Key Points
The surface protein CD33 is amenable to multiple modes of editing without impact on hematopoietic cell reconstitution.
CD33-directed CAR T cells specifically eliminate CD33+ cells while sparing CD33-modified stem cell progeny.
Visual Abstract
The treatment of monogenetic disorders, such as hemoglobinopathies and lysosomal storage diseases, has markedly improved with the advent of cell and gene therapies, particularly allogeneic or gene-modified autologous stem cell transplantations. However, therapeutic efficacy is reliant on maintaining engraftment above a critical threshold. To maintain such engraftment levels, we and others have pursued approaches to shield edited cells from antibody or chimeric antigen receptor (CAR) T-cell–mediated selection. Here, we focused on CD33, which is expressed early on hematopoietic stem and progenitor cells (HSPCs) as well as on myeloid progenitors. Rhesus macaques were engrafted with HSPCs edited to ablate CD33 using either CRISPR/CRISPR-associated protein 9 or adenine base editor. Both editing strategies showed similar post-transplant recovery kinetics and yielded equivalent levels of engraftment. We then created a V-set domain–specific CAR construct (CAR33), validated its functionality in vitro, and treated both animals with autologous CAR33 T cells. CAR33 T cells expanded after infusion and caused specific depletion of CD33WT but not CD33null progeny, leading to a transient enrichment for gene-edited cells in the blood. No depletion was seen in the bone marrow stem cell compartment with CD34+CD90+ HSCs expressing lower levels of CD33 in comparison to monocytes. Thus, we show proof of concept and safety of an epitope editing–based enrichment/protection strategy in macaques.
Introduction
The treatment of monogenetic diseases has been revolutionized by the advent of gene and cell therapy. For instance, last year alone, the US Food and Drug Administration has approved 2 new therapeutics for the treatment of sickle cell disease, marking the first time that autologous hematopoietic stem cell transplantation (auto-HSCT) has had curative potential for this disease.1 However, the treatment of sickle cell disease and other diseases is reliant on maintenance of therapeutic cells above a critical threshold, generally accepted to be 20%.2-4 Should engraftment wane below this therapeutic threshold, the only current recourse to maintain efficacy is repeated HSCT. To avoid a secondary transplant, our group has investigated cointroducing either transgenes (eg, methylguanyl-methyltransferase P140K) and/or editing cell surface markers such as CD33 and using them as “selection handles” to allow for the enrichment of gene-modified cells after HSCT.5-8 We have demonstrated the feasibility of this approach with lentiviral overexpression of the inhibitor resistant P140K variant of methylguanyl-methyltransferase paired with methylating chemotherapeutics to achieve enrichment of gene-modified cells to >90% of all HSCs.5 However, the reliance on genotoxic and nonspecific chemotherapeutics for enrichment is suboptimal, prompting us to explore more targeted enrichment strategies.
Recently, multiple groups reported the use of base editors (BEs) to protect surface antigens from epitope-specific targeting strategies such as chimeric antigen receptor (CAR) T cells and bispecific T-cell engagers.9,10 Our group, and others, have explored this technique using CRISPR/CRISPR-associated protein 9 (Cas9) and adenine BEs (ABEs) to edit the siglec-family protein CD33.6,7,11,12 Editing of CD33 has been shown to have no impact on HSC differentiation potential and confer selective resistance to anti-CD33 therapeutics such as CAR T cells (CAR33) and antibody-drug conjugates. Although the combination of CD33 editing and targeting has primarily been investigated in the context of acute myeloid leukemia (AML) treatment,13 we propose that this strategy could serve as an attractive alternative to genotoxic regimens to facilitate the selection and enrichment of gene-modified HSCs. Here, we characterize the safety and efficacy of 2 CD33 editing strategies using CRISPR/Cas9 and ABE in 2 rhesus macaques (RMs).
Materials and methods
Autologous NHP transplants
Healthy juvenile RMs were housed at the University of Washington National Primate Research Center under conditions approved by the American Association for the Accreditation of Laboratory Animal Care. All experimental procedures performed were reviewed and approved by the institutional animal care and use committee of the Fred Hutchinson Cancer Center (Fred Hutch) and University of Washington (protocol no. 3235-01). This study was carried out in strict accordance with the recommendations in the guide for the care and use of laboratory animals of the National Institutes of Health, and monkeys were randomly assigned to the study.
Autologous nonhuman primate (NHP) transplantation, priming (mobilization), collection of cells, and genetic engineering were conducted consistent with our previously published protocols.14-16 In parallel to cell processing, macaques were conditioned with myeloablative total body irradiation (TBI) of 1020 cGy from a 6-MV X-ray beam of a single-source linear accelerator located at the Fred Hutch South Lake Union Facility (Seattle, WA); irradiation was administered as a fractionated dose over the 2 days before cell infusion. During irradiation, animals were housed in a specially modified cage that provided unrestricted access for the irradiation while simultaneously minimizing excess movement. The dose was administered at a rate of 7 cGy/min delivered as a midline-tissue dose. Granulocyte colony-stimulating factor was administered daily from the day of cell infusion until the animals began to show onset of neutrophil recovery. Supportive care, including antibiotics, electrolytes, fluids, and transfusions, was given as necessary, and blood counts were analyzed daily to monitor hematopoietic recovery.
Please see the supplemental Materials and methods for details on animals use and handling protocols.
