United States and Europe), the number of patients receiving allo-HCTs from 1997 to 2017 increased from 4751 to 17 155, representing a 360% increase.3
Because of the need for acute and chronic care, including conditioning regimen administration and ongoing management of complications, allo-HCT is one of the most resource-intense medical procedures,4,5 with care spanning years for surviving patients, thereby posing a significant financial burden on the health care system. Allo-HCT has been associated with a high incidence of post-HCT complications, including graft-versus-host disease (GVHD) and infectious events.6,7 Among patients with commercial insurance and with Medicare supplemental insurance in the United States, the mean cancer-related health care costs in the 6 months before diagnosis and time from diagnosis to HCT combined were estimated at ∼$400 000 and $200 000 (in 2018 US dollars [USD]), respectively.8 Furthermore, at 100 days after HCT, the median total health care cost was ∼$250 000 for patients receiving nonmyeloablative or reduced-intensity conditioning regimens and $290 000 for patients receiving myeloablative conditioning regimens (in 2013 USD)5; in another claims-based study examining the 12 months after the first medical claim for HCT, the all-cause costs were estimated at ∼$540 000 for patients with complications and $325 000 for patients without complications (in 2015 USD).9
Previous studies have examined the financial burden of US patients receiving allo-HCT, focusing on costs related to either the pre-HCT or post-HCT period.5,8-16 Additionally, a few studies have evaluated the financial impact of post-HCT complications, including GVHD17-21 or viral infections,22-25 on driving the associated health care costs. Only 1 previous study had examined health care costs 6 months before and 12 months after HCT for patients with hematologic malignancies who had undergone allo-HCT compared with costs for control patients with hematologic malignancies who did not receive allo-HCT26; however, although this study reported the costs of prescriptions filled to prevent or mitigate complications of allo-HCT, it did not capture the actual rates of complications or the total health care costs. This retrospective study was conducted to quantify the health care costs from a US payer’s perspective during the pretransplant period and up to 100 days after transplantation and to assess real-world complication rates for allo-HCT among commercially insured patients in the United States.
Methods
Data source
This was a retrospective, observational study that identified patients with an allo-HCT using health insurance claims data from the Merative MarketScan Commercial Claims and Encounters database from 1 January 2016 to 30 June 2020. Allo-HCT was identified based on billing codes (supplemental Table 1) using Current Procedural Terminology, Healthcare Common Procedure Coding System, or International Classification of Diseases (ICD), 10th Revision, Procedure Coding System.
Study population
Patients aged between 12 and 64 years who received an inpatient allo-HCT and met the following inclusion criteria were included: (1) had at least 1 diagnosis of hematologic malignancy (ie, lymphoma; multiple myeloma and malignant plasma cell neoplasm; leukemia; other and unspecified malignant neoplasms of lymphoid, hematopoietic, and related tissue; and myelodysplastic syndrome; ICD-10 clinical modification [ICD-10-CM] codes C81-C96 and D46) at or within 6 months before the index date (date of the first administration of allo-HCT); (2) had at least 6 months of continuous enrollment before and at least 1 month of continuous enrollment after the index date; (3) had no diagnosis of chronic lymphocytic leukemia or non–in situ, nonhematologic primary cancers on or within 6 months before the index date; and (4) had no autologous HCT procedure on or within 6 months before the date of the first administration of allo-HCT. Patients on capitated health plans (ie, health maintenance organizations) were excluded from the analysis of health care costs but were included for assessments of other end points.
Study design
This study was specifically designed to describe the health care costs as well as specific cost drivers from a US payer’s perspective during the different periods before and after transplant for allo-HCT recipients among commercially insured patients in the United States. Patients’ out-of-pocket costs were not included in this analysis. The 6 months before, but not including, the index date (the administration date of the first allo-HCT) were considered the baseline period. The 100 days before the index date were the pretransplant period; 14 days before the index date were the conditioning period; and 100 days after, and including, the index date were the posttransplant period. The 14 days before transplant to 100 days after transplant was the transplant period. The full-observation period was from the index date to 1 year thereafter (Figure 1).
