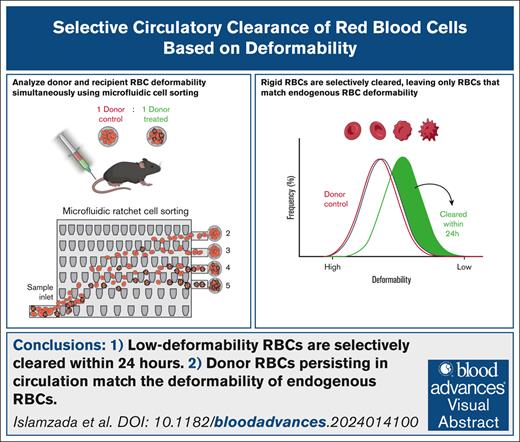
Key Points
Subpopulations of donor RBCs with low deformability are selectively cleared within 24 hours of transfusion in a murine model.
Donor RBCs that persist in circulation match the deformability distribution of endogenous RBCs.
Visual Abstract
Donated red blood cells (RBCs) collected for blood transfusions progressively lose their deformability due to natural aging and cold storage in blood bags. This loss accelerates circulatory clearance via mechanical sensing by the spleen, leading to RBC retention and entrapment. Although reduced deformability is known to shorten RBC circulation time, the extent to which splenic clearance distinguishes and removes RBCs with altered deformability is poorly understood. Here, we show that subpopulations of donor RBCs with a deformability distribution distinct from endogenous recipient’s RBCs are selectively and specifically cleared from circulation within 24 hours of infusion in a mouse model, whereas donor RBCs with a deformability distribution similar to endogenous recipient RBCs persist and undergo normal clearance. We performed this study by treating murine donor RBCs with the mild catalase inhibitor aminotriazole to generate donor RBCs with a widened range of deformability. These cells were then fluorescently labeled and infused into syngeneic recipients. Using a microfluidic device capable of deformability-based sorting of RBCs, we concurrently measured the deformability distribution of donor RBCs pretransfusion and posttransfusion, along with endogenous recipient RBCs. Our findings provide direct evidence that RBCs with deformability profiles distinct from endogenous recipient RBCs are selectively and specifically cleared from circulation.
Introduction
In healthy humans, red blood cells (RBCs) circulate for an average of 120 days before reaching senescence and being cleared by the reticuloendothelial system. RBCs stored before being used in transfusion are known to degrade via the storage lesion, which may contribute to premature circulatory clearance and reduced overall circulation time for transfused RBCs.1,2 Understanding the mechanisms of RBC circulatory clearance could have significant implications in transfusion medicine, such as enabling the development of biomarkers to identify long-circulating donor RBCs, which would be of particular benefit to chronic transfusion recipients.
Deformability has long been understood to be a key factor in the circulatory clearance of RBCs.3-12 This understanding stems from the fact that these 8-μm discoid-shaped cells must deform hundreds of times as they transit through the microvasculature (∼2.5 μm in diameter),13 as well as through the endothelial clefts of the spleen (∼1 μm).14 The remarkable deformability of RBCs diminishes with natural aging and cold storage through multiple chemical processes, including lipid peroxidation,15-17 membrane asymmetry, Band 3 clustering,18 and slowed metabolism due to adenosine triphosphate and 2,3-diphosphoglycerate depletion.19 These changes collectively contribute to the loss of cell deformability, which then causes circulatory clearance through splenic mechanical sensing. Previous studies have shown that RBCs with reduced deformability, caused by physical, chemical, or pathological alterations, result in splenic retention and entrapment. Specifically, several studies have associated altered RBC deformability with hematological disease and reduced transfusion efficacy.20-23 The prevailing explanation for these findings is that RBCs are selectively removed from the circulation by the spleen, as demonstrated previously using ex vivo splenic perfusion models.14,24 These studies also showed that decreased cell deformability, induced by a reduction of RBC surface area, results in lower survivability after infusion into mice.25-27 Despite these insights, however, it remains unclear to what extent splenic clearance distinguishes donor RBCs based on their deformability relative to normally circulating RBCs.
