Key Points
BCR-ABL drugs differentially modulate parasite and thrombin-induced activation of human brain endothelial cells.
Nilotinib reduces vascular pathology in mice with experimental CM.
Visual Abstract
Cerebral malaria (CM), a life-threatening complication of Plasmodium falciparum infection, is characterized by the sequestration of infected erythrocytes in the brain microvasculature. Our study investigated the potential of repurposing tyrosine kinase inhibitors targeting BCR-ABL1 (BCR-ABL drugs), which are also known to be effective against P falciparum blood-stage parasites, for mitigating inflammation and blood-brain barrier breakdown in CM. Our analysis demonstrated differential protective effects of BCR-ABL drugs on primary human brain microvascular endothelial cells exposed to thrombin or a P falciparum-infected erythrocyte challenge. Bosutinib attenuated both thrombin- and parasite-induced barrier alterations, whereas nilotinib was only effective against thrombin, and imatinib protected against neither. Bosutinib’s barrier protective effect was associated with reduced interendothelial gap formation and decreased phosphorylation of the adherens junction protein VE-cadherin and the focal adhesion protein paxillin. In the mouse experimental CM model, nilotinib showed superior efficacy over imatinib and bosutinib. In mice, nilotinib led to fewer brain hemorrhages and less vascular congestion than the antimalaria drug artesunate at similar levels of parasitemia control. Our findings provide important mechanistic insight into the activities of BCR-ABL drugs to suppress endothelial barrier disruptive signaling in vitro and to protect in a mouse model of CM. These findings can inform the repurposing of these drugs in malaria treatment, particularly for managing cerebral complications.
Introduction
Vascular dysfunction, characterized by endothelial activation and vascular leak, is observed with hyperinflammatory infectious diseases.1-3 However, there are limited therapeutic approaches for repairing and stabilizing the endothelial barrier following inflammatory damage. Cerebral malaria (CM) is a severe complication of Plasmodium falciparum infection that kills hundreds of thousands of African children each year4 and can lead to long-term neurologic and cognitive impairments in survivors.5,6 Despite the success of the fast-acting antimalaria drug artesunate in improving patient survival,7 there remains a substantial need to identify adjunctive drug therapies to attenuate hyperinflammatory injury present in severe malaria.
The pathology of CM is characterized by the sequestration of P falciparum–infected erythrocytes (Pf-IEs) in the brain microvasculature,8,9 which leads to blood flow obstruction, ischemia, and brain swelling.10 This sequestration promotes endothelial dysfunction and blood-brain barrier disruption through a combination of parasite factors,11-15 dysregulation of coagulation,16 and cytokine storm.2,17,18 Moreover, CM isolates adhere to endothelial protein C receptor (EPCR),19-24 a crucial anti-inflammatory receptor on blood vessels. Parasite binding disrupts its interaction with protein C and impairs the regulation of thrombin-induced signaling in endothelial cells.25-27 Postmortem brain histology revealed co-localization of thrombin, fibrin deposits, and Pf-IEs in cerebral microvessels,28,29 accompanied by EPCR loss,16 which point to complex interactions between thrombin and secreted parasite inflammatory stimuli that drive endothelial dysfunction in CM.
Host-directed therapies for malaria represent a promising strategy to overcome parasite drug resistance and counteract inflammation in severe malaria.2,30-32 Recent studies have demonstrated that BCR-ABL1 tyrosine kinase inhibitors (hereafter referred to as BCR-ABL drugs), originally developed for chronic myelogenous leukemia treatment,33 effectively inhibit P falciparum growth in erythrocytes.34-36 Moreover, imatinib has shown safety and efficacy in accelerating P falciparum clearance in human adults with uncomplicated malaria.37 Beyond antiparasitic effects, BCR-ABL drugs are being investigated for their potential to address endothelial dysfunction and vascular leak in murine models of sepsis.38-40 Some BCR-ABL drugs have demonstrated the ability to attenuate thrombin-induced permeability in primary human brain microvascular endothelial cells (HBMECs)41 and to exhibit anti-inflammatory activity in the mouse experimental CM (ECM) model.42,43 Taken together, these findings suggest that modulating kinase signaling pathways may offer a dual benefit by enhancing parasitemia control while simultaneously reducing inflammatory damage to blood vessels.
This study evaluated BCR-ABL drugs using primary HBMEC in vitro assays and a mouse ECM model.44,45 Our analysis revealed different barrier protective activities among the BCR-ABL drug family members against thrombin and Pf-IE stimuli. Notably, nilotinib diminished brain vascular pathology in mice, both as a monotherapy and in combination with the antimalarial drug artesunate.
Methods
P falciparum culture and 2-cycle in vitro growth inhibition assay
P falciparum 3D7 and IT4var19 (DC8-EPCR) parasites were cultured and synchronized according to standard procedures.19 Lysates of uninfected red blood cells or purified mature stage P falciparum parasite IEs were prepared by 5 freeze-thaw cycles using dry ice and a 37°C water bath. Lysates from 2 × 106 IEs per cm2 were used for HBMECs monolayer for xCELLigence and western blot assays. Enrichment of mature schizont-IEs was achieved using Low-Density and Magnetic Activated Cell Sorting (LD-MACS) magnetic purification (25 LD columns, Miltenyi Biotec, Germany). For parasite-drug viability assays, we used a 2-cycle P falciparum growth inhibition assay. Ring-stage (12-hour) Pf-IEs were treated with BCR-ABL drugs at different concentrations. To measure parasite viability, parasitemia was evaluated using flow cytometry after 60 hours of drug treatment (72-hour time point) using SYBR Green I DNA stain (Invitrogen), and the data were subsequently analyzed using FlowJo software. To assess parasite morphology after the drug treatments, blood smears were fixed with methanol, stained with 5% Giemsa, and images were acquired using brightfield microscopy at magnification ×100 with a Keyence microscope (Keyence Corporation).
Human brain microvascular endothelial cell culture
Primary HBMECs (Cell Systems; catalog no. ACBRI 376) were cultured on rat tail collagen type I (5 μg/cm2) in Endothelial Cell Growth Medium 2 (EGM-2) Basal Medium (Lonza) supplemented with Endothelial Cell Growth Medium-2MV (EGM-2MV) Microvascular Endothelial Cell Growth Medium SingleQuots (Lonza) and 5% fetal calf serum at 37°C and 5% CO2. HBMECs were obtained at passage 3 and used until passage 9.
xCELLigence barrier assay
HBMECs at 10 000 to 12 000 cells per well were grown to confluency for 3 days in rat collagen type I precoated xCELLigence 96 PET E-plates (Agilent Technologies). For thrombin perturbation, cells were treated with 5 nM thrombin (Sigma-Aldrich) for 7 minutes, followed by the addition of 0.5 μM kinase inhibitors (KIs; Selleck Chemicals; supplemental Table 1) in triplicate wells. For parasite perturbations, KIs (0.5 μM) and lysates prepared from 2 × 106/cm2 mature-stage 3D7 Pf-IEs were added together to the HBMEC monolayer. Alternatively, magnet-purified schizont stage IT4var19-IEs and KIs (0.5 μM) were added at the same time to the HBMEC monolayer. To record change in barrier properties, the cell index was measured every minute for the first 2 hours and, thereafter, every 5 minutes for 4 hours. The cell index was monitored and analyzed as detailed in the supplemental Methods.
Immunofluorescence microscopy
HBMECs were seeded at 12 000 cells per well in collagen type I–coated 8-chamber slides (Corning BioCoat). For thrombin perturbation, the cells were treated with 5 nM thrombin for 7 minutes, followed by the addition of KIs (0.5 μM). After the indicated times, HBMECs were fixed in 3.7% paraformaldehyde for 30 minutes, followed by 3 phosphate-buffered saline (PBS) washes. HBMECs were immunofluoretically stained for VE-cadherin, phalloidin, and DAPI (4′,6-diamidino-2-phenylindole) in different treatment conditions and analyzed as detailed in the supplemental Methods.