Patient demographics (including age, sex, region of residence, type of insurance plan, and year of index date) and clinical characteristics (including type of primary malignancy, comorbidity status by National Cancer Institute Comorbidity Index [NCICI], type of prior treatment, conditioning regimen, and donor source) were reported for all patients during the baseline period. The NCICI was selected to calculate an HCT score because the required information was available in the claims data. Although the HCT-specific comorbidity index is specific to patients undergoing allo-HCT, it requires laboratory values that are not available in the health insurance claims data used in this analysis. However, there are large overlaps between the NCICI and HCT-specific comorbidity index across comorbidities, suggesting that an NCICI score is relevant to this population. After allo-HCT, the presence of complications was assessed based on ICD-10-CM and included (1) GVHD, (2) cytomegaloviral (CMV) infection, (3) other infections (ie, bacterial, non-CMV, or fungal); (4) malignant relapse, and (5) other complications (ie, end organ damage or mucositis).
All-cause health care resource use (inpatient and intensive care unit [ICU]-level care, including bone marrow transplant [BMT] unit, outpatient, and emergency room [ER] visits) was summarized for all patients during the pretransplant, transplant, and posttransplant periods. The proportion of patients with readmission was summarized during the posttransplant period only. Health care costs, defined as the amount paid by the payer to the health care provider, were quantified among all patients in noncapitated health plans and included medical (both ICU and non–ICU-level care, including BMT unit; outpatient and ER visits; and other costs) and outpatient pharmacy; these were inflated to 2020 USD using the Personal Consumption Expenditures health index.
During the full-observation period, all-cause health care costs were assessed among the subset of patients with noncapitated health plans with ≥100 days of follow-up after transplant. These included health care costs (medical and outpatient pharmacy) and annualized health care costs, defined as the sum of health care costs divided by the sum of patient follow-up. These results were further stratified based on (1) patients’ chronic GVHD (cGVHD) status (≥1 diagnosis code for cGVHD [ICD-10-CM: D89.81]) during the full-observation period from days 101 to 365, and (2) patients’ length of primary hospitalization (ie, 1-30 days, 31-59 days, or ≥60 days).
All-cause inpatient costs during the posttransplant period were predicted based on inpatient length of stay (LOS), adjusted for baseline characteristics (including age, sex, type of malignancy, transplant type, prior antineoplastic treatments, and comorbidities) and posttransplant complications (GVHD, CMV infection, other infections, and transfusion). The incremental cost of 1 additional day in the hospital was defined as the difference between predicted inpatient costs of staying in the hospital for N days and N − 1 days; incremental costs of every 10th day, up to 100th day, in the hospital were estimated.
Statistical analysis
For descriptive analyses, mean and standard deviations (SDs) or medians and interquartile ranges (IQRs) were reported for all continuous variables; for categorical variables, frequencies and percentages were reported. Health care resource use costs were reported as means, SDs, medians, and ranges.
A generalized linear model with a gamma distribution was used to predict inpatient costs and incremental cost of each additional day in the hospital based on inpatient LOS during the posttransplant period. This approach estimates the inpatient costs based on the fitted generalized linear model by varying inpatient LOS while keeping other patient characteristics the same as observed in the sample. The predicted incremental daily inpatient cost for a given day (eg, 10th day) is the difference between the 2 predicted costs on subsequent days (eg, the difference between the inpatient cost for a LOS of 10 days and the inpatient cost for a LOS of 9 days) and can be interpreted as the additional inpatient cost of staying in the hospital for “N” days compared with the same population, had the patient stayed in the hospital for N = 1 days.
Results
Patient characteristics
A total of 1082 patients with allo-HCT were included in the analysis (supplemental Figure 1). The mean (±SD) age at index allo-HCT was 47.9 (±14.4) years; more than half (57.2%) of the patients were male (Table 1). The most common region of residence was the South (39.2%), followed by Northeast (24.0%). Nearly half (47.7%) of the patients had a preferred provider organization insurance.