In this study, we investigated whether the clearance of donor mouse RBCs depends on how closely their RBC deformability profile matches that of the endogenous recipient RBCs. We showed that donor RBCs with deformability distributions distinct from those of recipient RBCs were rapidly cleared, whereas donor RBCs with deformability distributions similar to those of endogenous recipient RBCs persisted and underwent normal clearance. To generate donor RBCs with a broader range of deformability, we chemically degraded these cells using aminotriazole (AMT), an irreversible catalase inhibitor that induces oxidative stress and rigidifies RBCs.28 These donor RBCs were then infused into syngeneic mice to evaluate 2 possible models of circulatory clearance. In Model 1, the circulatory clearance of donor RBCs is independent of RBC deformability, which would result in the deformability distribution of transfused RBCs being unchanged posttransfusion (Figure 1A). In Model 2, rigid RBCs are selectively eliminated from circulation, which would result in posttransfusion circulating donor RBCs that have a more deformable profile than the pretransfusion donor RBCs (Figure 1B). Using the microfluidic ratchet device to assess RBC deformability, we observed selective removal of rigidified RBCs, consistent with Model 2. In fact, the selective removal of RBCs based on deformability produces a circulating donor RBC population with a deformability distribution that matches that of endogenous recipient RBCs. Our findings suggest that the murine circulatory system actively regulates the deformability distribution of circulating RBCs by selectively purging cells that fail to provide the deformability required for optimal circulatory function.
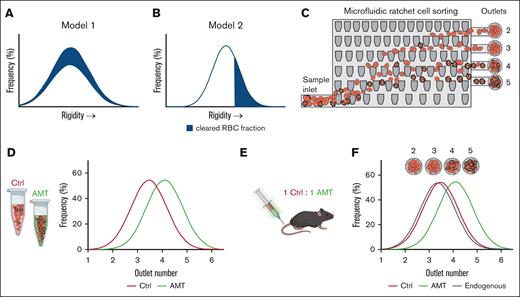
Study overview. (A-B) Potential models for circulatory clearance of donor RBCs as a function of cell deformability. If circulatory clearance is independent of deformability, then the deformability distribution of circulating cells remains unchanged (A). If circulatory clearance is dependent on cell deformability, the more rigid cells are preferentially removed (B). (C) The microfluidic ratchet device sorts RBCs based on deformability. Cells are typically distributed between outlets 2 and 5. (D-F) Experimental plan. Mouse RBCs are chemically treated using AMT to alter their deformability distribution relative to the control (D). Both control and AMT-treated cells are labeled with long-lasting fluorescent dyes and transfected into recipient mice (D-E). RBCs sampled from recipients are sorted using a microfluidic ratchet device to concurrently measure the deformability distribution of control donor RBCs, AMT-treated donor RBCs, and endogenous RBCs circulating in the recipient mice (F). Ctrl, control.
Study overview. (A-B) Potential models for circulatory clearance of donor RBCs as a function of cell deformability. If circulatory clearance is independent of deformability, then the deformability distribution of circulating cells remains unchanged (A). If circulatory clearance is dependent on cell deformability, the more rigid cells are preferentially removed (B). (C) The microfluidic ratchet device sorts RBCs based on deformability. Cells are typically distributed between outlets 2 and 5. (D-F) Experimental plan. Mouse RBCs are chemically treated using AMT to alter their deformability distribution relative to the control (D). Both control and AMT-treated cells are labeled with long-lasting fluorescent dyes and transfected into recipient mice (D-E). RBCs sampled from recipients are sorted using a microfluidic ratchet device to concurrently measure the deformability distribution of control donor RBCs, AMT-treated donor RBCs, and endogenous RBCs circulating in the recipient mice (F). Ctrl, control.