Handling of HSPCs ex vivo
Primed NHP bone marrow (BM) was harvested, enriched, and cultured as previously described.16,17 Briefly, animals were mobilized by daily administration of granulocyte colony-stimulating factor and stem cell factor at 100 mg/kg per day and 50 mg/kg per day, respectively, for 4 days. On the fifth day, primed marrow was collected by aspiration from all 4 long bones. Before enrichment of CD34+ cells, red cells were lysed in ammonium chloride lysis buffer, and white blood cells were incubated for 30 minutes with the 12.8 immunoglobulin M anti-CD34 antibody and then washed and incubated for another 30 minutes with magnetic-activated cell-sorting anti–immunoglobulin M microbeads (Miltenyi Biotec). The cell suspension was run through magnetic columns enriching for CD34+ cell fractions with a purity of 60% to 80% confirmed by flow cytometry. Enriched CD34+ cells were cultured in StemSpan serum-free expansion medium II (STEMCELL Technologies) supplemented with penicillin and streptomycin (100 U/mL; ThermoFisher Scientific), stem cell factor (PeproTech), thrombopoietin (PeproTech), and Fms-related tyrosine kinase 3 ligand (Miltenyi Biotec) (100 ng/mL for each cytokine).
Isolated CD34+ cells were edited as previously described.8 Briefly, Cas9 purified protein and chemically modified guide RNA were incubated together at room temperature for 10 minutes to form functional ribonucleoproteins (RNPs). RNPs were introduced to CD34+ cells by electroporation of 3 million cells per 2-mm cuvette. For base editing, ABE messenger RNA and single guide RNA (sgRNA) were cointroduced by electroporation of 3 × 106 cells per 2-mm cuvette. Edited cells were recovered in medium overnight at 37°C before infusion into animals.
FACS
Antibodies used for analysis and sorting of NHP cells are listed in supplemental Tables 1-3. Dead cells and debris were excluded by forward scatter/side scatter gating (supplemental Figures 1 and 2). Flow cytometric analysis was performed on the Symphony S2, fluorescence-assisted cell sorting (FACS) Aria IIu, and Canto II (BD Biosciences). Cells for in vitro assays and autologous NHP transplants were sorted after CD34 enrichment using the Symphony S6 cell sorter (BD Biosciences) and sort purity assessed by recovery of sorted cells.
CFC assay
For colony-forming cell (CFC) assays, 1000 to 1200 sorted cells were seeded into 3.5 mL ColonyGEL 1402 (ReachBio). Hematopoietic colonies were scored after 12 to 14 days. Arising colonies were identified as colony-forming unit (CFU) granulocyte, CFU macrophage, CFU granulocyte macrophage, and burst-forming unit erythrocyte. Colonies consisting of erythroid and myeloid cells were scored as CFU-mix. Colony-forming potential was calculated following the equation: (number of colonies formed/number of cells plated) × 100.
ddPCR
Two sets of primers and probes were selected based on 3 CD33 sequences provided (human, rhesus, and pseudogene), which share the same forward primer and probe. The reverse primer CD33Rev1 1210-1189 is specific to rhesus CD33. Duplex rhesus CD33 digital droplet polymerase chain reaction (ddPCR) is performed with CD33Fwd723-740, CD33Probe760-774, CD33Rev1 1210-1189 (rhesus), and rhesus RPP30 primer and probe set. The primer and probe sequences are all listed in supplemental Table 4. The DNA ddPCR was performed on a Bio-Rad QX200 instrument with ddPCR supermix for probe (Bio-Rad). The DNA droplets were generated with an automated droplet generator. The thermal condition of ddPCR were 95°C for 10 minutes, then 40 cycles at 94°C for 30 seconds, 60°C for 1 minute, followed by 98°C for 10 minutes. The copy number of CD33 and RPP30 per reaction were analyzed and calculated with QuantaSoft Analysis Pro Software (Bio-Rad).
Production of CAR constructs
The variant heavy chain and variant light chain amino acid sequences were adapted from a known NHP/human cross-reactive antibody and synthesized as an single-chain variable fragment (scFv) with a G4S linker.18-20 Our designed anti-CD33 scFv was synthesized as a gene block (Integrated DNA Technologies) and introduced into our humanized second-generation 41BBζ CAR lentivirus backbone by Gibson cloning for overexpression in human T cells. For NHP work, CAR33 was transferred to a simian immunodeficiency virus (SIV) overexpression vector by Gibson cloning.
CAR33 T-cell manufacturing
CAR33-expressing, self-inactivating lentivirus was produced by the Fred Hutch Preclinical Vector Production Core using a third-generation packaging system pseudotyped with Cocal envelope. Vectors were titered by the transduction of HT1080 cells and measurement of lentiviral integration by quantitative PCR (qPCR). CAR T cells were manufactured ex vivo using an optimized protocol as described. Briefly, peripheral blood mononuclear cells (PBMCs) were collected from each NHP by leukapheresis. CD4+ and CD8+ T cells were isolated from cryopreserved PBMCs by bead-based selection (STEMCELL Technologies), followed by stimulation with artificial antigen-presenting cells (generous gift from James Riley). Three days later, the CD4+ and CD8+ T cells were transduced separately with CAR33 lentiviral vector at a multiplicity of infection of 10.21,22 Transduction was carried out by coincubating lentivirus with cells (4 × 106 cells per mL) and protamine sulfate (4 mg/mL) and rotating the transduction reaction for 4 hours at 37°C. The next day, the transduced CD4+ and CD8+ T cells were pooled at a 1:1 ratio and seeded into G-REX100 flasks (Wilson Wolf, Saint Paul, MN) for 7 to 8 days of expansion.