Patient characteristics at allogeneic HCT
| Patient characteristics . | All patients (N = 1082) . |
|---|---|
| Demographic characteristics | |
| Age, y, mean ± SD | 47.9 ± 14.4 |
| Age category, n (%) | |
| 12-17 | 55 (5.1) |
| 18-39 | 228 (21.1) |
| 40-64 | 799 (73.8) |
| Male, n (%) | 619 (57.2) |
| Region, n (%) | |
| South | 424 (39.2) |
| Northeast | 260 (24.0) |
| North Central | 236 (21.8) |
| West | 162 (15.0) |
| Insurance plan type, n (%) | |
| PPO | 537 (47.7) |
| HDHP | 244 (22.6) |
| POS | 151 (14.0) |
| HMO | 114 (10.5) |
| Comprehensive | 31 (2.9) |
| EPO | 15 (1.4) |
| Unknown | 11 (1.0) |
| Year of index date, n (%) | |
| 2016 | 147 (13.6) |
| 2017 | 294 (27.2) |
| 2018 | 297 (27.4) |
| 2019 | 263 (24.3) |
| 2020 | 81 (7.5) |
| Clinical characteristics | |
| Types of primary malignancy, n (%) | |
| Acute myeloid leukemia | 465 (43.0) |
| Myelodysplastic syndrome | 176 (16.3) |
| Acute lymphocytic leukemia | 159 (14.7) |
| Lymphoma | 132 (12.2) |
| Chronic myeloid leukemia | 34 (3.1) |
| Other | 116 (10.7) |
| NCICI, mean ± SD | 1.5 ± 1.6 |
| Prior chemotherapy treatment, n (%) | 847 (78.3) |
| Prior CAR-T infusion, n (%) | 2 (0.2) |
| Donor source, n (%) | |
| Peripheral blood | 851 (78.7) |
| Bone marrow | 117 (10.8) |
| Cord blood | 34 (3.1) |
| Unspecified | 80 (7.4) |
| Patient characteristics . | All patients (N = 1082) . |
|---|---|
| Demographic characteristics | |
| Age, y, mean ± SD | 47.9 ± 14.4 |
| Age category, n (%) | |
| 12-17 | 55 (5.1) |
| 18-39 | 228 (21.1) |
| 40-64 | 799 (73.8) |
| Male, n (%) | 619 (57.2) |
| Region, n (%) | |
| South | 424 (39.2) |
| Northeast | 260 (24.0) |
| North Central | 236 (21.8) |
| West | 162 (15.0) |
| Insurance plan type, n (%) | |
| PPO | 537 (47.7) |
| HDHP | 244 (22.6) |
| POS | 151 (14.0) |
| HMO | 114 (10.5) |
| Comprehensive | 31 (2.9) |
| EPO | 15 (1.4) |
| Unknown | 11 (1.0) |
| Year of index date, n (%) | |
| 2016 | 147 (13.6) |
| 2017 | 294 (27.2) |
| 2018 | 297 (27.4) |
| 2019 | 263 (24.3) |
| 2020 | 81 (7.5) |
| Clinical characteristics | |
| Types of primary malignancy, n (%) | |
| Acute myeloid leukemia | 465 (43.0) |
| Myelodysplastic syndrome | 176 (16.3) |
| Acute lymphocytic leukemia | 159 (14.7) |
| Lymphoma | 132 (12.2) |
| Chronic myeloid leukemia | 34 (3.1) |
| Other | 116 (10.7) |
| NCICI, mean ± SD | 1.5 ± 1.6 |
| Prior chemotherapy treatment, n (%) | 847 (78.3) |
| Prior CAR-T infusion, n (%) | 2 (0.2) |
| Donor source, n (%) | |
| Peripheral blood | 851 (78.7) |
| Bone marrow | 117 (10.8) |
| Cord blood | 34 (3.1) |
| Unspecified | 80 (7.4) |
Demographic and clinical characteristics of patients identified using the Merative MarketScan Commercial Claims and Encounters database between 1 January 2016 and 30 June 2020.
CAR-T, chimeric antigen receptor T cell; EPO, exclusive provider organization; HDHP, high-deductible health plan; HMO, health maintenance organization; POS, point of service; PPO, preferred provider organization.
The most common type of primary malignancy was acute myeloid leukemia (43.0%), followed by myelodysplastic syndrome (16.3%) and acute lymphocytic leukemia (14.7%). The mean (±SD) NCICI score was 1.5 (±1.6); the most common comorbidities were chronic obstructive pulmonary disease (15.8%), mild liver disease (15.5%), and congestive heart failure (11.7%). The majority (78.3%) of patients had received prior chemotherapy during the baseline period. The most common type of allo-HCT graft was peripheral blood (78.7%), followed by bone marrow (10.8%) and cord blood (3.1%).