Western blot
HBMECs were seeded in 12-well plates at 24 000 cells per well and grown until confluence for 3 days. For thrombin perturbation, cells were treated with 5 nM thrombin for 7 minutes, followed by treatment with KIs (0.5 μM). For parasite perturbations, KIs (0.5 μM) and lysates prepared from 2 × 106/cm2 mature-stage 3D7 Pf-IEs were added together to the HBMEC monolayer. After incubation, HBMECs were lysed, proteins were separated by electrophoresis, and transferred to polyvinylidene fluoride (PVDF) membranes for antibody staining. The blots were imaged using Bio-Rad ChemiDoc imaging system, and signals were quantified by densitometry using ImageJ v1.53q (National Institutes of Health; https://imagej.nih.gov/ij/). Western blots were analyzed as detailed in the supplemental Methods.
Assessments of BCR-ABL drugs in the mouse ECM model
C57BL/6 female mice at 7 to 10 weeks old were purchased from the Jackson Laboratories and were acclimated for at least 3 days before experimental manipulation. The parasite strain P berghei ANKA (PbANKA) was supplied by Tracey J. Lamb.42 The mice were infected intraperitoneally with 0.5 × 106 PbANKA. Body weights and blood smear parasitemia were monitored daily. Clinical scores were assessed twice a day starting on day 5 after infection using an adapted 20-point rapid murine coma and behavior scale (RMCBS).46 As a humane end point, mice were euthanized at an RMCBS of ≤5, because they were likely to die within hours.46 Imatinib (STI571 mesylate, Selleckchem) was diluted to a final concentration of 150 mg/kg in PBS and administered by oral gavage (OG) in a 100 μL suspension in PBS. Bosutinib (SKI-606, Selleckchem) was reconstituted in a solution buffer (2% dimethyl sulfoxide [DMSO], 30% polyethylene glycol, 5% Tween in Milli-Q water) to 50 mg/mL and administered to mice via intraperitoneal (IP) injections at a dose of 100 mg/kg per day in a 100 μL suspension of 1× PBS. Nilotinib (AMN-107, Selleckchem) was dissolved in PBS and given via OG at a dose of 100 mg/kg per day in a 100 μL suspension. Control mice received either the nilotinib vehicle (PBS) through OG or the bosutinib vehicle (2% DMSO, 30% polyethylene glycol, 5% Tween in Milli-Q water) through IP injection. Artesunate (A3731, Sigma) was resuspended in PBS and provided to infected mice from day 6 to 10 after infection in the late-stage treatment regimen at 5 mg/kg per day through IP injection. For the nilotinib + artesunate group, nilotinib was provided through OG and artesunate through IP injection. For bosutinib + artesunate group, both were provided through IP injections.
To measure organ edema, mice were injected intravenously with 200 μL of 1% Evans blue dye (Sigma Aldrich) through the retroorbital plexus under anesthesia 45 minutes before euthanasia. Perfused brains and lungs were collected, and Evans blue dye was extracted and quantified by spectrophotometry as detailed in the supplemental Methods.
For histologic analysis, the brain and lungs were collected from infected or uninfected mice, treated or untreated with nilotinib or bosutinib on their own or coadministered with artesunate, on the seventh or eighth day after infection. Tissue collection, preparation, and analysis are detailed in the supplemental Methods.
All mice experiments were approved by the Seattle Children’s Institutional Animal Care and Use Committee under protocol ACUC00649.
Statistical analysis
Statistical analysis was performed using Prism software (GraphPad Software Inc., version 8). Data are presented as mean ± standard deviation. A P value ≤.05 was considered statistically significant. Survival curves were analyzed using log-rank tests (Mantel Cox tests). For comparisons between multiple groups, the data were compared using analysis of variance. To assess the differences between groups and to adjust for multiple comparisons, the Dunnett test was used to compare all groups against a single group.
Results
Bosutinib and nilotinib attenuate thrombin-induced barrier disruption in HBMECs with different kinetics
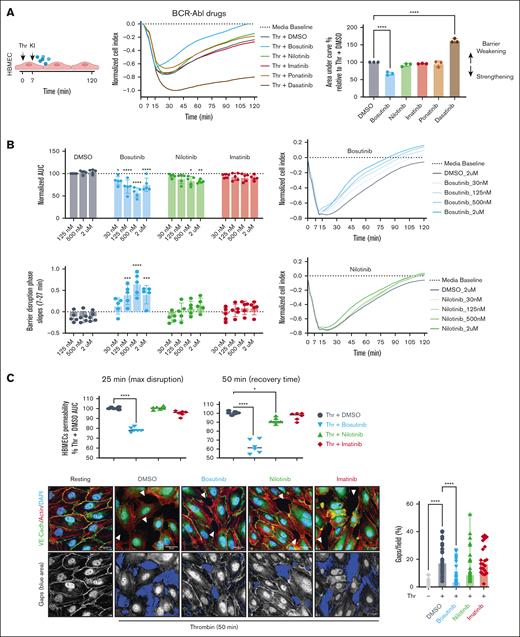
Previously, we found that BCR-ABL drugs can variably alter thrombin-induced barrier disruption in HBMECs.41 Here, we expanded on this study by comparing the 5 clinically approved BCR-ABL drugs. HBMECs were treated with 5 nM thrombin, and the barrier changes were evaluated using the xCELLigence system, which reports cell index as a proxy for barrier integrity. Thrombin induced an acute cell index drop with a maximum barrier disruption peak between 20 and 30 minutes after exposure, followed by recovery over the next 2 hours (Figure 1A). The KIs were added to HBMECs 7 minutes after thrombin treatment and barrier activity was evaluated by calculating the area under the curve for the cell index and then normalizing to the area under the curve for the DMSO vehicle control (Figure 1A). As before,41 bosutinib was protective, imatinib was neutral, and dasatinib worsened thrombin-induced disruption. By comparison, nilotinib was mildly protective and ponatinib was weakly protective (Figure 1A).
Bosutinib and nilotinib can attenuate Thr-induced barrier disruption in HBMECs. (A) Left, schematic overview. An xCELLigence assay was used to measure thrombin (Thr)-induced barrier disruption in an HBMEC monolayer. Traces were normalized to media control. KIs (0.5 μM) were added 7 minutes after Thr to assess barrier protective activity. Right: the total change in the cell index is summarized as the area under the curve (AUC) quantification relative to that of DMSO treatment (100%). The data are presented as mean ± standard deviation (SD; n = 3, each done in duplicate). (B) Dose titration of bosutinib, nilotinib, and imatinib in the xCELLigence assay in Thr-treated HBMECs. The data are presented as mean ± SD (n = 5, each done in triplicate). (C) Top, quantification of Thr-induced barrier permeability at the maximum disruption time point (25 minutes) and at a mid-recovery time point (50 minutes), determined using xCELLigence assay. The data are presented as means ± SD (n = 3, each done in duplicate). Bottom left, representative confocal images of HBMECs under resting or Thr-activated conditions at 50 minutes in the presence or absence of KIs (0.5 μM). The fluorescence of VE-cadherin (green), phalloidin (red), and DAPI (blue) are shown. Gaps are indicated as white arrowheads. Bottom right panel: the highlighted blue areas represent gaps in the monolayer. ImageJ quantification showing the percentage intercellular gaps per field, calculated from 8 uniformly sampled fields (n = 3). A 1-way analysis of variance (ANOVA), followed by Dunnett multiple comparison test, was used to analyze the data. ∗P < .05; ∗∗∗∗P < .0001.
