Key Points
PRMT5 is overexpressed in T, NK, monocytes, and dendritic cells in a murine model of secondary HLH.
PRMT5 inhibition reduces clinical signs of HLH, including physical traits, cellular populations, and inflammatory cytokines in this model.
Visual Abstract
Hemophagocytic lymphohistiocytosis (HLH) is a rare but aggressive and potentially lethal hyperinflammatory syndrome characterized by pathologic immune activation and excessive production of proinflammatory cytokines leading to tissue damage and multisystem organ failure. There is an urgent need for the discovery of novel targets and development of therapeutic strategies to treat this rare but deadly syndrome. Protein arginine methyltransferase 5 (PRMT5) mediates T-cell–based inflammatory responses, making it a potential actionable target for the treatment of HLH. Using CPG-1826 and anti–interleukin-10R (IL-10R) antibody, we induced murine secondary HLH in vivo with a marked expansion of splenic myeloid cell subsets and concurrent reduction of T- and natural killer (NK)–cell populations. PRMT5 expression was significantly upregulated in splenic T and NK lymphocytes, monocytes, and dendritic cells in mice with HLH (P < .05). Treatment with PRT382, a potent and selective PRMT5 inhibitor, significantly reduced physical signs of secondary HLH, including splenomegaly, hepatomegaly, and anemia (P < .0001 in each case), when compared with untreated mice. Inflammatory cytokines known to drive hyperinflammation in HLH, including interferon-γ and IL-6 were reduced to healthy levels with PRT382 treatment (P > .999 for both). PRT382 treatment also reduced the expansion of myeloid cell populations (P < .0001) in mice with HLH, compared with untreated mice, while restoring T- and NK-cell numbers (P < .001 for both). These results identify PRMT5 as a promising target for the management of secondary HLH and justify further exploration in this and other models of hyperinflammation.
Introduction
Hemophagocytic lymphohistiocytosis (HLH) is a rare but potentially fatal hyperinflammatory syndrome characterized by dysregulated immune activation, excessive production of proinflammatory cytokines, and hyperactivation of T cells and macrophages leading to tissue damage and multisystem organ failure.1 Of the many disorders classified within uncontrolled hyperinflammation, there are 2 major subtypes of HLH: primary HLH caused by mutations leading to ineffective cellular cytotoxicity and secondary HLH, which includes macrophage activation syndrome (MAS).1 Secondary HLH affects adult patients and can be triggered by a variety of infections, malignancies, or immunomodulatory cancer therapies, such as chimeric antigen receptor T-cell therapy.2-5 Although primary HLH is a rare disease primarily affecting pediatric patients (∼100 cases per year in the United States), secondary HLH affecting adults is becoming a more common finding now that diagnostic guidelines have been developed, with 25 to 50 new cases per year diagnosed at The Ohio State University Medical Center.6
An initial diagnosis of HLH is frequently established at the time when patients present with a severe illness requiring critical care, where treatment goals include both control of the triggering condition and depletion of the immune cells contributing to the overactive inflammation.7 Elimination of activated cells with the chemotherapeutic agent, etoposide, was the first line of treatment to demonstrate success in this disease in the 1980s.8 Etoposide is currently used along with other agents including corticosteroids; IV immunoglobulin; antithymocyte globulin; alemtuzumab, a monoclonal antibody (mAb) specific for CD52 to deplete lymphocytes and monocytes; and a modified doxorubicin/etoposide/methylprednisolone regimen that has been used with mixed results.2,7
Recent efforts have focused on immunomodulatory approaches that target the pathophysiologic mechanisms driving inflammation in HLH. The only immunomodulatory therapy approved by the Food and Drug Administration to treat pediatric primary HLH is emapalumab, an anti–interferon gamma (IFN-γ) mAb.9 Emapalumab is used to bridge children with primary HLH to allogeneic stem cell transplantation; however, its utility in adult patients with secondary HLH is limited. Although allogeneic stem cell transplantation can be curative for pediatric patients, transplant-associated mortality in adults is exceptionally high.10 Other efforts include the off-label but first line use of interleukin-1 (IL-1) inhibitors11-13 and the inhibition of Janus kinases (JAK1/2) with ruxolitinib, which is currently undergoing clinical investigation.14 Current approaches to treatment of HLH in adults offer transient improvement at the expense of toxicity, leading to vulnerability to infection, metabolic dysregulation, and mortality rate approaching 50%.15,16 The poor prognosis facing adults with HLH emphasizes the unmet need for discovery of novel targets and therapeutic strategies directed at pathologic mechanisms.
Protein arginine methyltransferase 5 (PRMT5) catalyzes the addition of symmetric dimethyl groups on arginine (R) residues of histone (H3R8me2s; H4R3me2s) and nonhistone proteins.17 The symmetric dimethylation of arginine activity of PRMT5 regulates many cellular functions, including alternative splicing, epigenetic control of gene expression, and survival/growth and death pathways orchestrated by p53,18,19 NF-κB/p65,20,21 B-cell lymphoma 6,22 protein kinase B,23,24 and E2 promoter binding factor 1.25-27 PRMT5 expression is tightly regulated in normal tissues but high and sustained in inflammatory conditions, such as cancer and autoimmunity.28 PRMT5 mediates inflammatory T-cell responses in experimental autoimmune encephalomyelitis29 and acute graft-versus-host disease models,29,30 affects NF-κB–driven transcription of inflammation-related target genes, such as tumor necrosis factor-α and IL-1α,20,31,32 and promotes autoimmune-related inflammation.29,33-36 Mechanistic studies have revealed that PRMT5 promotes Th17 CD4 T-cell differentiation through the induction of cholesterol biosynthetic pathway enzymes, leading to activation of retinoic acid-related orphan receptor γt,37 and promoting T-cell and monocyte activation through initiation of types I and III IFN signaling.38 Of particular interest is the role PRMT5 plays in mediating the activation of T cells29 and macrophages.39
Given the accumulating data on PRMT5 involvement in driving inflammation in multiple disease contexts, we hypothesized that PRMT5 activity would be relevant to hyperinflammatory loops that are encountered in secondary HLH and MAS. Using an inducible murine model of secondary HLH,40-42 we found expression of PRMT5 in multiple cell types, including T, NK, and myeloid cells. PRMT5 expression was significantly upregulated with HLH induction in dendritic and NK cells with a trend in CD8 T cells. This led us to test PRMT5 inhibition in this model. Treatment with low-dose PRT328, a highly selective PRMT5 inhibitor,43 reduced the clinical hallmarks of HLH, completely blocked IFN-γ cytokine production, and blocked expansion of inflammatory myeloid cell subsets in the murine model of secondary HLH. These results provide promising insight identifying PRMT5 as an actionable target for the management of HLH that requires further investigation in humans.