Complications after allo-HCT
During the posttransplant period (first 100 days), 51.7% of patients experienced acute GVHD (Figure 2). Among posttransplant infections, 20.8% had CMV infection; other, non-CMV infections were bacterial (45.4%), fungal (15.1%), or other non-CMV viral (9.3%). Other posttransplant complications that generated expenditures within the time periods of interest included mucositis (29.5%), malignant relapse (23.8%), and acute renal failure (23.2%). Less than 10% of patients experienced acute respiratory failure and acute heart failure.
Proportion of patients with posttransplant complications (within 100 days after transplant). Rates of post-HCT complications occurring 100 days after HCT using medical billing codes are shown.
Proportion of patients with posttransplant complications (within 100 days after transplant). Rates of post-HCT complications occurring 100 days after HCT using medical billing codes are shown.
Of the 816 patients in the full-observation study period from index date until 1 year after transplant, 517 (63.4%) patients experienced cGVHD and 356 (43.6%) patients had a relapse.
Health care resource use
During the pretransplant period, the majority (70.1%) of patients had at least 1 inpatient admission; of these, the median (range) inpatient LOS was 23.0 (1.0-100.0) days (Table 2). During the posttransplant period, the median (range) inpatient LOS was 30.5 (1.0-100.0) days, and 30.7% of the patients experienced a subsequent readmission. Across the transplant period, the median (range) inpatient LOS was 31.0 (1.0-104.0) days, of which 28.0 (1.0-100.0) days were reported as primary hospitalization. During the full-observation study period, 59.4%, 34.9%, and 5.6% had a length of primary hospitalization LOS between 1 and 30 days, 31 and 59 days, and ≥60 days, respectively.
All-cause health care resource use across time periods
| Health care resource use . | Before transplant (100 d) . | After transplant (100 d) . | Transplant period (14 d before to 100 d after) . |
|---|---|---|---|
| Inpatient admission, n (%) | 759 (70.1) | 1082 (100.0) | 1082 (100.0) |
| Number of admissions, median (range) | 1.0 (0.0-8.0) | 1.0 (1.0-5.0) | 1.0 (1.0-6.0) |
| Inpatient days (among those with ≥1 admission), median (range) | 23.0 (1.0-100.0) | 30.5 (1.0-100.0) | 31.0 (1.0-104.0) Primary hospitalization: 28 (1-100) |
| ICU-level care, including BMT unit, n (%) | 250 (23.1) | 462 (42.7) | 477 (44.1) Primary hospitalization: 413 (38.2) |
| Number of stays, median (range) | 0.0 (0.0-5.0) | 0.0 (0.0-5.0) | 0.0 (0.0-5.0) Primary hospitalization: 0 (0-1) |
| ICU days (among those with ≥1 stay), median (range) | 9.0 (1.0-68.0) | 28.0 (1.0-100.0) | 29.0 (1.0-101.0) Primary hospitalization: 27.0 (1.0-100.0) |
| Number of outpatient visits, median (range) | 26.0 (0.0-83.0) | 20.0 (0.0-81.0) | 25.0 (0.0-90.0) |
| ER visit, n (%) | 258 (23.8) | 152 (14.0) | 170 (15.7) |
| Number of visits, median (range) | 0.0 (0.0-22.0) | 0.0 (0.0-38.0) | 0.0 (0.0-40.0) |
| Health care resource use . | Before transplant (100 d) . | After transplant (100 d) . | Transplant period (14 d before to 100 d after) . |
|---|---|---|---|
| Inpatient admission, n (%) | 759 (70.1) | 1082 (100.0) | 1082 (100.0) |
| Number of admissions, median (range) | 1.0 (0.0-8.0) | 1.0 (1.0-5.0) | 1.0 (1.0-6.0) |
| Inpatient days (among those with ≥1 admission), median (range) | 23.0 (1.0-100.0) | 30.5 (1.0-100.0) | 31.0 (1.0-104.0) Primary hospitalization: 28 (1-100) |
| ICU-level care, including BMT unit, n (%) | 250 (23.1) | 462 (42.7) | 477 (44.1) Primary hospitalization: 413 (38.2) |
| Number of stays, median (range) | 0.0 (0.0-5.0) | 0.0 (0.0-5.0) | 0.0 (0.0-5.0) Primary hospitalization: 0 (0-1) |
| ICU days (among those with ≥1 stay), median (range) | 9.0 (1.0-68.0) | 28.0 (1.0-100.0) | 29.0 (1.0-101.0) Primary hospitalization: 27.0 (1.0-100.0) |
| Number of outpatient visits, median (range) | 26.0 (0.0-83.0) | 20.0 (0.0-81.0) | 25.0 (0.0-90.0) |
| ER visit, n (%) | 258 (23.8) | 152 (14.0) | 170 (15.7) |
| Number of visits, median (range) | 0.0 (0.0-22.0) | 0.0 (0.0-38.0) | 0.0 (0.0-40.0) |
Health care resource use associated with inpatient, ICU, outpatient, and ER admissions across the pretransplant, posttransplant, and full-observation periods are shown.