Bosutinib and nilotinib can attenuate Thr-induced barrier disruption in HBMECs. (A) Left, schematic overview. An xCELLigence assay was used to measure thrombin (Thr)-induced barrier disruption in an HBMEC monolayer. Traces were normalized to media control. KIs (0.5 μM) were added 7 minutes after Thr to assess barrier protective activity. Right: the total change in the cell index is summarized as the area under the curve (AUC) quantification relative to that of DMSO treatment (100%). The data are presented as mean ± standard deviation (SD; n = 3, each done in duplicate). (B) Dose titration of bosutinib, nilotinib, and imatinib in the xCELLigence assay in Thr-treated HBMECs. The data are presented as mean ± SD (n = 5, each done in triplicate). (C) Top, quantification of Thr-induced barrier permeability at the maximum disruption time point (25 minutes) and at a mid-recovery time point (50 minutes), determined using xCELLigence assay. The data are presented as means ± SD (n = 3, each done in duplicate). Bottom left, representative confocal images of HBMECs under resting or Thr-activated conditions at 50 minutes in the presence or absence of KIs (0.5 μM). The fluorescence of VE-cadherin (green), phalloidin (red), and DAPI (blue) are shown. Gaps are indicated as white arrowheads. Bottom right panel: the highlighted blue areas represent gaps in the monolayer. ImageJ quantification showing the percentage intercellular gaps per field, calculated from 8 uniformly sampled fields (n = 3). A 1-way analysis of variance (ANOVA), followed by Dunnett multiple comparison test, was used to analyze the data. ∗P < .05; ∗∗∗∗P < .0001.
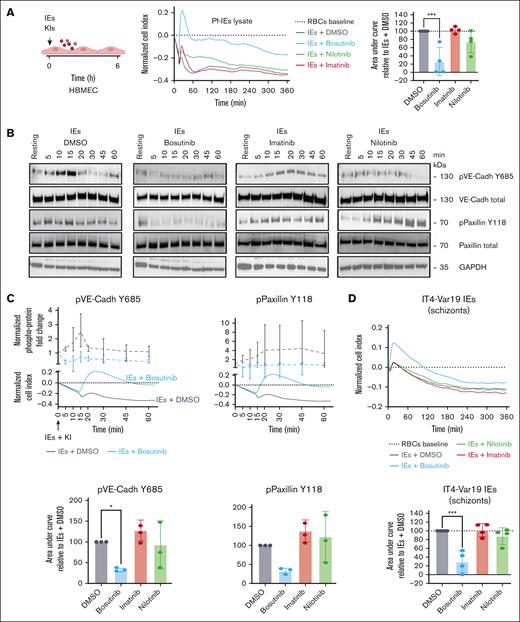
To further investigate the mechanism(s) of drug action, we performed dose-titration experiments for bosutinib, nilotinib, and imatinib. In vivo, bosutinib achieves therapeutic plasma concentrations of 100 to 300 nM in humans as opposed to 1 to 2 μM for nilotinib in plasma.47,48 Dose-response studies demonstrated that bosutinib was more effective at attenuating thrombin disruption than either nilotinib or imatinib across concentrations ranging from 30 nM to 2 μM (Figure 1B; supplemental Figure 1). The barrier-protective effects of nilotinib reached statistical significance only at higher concentrations (Figure 1B; supplemental Figure 1). Among the BCR-ABL inhibitors tested, bosutinib involved a 2-step mechanism with blunting of barrier disruption and expedited recovery (Figure 1B-C), conferring a V-shaped recovery slope. By comparison, nilotinib had a single-step mechanism of expediting barrier recovery (Figure 1B-C; supplemental Figure 1). We further investigated BCR-ABL drugs in cytokine storm–associated vascular injury using sequential tumor necrosis factor exposure, followed by thrombin-mediated barrier disruption. Interestingly, nilotinib treatment conferred enhanced barrier protection to thrombin challenge alone and was equally as effective as bosutinib, whereas imatinib showed no significant activity (supplemental Figure 1C). To visualize these effects, we conducted confocal microscopy analysis using immunofluorescent labeling of VE-cadherin, the major adherens junction protein, combined with phalloidin staining to label the actin cytoskeleton. Thrombin-treated HBMECs had substantial gaps in the endothelial monolayer at the 50-minute timepoint, which were associated with the loss of circumferential VE-cadherin and actin stress fibers (Figure 1C). Although actin stress fiber formation persisted across all drug treatments, bosutinib uniquely reduced thrombin-induced gap formation at 50 minutes, an effect not observed with nilotinib or imatinib (Figure 1C). Taken together, this analysis shows that bosutinib and nilotinib were the most protective against host proinflammatory mediators and that bosutinib exhibited faster barrier protective action than the other BCR-ABL drugs in thrombin-treated HBMECs.
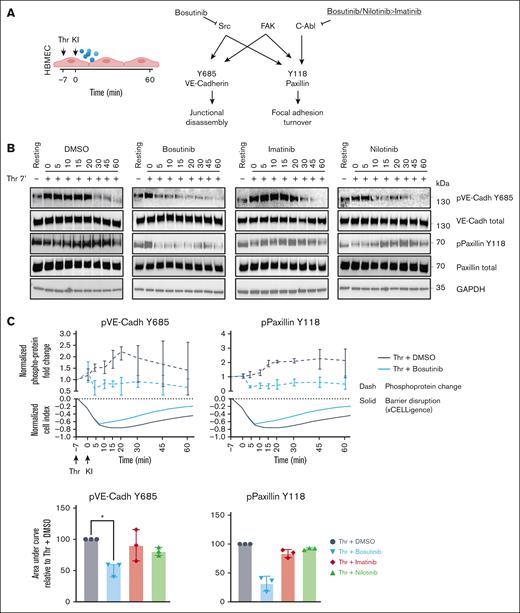
Bosutinib attenuates phosphorylation of adherens junctions and focal adhesions during thrombin-induced endothelial gap formation
Phosphorylation of key junctional proteins, like VE-cadherin (Y685)49-51 and the focal adhesion protein paxillin (Y118),52,53 have been associated with endothelial barrier disruption. We investigated whether bosutinib, which inhibits both ABL and the c-Src kinases responsible for these phosphorylations (Figure 2A), is more effective than nilotinib and imatinib at reducing phosphorylation at these sites. We probed kinase phosphorylation during barrier disruption by western blotting, and the phosphoprotein levels were normalized to total protein levels. HBMECs were pretreated with thrombin for 7 minutes, followed by addition of the KIs. Thrombin induced a transient ∼2.3-fold increase in VE-cadherin (Y685) phosphorylation, peaking at 20 to 30 minutes after thrombin exposure (Figure 2B-C). Paxillin (Y118) phosphorylation exhibited a slower onset but was sustained over 60 minutes (Figure 2B-C). Bosutinib (ABL + c-Src inhibitor)54 substantially reduced thrombin-induced phosphorylation at both VE-cadherin (Y685) and paxillin (Y118), which coincided with its rapid barrier protective effect observed in the xCELLigence assay (Figure 2C). Conversely, nilotinib and imatinib, which inhibit ABL but not c-Src,54 did not attenuate phosphorylation at either site (Figure 2B-C; supplemental Figure 2).
Bosutinib attenuates phosphorylation of adherens junctions and focal adhesions during Thr-induced endothelial barrier disruption. (A) A schematic overview of the target selectivity of BCR-ABL drugs against 3 kinases that phosphorylate VE-cadherin (Y685) and paxillin (Y118). (B) Thrombin (Thr)-induced temporal phosphorylation kinetics of VE-cadherin (Y685) and paxillin (Y118) were probed using immunoblotting, along with their respective total proteins. GAPDH was used as a loading control (n = 3). (C) Upper panel, Thr-induced fold change of the indicated phosphoprotein in the presence of DMSO vehicle control or bosutinib is plotted above the respective xCELLigence traces. ImageJ quantification of phosphoproteins levels in panel B was adjusted to the total protein amounts, and fold change was calculated by normalizing to the resting state (nontreated, media only). The data are presented as means ± SD (n = 3). Lower panel, quantification of the KI effect on phosphorylation (total protein normalized) in the presence of KIs or DMSO control (100%). The bars represent the median AUC ± SD (n = 3). A 1-way ANOVA, followed by Dunnett multiple comparison test, was used to analyze the data. ∗P < .05. FAK, focal adhesion kinase; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; pVE-Cadh, protein VE-cadherin.