Methods
Deformability assessment using the microfluidic ratchet device
Our study used a microfluidic ratchet device to perform high-throughput RBC deformability profiling of endogenous and transfused RBCs, before and after transfusion. The microfluidic ratchet device has been used previously to sort RBCs based on deformability and measure RBC deformability.29,30 The operation of this device involves deforming RBCs through a matrix of tapered constrictions (Figure 1C). The openings of the constrictions are smaller than those of RBCs, ranging from 1.5 to 7.5 μm in the lateral direction and 4 μm in thickness. The openings of the tapered constrictions are constant along the horizontal direction in the matrix and progressively reduce from the bottom to the top of the matrix. The geometry of these constrictions enables the application of progressively greater deformation forces as individual RBCs travel upward through the matrix. The applied deformation force on each cell is dictated by the overall fluid flow through the matrix, which is determined by the pressure-driven flow through the precisely defined microchannels leading into the matrix. To sort RBCs based on deformability, an oscillatory pressure-driven flow is introduced vertically through the matrix, whereas a constant horizontal pressure-driven flow is introduced horizontally through the matrix. The cell sample is introduced from the bottom-left corner of the matrix, where it flows through the matrix in a diagonal pattern, with each cell ultimately reaching a limiting constriction and sorted into 1 of the 12 outlets. Most RBCs are distributed in outlets 2 to 5. After sorting, the distribution of RBCs can be determined by counting the number of cells at each outlet using microscopy. The count from each outlet is then divided by the total RBC count to obtain the percentage of cells at each outlet (Figure 1F).
The microfluidic ratchet device was fabricated using replica molding of polydimethylsiloxane silicone as previously described.30 The master mold for the microfluidic ratchet device was prepared using photolithographic microfabrication. The master mold was then used to create a secondary polyurethane mold using Smooth-Cast urethane resin (Smooth-Cast ONYX SLOW, Smooth-On), as described previously.29,30 This secondary mold was then used to fabricate single-use microfluidic devices using PDMS silicone (Sylgard-184, Ellsworth Adhesives).
To measure RBC deformability, the prepared RBC samples (see subsequent sections) were suspended in 1% hematocrit in Hanks' Balanced Salt Solution (HBSS) and Pluronic buffer, and sorted based on their deformability into 12 outlets. Cells from outlets 2, 3, 4, 5, and 6 were transferred to a 96-well flat-bottom plate. RBCs were imaged using a Nikon Ti-2 inverted microscope and counted using the Nikon Elements AR software. The deformability of endogenous, control, and AMT-treated RBC subpopulations was calculated by quantifying the number of fluorescently labeled and unlabeled RBCs in each fraction using the ImageJ software. The cumulative distribution of RBCs in the outlets was calculated, and the outlet where 50% of the most deformable cells were collected was described as the rigidity score (RS).
Mouse models, transfusions, and blood sampling
This study was completed in accordance with The University of British Columbia Animal Research Ethics Board under study A18-0362. To avoid the confounding effect of sex-mismatched transfusion, we obtained only female C57BL/6J mice between the ages of 6 to 10 weeks (Jackson Laboratories) as both donors and recipients in the study. Donor mice (N = 7) were euthanized using isoflurane and carbon dioxide. A blood sample was then collected by terminal bleeding via cardiac puncture using a heparinized syringe (BD) and a 25-gauge needle (BD). Blood from multiple donors was pooled and transferred to an EDTA tube. Transfusion recipient mice (N = 7) were weighed to determine the volume of transfusion at 10% of the estimated circulating blood volume.
The transfusions were performed via tail vein injection. After transfusion, RBCs were sampled from recipients via a tail vein prick using a 27-gauge needle. Blood samples were obtained 24 hours posttransfusion, followed by weekly administration for 5 weeks. The animals were weighed and monitored daily for any adverse effects. None were observed posttransfusion. At each sampling time point, ∼30 μL of blood was drawn from the recipient mice via a tail vein poke into an EDTA tube. The RBCs were then washed in HBSS and Pluronic buffer, as described later and assessed for RBC deformability and percentage of remaining transfused RBCs.