Chromium release assay
Chromium release assay performed as previously described.23 Briefly, target cells were incubated for 2 hours with chromium-51, washed, and then exposed to CAR T cells for 4 hours at indicated effector-to-target ratio or to media alone (spontaneous release) or detergent containing triton X-100 (maximum release). Supernatant was then harvested, and γ-scintillation quantified by TopCount (Perkin Elmer). Cytotoxicity = (γ count CAR T-cell – γ count spontaneous)/(γ count max – γ count spontaneous) × 100%.
xCELLigence assay
Target CD33+ and CD33− LLCMK2 cells were plated in 96-well plates. Cells were allowed to adhere for 24 hours, then normalized to set a baseline cell index. CAR33 or untransduced T cells were then added at indicated ratios and cell index was measured for 72 hours.
SIV qPCR
To quantify the distribution of CAR33 after infusion, genomic DNA was isolated from peripheral blood (PB) and BM of treated NHPs and used as a template for qPCR. TaqMan universal PCR master mix (Applied Biosystems) and TaqMan copy number reference assay RNaseP (Applied Biosystems) were used in combination with an SIV-specific primer pair and probe (supplemental Table 4). The final gene marking value per sample was then corrected for the amount of DNA loaded per reaction and the dilution factor.
Determination of antigen density
Antigen density was evaluated by flow cytometry using the Quantibrite phycoerythrin (PE) fluorescence quantitation kit (BD Biosciences). Briefly, cells were stained with our HSPC panel and a PE-conjugated anti-CD33 antibody. Cells were analyzed on a Symphony S2 cytometer (BD Biosciences) alongside standardized PE quantitative beads. CD33 antigen density was determined by referencing the PE mean fluorescent intensity against a standard curve generated from the bead standards.
Human PBMCs and HSPCs were acquired from the center for cellular excellence in hematology at the Fred Hutchinson Cancer Center. All acquired human samples were pathogen tested and deidentified in accordance with their institutional review board.
Results
Efficient knockdown of CD33 using CRISPR/Cas9 and ABE/Cas9
Two male NHPs were engrafted with autologous HSCs modified to abrogate anti-CD33 binding. In animal A17039 the V-set domain encoded by exon 2 was excised using CRISPR/Cas9 with 2 guide RNAs flanking exon 2, as reported previously.8 In the second animal, A18038, ABE was used to edit the splice acceptor site before exon 2, similarly aiming to omit exon 2 from the mature CD33 protein. We confirmed editing by ddPCR and next-generation sequencing (NGS) for A17039 and A18038, respectively. Lastly, we investigated whether the CRISPR/Cas9- and ABE/Cas9-mediated mutations to CD33 affected the differentiation potential of HSPCs. To this end, we using FACS to isolate CD34 subsets enriched for HSCs (CD34+CD90+), erythromyeloid progenitors (EMPs; CD34+CD90−), and lymphomyeloid progenitors (LMPs, CD34+ CD45RA+), which were subsequently plated in CFC assays (Figure 1A). Edited cells attained erythroid, granulocytic, and monocytic lineages, and were not impaired in their total colony-forming capacity. We then harvested single colonies from these CFC assays and assessed the successful editing of cells by PCR amplification (A17039) or NGS (A18038; Figure 1B). We found that CRISPR/Cas9 led to primarily monoallelic editing, whereas ABE/Cas9 primarily resulted in biallelic editing. Validation of the safety and efficacy of our CD33-editing strategies in vitro cleared the way for the autologous transplantation of ex vivo gene-modified cells.
HSPCs are amenable to both CRISPR/Cas9 and ABE/Cas9-mediated CD33 editing, engrafting long-term and maintaining multilineage potential. (A) CFC assay of transplanted HSPCs. Sorted on (1) CD34+, (2) CD34+CD90+, (3) CD34+CD90−, and (4) CD34+CD45RA+. (B) Editing of single colonies collected from CFC assay. (C) Schematic of BM transplant and tracking of hematologic recovery. (D) Representative table of transplant information. (E) Hematologic recovery of A17039 and A18038 as measured by time to recovery of platelets and neutrophils. Vertical dashed line represents day of transplant. Horizontal lines represent threshold of recovery (solid line) and healthy blood ranges (dashed lines). (F) Recovery of total WBC compartment with CD33-edited cells (top) and the granulocyte compartment with CD33-modified cells (bottom) measured by ddPCR/NGS or FACS, respectively. BFU-E, burst forming unit erythroid; G, granulocyte; GEMM, granulocyte, erythrocyte, macrophage, megakaryocyte; GM, granulocyte, macrophage; M, macrophage; QC, quality control; WBC, white blood cell; WT, wild type.
HSPCs are amenable to both CRISPR/Cas9 and ABE/Cas9-mediated CD33 editing, engrafting long-term and maintaining multilineage potential. (A) CFC assay of transplanted HSPCs. Sorted on (1) CD34+, (2) CD34+CD90+, (3) CD34+CD90−, and (4) CD34+CD45RA+. (B) Editing of single colonies collected from CFC assay. (C) Schematic of BM transplant and tracking of hematologic recovery. (D) Representative table of transplant information. (E) Hematologic recovery of A17039 and A18038 as measured by time to recovery of platelets and neutrophils. Vertical dashed line represents day of transplant. Horizontal lines represent threshold of recovery (solid line) and healthy blood ranges (dashed lines). (F) Recovery of total WBC compartment with CD33-edited cells (top) and the granulocyte compartment with CD33-modified cells (bottom) measured by ddPCR/NGS or FACS, respectively. BFU-E, burst forming unit erythroid; G, granulocyte; GEMM, granulocyte, erythrocyte, macrophage, megakaryocyte; GM, granulocyte, macrophage; M, macrophage; QC, quality control; WBC, white blood cell; WT, wild type.