Materials and methods
Murine secondary HLH model
All mouse studies were conducted at The Ohio State University with ethics approval (2009A0094-R4; principal investigator: R.A.B.). Secondary HLH was induced in 6- to 12-week-old female C57BL/6-J (The Jackson Laboratory) mice through intraperitoneal injections of 50 μg of CpG-1826 (Integrated DNA Technologies) and 200 μg of anti–IL-10R antibody (Bio X Cell, clone 1B1.3A), on days 0, 2, 4, and 7.41,42 Mice were treated through oral gavage, as previously described,44,45 with 0.25, 1, or 2 mg/kg PRT382 (Prelude Therapeutics) starting on day 0 for the dose-finding experiment. Alternatively, PRT382 1 mg/kg daily treatment was started on day 0 (9 days of treatment), 2 (7 days of treatment), or 4 (5 days of treatment) as indicated. Dosing was continued to day 8. Mice were treated with ruxolitinib 90 mg/kg twice daily on days 4 to 8, by oral gavage, as previously described.46 Mice were euthanized on day 9 due to a portion of mice consistently reaching early removal criteria before day 10, the previously described end point for this model.41,42 Disease progression was monitored by daily weights and signs of disease, such as weight loss, ruffled fur, inactivity, and splenomegaly. Upon euthanasia, blood from each mouse was collected and a necropsy was performed. Whole mouse, liver, and spleen were weighed. Blood and spleens were processed for secondary analysis.
Flow cytometry
For all assays except IFN-γ intracellular flow cytometry, spleens were processed into single-cell suspension in fixative solution (phosphate-buffered saline containing 1% polyformaldehyde [Fisher Scientific, 28908]). Cells were incubated in a fixative solution at room temperature for 40 to 60 minutes and then stained for flow cytometric analysis. For IFN-γ measurements, fresh splenocytes were processed into a single-cell suspension and were incubated in 5 mL of RPMI 1640 media with 10% fetal bovine serum (Thermo Fisher, 10438-026 Lot 2318524RP), 2 mM L-glutamine (Life Technologies, 25030081), 50 U/mL penicillin, 50 μg/mL streptomycin (Life Technologies, 15140122), 50 μM β-mercaptoethanol (Life Technologies, 21985023), 1% insulin-transferrin-selenium (Life Technologies, 41400045), and 1× Brefeldin A (Invitrogen, 50-112-9757). Cells were incubated in this media for 6 hours. Cells were then fixed using phosphate-buffered saline containing 1% paraformaldehyde (Fisher Scientific, 28908).
Fixed splenocytes were washed in phosphate-buffered saline and pelleted at 300g for 10 minutes at 4°C. One million cells per mouse were used for flow cytometry staining using the panel specified in supplemental Table 1. Mouse samples were treated with TruStain FcX PLUS anti-mouse CD16/32 (BioLegend, clone S17011E), stained with live/dead and cell surface markers simultaneously for 30 minutes on ice protected from light, washed once, and pelleted at 450g for 10 minutes at room temperature. Intracellular staining for PRMT5 or IFN-γ was performed after permeabilization with the permeabilization buffer (10×) kit (Invitrogen, 00-8333) according to the manufacturer’s instructions. Samples were fixed with 100 μL of intracellular fixation buffer (Invitrogen, 00-8222) overnight at 4°C protected from light. Samples were permeabilized and stained with anti-PRMT5 antibody (Abcam, ab109451) on ice and then washed and pelleted. A 5-μL donkey serum (Jackson ImmunoResearch Laboratories, NC9624464) was added, and the samples were stained with phycoerythrin anti-rabbit antibody (BioLegend, 406421). Finally, samples were fixed again in 1.6% paraformaldehyde (Thermo Scientific, 28908), stored at 4°C, and ran on the BD LSR Fortessa Cell Analyzer (BD Biosciences, 649225). Data were analyzed in Kaluza and Cytobank. Fluorescence minus one reactions were used to determine appropriate gating for each marker. Gating schemas can be found in supplemental Figure 1. Dimensional reduction and clustering were performed in Cytobank. viSNE generated tSNE plots were used to visualize heatmaps of marks and PRMT5 expression.
Plasma analysis
Plasma from each mouse sacrificed on day 9 was collected by centrifuging blood from EDTA-containing tubes (BD 365974) at 12 000g to 15 000g for 2 to 10 minutes at room temperature, then collecting the serum layer, and freezing it at −80°C. Plasma samples were analyzed using the IFN-γ mouse enzyme-linked immunosorbent assay (ELISA) kit (Thermo Fisher Scientific, KMC4021 or 88-7314), IL-18 mouse ELISA kit (Thermo Fisher Scientific, 88-50618), IL-1ß mouse ELISA kit (BioLegend, 432615), IL-6 mouse ELISA kit (BioLegend, 431315), and ferritin mouse ELISA kit (Abcam, AB157713), according to the manufacturer’s instructions. Plates were read at an absorbance of 450 nm, and the background was subtracted (570 nm) as directed. The concentration of cytokines was quantified using the standard curve.