HRU, health care resource use.
Compared with the pretransplant period, more patients were admitted to ICU-level care, including BMT units (42.7% vs 23.1%) during the posttransplant period; the median (range) ICU LOS was ∼3 times as long (28.0 [1.0-100.0] days vs 9.0 [1.0-68.0] days). Across the transplant period, 44.1% of patients were admitted to ICU-level care, including BMT units; the median (range) LOS was 29.0 (1.0-101.0) days. Among those with ICU/BMT unit-level care (477 patients) during the transplant period, 86.6% were admitted during their primary hospitalization for a median (range) of 27.0 (1.0-100.0) days.
The median (range) number of outpatient visits was higher in the pretransplant period compared with the posttransplant period (26.0 [0.0-83.0] vs 20.0 [0.0-81.0]); these data were unadjusted for observation time and did not account for time spent in other settings, including inpatient setting. During the pretransplant period, nearly a quarter (23.8%) of patients had at least 1 ER visit, with a median (range) of 0.0 (0.0-22.0) visits. Fewer patients (14.0%) had an ER visit during the posttransplant period, with a median (range) of 0.0 (0.0-38.0) visits.
Health care costs
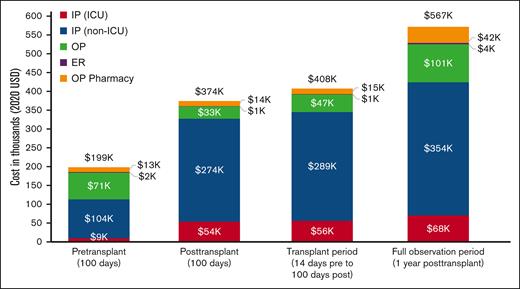
Patients on capitated health plans (ie, health maintenance organization and point of service with capitation) were excluded from analysis of health care costs because these plan types allow payment of a flat fee for each patient, which does not reflect the actual services patients received. A total of 937 patients were included in the analyses of health care costs (Figure 3; supplemental Table 2). Compared with the pretransplant costs, the median (IQR) posttransplant costs were ∼2 times higher ($306 133 [$214 519-$439 557] vs $155 319 [$86 115-$263 286]), mainly because of increased costs accrued in an inpatient setting ($260 312 [$182 619-$381 510] vs $55 707 [$0-167 668]).
Mean health care costs across time periods. Mean all-cause health care costs accrued across the pretransplant, posttransplant, and full-observation periods are shown. Note: the full-observation period costs were assessed among a subgroup of patients with ≥100 days of follow-up after transplant. IP, inpatient; OP, outpatient.
Mean health care costs across time periods. Mean all-cause health care costs accrued across the pretransplant, posttransplant, and full-observation periods are shown. Note: the full-observation period costs were assessed among a subgroup of patients with ≥100 days of follow-up after transplant. IP, inpatient; OP, outpatient.