Chemical treatment of RBCs
Mouse RBCs were treated with AMT (MilliporeSigma) to achieve a measurable reduction in RBC deformability without inducing hemolysis. The AMT concentrations used were identified through a literature review and validated experimentally (supplemental Figure 1). RBCs from donor mice were pooled and chemically treated with 25 mM of AMT. Donor mice were euthanized as described earlier and blood was collected immediately via cardiac puncture. Approximately 500 μL of blood was collected from each donor mouse. Blood samples from donor mice were then pooled and washed 3× in HBSS and Pluronic F127 buffer (MilliporeSigma) at a spin speed of 400g for 10 minutes for the first wash, followed by 400g for 5 minutes for subsequent washes. The washed RBCs were then suspended in 20% hematocrit, either treated with 25 mM AMT (MilliporeSigma), or phosphate-buffered saline as a control. RBCs were then incubated for 2.5 hours at 37°C on a tube revolver (Thermo Fisher Scientific). After incubation, the samples were washed 3× with HBSS and Pluronic F127 buffer to wash out the remaining AMT.
After washing, the RBC samples were counted using a hemocytometer in preparation for staining using PKH fluorescent dyes (Sigma-Aldrich). The control RBCs were stained with PKH-26 (red). AMT-treated RBCs were stained with PKH-67 (green). Following the manufacturer’s instructions, 1 μL of stain was used for every 2 × 106 cells. After staining and washing, the control and AMT-treated RBCs were suspended in HBSS at 50% hematocrit and mixed in a 1:1 ratio for transfusion. Samples of unstained and stained RBCs from both the control and treatment groups were also assessed for deformability before transfusion to ensure that the staining protocol did not alter RBC deformability. No changes in RBC deformability were found after PKH-26 or PKH-67 staining.
Data analysis
We derived an RS from the cumulative distribution of the RBCs to compare the deformability of different samples, as described previously.29 Specifically, we plotted the distribution of RBCs for each sample as a cumulative distribution, where cells in the lower-number outlets were more deformable and cells in higher-number outlets were more rigid. RS is calculated as the outlet where the cumulative distribution function crosses 50%. Fractional outlet values were obtained via linear interpolation between data points in the cumulative distribution. RS data were analyzed using GraphPad Prism v10.0. The means and standard deviations are plotted unless otherwise stated. Linear regression with 95% confidence intervals was used to calculate the correlations between data sets. Data sets were compared using analysis of variance with Geisser-Greenhouse correction and Tukey correction for multiple comparisons.
Results
Approach
We investigated the effect of RBC deformability on circulatory clearance by concurrently profiling the deformability of normal and low-deformability donor RBCs as well as endogenous recipient RBCs at multiple time points pretransfusion and posttransfusion. Both normal- and low-deformability donor RBCs were labeled using durable membrane fluorescent dyes and profiled using a microfluidic ratchet device (Figure 1D). These cells were transfused into recipient mice and sampled periodically for up to 5 weeks posttransfusion (Figure 1E-F). Each sample was sorted using a microfluidic ratchet. The deformability distribution of the endogenous RBCs was determined by counting the number of cells at each outlet using bright-field microscopy. The deformability distributions of the circulating transfused RBCs were determined by counting the cells at each outlet using fluorescence microscopy. The deformability distributions of RBCs acquired pretransfusion, as well as for circulating RBCs collected at multiple time points posttransfusion, were then used to infer the deformability of RBCs cleared from circulation.