Bosutinib attenuates phosphorylation of adherens junctions and focal adhesions during Thr-induced endothelial barrier disruption. (A) A schematic overview of the target selectivity of BCR-ABL drugs against 3 kinases that phosphorylate VE-cadherin (Y685) and paxillin (Y118). (B) Thrombin (Thr)-induced temporal phosphorylation kinetics of VE-cadherin (Y685) and paxillin (Y118) were probed using immunoblotting, along with their respective total proteins. GAPDH was used as a loading control (n = 3). (C) Upper panel, Thr-induced fold change of the indicated phosphoprotein in the presence of DMSO vehicle control or bosutinib is plotted above the respective xCELLigence traces. ImageJ quantification of phosphoproteins levels in panel B was adjusted to the total protein amounts, and fold change was calculated by normalizing to the resting state (nontreated, media only). The data are presented as means ± SD (n = 3). Lower panel, quantification of the KI effect on phosphorylation (total protein normalized) in the presence of KIs or DMSO control (100%). The bars represent the median AUC ± SD (n = 3). A 1-way ANOVA, followed by Dunnett multiple comparison test, was used to analyze the data. ∗P < .05. FAK, focal adhesion kinase; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; pVE-Cadh, protein VE-cadherin.
BCR-ABL drugs differ in attenuating parasite-induced permeability in primary HBMEC
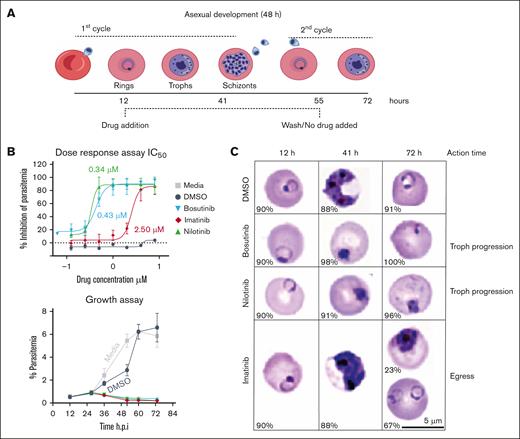
To study BCR-ABL drugs against parasite inflammatory factors, we assessed bosutinib, nilotinib, and imatinib in a 2-cycle P falciparum growth inhibition assay (Figure 3A; supplemental Figure 3). All the KIs displayed nanomolar to micromolar range potency against asexual parasite growth (Figure 3B-C). Bosutinib and nilotinib prevented trophozoite progression, whereas imatinib targeted the late schizont stage (Figure 3C), consistent with previous findings that BCR-ABL drugs can kill P falciparum blood stage parasites and that imatinib inhibits IE rupture and the release of invasive merozoites.35,36
BCR-ABL drugs suppress P falciparum growth in RBCs. (A) Schematic of the 2-cycle P falciparum blood stage growth inhibition assay. (B) Upper panel, 2-fold dose-response curve ranging from 0.1 to 8 μM, followed by a media wash at 55 hours. Parasitemia was measured using SYBR staining with flow cytometry, which was performed at 72 hours. The half-maximal inhibitory concentrations (IC50) of the drugs are indicated. Lower panel, P falciparum asexual intraerythrocytic growth assay. At the ring stage (∼12 h after invasion), media only treatment or treatment with 4 μM KIs or DMSO (vehicle) was applied. The parasites were grown for 2 consecutive growth cycles and the percentage parasitemia was determined using SYBR staining with flow cytometry every 12 hours after infection. The data are presented as means ± SD (n = 3). (C) Representative bright field images of Giemsa-stained blood smears for parasite maturation during drug assay. The percentage of the representative parasite stage is shown at the bottom left corner of each Giemsa-stained image for the respective time point and treatment condition. Scale bar = 5 μm. RBCs, red blood cells.
BCR-ABL drugs suppress P falciparum growth in RBCs. (A) Schematic of the 2-cycle P falciparum blood stage growth inhibition assay. (B) Upper panel, 2-fold dose-response curve ranging from 0.1 to 8 μM, followed by a media wash at 55 hours. Parasitemia was measured using SYBR staining with flow cytometry, which was performed at 72 hours. The half-maximal inhibitory concentrations (IC50) of the drugs are indicated. Lower panel, P falciparum asexual intraerythrocytic growth assay. At the ring stage (∼12 h after invasion), media only treatment or treatment with 4 μM KIs or DMSO (vehicle) was applied. The parasites were grown for 2 consecutive growth cycles and the percentage parasitemia was determined using SYBR staining with flow cytometry every 12 hours after infection. The data are presented as means ± SD (n = 3). (C) Representative bright field images of Giemsa-stained blood smears for parasite maturation during drug assay. The percentage of the representative parasite stage is shown at the bottom left corner of each Giemsa-stained image for the respective time point and treatment condition. Scale bar = 5 μm. RBCs, red blood cells.
Because these drugs exhibited antiparasitic growth effects against blood-stage Pf-IEs, we first employed lysates of schizont-stage Pf-IEs (Pf-IE lysates) to investigate the potential protective effects of BCR-ABL drugs against Pf-IE–induced endothelial barrier disruption.15 Consistent with previous findings,55,56 Pf-IE lysates induced a mild and gradual decline in barrier integrity over several hours (cell index: Pf-IE lysate −0.3 maximum drop at 6 hours) when compared with control red blood cell lysate (Figure 4A; supplemental Figure 4). Among the BCR-ABL inhibitors, bosutinib demonstrated substantial efficacy in blunting Pf-IE lysate–induced barrier disruption, whereas nilotinib and imatinib showed no protective effect (Figure 4A). Temporal western blot analysis revealed that Pf-IE lysates triggered a transient ∼2.3-fold increased phosphorylation of VE-cadherin (Y685) and a sustained ∼4.5-fold increase in paxillin (Y118) phosphorylation (Figure 4B-C). Notably, bosutinib substantially attenuated phosphorylation at both sites, whereas nilotinib and imatinib did not (Figure 4B-C; supplemental Figure 4).
Bosutinib can attenuate parasite-induced barrier disruption in HBMECs. (A) Schematic overview of the parasite-permeability assay. Representative xCELLigence recordings of HBMECs treated with lysates from 3D7 Pf-IEs. The traces were normalized to that of uninfected RBC lysates. KIs (0.5 μM) were added at the same time as the parasite lysate. Right, total change in cell index is summarized as the AUC relative to that of DMSO (100%). The data are presented as the mean ± SD (n = 4, done in duplicate). ∗P < .05 determined using a 1-way ANOVA, followed by Dunnett multiple comparison test (compared with the DMSO/Pf-IEs lysate group). (B) 3D7 Pf-IE lysate–induced phosphorylation of VE-cadherin (Y685) and paxillin (Y118) were probed using immunoblotting, along with their respective total proteins. GAPDH was used as a loading control. The western blot images are representative of 3 independent biologic replicates. (C) Upper panel, the 3D7 Pf lysate–induced fold change of the indicated phosphoproteins levels, adjusted to the total protein amounts (dashed lines), is plotted above the Pf lysate-induced change in the cell index (solid lines), measured using the xCELLigence platform in panel (A). Fold change was calculated by normalizing the data against the data of the resting state (nontreated, media only). Lower panel, The AUC was calculated for the temporal phosphoprotein kinetics (total protein normalized) in the presence of KIs or for the DMSO control (100%). Bars represent the mean AUC ± SD (n = 3). ∗∗∗P < .001; ∗P < .05 determined using 1-way ANOVA, followed by Dunnett multiple comparison test (compared with DMSO control). (D) Upper panel, representative recording of xCELLigence barrier response to schizont-stage IT4var19-IEs (after normalization to uninfected RBCs). DMSO or KIs (0.5 μM) were added after 1 hour of co-culture of purified schizont-stage IEs or RBCs with HBMECs. Lower panel, quantification of barrier protective activity of KIs against schizont-induced barrier disruption. The data are presented as mean ± SD (n = 4, each done in triplicate). ∗∗∗P < .001 determined using a 1-way ANOVA, followed by Dunnett multiple comparison test (compared with the DMSO/Pf-IEs group). GAPDH, glyceraldehyde-3-phosphate dehydrogenase; pVE-Cadh, protein VE-cadherin; RBCs, red blood cells.