CRISPR/Cas9-edited cells engraft and persist long-term in the PB and BM
Edited HSPCs cells were transplanted after TBI (1020 cGy) to assess engraftment potential and investigate their capacity for multilineage differentiation (Figure 1C). A total of 3.5 × 106/kg and 5 × 106/kg HSPCs cells were infused into A17039 and A18038, respectively (Figure 1D). In comparison to historic controls, both CRISPR/Cas9- and ABE/Cas9-mediated editing did not adversely affect hematopoietic reconstitution as measured by the time to recovery for neutrophils (A17039, day 10; A18038, day 16) and platelets (A17039, day 38; A18038, day 40; Figure 1E).15,24,25 Total white blood cell counts were within normal range in both animals 1 to 2 months after transplant and remained stable (supplemental Figure 1). Detailed analysis of PB composition by flow cytometry demonstrated recovery in all assessed immune compartments in both animals including granulocytes, monocytes, natural killer cells, T cells, and B cells (supplemental Figures 2 and 3). Granulocytes were the first population to recover after transplantation, with T cells and B cells returning to pretransplantation levels by 2 months after transplant.
Longitudinal retention of editing was assessed both by flow cytometric analysis of CD33 expression on the surface of granulocytes as well as by genomic analysis using ddPCR in A17039 and NGS in A18038. Surface CD33 expression revealed an initial spike in the CD33null population of the granulocyte compartment followed by long-term stabilization (75%-40% in A17039; 100%-20% in A18038; Figure 1F). This decrease in CD33 positivity was corroborated by our editing readouts, which initially showed editing rates of 60% and 80% in A17039 and A18038, respectively, which stabilized at 20% in both animals long-term (Figure 1F).
Ex vivo validation of a CD33-directed CAR
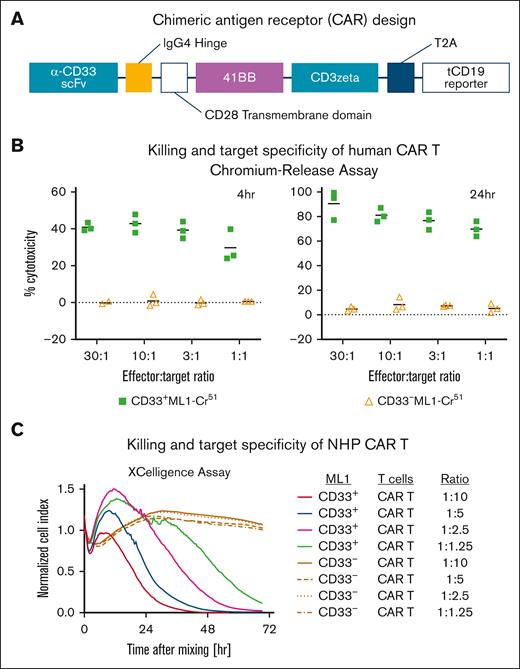
Next, we set out to develop a novel, human/NHP cross-reactive CD33-directed CAR construct. We grafted a human/NHP cross-reactive CD33-specific scFv onto a second-generation CAR backbone with a 41BB costimulatory domain and truncated CD19 (tCD19) reporter connected by a cleavable T2A sequence (Figure 2A). Delivery of our CAR construct was accomplished with lentivirus in human T cells and an SIV-derived vector for RM T cells. Successful transduction was assessed by flow cytometric analysis of tCD19 expression.
In vitro validation of a human/NHP cross-reactive CD33-directed CAR construct. (A) Representative schematic of CAR33 design with bicistronic tCD19 reporter. (B) CRA results of human CAR33 T cells cultured with CD33+ or CD33− ML-1 cells. (C) xCELLigence killing assay of macaque CAR33 T cells cultured with CD33+/− LLCMK2 cells. IgG4, immunoglobulin G4.
In vitro validation of a human/NHP cross-reactive CD33-directed CAR construct. (A) Representative schematic of CAR33 design with bicistronic tCD19 reporter. (B) CRA results of human CAR33 T cells cultured with CD33+ or CD33− ML-1 cells. (C) xCELLigence killing assay of macaque CAR33 T cells cultured with CD33+/− LLCMK2 cells. IgG4, immunoglobulin G4.
To validate CAR activity and CD33 specificity, we used 2 cytotoxicity assays: chromium release (CRA) for primary human T cells and xCELLigence for RM T cells. Assessment by CRA demonstrated up to 40% cytotoxicity by 4 hours of coculture with a CD33+ ML-1 cell line and 100% cytotoxicity after 24 hours of coculture (Figure 2B). Importantly, there was little cytotoxicity (<10%) observed in conditions in which CAR33 T cells were cocultured with CD33− ML-1 cells, confirming the specificity of killing. To validate our CAR construct in RM T cells, we used the xCELLigence impedance-based killing assay. We similarly observed a dose- and time-dependent killing of CD33+ but not CD33− RM–derived LLCMK2 cell line (Figure 2C). Demonstration of the efficacy and specificity of our CAR construct in vitro paved the way for application in vivo.