AMT decreases mouse RBC deformability
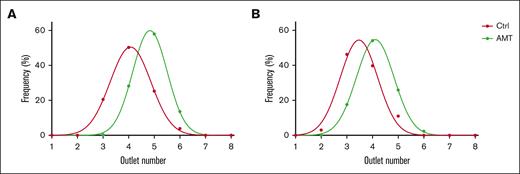
To obtain a reliable source of murine RBCs with reduced deformability, we treated donor RBCs with 25 mM AMT. AMT inhibits catalase,31 which is the main intraerythrocytic mechanism for degrading hydrogen peroxide.32 Hydrogen peroxide generated passively through metabolism accumulates in RBCs and produces oxidative stress that irreversibly reduces cell deformability.11 We performed AMT treatment on RBCs from 2 groups of mice (N = 7 total) and used the microfluidic ratchet to assess changes in the distribution of RBC deformability (Figure 2). AMT-treated RBCs were distributed in higher-number outlets, which was consistent with reduced deformability.
AMT treatment reduces murine RBC deformability. Deformability distributions of control and AMT-treated mouse RBCs. Data are shown for 2 pooled donor groups with n = 3 and n = 4, respectively. Ctrl, control.
AMT treatment reduces murine RBC deformability. Deformability distributions of control and AMT-treated mouse RBCs. Data are shown for 2 pooled donor groups with n = 3 and n = 4, respectively. Ctrl, control.
Selective circulatory clearance of RBCs based on deformability
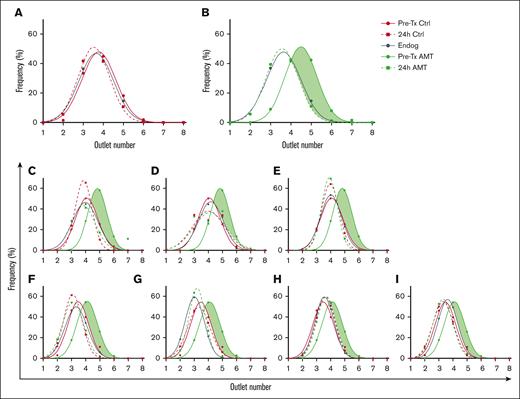
To elucidate the role of cell deformability in circulatory clearance, we infused control and AMT-treated donor RBCs in a 1:1 ratio into syngeneic recipient mice. We measured the deformability distribution after sorting donor RBCs at pretransfusion and posttransfusion, as well as endogenous recipient RBCs (Figure 3). For control donor RBCs, the pretransfusion and posttransfusion deformability distributions were nearly identical to each other and endogenous recipient RBCs (Figure 3A). For AMT-treated donor RBCs, the pretransfusion RBCs were significantly more rigid than endogenous recipient RBCs; however, at 24 hours posttransfusion, the deformability distribution of AMT-treated donor RBCs remaining in circulation was identical to that of endogenous recipient RBCs (Figure 3B). This result suggests that the population of rigid donor RBCs, shown as green shading in Figure 3B, was selectively eliminated from circulation. The observed selective clearance of rigid RBCs was consistent in all recipient mice in our study (Figure 3C-I). Together, these results confirm that the murine circulatory system selectively clears RBCs based on loss of deformability.
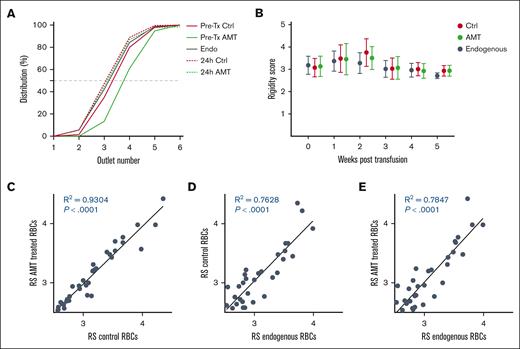
Deformability distribution after sorting of pretransfusion and posttransfusion donor RBCs, and endogenous recipient RBCs. In each graph, dots indicate the fraction of cells sorted into each outlet for each sample, whereas lines are Gaussian fits to show the distribution. (A) Deformability distribution of pretransfusion control donor RBCs (Pre-Tx Ctrl), 24-hour posttransfusion control donor RBCs (24-hour Ctrl), and endogenous recipient RBCs (Endog). (B) Deformability distribution of pretransfusion AMT-treated donor RBCs (Pre-Tx AMT), 24-hour posttransfusion AMT-treated donor RBCs (24-hour AMT), and endogenous recipient RBC (Endog). The shaded green area indicates the subtraction between the deformability distribution of AMT-treated and control donor RBCs, which is the population of RBCs selectively cleared from circulation. Data averaged from N = 7 recipients. (C-I) Deformability distributions of pretransfusion and posttransfusion donor RBCs, and endogenous recipient RBCs from individual mice.