Bosutinib can attenuate parasite-induced barrier disruption in HBMECs. (A) Schematic overview of the parasite-permeability assay. Representative xCELLigence recordings of HBMECs treated with lysates from 3D7 Pf-IEs. The traces were normalized to that of uninfected RBC lysates. KIs (0.5 μM) were added at the same time as the parasite lysate. Right, total change in cell index is summarized as the AUC relative to that of DMSO (100%). The data are presented as the mean ± SD (n = 4, done in duplicate). ∗P < .05 determined using a 1-way ANOVA, followed by Dunnett multiple comparison test (compared with the DMSO/Pf-IEs lysate group). (B) 3D7 Pf-IE lysate–induced phosphorylation of VE-cadherin (Y685) and paxillin (Y118) were probed using immunoblotting, along with their respective total proteins. GAPDH was used as a loading control. The western blot images are representative of 3 independent biologic replicates. (C) Upper panel, the 3D7 Pf lysate–induced fold change of the indicated phosphoproteins levels, adjusted to the total protein amounts (dashed lines), is plotted above the Pf lysate-induced change in the cell index (solid lines), measured using the xCELLigence platform in panel (A). Fold change was calculated by normalizing the data against the data of the resting state (nontreated, media only). Lower panel, The AUC was calculated for the temporal phosphoprotein kinetics (total protein normalized) in the presence of KIs or for the DMSO control (100%). Bars represent the mean AUC ± SD (n = 3). ∗∗∗P < .001; ∗P < .05 determined using 1-way ANOVA, followed by Dunnett multiple comparison test (compared with DMSO control). (D) Upper panel, representative recording of xCELLigence barrier response to schizont-stage IT4var19-IEs (after normalization to uninfected RBCs). DMSO or KIs (0.5 μM) were added after 1 hour of co-culture of purified schizont-stage IEs or RBCs with HBMECs. Lower panel, quantification of barrier protective activity of KIs against schizont-induced barrier disruption. The data are presented as mean ± SD (n = 4, each done in triplicate). ∗∗∗P < .001 determined using a 1-way ANOVA, followed by Dunnett multiple comparison test (compared with the DMSO/Pf-IEs group). GAPDH, glyceraldehyde-3-phosphate dehydrogenase; pVE-Cadh, protein VE-cadherin; RBCs, red blood cells.
To simulate sequestered Pf-IEs, we used MACS-purified schizont-stage IT4var19-IEs (DC8-EPCR binder)19 in co-culture with an HBMEC monolayer. After 1 hour of incubation, the KIs or DMSO vehicle control was added. This model similarly induced moderate and gradual barrier disruption (Figure 4D). Consistent with the lysate experiments, bosutinib significantly reduced schizont-IE–induced barrier disruption, whereas imatinib and nilotinib remained ineffective (Figure 4D).
In vivo efficacy of nilotinib and bosutinib in a mouse CM model
To assess the in vivo efficacy of BCR-ABL drugs, we used the mouse ECM model. Control PbANKA-infected C57BL/6 mice began to lose weight by day 5 after infection and experienced a decline in the 20-point RMCBS used to monitor infection46 by days 6 or 7 (supplemental Figure 5). By day 7, ∼50% of the mice reached the euthanasia cutoff (RMCBS score ≤5), which increased to 85% by day 8 and to 92% by day 9 (supplemental Figure 5). Hematoxylin and eosin–stained sections from mice with RMCBS scores (≤10) showed both brain pathology (brain hemorrhages and vascular congestion) and lung pathology (areas with alveolar edema, lung hemorrhage, congestion, cellular infiltration, and hyaline membrane; Figure 5A; supplemental Figures 6 and 7). Evans blue perfusion studies on day 7 after infection demonstrated 3 distinct phenotypes, namely (1) severe pulmonary edema with variable cerebral edema, (2) moderate cerebral edema with minimal pulmonary involvement, and (3) mild to moderate edema in both organs (Figure 5B). These findings indicate that a PbANKA infection induces complex multi-organ pathology.
BCR-ABL drugs attenuate ECM pathology and protect mice when provided before ECM symptoms develop. (A) Schematic overview of the ECM assay. Representative hematoxylin and eosin images of the brain and lung of uninfected (Un) and PbANKA-infected (Inf) mice euthanized on day 7 after infection with RMCBS score <8 (20×; scale bar = 50 μm). The arrowhead indicates brain hemorrhage. (B) Unsupervised hierarchical cluster map of brain and lung edema measured by Evans blue dye on day 7 after infection (gray scale) in comparison with the RMCBS clinical score (blue scale). (C) Schematic overview of the early-infection drug regimen (days 4 to 7). (D) Survival curves (mice were euthanized at an RMCBS score of ≤5; n = 18 Inf; 17 nilotinib [Nil]; 18 bosutinib [Bos]; 18 imatinib [Ima]). ∗P < .05; ∗∗∗∗P < .0001 determined using a log-rank test (Mantel Cox test) in comparison with the Inf group. (E) Parasitemia curves (n = 18 Inf; 17 Nil; 18 Bos; 18 Ima). (F) Brain edema measured by Evans blue assay on day 7 after infection (n = 6 Un; 12 Inf; 11 Nil; 11 Bos; 4 Ima). ∗∗∗∗P < .0001 determined using 1-way ANOVA, followed by Dunnett multiple comparison test (compared with the Inf group). (G) Lung edema measured by Evans blue assay on day 7 after infection (n = 6 Un; 12 Inf; 11 Nil; 11 Bos; 4 Ima). ∗P < .05; ∗∗P < .01 determined using a 1-way ANOVA, followed by Dunnett multiple comparison test (compared with the Inf group).
BCR-ABL drugs attenuate ECM pathology and protect mice when provided before ECM symptoms develop. (A) Schematic overview of the ECM assay. Representative hematoxylin and eosin images of the brain and lung of uninfected (Un) and PbANKA-infected (Inf) mice euthanized on day 7 after infection with RMCBS score <8 (20×; scale bar = 50 μm). The arrowhead indicates brain hemorrhage. (B) Unsupervised hierarchical cluster map of brain and lung edema measured by Evans blue dye on day 7 after infection (gray scale) in comparison with the RMCBS clinical score (blue scale). (C) Schematic overview of the early-infection drug regimen (days 4 to 7). (D) Survival curves (mice were euthanized at an RMCBS score of ≤5; n = 18 Inf; 17 nilotinib [Nil]; 18 bosutinib [Bos]; 18 imatinib [Ima]). ∗P < .05; ∗∗∗∗P < .0001 determined using a log-rank test (Mantel Cox test) in comparison with the Inf group. (E) Parasitemia curves (n = 18 Inf; 17 Nil; 18 Bos; 18 Ima). (F) Brain edema measured by Evans blue assay on day 7 after infection (n = 6 Un; 12 Inf; 11 Nil; 11 Bos; 4 Ima). ∗∗∗∗P < .0001 determined using 1-way ANOVA, followed by Dunnett multiple comparison test (compared with the Inf group). (G) Lung edema measured by Evans blue assay on day 7 after infection (n = 6 Un; 12 Inf; 11 Nil; 11 Bos; 4 Ima). ∗P < .05; ∗∗P < .01 determined using a 1-way ANOVA, followed by Dunnett multiple comparison test (compared with the Inf group).