CAR33 T cells efficiently activate and expand in vivo
Autologous T cells were harvested from A17039 and A18038, expanded ex vivo, and transduced with an SIV-packaged CAR construct. CAR identity was confirmed by flow cytometric assessment of tCD19 and transduction rates were confirmed to be >10%. Both A17039 and A18038 were infused with 52.2 × 106/kg (14.4% CAR+) T cells and 71.1 × 106/kg (33.7% CAR+) T cells, 730 and 609 days after transplantation, respectively (Figure 3A-B). After infusion, rapid contraction of the granulocyte compartment was observed. This coincided with expansion of T cells in both animals, reaching peaks of 17,560 and 1564 cells/ per μL at 8 and 7 days after transplantation, respectively (Figure 3D). Matching the differential expansion of T cells, A17039 experienced a markedly increased cytokine response concomitantly to T-cell expansion, with interleukin-6 (IL-6) and C-reactive protein (CRP) spiking to 200 pg/mL and 15 mg/mL 3 and 6 days after infusion, respectively (Figure 3C). This elevated cytokine response occurred despite treatment with anakinra, dexamethasone, and tocilizumab. T-cell proliferation and cytokine response paired with constitutional symptoms, with A17039 experiencing elevated temperature as well as bouts of lethargy. A18038 displayed a different response to CAR33 treatment. Pairing with a less pronounced expansion of T cells in the PB, we observed no increase of IL-6 and a slightly reduced peak in CRP (10 mg/mL). Correspondingly, A18038 did not experience the elevated temperatures or lethargy experienced by A17039. Neither animal displayed any other signs of CAR-related toxicities such as nephrotoxicity (blood urea nitrogen and creatinine) or hepatotoxicity (alanine aminotransferase and aspartate aminotransferase).
CAR33 expands in vivo and demonstrated divergent toxicity profiles in 2 RMs. (A) Representative schematic of CAR33 production and treatment of 2 RMs. (B) Values of total T cells and CAR33 T cells infused per kilogram bodyweight for both macaques and days after transplant (DPT) to CAR treatment. (C) Post-CAR cytokine response in both treated animals as measured by IL-6 and CRP. Colored X’s indicate treatment with toci (green), dexa (blue), and anakinra (orange). (D) Distribution of PB immune lineages before and immediately after CAR33 treatment. Population proportions determined by flow cytometry and matched to absolute cell counts determined by complete blood counts. Dex, dexamethasone; DPT, days post-transplant; NK cell, natural killer cell; toci, tocilizumab.
CAR33 expands in vivo and demonstrated divergent toxicity profiles in 2 RMs. (A) Representative schematic of CAR33 production and treatment of 2 RMs. (B) Values of total T cells and CAR33 T cells infused per kilogram bodyweight for both macaques and days after transplant (DPT) to CAR treatment. (C) Post-CAR cytokine response in both treated animals as measured by IL-6 and CRP. Colored X’s indicate treatment with toci (green), dexa (blue), and anakinra (orange). (D) Distribution of PB immune lineages before and immediately after CAR33 treatment. Population proportions determined by flow cytometry and matched to absolute cell counts determined by complete blood counts. Dex, dexamethasone; DPT, days post-transplant; NK cell, natural killer cell; toci, tocilizumab.
Next, we investigated the expansion of CAR T cells in the PB and BM to determine whether CARs could act on CD33+ HSPCs. To accomplish this, we used qPCR to assess the expansion of SIV amplicons as a proxy for expansion of transduced T cells. We observed peak viral long terminal repeat signal 7 days after CAR infusion, coinciding with the peak in T cells assessed by flow cytometry (supplemental Figure 4). To determine distribution of CAR33 after infusion, qPCR was performed on BM draws on days 30 and 8 for A17039 and A18038, respectively. SIV amplicons were observed in the BM draw from A18038 at day 8, coinciding with peak SIV in the PB (supplemental Figure 5). However, BM from A17039 was collected at day 30, and no SIV was observed above baseline.
CAR33 selectively spares CD33 edited progeny
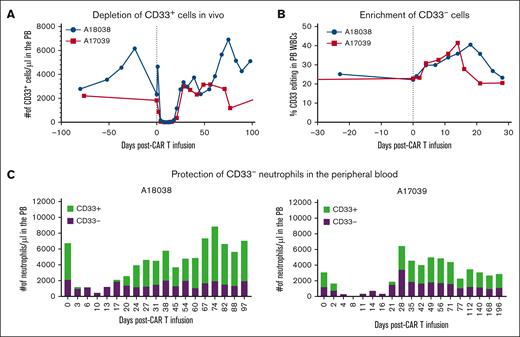
With the activity of CAR33 verified in vivo, we investigated whether CD33 editing conferred resistance to CAR killing. Assessment of CD33 populations within the granulocytic compartment revealed a heterogenous mix of CD33+ and CD33null granulocytes after HSCT, consistent with a heterogeneously edited HSC compartment. After CAR33 infusion, both animals showed a total elimination of CD33+ events in the PB (Figure 4A). Examining the impact of CAR33 treatment on the granulocytic compartment, we observed ablation of CD33+ granulocytes with sparing of CD33null granulocytes (Figure 4C). We further postulated that if the CD33null compartment within the granulocytes is primarily composed of CD33-edited cells, selection for that population would coincide with enrichment of gene editing. We assessed this using ddPCR and NGS in A17039 and A18038, respectively, and observed that enrichment of editing from ∼20% in both animals to up to 40% editing (Figure 4B). Approximately 21 days after CAR infusion, CD33+ granulocytes reemerged and returned to pretreatment levels in both animals. This tracked with return to pre-CAR levels of editing in the PB, indicating that selection was exclusive to CD33+ populations in the PB.
CAR33 treatment led to complete ablation of CD33+ events while sparing CD33null cells. (A) Depletion of CD33+ granulocytes in the PB in both treatment animals. Scaled proportion of CD33+ events by flow cytometry with absolute cell counts by complete blood counts (CBC). (B) Measured CD33 editing in bulk WBCs before and immediately after CAR33 treatment. Editing measured by ddPCR (A17039) and MiSeq (A18038). (C) Proportions of CD33+ and CD33− granulocytes after CAR33 treatment. Granulocyte identity and CD33 positivity determined by flow cytometry and scaled using absolute cell counts from CBC.