Deformability distribution after sorting of pretransfusion and posttransfusion donor RBCs, and endogenous recipient RBCs. In each graph, dots indicate the fraction of cells sorted into each outlet for each sample, whereas lines are Gaussian fits to show the distribution. (A) Deformability distribution of pretransfusion control donor RBCs (Pre-Tx Ctrl), 24-hour posttransfusion control donor RBCs (24-hour Ctrl), and endogenous recipient RBCs (Endog). (B) Deformability distribution of pretransfusion AMT-treated donor RBCs (Pre-Tx AMT), 24-hour posttransfusion AMT-treated donor RBCs (24-hour AMT), and endogenous recipient RBC (Endog). The shaded green area indicates the subtraction between the deformability distribution of AMT-treated and control donor RBCs, which is the population of RBCs selectively cleared from circulation. Data averaged from N = 7 recipients. (C-I) Deformability distributions of pretransfusion and posttransfusion donor RBCs, and endogenous recipient RBCs from individual mice.
Deformability distribution of donor RBCs in circulation over 35 days
To assess the deformability distribution of transfused RBCs over their circulatory lifetime, we first established a measure of the median deformability of each sample called the RS. The RS is obtained by plotting the deformability distribution as a normalized cumulative distribution and then finding an interpolated outlet number at the 50% crossover line. By plotting these cumulative distribution curves, we obtained the RS values for pretransfusion control (3.31), pretransfusion AMT-treated (3.99), endogenous (3.18), 24-hour posttransfusion control (3.07), and posttransfusion AMT-treated RBCs (3.13). As expected, only pretransfusion AMT-treated RBCs had a significantly greater RS value, whereas all others had nearly identical RS values (Figure 4A).
Deformability distribution of pretransfusion and posttransfusion donor and recipient RBCs over 56 days. (A) Deformability distributions are shown as cumulative distributions for pretransfusion control, pretransfusion AMT-treated, 24-hour posttransfusion control, and 24-hour posttransfusion AMT-treated donor RBCs, as well as endogenous recipient RBCs. A measure of the median deformability of each sample, referred to as the RS, can be obtained from the 50% crossover line of the interpolated cumulative distribution graph. (B) RS for posttransfusion control and AMT-treated donor RBCs as well as endogenous recipient RBCs over 35 days. (C-E) Correlations between the RS for posttransfusion AMT-treated donor RBCs, posttransfusion control donor RBCs, and endogenous RBCs sampled from individual mice at all time points.
Deformability distribution of pretransfusion and posttransfusion donor and recipient RBCs over 56 days. (A) Deformability distributions are shown as cumulative distributions for pretransfusion control, pretransfusion AMT-treated, 24-hour posttransfusion control, and 24-hour posttransfusion AMT-treated donor RBCs, as well as endogenous recipient RBCs. A measure of the median deformability of each sample, referred to as the RS, can be obtained from the 50% crossover line of the interpolated cumulative distribution graph. (B) RS for posttransfusion control and AMT-treated donor RBCs as well as endogenous recipient RBCs over 35 days. (C-E) Correlations between the RS for posttransfusion AMT-treated donor RBCs, posttransfusion control donor RBCs, and endogenous RBCs sampled from individual mice at all time points.