Subsequently, we administered daily treatments of nilotinib, bosutinib, or imatinib to PbANKA-infected mice from days 4 to 7 after infection (early treatment regimen; Figure 5C). Nilotinib conferred the strongest protection (90%) when compared with bosutinib (25%) and imatinib (30%; Figure 5D). Although all the cohorts exhibited characteristic weight loss by day 4, nilotinib-treated mice recovered the most rapidly (supplemental Figure 8). Nilotinib most effectively suppressed parasitemia (Figure 5E) and reduced brain and lung edema (Figure 5F-G). Bosutinib-treated mice exhibited multiple day–delayed parasitemia suppression but had reduced organ edema by days 7 to 8 despite suboptimal parasite control (Figure 5F-G). Imatinib showed no effect on PbANKA parasitemia (Figure 5E). The reduced half-lives of BCR-ABL inhibitors in C57BL/6 mice (1-3 hours) as opposed to in humans may account for their reduced antiparasitic efficacy.57 These data demonstrate that nilotinib and bosutinib reduce brain and lung edema in the early-intervention ECM model through distinct mechanisms of action.
Nilotinib rescues mice and reduces brain and lung pathology in a late-stage treatment regimen
In a clinical setting, BCR-ABL drugs would be used in combination with the existing front-line antimalaria treatment artesunate. To explore BCR-ABL drugs in this context, mice were administered nilotinib or bosutinib on days 6 to 10 after infection (late-stage treatment; Figure 6A), coinciding with initial RMCBS score deterioration. Four experimental mice cohorts were compared, namely (1) infection control, (2) subtherapeutic dose of artesunate alone (5 mg/kg per day), (3) nilotinib or bosutinib alone, and (4) nilotinib or bosutinib as adjunctive therapy with artesunate (nilotinib or bosutinib + artesunate). Nilotinib conferred stronger protection than bosutinib as a monotherapy (44% vs 16%; Figure 6B; supplemental Figure 9). In addition, some mice in the bosutinib treatment group experienced fatal internal bleeding and suddenly died without a decline in the RMCBS score, prompting subsequent analyses to focus exclusively on nilotinib. Although individual nilotinib or artesunate treatments each conferred ∼44% protection, combination therapy enhanced survival to 78% (Figure 6B). The combination treatment cohort of nilotinib + artesunate exhibited accelerated parasite clearance (Figure 6C-E). To investigate the effect of BCR-ABL drugs on organ edema, we performed an Evans blue perfusion assay on day 7 or day 8 after infection, concurrent with significant RMCBS score decline (≤ 5) in control mice (Figure 6F; supplemental Figure 8). At assessment, both artesunate and the nilotinib + artesunate treatment groups had lower parasitemia than the nilotinib alone or infection control mice (Figure 6G). The combined nilotinib + artesunate treatment reduced brain edema (P = .01) and showed a trend toward reduced lung edema that did not achieve statistical significance (P = .0572; Figure 6H-I).
Coadministration of nilotinib with artesunate increased survival and decreased brain edema. (A) Schematic overview of the late-stage treatment regimen (days 6-10) as highlighted by the gray box. (B) Survival curves (mice were euthanized at an RMCBS score ≤ 5; n = 18 per group. ∗P < .05; ∗∗∗∗ P < .0001 analyzed using log-rank tests [Mantel Cox tests] in comparison with the infected group). (C) Parasitemia curves (n = 18 per group). (D) Parasitemia in individual mice on days 7 to 11 after infection. ∗P < .05; ∗∗P < .01 determined using 2-way ANOVA, followed by Tukey multiple comparison test. (E) Giemsa-stained blood smears were used to determine the parasite life stages on day 8 after infection in the no treatment and nilotinib-treated PbANKA-infected mice. (F) Schematic overview of Evans blue dye assay conducted on day 7 or 8. (G) Parasitemia measured on the day of Evans blue dye assay (n = 9 Un; 17 Inf; 18 Art; 18 Nil+Art). ∗ P < .05; ∗∗P < .01 determined using 1-way ANOVA, followed by Dunnett multiple comparison test (compared with the infected group). (H) Brain edema measured by Evans blue assay on day 7 or 8 after infection (n = 9 Un; 17 inf; 18 Art; 18 Nil+Art). ∗P < .05; ∗∗P < .01 determined using 1-way ANOVA, followed by Dunnett multiple comparison test (compared with the infected group). (I) Lung edema measured by Evans blue assay on day 7 or 8 after infection (n = 9 Un; 17 Inf; 18 Art; 18 Nil+Art). ∗∗P < .01 determined using 1-way ANOVA, followed by Dunnett multiple comparison test (compared with the infected group). Art, artesunate.
Coadministration of nilotinib with artesunate increased survival and decreased brain edema. (A) Schematic overview of the late-stage treatment regimen (days 6-10) as highlighted by the gray box. (B) Survival curves (mice were euthanized at an RMCBS score ≤ 5; n = 18 per group. ∗P < .05; ∗∗∗∗ P < .0001 analyzed using log-rank tests [Mantel Cox tests] in comparison with the infected group). (C) Parasitemia curves (n = 18 per group). (D) Parasitemia in individual mice on days 7 to 11 after infection. ∗P < .05; ∗∗P < .01 determined using 2-way ANOVA, followed by Tukey multiple comparison test. (E) Giemsa-stained blood smears were used to determine the parasite life stages on day 8 after infection in the no treatment and nilotinib-treated PbANKA-infected mice. (F) Schematic overview of Evans blue dye assay conducted on day 7 or 8. (G) Parasitemia measured on the day of Evans blue dye assay (n = 9 Un; 17 Inf; 18 Art; 18 Nil+Art). ∗ P < .05; ∗∗P < .01 determined using 1-way ANOVA, followed by Dunnett multiple comparison test (compared with the infected group). (H) Brain edema measured by Evans blue assay on day 7 or 8 after infection (n = 9 Un; 17 inf; 18 Art; 18 Nil+Art). ∗P < .05; ∗∗P < .01 determined using 1-way ANOVA, followed by Dunnett multiple comparison test (compared with the infected group). (I) Lung edema measured by Evans blue assay on day 7 or 8 after infection (n = 9 Un; 17 Inf; 18 Art; 18 Nil+Art). ∗∗P < .01 determined using 1-way ANOVA, followed by Dunnett multiple comparison test (compared with the infected group). Art, artesunate.
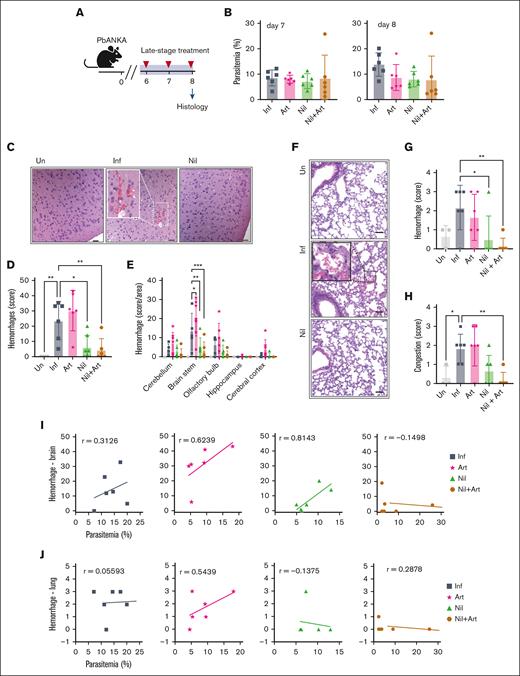
Histopathologic evaluation of vascular pathology was performed on day 8 after infection (Figure 7A). Despite comparable parasitemia between the nilotinib and artesunate cohorts (Figure 7B), nilotinib treatment significantly reduced brain and lung hemorrhages (Figure 7C-G). In addition, the nilotinib + artesunate group exhibited reduced lung congestion (Figure 7H). The vascular protective effect of nilotinib against hemorrhages was particularly prominent in the brain stem (Figure 7E). Nilotinib-treated mice had fewer brain and lung hemorrhages than the artesunate or control-infected mice at both low and high levels of parasitemia (Figure 7I-J). The combination therapy nilotinib + artesunate group exhibited minimal brain and lung hemorrhages when compared with the other treatment groups (Figure 7I-J). Collectively, these findings demonstrate nilotinib’s ability to attenuate brain and lung hemorrhages, both as a monotherapy or when coadministered with an antimalaria drug.