CAR33 treatment led to complete ablation of CD33+ events while sparing CD33null cells. (A) Depletion of CD33+ granulocytes in the PB in both treatment animals. Scaled proportion of CD33+ events by flow cytometry with absolute cell counts by complete blood counts (CBC). (B) Measured CD33 editing in bulk WBCs before and immediately after CAR33 treatment. Editing measured by ddPCR (A17039) and MiSeq (A18038). (C) Proportions of CD33+ and CD33− granulocytes after CAR33 treatment. Granulocyte identity and CD33 positivity determined by flow cytometry and scaled using absolute cell counts from CBC.
CD33 expression is limited on RM HSCs
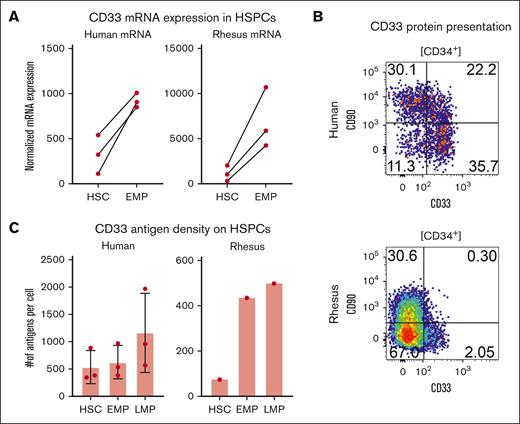
We sought to determine the reason for the transience of CD33 editing enrichment. To this end, we performed bulk RNA sequencing of our previously defined HSPC subsets. We found that there was a broadly conserved cd33 expression pattern between human and RM samples, with HSCs expressing fewer transcripts of cd33 than EMPs (Figure 5A). In addition to this, we appreciated a log-fold difference in cd33 expression in both HSCs and EMPs between RMs and humans. Next, we sought to determine whether this disparity in transcripts corelated to a disparity in CD33 protein expression (Figure 5B-C). Although there was a consistent relationship across species in which LMPs had the highest density of CD33 and HSCs had the lowest, there were species-specific differences in CD33 density, with all RM HSPC subsets (HSCs, LMPs, and EMPs) displaying significantly lower CD33 than human samples.
CD33 is differentially expressed in RM but not human HSPCs. (A) mRNA expression from bulk sequencing of HSCs and EMPs from 3 human donors (left) and 3 macaque donors (right) normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) transcripts. (B) Representative flow plot of the canonical HSC marker CD90 against CD33 in human (top) and rhesus (bottom). (C) Calculated antigen per cell on HSCs, EMPs, and LMPs in human (left) and rhesus (right). mRNA, messenger RNA.
CD33 is differentially expressed in RM but not human HSPCs. (A) mRNA expression from bulk sequencing of HSCs and EMPs from 3 human donors (left) and 3 macaque donors (right) normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) transcripts. (B) Representative flow plot of the canonical HSC marker CD90 against CD33 in human (top) and rhesus (bottom). (C) Calculated antigen per cell on HSCs, EMPs, and LMPs in human (left) and rhesus (right). mRNA, messenger RNA.
Discussion
Here, we demonstrate that CRISPR/Cas9 as well as ABE/Cas9 can both efficiently ablate CD33 expression in the hematopoietic system of NHPs. Most importantly, short-term neutrophil recovery and long-term multilineage differentiation of CD33null HSCs were not affected. To enrich for CD33null cells, we further validated a human/NHP cross-reactive CAR33 construct and demonstrated selective depletion of CD33-expressing cells ex vivo as well as in vivo. Our results provide evidence in the preclinical NHP model that CAR33 T cells selectively spare CD33null granulocytes, whereas enrichment of long-term persisting HSCs was not accomplished because of a rhesus-specific lack of CD33 on HSCs.
Ongoing clinical trials have demonstrated the safety and efficacy of CD33 modification in the allogeneic transplant setting26,27; however, we investigated the ability to use this strategy to enrich for gene-edited cells using CAR T cells recognizing and specifically killing remaining CD33-expressing cells. We sought to compare the editing efficiency and engraftment potential of autologous HSCs edited with CRISPR/Cas9 or ABE/Cas9. We found that editing was comparable, with rates of 60% and 80% in 1 animal treated with CRISPR/Cas9 and ABE/Cas9, respectively. These findings were corroborated by flow cytometry, which showed a distinct edited population of CD33null HSPCs after electroporation. Both methodologies of CD33 editing did not have any apparent impact on the colony-forming potential or multilineage differentiation capacity of cells. Interestingly, we did see a difference in the composition of edited HSPC products in vitro, with CRISPR/Cas9 yielding a primarily monoallelic edited product, whereas most ABE/Cas9 edited HSPCs were biallelically edited. This observation could be explained, in part, by double-strand DNA breaks induced by CRISPR/Cas9, leading to increased cell stress and genomic rearrangements in biallelically edited cells as compared with those with 1 modified allele.28,29 Meanwhile, ABE/Cas9 is not reliant on double-strand DNA breaks, thus potentially affording higher rates of biallelic editing efficiency while sparing cellular fitness. Importantly, although our aim was to specifically omit the second exon of CD33, given other groups’ prior reports on the ability to wholesale ablate CD33, it is possible that our infusion product was instead a mix of CD33-edited and CD33-null cells.