We used this approach to assess the deformability distribution of posttransfusion control, posttransfusion AMT-treated, and endogenous RBCs in circulation in recipient mice over 35 days, which captures the bulk of the expected 45- to 55-day lifespan of murine RBCs (Figure 4B).33,34 The deformability distribution of all 3 populations of RBCs was highly similar and consistent over their entire circulatory lifetime when averaged over the 7 recipient mice. We further assessed the correlation between deformability distributions of posttransfusion control, posttransfusion AMT-treated, and endogenous RBCs sampled from individual recipient mice at each time point (Figure 4C-E). We found that the RS values for these 3 groups of RBCs were highly correlated despite the variability in the RS value of recipient RBCs. These results show that the deformability distribution of transfused RBCs in circulation matches the deformability distribution of endogenous RBCs. In other words, the murine circulatory system strictly and specifically regulates the deformability distribution of RBCs that are allowed to circulate.
Clearance of control and AMT-treated donor RBCs over 35 days
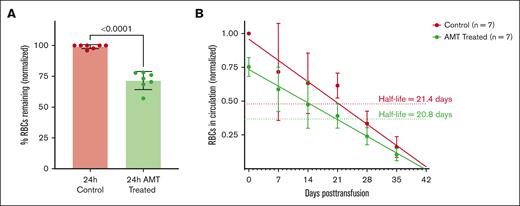
Because control and AMT-treated RBCs were transfused into recipients at a 1:1 ratio, we investigated the clearance of these RBCs by counting both cell types from each recipient blood sample. At 24 hours posttransfusion, 27.5% of AMT-treated RBCs were immediately cleared from circulation (Figure 5A). These cleared cells are the rigid RBCs indicated by the green shading in Figure 3. After 24 hours, both control and AMT-treated RBCs appeared to be cleared at a similar rate with half-lives of 21.4 days and 20.8 days, respectively (Figure 5B). This result confirms that (1) after removing the low-deformability RBCs, the remaining RBCs are cleared normally and (2) other than reducing deformability for some RBCs, AMT does not otherwise affect the clearance rate of donor RBCs.
Clearance of control and AMT-treated donor RBCs after transfusion. Transfused control and AMT-treated donor RBCs in circulation normalized to the mean number of control RBCs at 24 hours (A; N = 7) and over 35 days (B). After the initial clearance of 27.5% of the less deformable RBCs in the AMT-treated sample, the control and remaining AMT-treated RBCs are cleared with a half-life of 21.4 and 20.8 days, respectively.
Clearance of control and AMT-treated donor RBCs after transfusion. Transfused control and AMT-treated donor RBCs in circulation normalized to the mean number of control RBCs at 24 hours (A; N = 7) and over 35 days (B). After the initial clearance of 27.5% of the less deformable RBCs in the AMT-treated sample, the control and remaining AMT-treated RBCs are cleared with a half-life of 21.4 and 20.8 days, respectively.
Discussion
This study investigated the extent to which RBC deformability drives circulatory clearance. We addressed this question by monitoring the biophysical properties of transfused donor RBCs treated using AMT, which irreversibly reduces RBC deformability through intracellular oxidative stress resulting from catalase inhibition.28 Using the microfluidic ratchet device,29,30,35 we concurrently measured the deformability distribution of endogenous and transfused RBCs in the same recipients, which were fluorescence-labelled to distinguish control and rigidified RBC subpopulations. We also tracked the relative abundance of these cells at multiple time points posttransfusion. Through this analysis, we found that rigidified RBCs were rapidly cleared within the first 24 hours posttransfusion, but not control RBCs. Importantly, after selectively clearing the rigid RBCs from the circulation, the remaining RBCs maintained the same deformability distribution as the endogenous cells and were cleared at a normal rate. Together, these findings show that transfused RBCs are removed from circulation in a deformability-dependent manner and that this removal process produces an RBC population that matches the deformability distribution of endogenous RBCs in the recipient.