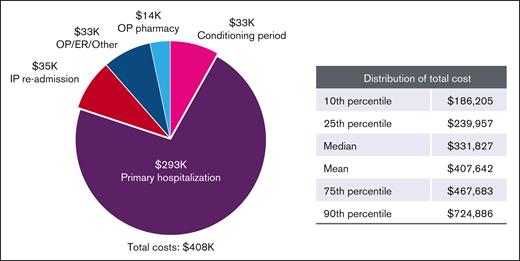
Across the transplant period, the median [IQR] costs were $331 827 ($239 957-$467 683; Figure 4; supplemental Table 2). The largest driver of health care costs was primary hospitalizations ($239 079 [$172 729-$342 588]); together with readmission costs ($34 518), the costs accrued in an inpatient setting account for 80% of costs across the transplant period. Median (IQR) costs associated with the conditioning period were $12 100 ($5070-$25 910).
Transplant period costs (in thousands, 2020 USD), based on type and setting. Health care costs accrued based on type in the transplant period are shown.
Transplant period costs (in thousands, 2020 USD), based on type and setting. Health care costs accrued based on type in the transplant period are shown.
A total of 816 patients in noncapitated health plans with ≥100 days of follow-up after transplant were included in the analysis of all-cause costs during the full-observation period from index date to 1 year after transplant (supplemental Table 3; average follow-up time [±SD]: 9.6 months [±3.0]). Among these patients, the median (IQR) per-patient health care cost to a third-party payer from index date until 1 year after transplant was $447 500 ($292 954-$658 775). When annualized, the median (IQR) costs increased to $586 312 ($377 249-$1 006 582) per patient per year. Two sensitivity analyses were conducted to examine the potential impact of (1) having cGVHD and (2) long LOS during primary hospitalization; both were associated with higher 1-year costs. When stratified by cGVHD status, median all-cause health care costs were $474 507 and $416 586 for patients with and without cGVHD, respectively. All-cause health care costs increased with length of primary hospitalization when stratified by length of primary hospitalization stay, with LOS durations of 1 to 30 days, 31 to 59 days, and ≥60 days incurring median costs of $389 349, $528 348, and $847 673, respectively.
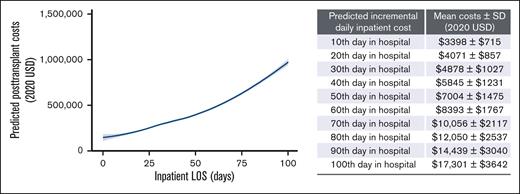
Increased predicted inpatient costs were associated with longer observed inpatient LOS. In addition, the predicted incremental costs per additional day in an inpatient setting increases as inpatient LOS increases, after adjusting for baseline demographic and clinical characteristics (Figure 5; supplemental Table 2). Specifically, staying in the hospital for 10 days was associated with an additional mean (±SD) of $3398 (±$715; median: $3381) compared with staying in the hospital for 9 days, indicating an incremental cost of $3398 (±$715) on the 10th day in the hospital. The incremental mean (±SD) of an additional inpatient day increased to a mean of $4071 (±$857; median: $4071), $7004 (±$1475; median: $7004), and $17 301 (±$17 301; median $17 217) on the 20th, 50th, and 100th day, respectively.
Predicted inpatient costs based on inpatient LOS. Predicted inpatient costs in the 100 days after HCT based on inpatient LOS after adjusting for baseline demographic and clinical characteristics are shown. Note: inpatient costs were predicted based on a generalized linear model with gamma distribution, including LOS, baseline characteristics (ie, age, sex, type of malignancy, transplant type, prior antineoplastic treatments, and comorbidities) and posttransplant complications (ie, GVHD, CMV infection, other infections, and transfusion).
Predicted inpatient costs based on inpatient LOS. Predicted inpatient costs in the 100 days after HCT based on inpatient LOS after adjusting for baseline demographic and clinical characteristics are shown. Note: inpatient costs were predicted based on a generalized linear model with gamma distribution, including LOS, baseline characteristics (ie, age, sex, type of malignancy, transplant type, prior antineoplastic treatments, and comorbidities) and posttransplant complications (ie, GVHD, CMV infection, other infections, and transfusion).