Complete blood count analysis
Complete blood count analysis was performed on whole blood from EDTA-containing tubes using the Element HT5 Auto Hematology analyzer (Heska), according to the manufacturer’s instructions.
Statistical analysis
Data were analyzed with 1-way or 2-way analysis of variances (ANOVA) with Tukey correct for multiple comparisons, Kruskal-Wallis test with Dunn test for multiple comparisons, or unpaired Student t tests as applicable. Error bars show standard deviation of the data (∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001).
Results
PRMT5 is upregulated in lymphoid and myeloid subsets in the murine model of secondary HLH
To study HLH pathogenesis in a preclinical setting, we used a mouse model of secondary HLH, where lethal hyperinflammation is triggered by introduction of CpG-1826 oligonucleotide, a potent stimulator of toll-like receptor 9 (TLR9), along with an anti–IL-10 receptor mAb.40,42 The resulting insult leads to the development of a cytokine storm, hematologic dysfunction, myeloid cell expansion and activation, and multisystem organ failure similar to that observed in patients with untreated secondary HLH.40,42 At day 9, splenocyte immune populations were analyzed by flow cytometry (gating strategies shown in supplemental Figure 1). We observed a significant expansion of total CD11b+ myeloid cells, including a significant expansion of monocytes and neutrophils in the spleens of mice with induced secondary HLH (P < .05 in all comparisons but dendritic cells; Figure 1A). Percentages of CD4 and CD8 T cells and NK cells were concurrently significantly reduced relative to healthy mice (P < .001, P <.05, and P <.01, respectively) (Figure 1A). Monocytes in HLH-induced mice significantly upregulated CD44 (P < .0001) consistent with an activated state (Figure 1B).
PRMT5 expression is present and increases in myeloid and lymphoid mononuclear cell subsets after HLH induction. (A) Percentage of major immune cell types of all singlets from splenocytes of healthy C57bl/6 mice or those with induced HLH. (B) Mean fluorescent intensity (MFI) of CD44 staining in monocytes (Ly6C+Ly6G–) of healthy C57bl/6 mice or those with induced HLH. (C) t-SNE grouping of CD4, CD8, and NK1.1+ splenocytes from a healthy or HLH-induced C57bl/6 mouse. Heat maps reveal expression of key marks that allow for subset identification and PRMT5 expression. (D) MFI of PRMT5 in CD4 T cells, CD8 T cells, and NK cells of healthy C57bl/6 mice or those with induced HLH. Error bars show standard deviation. Multiple unpaired t tests with the Holm-Šídák method were used in panels A and D, whereas a Student t test was used in panel B to determine statistical significance. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. DCs, dendritic cells; ns, no significance; TX, treatment.
PRMT5 expression is present and increases in myeloid and lymphoid mononuclear cell subsets after HLH induction. (A) Percentage of major immune cell types of all singlets from splenocytes of healthy C57bl/6 mice or those with induced HLH. (B) Mean fluorescent intensity (MFI) of CD44 staining in monocytes (Ly6C+Ly6G–) of healthy C57bl/6 mice or those with induced HLH. (C) t-SNE grouping of CD4, CD8, and NK1.1+ splenocytes from a healthy or HLH-induced C57bl/6 mouse. Heat maps reveal expression of key marks that allow for subset identification and PRMT5 expression. (D) MFI of PRMT5 in CD4 T cells, CD8 T cells, and NK cells of healthy C57bl/6 mice or those with induced HLH. Error bars show standard deviation. Multiple unpaired t tests with the Holm-Šídák method were used in panels A and D, whereas a Student t test was used in panel B to determine statistical significance. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. DCs, dendritic cells; ns, no significance; TX, treatment.
PRMT5 inhibition reduces pathology in a murine model of secondary HLH. (A) Schema of mouse studies. HLH was induced with CPG-1826 and anti–IL-10R on days 0, 2, 4, and 7. PRT382 was dosed daily starting on day 0 (9 days of treatment [TX]), 2 (7 days of TX), or 4 (5 days of TX). Ruxolitinib (Rux) was given twice daily from day 4 to 8. Mice were sacrificed on day 9 and samples were processed for additional analysis. (B) Average weight of each cohort as a percentage change from day 0 in healthy mice, induced HLH with no TX, induced HLH with Rux TX, or HLH with PRT382 TX started on day 0, 2, or 4. Average spleen (C) and liver (D) mass as a percentage of mouse body weight in each cohort determined on necropsy. (E) RBC count, (F) hemoglobin concentration, and (G) lymphocyte count from complete blood counts on blood on day 9. Error bars show standard deviation. A Kruskal-Wallis test with Dunn test for multiple comparisons was used in panel B and 1-way analysis of variances (ANOVAs) with Tukey multiple comparisons were used in panels C-G to determine significance. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. RBC, red blood cell.
PRMT5 inhibition reduces pathology in a murine model of secondary HLH. (A) Schema of mouse studies. HLH was induced with CPG-1826 and anti–IL-10R on days 0, 2, 4, and 7. PRT382 was dosed daily starting on day 0 (9 days of treatment [TX]), 2 (7 days of TX), or 4 (5 days of TX). Ruxolitinib (Rux) was given twice daily from day 4 to 8. Mice were sacrificed on day 9 and samples were processed for additional analysis. (B) Average weight of each cohort as a percentage change from day 0 in healthy mice, induced HLH with no TX, induced HLH with Rux TX, or HLH with PRT382 TX started on day 0, 2, or 4. Average spleen (C) and liver (D) mass as a percentage of mouse body weight in each cohort determined on necropsy. (E) RBC count, (F) hemoglobin concentration, and (G) lymphocyte count from complete blood counts on blood on day 9. Error bars show standard deviation. A Kruskal-Wallis test with Dunn test for multiple comparisons was used in panel B and 1-way analysis of variances (ANOVAs) with Tukey multiple comparisons were used in panels C-G to determine significance. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. RBC, red blood cell.