Despite this observed editing difference in the infusion products, the 2 animals treated with the CRISPR/Cas9 and ABE/Cas9-edited products showed virtually identical recovery kinetics. Both animals achieved neutrophil recovery (10 days) and platelet recovery (40 days) as well as complete, multilineage reconstitution of the PB and BM by 3 to 4 months after transplant. Looking specifically at CD33, we observed initial discrepancies between our 2 animals, with the ABE/Cas9 edited animal demonstrating a higher level of editing (60% to 80%) and more prominent CD33null granulocyte compartment (75% to 100%). However, 1-year after transplant, CD33 protein and editing levels stabilized to 40% to 20% CD33null and 20% edited for A17039 and A18038, respectively. This long-term decline from peak editing levels immediately after transplant is consistent with prior studies from our group, and is owed to our use of a nonlethal TBI regimen and longitudinal equilibration between remaining endogenous HSCs and transplanted, gene-modified cells.30 Separate investigation of individual lineages within the PB and BM showed that neither CD33 editing modality led toward skewing to any lineage. These findings corroborate what we, and several other groups, have observed in human and NHP hematopoiesis when editing with CRISPR/Cas9.7,11,12 Additionally, our study directly assesses the impact of CD33 modification by ABE/Cas9, demonstrating the safety and efficacy of this approach as compared with the established CRISPR/Cas9 editing strategy.
With A17039 and A18038 engrafted with CD33null populations, we turned our attention toward the validation of human/NHP cross-reactive CAR33 constructs. We converted a known cross-reactive antibody into an scFv and grafted it to a second-generation CAR backbone. We validated the activity of this CAR33 construct in primary human T cells by CRA and RM T cells by xCELLigence. Both assays demonstrated dose-dependent and time-dependent killing kinetics, with little to no killing of CD33null targets. With a cross-reactive CAR33 construct in hand, we turned to apply it to our CD33-edited NHPs.
Treatment of A17039 and A18038 with autologous CAR33 T cells led to divergent responses, mirroring the variable responses observed in patients. A17039 experienced profound T-cell expansion and cytokine (IL-6 and CRP) response reminiscent of clinical cytokine release syndrome (CRS). In addition to apparent CRS by cell and cytokine readouts, A17039 displayed elevated temperatures and bouts of lethargy also in line with clinical CRS, despite treatment with dexamethasone, anakinra, and tocilizumab. A18038, in contrast, experienced a mild reaction to CAR33 infusion, punctuated by far less T-cell expansion, undetectable elevation of IL-6, and no constitutional manifestations of CRS. A18038 did experience elevation of CRP, however not to the extent of A17039. This differential T-cell expansion was reflected, too, in qPCR of PB, which demonstrated significantly higher CAR marking at 7 days after infusion in A17039 than in A18038, aligning with the observed spike in T cells by complete blood count (CBC). The divergent responses observed in these 2 RMs emulates the variable responses seen with CAR T-cell products in the clinic.31 Previous work by several groups32-35 has implicated several independent biological factors in the development of CRS including marrow tumor burden, lymphodepletion, and high CAR T-cell dose. Yet in our NHP model lacking any tumor burden, controlled for pretreatment CD33 expression, and lacking lymphodepletion before CAR T-cell administration, we observed divergent CAR T-cell expansions, highlighting the inherently stochastic nature of CAR T cells. In concordance with previously published results from clinical trials, the symptoms of CRS were associated with the higher CAR T-cell expansion in A17039 as well as elevated levels of IL-6 relative to A18038.36 However, the mechanisms underlying why 1 animal experienced profound T-cell expansion whereas the other did not, remains unclear.
Interestingly, despite the difference in CAR proliferation kinetics, the impact on the CD33+ populations in the PB were nearly identical. Granulocytes in both animals contracted as T cells expanded, reaching nadir 8 days and 11 days, respectively, after infusion. This depletion of CD33+ events in the PB persisted for 3 weeks after infusion before returning to pretreatment levels. These kinetics of CAR expansion and target killing are in line with previous NHP studies using CD20-directed CARs published by our group and others.22,37 Closer examination of the granulocytic compartment revealed that this depletion was specifically of CD33+ granulocytes, preferentially sparing the CD33null. To assess the impact of depletion of the CD33+ compartment on editing levels in the PB, we performed ddPCR and NGS on A17039 and A18038, respectively, and observed a transient increase in editing from 20% before CAR treatment to 40% and 30%, respectively. This increase in editing waned in line with the reemergence of the CD33+ compartment.
Given the expression of CD33 on HSCs in humans, we had anticipated a more long-lived increase in editing. This led us to comprehensively assess the expression of CD33 on HSCs, EMPs, and LMPs in human, RM, and PM. The Eaves laboratory had previously reported CD33 as a critical predictor of long-term engrafting potential of HSCs in human cord blood HSCs.13,38 However, we found that there are species-specific differences in the expression of cd33 messenger RNA, with RM HSPCs expressing significantly fewer transcripts than human HSPCs. We secondarily evaluated this relationship with a flow cytometry–based antigen density assay, which revealed that RM HSCs were uniquely CD33 dim, whereas human HSCs expressed moderate levels.