Discussion
This retrospective study provided a comprehensive and up-to-date real-world estimate of health care resource use and cost of care from a US payer perspective associated with allo-HCT among different treatment periods for patients with hematologic malignancies. Additionally, the study has a larger sample size than similar studies using US commercial claims5,8,26 and is based on a more recent time period. Consistent with data from previous studies examining the costs associated with the pre-HCT8,26 and post-HCT,5,14,26 patients with hematologic malignancies undergoing inpatient allo-HCT experienced significant economic burden, with primary hospitalization as the major driver of health care costs. As expected, our findings showed that the number of patients with ICU-level care, including BMT unit was numerically higher in the post-HCT compared with the pre-HCT period and could reflect hospital care assignments. Similar findings were noted in a retrospective matched-case control study using patient-level administrative claims databases from January 2011 to June 2013.26 From both the analysis of all-cause health care costs during the full-observation period by length of primary hospital stay and the inpatient cost prediction analysis, longer observed inpatient LOS was associated with higher predicted inpatient costs; this trend is consistent with that found in a study examining the National Inpatient Sample database.27 Thus, the costs of care to a US payer would be reduced by shortening the length of hospitalizations.
All-cause health care costs during the full-observation period were higher for patients with cGVHD compared with those without cGVHD. Previous studies showed that patients who develop acute GVHD18,19 or cGVHD21 are likely to incur higher expenses after HCT compared with those who do not develop GVHD. A previous study found that patients with multiple double-stranded DNA viral infections after allo-HCT had used more health resources, thereby incurring higher health care expenditures.25
Recently, a 2022 study estimated that over a lifetime, the average per-patient medical cost of allo-HCT was ∼$1.2 million USD,28,29 of which 37% to 53% of the costs were attributed to management of cGVHD, with expected lifetime quality-adjusted life years of ∼4.7. Accounting for the fact that several small-molecule inhibitors have recently been approved to clinically manage cGVHD, the future per-patient cost of allo-HCT was estimated to be $1.4 to $1.6 million USD, with expected quality-adjusted life years of 4.8.28 These data underscore the need for more effective therapies for patients with hematologic malignancies undergoing allo-HCT, including stem cell sources, which have lower rates of complications and reduce the need for extended inpatient care, thereby decreasing the overall cost of care.
Multiple limitations associated with any retrospective claims-based study must be considered. First, this analysis included patients with commercial insurance coverage and the results may not be generalizable to the overall population of US patients with hematologic malignancies who receive allo-HCT. The presence of diagnoses and procedures was identified using available diagnostic and procedure codes and may be subject to coding entry error or missingness, which can lead to under or overrepresentation of clinical outcomes or complications. To highlight this issue, we can confirm that the rate of reported complications in this study was mostly comparable with that from previously published studies using patient-level administrative claims databases in similar populations.9 We also note that the rate of acute GVHD observed in the study was consistent with historical data.30 In contrast, differences can be seen when comparing rates of complications in the claims database used in this analysis with rates reported in prospectively randomized clinical trials and observational settings. For example, acute renal failure was found to be 23.3% in this analysis; however, a recent meta-analysis assessing acute kidney injury (AKI) for patients undergoing HCT reported an overall incidence of AKI of 55.1% in this population, of which 8.3% was classified as severe AKI, and 7.2% of patients underwent renal replacement therapy.31 It is important to highlight possible reasons for these differences. Although data collected prospectively in clinical trials often represent a more selected population compared with real-world experiences, data from claims databases may reflect not only institutional coding of underlying diagnoses and procedures but can also be the consequence of coding of complications to maximize reimbursements because an increased number of diagnoses can result in increased payments. Thus, in this example, reporting acute renal failure may not always reflect the severe cases requiring renal replacement therapy, but rather reflects a clinical change that, if not reversed, can place a patient at risk for significant harm. Similar assessments can be reflected in other clinical situations, such as rates of infection or even disease status. Thus, we recognize that limitations of the data available in a claims database are linked to a lack of granularity with an inability to discriminate the severity of a complication that is more accurately captured in a clinical trial or detailed retrospective institutional analysis. As such, a claims database can be useful because it can represents a wide spectrum of patients who undergo a particular procedure for a particular indication.