Nilotinib reduces brain and lung hemorrhages in late-stage treatment intervention. (A) Schematic overview of the histology study conducted on day 8 after infection. (B) Parasitemia in mice on day 7 and 8 after infection (n = 6 mice per group). (C) Representative hematoxylin and eosin (H&E)–stained brain sections of uninfected (Un), infected (Inf), and nilotinib (Nil)-treated mice. Hemorrhage (white dashed box) in the cerebral cortex area on day 8 after infection (magnification ×10; scale bar = 100 μm; magnification ∼×3.6, boxed inset). (D) Total brain hemorrhage score. (E) Individual brain area hemorrhage score. (F) Representative H&E lung sections (magnification ×20; scale bar = 50 μm) showing hemorrhage area (black dashed box; magnification ∼×4, boxed inset). Lungs were collected on day 8 after infection. (G) Lung hemorrhage score. (H) Lung congestion score. (I) Correlation of parasitemia and brain hemorrhage score. (J) Correlation of parasitemia and lung hemorrhage score. A 1-way ANOVA, followed by Dunnett multiple comparisons test (compared with the infected group) was used to analyze the data. ∗P < .05; ∗∗P < .01; ∗∗∗P < .01.
Nilotinib reduces brain and lung hemorrhages in late-stage treatment intervention. (A) Schematic overview of the histology study conducted on day 8 after infection. (B) Parasitemia in mice on day 7 and 8 after infection (n = 6 mice per group). (C) Representative hematoxylin and eosin (H&E)–stained brain sections of uninfected (Un), infected (Inf), and nilotinib (Nil)-treated mice. Hemorrhage (white dashed box) in the cerebral cortex area on day 8 after infection (magnification ×10; scale bar = 100 μm; magnification ∼×3.6, boxed inset). (D) Total brain hemorrhage score. (E) Individual brain area hemorrhage score. (F) Representative H&E lung sections (magnification ×20; scale bar = 50 μm) showing hemorrhage area (black dashed box; magnification ∼×4, boxed inset). Lungs were collected on day 8 after infection. (G) Lung hemorrhage score. (H) Lung congestion score. (I) Correlation of parasitemia and brain hemorrhage score. (J) Correlation of parasitemia and lung hemorrhage score. A 1-way ANOVA, followed by Dunnett multiple comparisons test (compared with the infected group) was used to analyze the data. ∗P < .05; ∗∗P < .01; ∗∗∗P < .01.
Discussion
Our research corroborates previous findings that BCR-ABL drugs inhibit blood stage P falciparum growth34-36 with nilotinib and bosutinib preventing trophozoite progression and imatinib targeting late blood-stage parasites. Because coadministration of imatinib with antimalaria drugs accelerated parasite clearance and fever resolution in Vietnamese patients with uncomplicated malaria,37 we evaluated the potential anti-inflammatory properties of BCR-ABL drugs in severe malaria models.
Sequestration is central to P falciparum CM pathogenesis,9,58 triggering both systemic inflammation and localized cerebrovascular inflammation from both pathogen-associated molecular patterns, such as parasite histones11,12 and P falciparum histidine rich protein 2,14 and malaria damage–associated molecular patterns, including heme,59,60 uric acid,61,62 and extracellular vesicles.63 Although Pf-IEs can promote the disruption of brain endothelial cell monolayers,15 the underlying molecular mechanisms remain poorly understood. By using primary HBMECs, we observed that thrombin induced an acute, reversible barrier disruption and that Pf-IEs elicited a mild and gradual alteration in barrier phenotype. Among the 5 BCR-ABL drugs, bosutinib and nilotinib were the most effective at protecting HBMEC barrier integrity against tumor necrosis factor and thrombin, which are 2 key host inflammatory mediators in human CM. Our analysis showed that bosutinib exhibited a distinctive 2-step mechanism by both blunting thrombin disruption and accelerating barrier recovery. Conversely, nilotinib primarily hastened barrier recovery. Furthermore, only bosutinib proved effective against Pf-IE–induced barrier disruption, suggesting distinct mechanistic pathways. We found that bosutinib was uniquely able to prevent the phosphorylation of ABL- and c-Src–dependent sites in VE-cadherin and paxillin49,51,52 that was induced by both thrombin and parasite stimuli, consistent with a role of the Src-family of kinases in parasite-induced barrier disruption.64 Bosutinib’s protective mechanism extended to strengthening endothelial junctions and focal adhesions in thrombin-treated human umbilical vein endothelial cells via a mitogen-activated protein 4 kinase 4–dependent pathway.38 Taken together, these findings indicate commonalities in bosutinib’s protective effects against different permeability mediators through interference with kinase regulators of the adherens junctions and focal adhesions. In contrast, nilotinib showed limited impact on barrier disruption and primarily facilitated barrier recovery through undefined molecular mechanisms. The remaining BCR-ABL inhibitors displayed either minimal effects (imatinib and ponatinib) or exacerbated barrier disruption (dasatinib), likely reflecting their distinct polypharmacologic profiles.41,54 Overall, these findings highlight the diverse phenotypes of BCR-ABL drugs on both endothelial barrier function and in blood-stage P falciparum killing assays, underscoring the complexity of their interactions with host-parasite systems.
Given the absence of animal models of P falciparum CM, we used the mouse ECM model to evaluate in vivo drug efficacy. It should be noted that P berghei parasites lack the P falciparum erythrocyte membrane protein 1 (PfEMP1) cytoadhesion protein family, which leads to reduced accumulation of IEs in the mouse brain.65,66 Despite this limitation, the mouse ECM model has been extensively employed to investigate adjunctive therapies aimed at counteracting inflammation in CM.67-72 In mice, nilotinib demonstrated superior efficacy over bosutinib and imatinib, primarily attributable to nilotinib’s substantially greater effectiveness in controlling parasitemia. Nevertheless, bosutinib reduced brain and lung edema when administered in an early intervention regimen despite having no impact on parasitemia. However, when administered in a late infection treatment regimen, some mice experienced adverse bleeding side effects, which align with the known bosutinib-associated effects, including thrombocytopenia and platelet function effects.73,74 These findings warrant circumspection in the repurposing of bosutinib for severe malaria treatment. Future work is needed to understand what drives these different outcomes and whether an optimal dose regimen can be established in the ECM model to ensure both safety and efficacy in potential clinical applications. Although mice treated with nilotinib and artesunate had equivalent levels of parasitemia, nilotinib led to less brain hemorrhages and reduced vascular congestion, including fewer hemorrhages within the brain stem, a site of extensive vascular leak and neuronal apoptosis in the ECM model.75 This finding is particularly significant given that petechial brain hemorrhages are a characteristic pathologic feature observed in two-thirds of fatal pediatric CM cases.9,28 The in vivo mechanism(s) of action for nilotinib is likely to be multifaceted and intricate. CD8 T cells play a critical role in fatal brain edema in the mouse ECM model.75-79 Beyond parasitemia control and barrier restorative activity in a primary brain endothelial cell in vitro model, nilotinib can hamper in vitro CD8 T cell proliferation and effector function80,81 and targets a host tyrosine kinase receptor implicated in endothelial barrier integrity in the ECM model.42 Nilotinib’s potential to simultaneously influence multiple pathophysiological pathways suggest that it may represent a promising therapeutic approach for severe malaria. Further investigations are needed to delineate the in vivo mechanism(s) of action of nilotinib in the mouse ECM model.