To determine what role PRMT5 may play in the pathogenesis of HLH, we analyzed the expression levels of PRMT5 in splenocytes from C57bl/6 mice with induced secondary HLH by intracellular flow cytometry and compared those levels to what was observed in healthy mouse splenocytes. Dimensional reduction and clustering by viSNE showed that NK and CD8 T cells shift toward higher PRMT5 expression after HLH induction (Figure 1C). We observed that the level of PRMT5 expression, measured by the median fluorescence intensity, was significantly increased in total lymphocytic splenocytes and NK, CD4, and CD8 T cells (P < .05) (Figure 1D; supplemental Figure 2). Representative viSNE illustrations of the myeloid splenocyte populations revealed remarkable expansion in monocyte (CD11b+Ly6C+Ly6G–) and neutrophil (CD11b+Ly6CloLy6G+) populations in mice with secondary HLH (Figure 2A). viSNE clustering and median fluorescence intensity analysis also revealed an increase in the intensity of PRMT5 staining in monocytes and dendritic cells (CD11c+I-A/I-E+) after induction of HLH (P < .05; Figure 2). PRMT5 expression in monocytes increased and contributed to a significant total increase in all PRMT5+ myeloid cells (Figure 2; supplemental Figure 2). The increase of PRMT5 expression in monocytes was of particular interest as this cell type has been found to be a key marker in HLH diagnosis and classification.47,48 These results suggested that PRMT5 may play an active role in the pathophysiology of inflammation in the murine model of secondary HLH.
PRMT5 expression in monocytes and dendritic cells increases with the induction of HLH. (A) tSNE plot of dendritic cells (D), monocytes (M), and neutrophils (N), with heat maps showing identifying marks. D, CD11c+I-A/I-E+; M, Ly6C+Ly6G–; N, Ly6CloLy6G+. (B) Heat map of PRMT5 expression with the same clustering as shown in panel A. (C) Median fluorescent intensity (MFI) of total myeloid cells (D + M + N), monocytes, neutrophils, and dendritic cells positive for PRMT5 expression. Error bars show standard deviation. Multiple unpaired t tests with the Holm-Šídák method were used to determine statistical significance in panel C. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
PRMT5 expression in monocytes and dendritic cells increases with the induction of HLH. (A) tSNE plot of dendritic cells (D), monocytes (M), and neutrophils (N), with heat maps showing identifying marks. D, CD11c+I-A/I-E+; M, Ly6C+Ly6G–; N, Ly6CloLy6G+. (B) Heat map of PRMT5 expression with the same clustering as shown in panel A. (C) Median fluorescent intensity (MFI) of total myeloid cells (D + M + N), monocytes, neutrophils, and dendritic cells positive for PRMT5 expression. Error bars show standard deviation. Multiple unpaired t tests with the Holm-Šídák method were used to determine statistical significance in panel C. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
PRMT5 inhibition reduces physical hallmarks of secondary HLH
To target PRMT5 in the model of secondary HLH, we used PRT382, a selective and potent inhibitor of PRMT5.43 We used the previously reported JAK1/2 inhibitor, ruxolitinib, as a positive control for disease response, because it is known to have therapeutic activity in this model, reducing weight loss and improving organ condition.40 We tested daily low-dose PRT382 treatment ranging from 0.25 to 2 mg/kg starting on day 0 and found that the 1 mg/kg dose induced optimal reduction of splenomegaly and hepatomegaly without exacerbating mouse weight loss (supplemental Figure 3). Weight loss is a known toxicity of PRMT5 inhibition in murine models,49 but additional weight loss compared with the untreated mice was not observed at this low dose. On the basis of published kinetics, PRT382 was not predicted to accumulate over the 9-day dosing period when dosed at 1 mg/kg daily.43 This dose was used for all following experiments (Figure 3A). To determine whether PRMT5 inhibition could treat established inflammation of secondary HLH, initiation of PRT382 1 mg/kg treatment on days 0, 2, and 4 was compared. This resulted in mice receiving treatment for 9, 7, or 5 days, respectively. Secondary HLH induction caused significant weight loss in untreated mice; PRT382 started on day 4 and ruxolitinib treatment was able to attenuate some of this weight loss (Figure 3B). HLH induced the development of hepatomegaly and splenomegaly in untreated mice (Figure 3C-D), as previously described.40 Although induction of HLH more than doubled the mass of the spleen as a percentage of total body weight compared with healthy mice, PRT382 treatment started on day 0 or 2 reduced disease-associated splenomegaly back to baseline (Figure 3C). Ruxolitinib treatment was also able to reduce splenomegaly, as previously reported,40 though average spleen weights remained elevated in mice with HLH treated with ruxolitinib compared with control mice (Figure 3C). Induction of secondary HLH was also associated with hepatomegaly, whereas treatment with PRT382 initiated on day 0 or 2 significantly reduced liver weights compared with those in the untreated mice (Figure 3D). PRT382 started on day 4 or ruxolitinib treatment was not able to significantly reduce this biomarker (Figure 3D).
Additional relevant biomarkers of HLH-related inflammation were investigated. Secondary HLH/MAS is characterized by anemia, thrombocytopenia, lymphocytopenia, and elevated levels of the acute-phase reactant ferritin.40-42 We found that red blood cell counts and hemoglobin were significantly reduced with HLH induction and significantly improved with PRT382 administration in all treated cohorts (Figure 3E-F). The number of circulating lymphocytes was increased in all the PRT382 treatment groups (Figure 3G). Platelets reduced in mice with untreated HLH but trended toward recovery with PRT382 treatment, although the differences seen in the PRT382-treated cohorts compared with untreated mice were not significant which may reflect drug-related toxicity rather than lack of activity (supplemental Figure 4A). Plasma levels of the acute-phase reactant ferritin increased in mice with untreated secondary HLH, improved with ruxolitinib treatment, and revealed significant reduction in mice treated with PRT382 that was started on day 4, whereas earlier initiation of PRT382 on day 0 trended toward improvement and approached significance (P = .055; supplemental Figure 4B).