Our findings provide compelling preliminary data demonstrating the safety of CD33-modified auto-HSCT by both CRISPR/Cas9 and ABE/Cas9 editing, however we caveat this finding with the limited capacity of CAR33 T cells to select against CD33+ HSCs in our NHP model. This is most likely because of the relative antigen dearth of CD33 on HSCs compared with granulocytes, in line with the well-established reliance of CAR T cells on high antigen density for efficient killing.39 Another alternative explanation to our lack of long-lived enrichment could be because of limited persistence of CAR T cells. We observed peak CARs ∼7 days after infusion, with CARs becoming undetectable by qPCR or FACS 14 days after treatment, potentially driven by our lack of lymphodepletion before CAR administration or rejection of CAR T cells because of the use of a human CAR33 construct via humoral immune mechanisms. However, given the extent of T-cell expansion observed in A17039, we anticipate that any enrichment would have been observed despite this relatively limited expansion window. Despite the inability to attain long-term enrichment with CAR33 T cells, we cannot rule out the potential for this strategy in humans or using other CD33-directed therapies such as antibody-drug conjugates.40 Similarly, several publications have recently expanded the repertoire of antigens amenable to epitope editing to include CD45, CD117, and CD123, and CD135.9,10 These antigens are significantly more densely expressed than CD33 on HSCs, potentially making them viable strategies for CAR-based editing enrichment.41,42
In addition to our findings of the ability for CAR33 T cells to target CD33+ cells in NHPs and in vitro, they also stand in strong support of the safety and utility of CD33 editing in the context of AML treatment in both the autologous and allogeneic setting. Notably, we maintained CD33null granulocytes throughout CAR33 expansion in both animals, shielding a critical component of the innate immune system, typically a necessary casualty of CD33-directed chemotherapies.43,44 Similarly, the inherent protection of HSCs from CAR33 because of limited antigen density rules out the possibility of marrow failure because of CAR33 treatment in patients with AML, further bolstering arguments of the safety of this approach.
In conclusion, we have demonstrated the safety and efficacy of the previously established pairing of CD33-modified HSCT with CAR33 in our autologous, large animal NHP model. We have further demonstrated the limitations of CAR33 killing on RM HSCs, potentially because of insufficient antigen density. Finally, we demonstrated in vitro the potential of CAR33 T cells to eliminate CD33+ human HSPCs, confirming the viability of this strategy as a therapeutic for AML and potential method to enrich for gene-edited cells after transplant.
Acknowledgments
All nonhuman primate work was performed at the Washington National Primate Research Center (WaNPRC) by Veronica Nelson, Erica Wilson, Sarah Herrin, Chad Littlewood, and Christopher Wessel. Helen Crawford assisted with the formatting and preparation of this manuscript.
Research reported in this publication was supported by the National Institutes of Health (NIH)/National Cancer Institute (P30 CA015704 [specifically, Fred Hutch Genomics and Bioinformatics Core Facility, RRID:SCR_022606; and Fred Hutchinson Center (Fred Hutch) Flow Cytometry Core Facility, RRID:SCR_022613], R01 CA266556, and R21 CA245594); the NIH National Heart, Lung, and Blood Institute (awards R01 HL136135 and R01 HL151765); and by the Cell Manipulation Tools Core-Vector Production of Fred Hutch, which is funded by the National Institute of Diabetes and Digestive Diseases Cooperative Center of Excellence in Hematology (grant U54 DK106829). Work done at the WaNPRC was supported by the NIH (award P51OD010425).
Authorship
Contribution: S.R., O.H., R.B.W., and H.-P.K. designed the study; N.E.P., E.F., M.J.L., G.K., H.Z., K.R.J., S.R., O.H., S.F., C.J.T., G.S.L., J.T., Z.B., and K.S. performed longitudinal follow-up, CD34 enrichments, digital droplet polymerase chain reaction, chimeric antigen receptor (CAR) T-cell manufacturing, ex vivo CAR assays, and fluorescence-assisted cell sorting; N.E.P. and S.R. generated the figures.; H.-P.K. funded the study; N.E.P., S.R., R.B.W., and H.-P.K. wrote the manuscript; and all authors reviewed and edited the final manuscript.
Conflict-of-interest disclosure: S.R. is consultant to 48 Bio Inc and Ensoma Inc. C.J.T. has received research funding from Juno Therapeutics/Bristol Myers Squibb (BMS), Nektar Therapeutics, and 10x Genomics; serves on scientific advisory boards for Caribou Biosciences, T-CURX, Myeloid Therapeutics, ArsenalBio, Cargo Therapeutics, Celgene/BMS Cell Therapy, Differentia Bio, eGlint, and Advesya; is a data and safety monitoring board member for Kyverna; holds ad hoc advisory roles/consulting (last 12 months) for Prescient Therapeutics, Century Therapeutics, IGM Biosciences, AbbVie, Boxer Capital, Novartis, and Merck; holds stock options in Eureka Therapeutics, Caribou Biosciences, Myeloid Therapeutics, ArsenalBio, Cargo Therapeutics, and eGlint; has had a speaker engagement for Pfizer and Novartis within the last 12 months; and is an inventor on patents related to CAR T-cell therapy. R.B.W. received laboratory research grants and/or clinical trial support from Aptevo, Celgene/BMS, ImmunoGen, Janssen, Jazz, Kite, Kura, Pfizer, and Vor Biopharma; and has been a consultant to Wugen. H.-P.K. is or was a consultant to, and has or had ownership interests in, Rocket Pharmaceuticals, Homology Medicines, Vor Biopharma, and Ensoma Inc; and was a consultant to CSL Behring and Magenta Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Hans-Peter Kiem, Translational Science and Therapeutics Division, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, 1100 Fairview Ave N, D1-100, Seattle, WA 98109-1024; email: hkiem@fredhutch.org.
References
Author notes
N.E.P. and S.R. contributed equally as joint first authors to this study.
We have endeavored to make our “Materials and methods” as robust as possible to ensure replication. All data sets are available upon request from the corresponding author, Hans-Peter Kiem (hkiem@fredhutch.org). This manuscript does not include any large data sets (such as single-cell RNA sequencing) that would warrant uploading to a public database.
The full-text version of this article contains a data supplement.