Our study presents both strengths and limitations. A notable strength is the use of both a human in vitro brain endothelial model and the mouse ECM model, providing the evaluation of these drugs in a more complex environment. However, the ECM model, although valuable, does not perfectly replicate the human pathophysiology, especially for studying the Pf-IE driven inflammatory processes that are unique to human CM.2 Consequently, the extrapolation of our murine findings to human scenarios has limitations. Further research is necessary to elucidate the pharmacokinetics and safety profiles of BCR-ABL drugs in the context of mild human malaria infections37 and as candidate adjunctive therapies for pediatric CM.82 Likewise, subsequent investigations in the murine ECM model are warranted to discern whether nilotinib’s protective effect is primarily attributable to parasitemia control or potentially mediated by other anti-inflammatory mechanisms that may have translational relevance to human disease. In conclusion, these findings have significant implications for the repurposing of BCR-ABL drugs in malaria treatment and, simultaneously, may offer novel avenues to combat emerging drug-resistant parasites83 and mitigate the adverse effects of inflammation in CM.
Acknowledgments
The authors thank Corey Layzer for technical assistance, the University of Washington histology core for histology preparations and sectioning, and Tracey J. Lamb (University of Utah) for providing the P berghei ANKA parasites used in the experiments. Illustrative images were created with BioRender.com.
This work was funded by the National Institutes of Health grant RO1 AI48802 (J.D.S. and A.K.).
Authorship
Contribution: L.S.O., P.B., A.K., and J.D.S. conceptualized the study; L.S.O., P.B., V.I.P., L.W., R.J.R.X.F., and S.E. contributed to the methodology; L.S.O., P.B., and V.I.P. performed the investigation; A.K. and J.D.S. supervised the study; L.S.O., P.B., and J.D.S. wrote the original draft; and all the authors wrote and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Joseph D. Smith, Center for Global Infectious Disease Research, Seattle Children’s Research Institute, 1916 Boren Ave, Seattle, WA 98101; email: joe.smith@seattlechildrens.org.
References
Author notes
L.d.S.O. and P.B. contributed equally to this work.
Original data are available on request from the corresponding author, Joseph D. Smith (joe.smith@seattlechildrens.org).
The full-text version of this article contains a data supplement.

![BCR-ABL drugs attenuate ECM pathology and protect mice when provided before ECM symptoms develop. (A) Schematic overview of the ECM assay. Representative hematoxylin and eosin images of the brain and lung of uninfected (Un) and PbANKA-infected (Inf) mice euthanized on day 7 after infection with RMCBS score <8 (20×; scale bar = 50 μm). The arrowhead indicates brain hemorrhage. (B) Unsupervised hierarchical cluster map of brain and lung edema measured by Evans blue dye on day 7 after infection (gray scale) in comparison with the RMCBS clinical score (blue scale). (C) Schematic overview of the early-infection drug regimen (days 4 to 7). (D) Survival curves (mice were euthanized at an RMCBS score of ≤5; n = 18 Inf; 17 nilotinib [Nil]; 18 bosutinib [Bos]; 18 imatinib [Ima]). ∗P < .05; ∗∗∗∗P < .0001 determined using a log-rank test (Mantel Cox test) in comparison with the Inf group. (E) Parasitemia curves (n = 18 Inf; 17 Nil; 18 Bos; 18 Ima). (F) Brain edema measured by Evans blue assay on day 7 after infection (n = 6 Un; 12 Inf; 11 Nil; 11 Bos; 4 Ima). ∗∗∗∗P < .0001 determined using 1-way ANOVA, followed by Dunnett multiple comparison test (compared with the Inf group). (G) Lung edema measured by Evans blue assay on day 7 after infection (n = 6 Un; 12 Inf; 11 Nil; 11 Bos; 4 Ima). ∗P < .05; ∗∗P < .01 determined using a 1-way ANOVA, followed by Dunnett multiple comparison test (compared with the Inf group).](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/9/10/10.1182_bloodadvances.2024015364/2/m_blooda_adv-2024-015364-gr5.jpeg?Expires=1769104450&Signature=d6BA4Z~bTtDK83v~UdIgm~CjmG54cL9TzaW4QWRGFod5s67du-XQ9REUkTJGud4SxJ1uOZllHKJeH4MFaf4JX4zvZ~vmpOQ-GGCCjFkBClItC5XuyHoSOIp~P1oNeWhOKWF4tYdY8qgBdDeKJBG~JqOQFVKbBQbjJSFpW2z4BNEW3UAUh8E9fDlxhSZTHrYvvTesmSO2dLoBgM2EjDfejkFQ3-Hwe5-VKxJ3dW~8yqkFt8VdCWdqBpgx0I49O0-cSxCDZZsCSgunkF0HOxDmi4OjKRdEMWjCNoX5HZZLf8E~MTx5GEmwM439XoiUUOyCaeWfCC5vWFuwEbSLD9GwZw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Coadministration of nilotinib with artesunate increased survival and decreased brain edema. (A) Schematic overview of the late-stage treatment regimen (days 6-10) as highlighted by the gray box. (B) Survival curves (mice were euthanized at an RMCBS score ≤ 5; n = 18 per group. ∗P < .05; ∗∗∗∗ P < .0001 analyzed using log-rank tests [Mantel Cox tests] in comparison with the infected group). (C) Parasitemia curves (n = 18 per group). (D) Parasitemia in individual mice on days 7 to 11 after infection. ∗P < .05; ∗∗P < .01 determined using 2-way ANOVA, followed by Tukey multiple comparison test. (E) Giemsa-stained blood smears were used to determine the parasite life stages on day 8 after infection in the no treatment and nilotinib-treated PbANKA-infected mice. (F) Schematic overview of Evans blue dye assay conducted on day 7 or 8. (G) Parasitemia measured on the day of Evans blue dye assay (n = 9 Un; 17 Inf; 18 Art; 18 Nil+Art). ∗ P < .05; ∗∗P < .01 determined using 1-way ANOVA, followed by Dunnett multiple comparison test (compared with the infected group). (H) Brain edema measured by Evans blue assay on day 7 or 8 after infection (n = 9 Un; 17 inf; 18 Art; 18 Nil+Art). ∗P < .05; ∗∗P < .01 determined using 1-way ANOVA, followed by Dunnett multiple comparison test (compared with the infected group). (I) Lung edema measured by Evans blue assay on day 7 or 8 after infection (n = 9 Un; 17 Inf; 18 Art; 18 Nil+Art). ∗∗P < .01 determined using 1-way ANOVA, followed by Dunnett multiple comparison test (compared with the infected group). Art, artesunate.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/9/10/10.1182_bloodadvances.2024015364/2/m_blooda_adv-2024-015364-gr6.jpeg?Expires=1769104450&Signature=S3ZlDGVH68GKtWU4DDz7nWMDQi4-ds7NZQk6qGDdc3kXPU0ySIoML2Sp9o3VRDJvuIs1lt~vngfclxTOuD-RKk6FGHYa5ppGXsDTkXqCyRFvV35Uijp~QlWan~eyDyjSTNqReVCblOtyn9txECbGFHP9vyH8-zGabekCI3zKKByJPs7G8dM7RXgaWhIXZb3NILphtPgIwzRr8wAlFnFltB53NRHnKAlUSuTnLZOqnYsbLUvi6a93vqpbuO1SeCoTxiHaqGSU7yreZwO8dJ1blRiEn5A7~ULIGIe0VA9aU85SVdFTTBR3gtljok0m9t-LiFDCW0sx8ZGXx9twvrXHOg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)