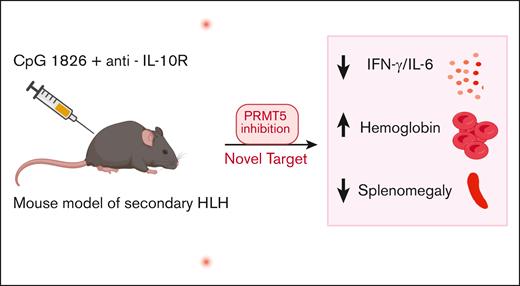
In this issue of Blood Advances, Brown-Burke et al1 investigated the role of Protein Arginine Methyltransferase 5 (PRMT5) inhibition, a protein arginine methyltransferase, as a therapeutic strategy for secondary hemophagocytic lymphohistiocytosis (sHLH). Their study demonstrated that targeting PRMT5 reduced hypercytokinemia, normalized hematological parameters, and improved clinical signs of sHLH in a murine model.1 Among posttranslational modifications, arginine methylation catalyzed by the PRMT family using S-adenosylmethionine plays a key role in several cellular processes.2 Global knockout of PRMT5 is embryonically lethal, and its deletion in hematopoietic cells leads to bone marrow aplasia.3 Within the immune compartment, PRMT5 is essential for T-cell proliferation upon activation, Regulatory T cell (Treg) maintenance, B-cell development, and antibody responses.4,5 Given its role in immune cell activation and growth, authors hypothesized that PRMT5 contributes to hyperinflammation by helping sustain the excessive immune response seen in HLH and inhibition of this pathway could help ameliorate manifestations of HLH.
To test PRMT5 inhibition in HLH, the authors used an established sHLH mouse model with repeated CpG and anti-interleukin-10 (IL-10) receptor antibody injections.1,6 Ruxolitinib, a JAK1/JAK2 inhibitor known to dampen inflammation and improve signs of HLH, was used as a comparison cohort.7 PRT382, a novel and selective PRMT5 inhibitor, improved splenomegaly, whereas ruxolitinib also reduced spleen weight but did not fully restore it to baseline. Beyond organ pathology, PRT382 significantly increased hemoglobin and lymphocyte counts, however, platelet recovery remained nonsignificant. Ferritin levels showed significant improvement when treatment was initiated at HLH induction, with a trend toward improvement when started later. Notably, improvements in signs of HLH following established inflammation suggest PRMT5 may be effective even in established disease, highlighting its clinical relevance. Moreover, PRMT5 inhibition decreased key inflammatory cytokines interferon-γ (IFN-γ) and IL-6 but had no effect on IL-1β or IL-18, whereas ruxolitinib therapy appeared to decrease IFN-γ, IL-6 and IL-18 levels in plasma (see figure). Although the study attempted to compare the effects of ruxolitinib and PRMT5 inhibition, many comparisons were made between ruxolitinib therapy initiated on day 4 vs PRMT5 inhibition initiated on day 0, making a true comparison of efficacy between these 2 therapies difficult.
sHLH is induced in mice through the administration of CpG-1826 and anti-IL-10R antibodies. Treatment with PRT382, a selective PRMT5 inhibitor, mitigates HLH-associated pathology, including splenomegaly, hepatomegaly, and anemia, while normalizing IFN-γ and IL-6 levels.
sHLH is induced in mice through the administration of CpG-1826 and anti-IL-10R antibodies. Treatment with PRT382, a selective PRMT5 inhibitor, mitigates HLH-associated pathology, including splenomegaly, hepatomegaly, and anemia, while normalizing IFN-γ and IL-6 levels.
PRMT5 plays a crucial role in maintaining cell survival and homeostasis in immune cells, but it has broader expression in nervous, muscular, hematopoietic, and reproductive systems.8 Due to its broad expression beyond the immune system, concerns persist regarding toxicity and the tolerability of treatment in the context of severe inflammatory conditions. In fact, early-generation PRMT5 inhibitors led to toxicities such as thrombocytopenia, neutropenia, and anemia.9 In this study, although hemoglobin improved with inhibition, therapy did not significantly improve platelet count, adding to concerns about its hematopoietic toxicity. One known side effect of PRMT5 inhibition is weight loss, and mice treated with PRT382 on day 0 experienced slightly more weight loss than untreated mice.1 However, this effect began to improve within a week. Although the authors demonstrated that PRMT5 inhibition improved myeloid expansion in the sHLH model, the exact mechanism by which PRMT5 inhibition results in improved inflammation in this sHLH is not fully understood. It is not known if the improvement in HLH parameters and myeloid expansion is primarily due to its direct effect on the myeloid or T-cell compartment or both. The lack of impact on IL-18 levels might suggest that the anti-HLH activity of PRMT5 inhibition may not be driven by the inhibition of inflammasome activity. This underscores the importance of studying PRMT5 inhibition in primary HLH, where T-cell and natural killer cell cytotoxic defect results in overwhelming immune activation.
PRMT5 as a potential therapeutic target is being extensively studied in preclinical tumor models. Although no PRMT5 inhibitor has yet received US Food and Drug Administration approval, ongoing phase 1 and 2 clinical trials provide critical insights into its feasibility and toxicity profile in humans.8,9 As a novel approach that is distinct from prior HLH treatments, PRMT5 inhibition warrants further preclinical studies in primary HLH, both alone and in combination with other therapies, to explore its potential in targeting multiple inflammatory pathways. If validated for efficacy and safety, the findings of Brown-Burke et al could significantly broaden the repertoire of targeted therapeutic strategies for mitigating this potentially fatal hyperinflammatory state.
Conflict-of-interest disclosure: S.C. serves on the advisory board of Sobi and Pharming. S.M. declares no competing financial interests.