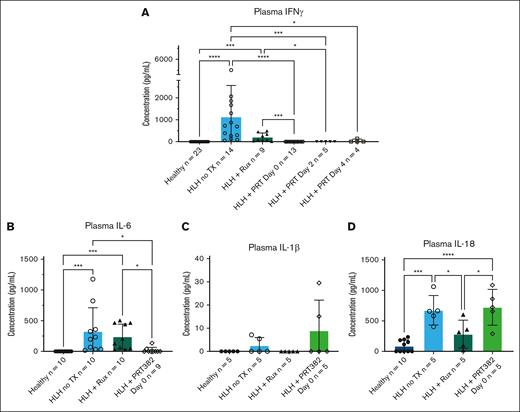
The murine model of secondary HLH induced by CpG-1826 is characterized by elevation of inflammatory cytokines in response to repeated TLR9 stimulation, including IFN-γ, IL-6, IL-1β, and IL-18.41,42 We observed that plasma levels of IFN-γ were highly elevated with secondary HLH induction, and PRT382 treatment initiated on days 0, 2, and 4 reduced these levels back to baseline observed in healthy mice (Figure 4A). IL-6 was also significantly increased with HLH induction and effectively reduced with PRT382 administration on day 0 (Figure 4B). Comparing untreated HLH mice with healthy controls and those treated with PRT382 started on day 0, we found an increase in plasma levels of IL-1β with HLH that was not ameliorated with PRT382 administration (not statistically significant) (Figure 4C). Similarly, IL-18 increased significantly in mice with untreated HLH and remained elevated with PRT382 administration started on day 0 (Figure 4D). Together, our data reveal that PRMT5 inhibition significantly reduced the hallmarks of HLH, including splenomegaly, hepatomegaly, anemia, thrombocytopenia, hyperferritinemia, and plasma hypercytokinemia, including elevation of IFN-γ and IL-6, but not the HLH-associated elevation in IL-1β or IL-18, in the murine model of secondary HLH.
Plasma levels of inflammatory cytokines are reduced with PRMT5 inhibition. (A) Concentration of IFN-γ in plasma as determined by ELISA in healthy mice, induced HLH with no TX, induced HLH with Rux TX, or HLH with PRT382 TX started on day 0 (9 days of treatment), 2 (7 days of treatment), or 4 (5 days of TX). Plasma levels of (B) IL-6, (C) IL-1β, and (D) IL-18 as determined by ELISA in healthy mice, induced HLH with no TX, induced HLH with Rux TX, or with PRT382 TX started on day 0. Error bars show standard deviation. One-way ANOVAs with Tukey multiple comparisons were used to determine significance. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
Plasma levels of inflammatory cytokines are reduced with PRMT5 inhibition. (A) Concentration of IFN-γ in plasma as determined by ELISA in healthy mice, induced HLH with no TX, induced HLH with Rux TX, or HLH with PRT382 TX started on day 0 (9 days of treatment), 2 (7 days of treatment), or 4 (5 days of TX). Plasma levels of (B) IL-6, (C) IL-1β, and (D) IL-18 as determined by ELISA in healthy mice, induced HLH with no TX, induced HLH with Rux TX, or with PRT382 TX started on day 0. Error bars show standard deviation. One-way ANOVAs with Tukey multiple comparisons were used to determine significance. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
PRT382 counteracts the expansion of inflammatory myeloid cell populations and inflammatory cytokine production in the murine model of secondary HLH
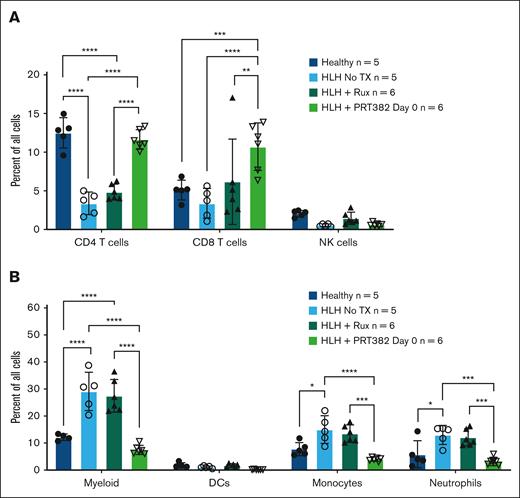
To explore the effects of PRMT5 inhibition on specific cell types in the HLH model, flow cytometry was performed on splenocytes at day 9 after treatment with ruxolitinib, PRT382 starting on day 0, or vehicle control, and compared with healthy C57bl/6 mice. Among lymphoid cell populations, HLH induced a reduction in the relative number of CD4 and CD8 T cells (Figure 5A). PRMT5 inhibition restored CD4 T-cell proportions to baseline levels (Figure 5A). Interestingly, CD8 T cells recovered beyond baseline proportions with PRT382 treatment (Figure 5A). Among the myeloid immune cell populations, HLH induced significant expansion of total CD11b+ myeloid cells, monocytes, and neutrophils (Figure 5B). In mice with HLH that were treated with PRT382, both monocyte and neutrophil populations were brought back to levels observed in healthy controls (Figure 5B).
Proportion of cells returned to normal levels with PRT382 TX. The percentage of all singlets that (A) CD4 T cells, CD8 T cells, and NK cells or (B) myeloid cells (D + M + N), dendritic cells, monocytes, and neutrophils make up in healthy mice, those with induced HLH, with Rux TX, or with PRT382 TX started on day 0. Error bars show standard deviation. A 2-way ANOVA with Sidak's multiple comparisons was used to determine significance. Note: no comparison between HLH no TX and HLH Rux was found to be significant. One outlier was removed from the healthy cohort for myeloid cells. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. DCs, dendritic cells.