The claims databases record diagnostic and procedure codes for reimbursement purposes, clinical diagnoses (including diagnosis of complications), or measures of severity of disease or complications (eg, severity of acute GVHD or cGVHD) beyond those resulting in specific billing codes and out-of-pocket costs incurred by patients were not considered in this analysis. Consequently, the full clinical incidence and impact of allo-HCT may be underestimated. The period that was selected coincided with the beginning of the global severe acute respiratory syndrome coronavirus 2 (COVID-19) pandemic early in 2020. Immunocompromised patients (eg, post–allo-HCT patients) infected with COVID-19 have higher risk of death.32 It is important to note that early death is inversely related to cost of care of allo-HCT. Because mortality data were not captured in this data set, the study period coinciding with the beginning of the pandemic further amplified the impact of shortened ICU LOS durations from early death. It is also possible that allo-HCT was deferred for many patients with low-risk hematologic malignancies during the pandemic, which might have resulted in the prioritization of patients with high-risk disease who received allo-HCT. Consequently, the claims-based data may potentially underestimate the full impact of the health care costs of allo-HCT. Different levels of service (ie, service provided at a transplant-specialty clinic vs an ICU) cannot be differentiated based on billing code data. Owing to common practice in the health care system, the transplant inpatient unit may be coded as ICU at some centers, which may overestimate the reported degree of ICU-level care, including BMT unit stays. Costs were measured from the payer’s perspective (ie, the amounts reimbursed by insurers) and do not include all costs incurred, such as out-of-pocket expenses and indirect costs because of productivity loss, which may underestimate the overall costs. Additionally, the impact of any new medications approved during the study time frame on costs was not assessed.
In conclusion, our study showed that primary hospitalization was the largest driver of health care costs after allo-HCT. These costs might have been attributed, in part, to high rates of post-HCT complications. Understanding the economic burden and specific cost drivers of allo-HCT across distinct treatment periods adds value to the wealth of currently existing medical literature. Decreasing the length of primary hospitalization and avoiding readmissions should reduce the cost of care for US patients undergoing allo-HCT. Novel therapies and stem cell sources that reduce the need for extended inpatient care with lower rates of complications may significantly reduce the cost of care for this population. Furthermore, it is critical to scrutinize health care costs of allo-HCT with the emergence of novel therapies related to pretransplant conditioning, graft engineering, and GVHD prophylaxis.
Acknowledgments
Analytic support was provided by Ahmed Noman of Analysis Group, Inc, which received consulting fees from Gamida Cell to conduct this study. Editorial and medical writing support was provided by Thai Cao of Envision Pharma, Inc, and funded by Gamida Cell.
Authorship
Contribution: M.L.E., Y.S., Q.L., A.A., and J.S. were involved in the data analysis; and all authors were involved in the concept/design of the study and data interpretation, and critically reviewed, revised, and approved the manuscript content for publication.
Conflict-of-interest disclosure: R.T.M. is an adviser or consultant for AlloVir, Artiva, CRISPR Therapeutics, Incyte, and Novartis; reports honoraria from Bristol Myers Squibb/Celgene, Incyte, Intellia, and Kite; receives research support from AlloVir and Novartis; participates in data and safety monitoring boards for Athersys, Novartis, Century Therapeutics, and VorPharma; and has a patent with Athersys. U.G. is an adviser or consultant for Astellas, Gamida Cell Ltd, Incyte, Jazz Pharmaceuticals, Mesoblast, and Novartis, and reports honoraria from Kite. R.M., R.S., H.S., and S.S. are employees of Gamida Cell Ltd. M.L.E., Y.S., Q.L., A.A., and J.S. are employees of Analysis Group Inc, which received consulting fees from Gamida Cell Ltd. for this research. This research was funded by Gamida Cell.
Correspondence: Richard T. Maziarz, Knight Cancer Institute, Oregon Health and Science University, 3181 SW Sam Jackson Park Rd, Mail code OC14HO, Portland, OR 97239; email: maziarzr@ohsu.edu.
References
Author notes
These data were presented in abstract form at the 2022 Tandem Meetings Transplantation and Cellular Therapy Meetings of American Society of Transplantation and Cellular Therapy and Center for International Blood & Marrow Transplant Research, Salt Lake City, UT, 23 to 26 April 2022.
Data are available on request from the corresponding author, Richard T. Maziarz (maziarzr@ohsu.edu), or medicalinformation@gamidacell.com.
The full-text version of this article contains a data supplement.