Proportion of cells returned to normal levels with PRT382 TX. The percentage of all singlets that (A) CD4 T cells, CD8 T cells, and NK cells or (B) myeloid cells (D + M + N), dendritic cells, monocytes, and neutrophils make up in healthy mice, those with induced HLH, with Rux TX, or with PRT382 TX started on day 0. Error bars show standard deviation. A 2-way ANOVA with Sidak's multiple comparisons was used to determine significance. Note: no comparison between HLH no TX and HLH Rux was found to be significant. One outlier was removed from the healthy cohort for myeloid cells. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. DCs, dendritic cells.
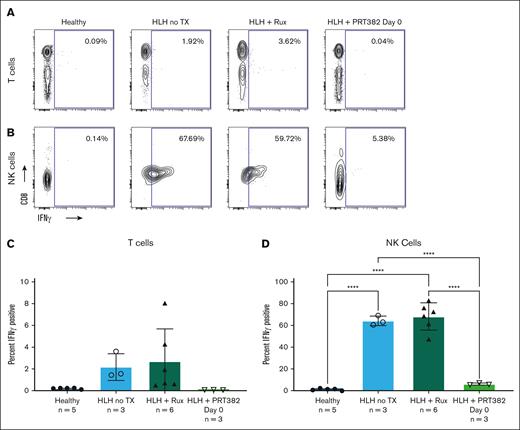
Using intracellular flow cytometry, we evaluated the levels of intracellular IFN-γ in T and NK cells. Intracellular IFN-γ was detected in unstimulated T and NK cells, and levels were increased in mice with untreated HLH, compared with healthy controls (Figure 6). The increase of intracellular IFN-γ in T cells was modest, possibly reflecting escape of intracellular cytokine during mouse organ processing. Ruxolitinib treatment failed to reduce the cytokine content in T or NK cells, but PRT382 brought IFN-γ levels back to those observed in healthy control mice (Figure 6). These results suggest that PRMT5 inhibition seems to be therapeutically active in the murine model of secondary HLH by reducing IFN-γ–driven inflammation.
PRMT5 inhibition reduces intracellular IFN-γ production in splenocytes derived from mice with HLH. Contours of intracellular IFN-γ in T (A) and NK (B) cells in healthy control mice, mice with HLH and no TX, mice with HLH treated with Rux, and mice with HLH treated with PRT382 started on day 0. Percentage of IFN-γ plus T cells (C) and NK (D) cells in each cohort. Error bars reveal standard deviation. A 1-way ANOVA with Tukey multiple comparisons was used to determine significance. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
PRMT5 inhibition reduces intracellular IFN-γ production in splenocytes derived from mice with HLH. Contours of intracellular IFN-γ in T (A) and NK (B) cells in healthy control mice, mice with HLH and no TX, mice with HLH treated with Rux, and mice with HLH treated with PRT382 started on day 0. Percentage of IFN-γ plus T cells (C) and NK (D) cells in each cohort. Error bars reveal standard deviation. A 1-way ANOVA with Tukey multiple comparisons was used to determine significance. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
Discussion
HLH is a life-threatening hyperinflammatory syndrome characterized by dysregulated immune activation resulting in tissue damage and end-organ failure. Current treatments for HLH are limited and include immunosuppressive therapy and chemotherapy, but these options are associated with significant toxicity and variable activity in adults.2 Here, we present evidence illustrating the proinflammatory role PRMT5 plays in the pathogenesis of secondary HLH with expression in key cell types in both the lymphoid and myeloid lineages. We demonstrated that inhibition of PRMT5 with a selective small molecule inhibitor PRT382 has promise as a therapeutic strategy in a preclinical of secondary HLH, resulting in the improvement of clinical features of the disease, including reduction of splenomegaly, hepatomegaly, anemia, thrombocytopenia, lymphocytopenia, hyperferritinemia, and inflammatory myeloid cell expansion. Diminished weight loss with PRT382 dosed at 0.25 mg/kg started on day 0 and 1 mg/kg started on day 4 resulted in improvement of the secondary HLH phenotype. We also saw reduction of plasma IFN-γ and IL-6 levels back to those of healthy control mice. The reduction in splenomegaly and plasma IFN-γ from PRT382 treatment was found even after treatment was delayed until 4 days after induction of inflammation. The differential response with weight loss and pathology suggests that PRMT5 inhibition is counteracting inflammation of secondary HLH in both a dose- and timing-dependent manner.
Our studies in mice with secondary HLH revealed that activated T- and NK-cell subsets express PRMT5. This observation is similar to that found in inflammatory diseases, such as experimental autoimmune encephalomyelitis and acute graft-versus-host disease, where PRMT5 has also been found to be a promising therapeutic target.29,30 Treatment of mice with secondary HLH with PRT382 leads to reversal of CD4 and CD8 T-cell lymphopenia and reduction in inflammatory myeloid, dendritic cells, monocytes, and neutrophil subsets similar to that found in control healthy mice. This was not found with ruxolitinib treatment.
Our results suggest that myeloid cell subsets contributing to hyperinflammation of HLH may be driven in part by PRMT5, an unexpected finding that has not been previously described. We observed an induction of PRMT5 expression in dendritic cells and monocytes of mice with secondary HLH. PRMT5 expression and influence have been well documented in lymphoid cell types, but this enzyme’s role in myeloid cells is poorly understood. PRMT5 has been documented as overexpressed and supportive in acute myeloid leukemia,50 including being hypothesized to be a target regulating the spliceosome in myeloid dysplastic syndrome and chronic myelomonocytic leukemia.49 As inflammatory monocytes have been implicated in multiple forms of autoimmunity and inflammation,51 our observations raise questions about the role of PRMT5 in myeloid cells in these disease conditions.
The use of the CpG-1826/IL-10R murine model of secondary HLH results in some limitation to our work. This model relies on stimulation of TLR9 and IL-10 receptor blockade to induce inflammation. Development of inflammation in the secondary HLH model relies on contributions from the T, B, and NK lymphocytes and myeloid cells.42 Therefore, this model does not fully recapitulate the pathologic mechanisms of primary HLH, which relies on ineffective T-cell–based cytotoxic granule production and the consequent inability of the adaptive cellular immune response to eliminate an antigen, leading to continuous stimulation. We are currently conducting studies in the murine model of primary HLH, in which inflammation is induced by infecting perforin-deficient mice with the lymphocytic choriomeningitis virus (LCMV).52 Although the perforin-knockout LCMV model relies on viral infection to develop inflammation of HLH, many features such as ineffective cytotoxic granule production, development of hypercytokinemia, and proinflammatory roles of T cells and myeloid cells are recapitulated, making this model a useful tool for the study of HLH pathology and investigation of targeted therapies.52 Our preliminary data gathered from the primary HLH model demonstrated similar increased expression of PRMT5 in immune cells on induction of disease and similar significant downregulation of plasma IFN-γ levels in mice treated with a PRMT5 inhibitor, suggesting a proinflammatory role of PRMT5 in the primary HLH pathophysiology.53
The use of only female mice also presents a limitation due to sex bias. This experimental limitation was necessary due to behavioral issues with male mice, resulting in injuries and wound healing that was a confounder for a model of autoinflammation. The sex bias is tempered by our communication with the authors of the article on effects of ruxolitinib in murine models of HLH40 who observed similar degrees of inflammation in male and female mice in the murine model of secondary HLH. Based on our group’s experience with PRMT5 studies and the use of PRMT5 inhibitors in murine models of Epstein-Barr virus (EBV)–driven lymphoma54 and mantle cell lymphoma (R.A. Baiocchi and L. Alinari, unpublished data, 2023), we have not observed any significant differences between male and female mice.
The mechanism of PRMT5 involvement in the inflammatory loops driving HLH is currently unknown; however, PRMT5 inhibition resulted in a drastic reduction of the levels of circulating IFN-γ levels and in IFN-γ production by T- and NK-cell populations in the murine model of secondary HLH. These results suggest that PRMT5 contributes significantly to the production of inflammatory mediators driving immune dysregulation in HLH. IFN-γ has been described as an important, but not exclusive, mediator of inflammation in the murine model of secondary HLH.41 We observed that PRMT5 inhibition leads to reduction in plasma IFN-γ and correction of anemia, which is driven by excess IFN-γ in the secondary HLH model.55 We also observed therapeutic effects of PRMT5 inhibition on IFN-γ–independent inflammatory markers, including thrombocytopenia, lymphocytopenia, and hyperferritinemia, suggesting a potent pleiotropic anti-inflammatory impact of PRMT5 inhibition in HLH. Finally, we observed a significant reduction of plasma IL-6 but not IL-1β or IL-18 in treated mice, indicating specific effects of PRMT5 inhibition on myeloid-derived cytokine production that likely did not involve the inflammasome. Additional experiments are underway to determine whether the suppressive effects of PRMT5 inhibition on inflammatory cytokine levels are direct or indirect and what stages of protein production may be affected.
Our findings also suggest that the therapeutic effects of PRMT5 inhibition with PRT382 may be distinct from the therapeutic impact of JAK1/2 inhibition with ruxolitinib in the murine model of secondary HLH. We observed that PRMT5 inhibition, but not JAK1/2 inhibition, reduces IFN-γ production in T and NK cells derived from spleens of mice with HLH. Ferritin was effectively reduced with ruxolitinib treatment but had a variable response with the duration of PRT382 treatment. Plasma cytokine levels also varied by treatment, although ruxolitinib more efficiently reduced IL-1β and IL-18, PRT382 had a greater impact on IFN-γ and IL-6 levels. We observed that PRMT5 is overexpressed in lymphoid and myeloid splenocyte subsets on secondary HLH induction, suggesting that PRMT5 inhibition might be able to additionally target inflammatory populations, if used in combination with ruxolitinib, which has been used previously to target inflammatory T-cell populations. CpG-1826, through TLR9 and NFκB signaling, is known to stimulate transcription of proinflammatory cytokines in myeloid cells.56 JAK1/2 and STAT molecules mediate signal transduction from the IFN-γ receptor to stimulate transcription of IFN-stimulated genes, such as IFN regulatory factor 1.57 PRMT5 in known to positively regulate NF-κB signaling and STAT phosphorylation in multiple cell types and may thus contribute to myeloid cell activation in secondary HLH.20,30,58 Our work suggests a rationale for exploring the combination of ruxolitinib and PRT382.
Recent work from Joly et al demonstrated that in a murine model of primary HLH, targeting single cytokines, including IL-1β, IL-6, or IL-18, had little to no therapeutic effect, but targeting multiple cytokine pathways through the combination of ruxolitinib and anti–IFN-γ had a synergistic effect on the disease phenotype.59 Although anti–IL-1β did not have an impact in the primary HLH model as reported by Joly et al, IL-1β inhibitors have efficacy in patients with secondary HLH/MAS,13 and our data suggest that PRMT5 inhibition reduces inflammation of secondary HLH in an IL-1β–independent manner; this provides a rationale for testing the therapeutic effects of combined IL-1β blockage with PRMT5 inhibition. Experiments to explore combined PRMT5 inhibition and cytokine targeting agents in murine models of primary and secondary HLH are in progress.
Our preliminary data provide supporting rationale for further exploration of PRMT5 as a promising target for treatment of HLH and for investigation of potential synergy between PRMT5 inhibition and JAK1/2 inhibition in murine models of HLH. These studies are currently underway using the murine models of primary and secondary HLH.
PRMT5 is an attractive target for secondary HLH also due to the study of PRMT5 inhibition for several triggers of this disease. For example, EBV is a known trigger of secondary HLH.3 Our previous work has demonstrated that PRMT5 is essential for EBV-driven B-cell transformation and that inhibition of this methyltransferase induces multiple immunogenic viral gene products that are prominent targets for adaptive T-cell immunity.21,54 Another promising target would be malignancy-associated HLH, as PRMT5 inhibition is currently being tested in numerous hematological malignancies, including non-Hodgkin lymphomas, acute myeloid leukemia, and myelodysplastic syndromes.49 PRMT5 is directly implicated in the pathogenesis of these diseases and may offer an opportunity to direct therapeutic impact on both malignant and hyperinflammatory conditions that are operable in malignancy-triggered HLH.
The results from the study have raised many questions about the role of PRMT5 in myeloid biology and pathology. As PRMT5 can modify many biologic processes ranging from transcriptional splicing, and signaling pathways to epigenetic control of gene expression, we will continue to explore PRMT5-related biology in each of the immune cell types we have identified to be relevant in this preclinical model. To complement this work, we are incorporating a second murine model of HLH which models primary HLH pathology through the knockout of PRF1 and infection with LCMV.52 We are also currently evaluating the expression profile of primary lymphoid and myeloid compartments in humans diagnosed with secondary HLH.
Acknowledgments
The authors thank Reena Shakya and University Laboratory Animal Resources staff for their help with the animal studies conducted at The Ohio State University. The authors thank Kim Nichols and Sabrin Albeituni for their generous help in setting up the secondary HLH murine model used in this study, and Rich Thompson for his consultation on statistical analyses. Prelude Therapeutics supported this study materially with the acquisition of PRT382.
This study was supported by funding from the Histiocytosis Association (P. Shindiapina), National Institutes of Health, National Cancer Institute grant 5P01CA214274-03 (R.A.B.), the Leukemia and Lymphoma Society (longitudinal functional genomics in mantle cell lymphoma therapy and drug resistance) (R.A.B.), the American Cancer Society (RSG RSG-22-053-01-IBCD [P.R.]), and The Ohio State University Comprehensive Cancer Center (CCSG P30 CA016058). F.B.-B. was supported by The Ohio State University Presidential Fellowship and Pelotonia Graduate Fellowship during this work. The authors acknowledge the support of the Histiocytosis Association for funding this research.
Authorship
Contribution: P. Shindiapina, R.A.B., and M.B.J. conceptualized and designed the study; P. Shindiapina, R.A.B., M.B.J., A.D.S., I.E.-A., P.R., N.B., K.V., and P. Scherle developed the methodology; F.B.-B., R.S., C.M., P.M., E. Brooks, A.W., I.H., S.L., A.S., A.Y., E.H.A., E. Baiocchi, S.F., A.M., I.E.-A., S.L.S., K.W., T.C., J.H.-M., S.S., H.L.K., J.W., S.K., K.S., C.-J.C., and P. Shindiapina acquired the data; F.B.-B., R.S., C.M., P.M., E. Brooks, E. Baiocchi, A.M., X.Z., I.E.-A., S.S., C.W., S.K., K.S., and P. Shindiapina analyzed and interpretated data, including statistical analysis, biostatistics, and computational analysis; and F.B.-B., R.S., E. Brooks, C.M., L.A., R.A.B., and P. Shindiapina wrote the manuscript.
Conflict-of-interest disclosure: P. Scherle, N.B., and K.V. report employment with Prelude Therapeutics. R.A.B. received research support for PRT drug product from Prelude Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Robert A. Baiocchi, Division of Hematology, College of Medicine, The Ohio State Univeristy, 400 W 12th Ave, 481A Wiseman Hall CCC, Columbus, OH 43210; email: robert.baiocchi@osumc.edu; and Polina Shindiapina, Division of Hematology, College of Medicine, The Ohio State University, 400 W 12th Ave, 481E Wiseman Hall CCC, Columbus, OH 43210; email: polina.shindiapina@osumc.edu.
References
Author notes
F.B.-B. and R.S. contributed equally to this study.
R.A.B. and P. Shindiapina contributed equally to this study.
Original data are available on request from the corresponding author, P. Shindiapina (polina.shindiapina@osumc.edu).
The full-text version of this article contains a data supplement.



![PRMT5 inhibition reduces pathology in a murine model of secondary HLH. (A) Schema of mouse studies. HLH was induced with CPG-1826 and anti–IL-10R on days 0, 2, 4, and 7. PRT382 was dosed daily starting on day 0 (9 days of treatment [TX]), 2 (7 days of TX), or 4 (5 days of TX). Ruxolitinib (Rux) was given twice daily from day 4 to 8. Mice were sacrificed on day 9 and samples were processed for additional analysis. (B) Average weight of each cohort as a percentage change from day 0 in healthy mice, induced HLH with no TX, induced HLH with Rux TX, or HLH with PRT382 TX started on day 0, 2, or 4. Average spleen (C) and liver (D) mass as a percentage of mouse body weight in each cohort determined on necropsy. (E) RBC count, (F) hemoglobin concentration, and (G) lymphocyte count from complete blood counts on blood on day 9. Error bars show standard deviation. A Kruskal-Wallis test with Dunn test for multiple comparisons was used in panel B and 1-way analysis of variances (ANOVAs) with Tukey multiple comparisons were used in panels C-G to determine significance. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. RBC, red blood cell.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/9/10/10.1182_bloodadvances.2024013651/2/m_blooda_adv-2024-013651-gr3.jpeg?Expires=1767721691&Signature=wvlsmO5As4NdMT7eKlwMAB6EfptK5pQ8G-DLb5lUGZVIBHgs6HD5p949ceNwcwCGjD1uK0ZPwEchC01jD8HaqcgfBkfpUzTkKNaynbV3oZ9k1a7ePHxwC5kmtQt~baG3LPsEijmWvKCKhV2tzmQhpoS1DGaZcZ8w6VS~9KEE9h6RAgCnlm2zQgNA81opTJDCH07QNHbFwjIGucZ7UwZP7BiRaeYQizU-~P0nu8Ju8SRysZW~YnVrdM5cgRFZa6j7Vx1QRBhGL7ZQLFsSkI~Vmyqkxk7OjrruMEuRzy2eYR1cbZ696f--pSdam7l-8uE8NbQlW7gRY8m9W9msPMewnw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)