A key challenge in analyzing the biophysical properties of transfused donor RBCs in recipient blood samples is that recipient RBCs greatly outnumber donor RBCs. Conventional methods for assessing RBC deformability, such as ektacytometry36-38 and micropore filtration,39-41 measure bulk deformability and may not be able to resolve deformability changes resulting from a small subpopulation of donor RBCs. Alternatively, single-cell methods, such as atomic force microscopy,42,43 optical tweezers,44-46 and micropipette aspiration47-49 can characterize only a few cells. Using a microfluidic ratchet device to sort RBCs based on deformability enables high-throughput profiling of single cells, thereby allowing us to analyze a subpopulation of transfused RBCs within a much larger population of recipient RBCs. This capability offers the major advantage of being able to concurrently measure the deformability and abundance of both donor and recipient RBCs to track the fate of transfused RBCs over time.
We began this study by considering 2 possible models for clearance of transfused RBCs (Figure 1A-B). In Model 1, RBC clearance was independent of cell deformability such that cells with different deformabilities were equally likely to be cleared. In Model 2, RBC clearance was dependent on cell deformability, such that the least deformable cells were selectively cleared. We treated RBCs with low-dose AMT to induce RBC rigidification. AMT irreversibly inhibits RBC catalase, which causes RBC rigidification by altering the oxidation status of these cells.28,31 RBCs sensitive to catalase inhibition are likely to be older RBCs that are closer to senescence. When we transfused chemically rigidified RBCs into mice, we observed that ∼25% of the transfused cells were rapidly cleared from circulation within the first 24 hours, with the result that the RBCs remaining in circulation had a nearly identical deformability distribution as endogenous RBCs. These results unequivocally support Model 2, in which the murine circulatory system selectively removes RBCs that do not match deformability distribution in the transfusion recipient. Notably, the surviving RBCs exhibited a stable deformability distribution and clearance rate for the following 35 days, which is consistent with previous reports.2
Although these results are conclusive for the mouse transfusion model presented here, future work could investigate the generalizability of these findings to humans. The mechanism for deformability-mediated RBC clearance could be confirmed by replicating this work in catalase-knockout mice,50 which would not be affected by AMT treatment and would, therefore, not exhibit a distinct circulatory time for treated cells. Furthermore, it is important to recognize that there are differences between mouse and human RBCs and circulatory physiology as well as differences in genetics between human transfusion recipients. Future work should explore these distinctions, which may give rise to differences in clearance mechanisms and tolerance of transfused RBCs.
In summary, our results show that low-deformability donor mouse RBCs are selectively removed from circulation within 24 hours of infusion into a recipient mouse and that the deformability distribution of the remaining RBCs converges to the deformability distribution of endogenous recipient RBCs. The implication of this finding is that low-deformability RBCs will be cleared rapidly after transfusion and should be avoided in recipients who require transfusion support over long periods. We envision that this work will inspire future efforts to develop deformability-based assays that can be integrated into routine testing of RBC units to estimate the circulation time of the product. Longer-circulating RBC units could be preferentially reserved for chronic transfusion recipients to reduce their transfusion frequency, thereby reducing transfusion-associated morbidities.
Acknowledgments
The authors thank The University of British Columbia Animal Care Services for their technical assistance.
This work was supported by grants from the Canadian Institutes of Health Research (322375, 362500, and 414861), Natural Sciences and Engineering Research Council of Canada (538818-19, 2020-05412, and 2020-00530), and Canadian Blood Services Graduate Fellowship Program (E.I. and E.S.L.), which is funded by the federal government (Health Canada) and the provincial and territorial ministries of health.
The views herein do not necessarily reflect the views of Canadian Blood Services or the federal, provincial, or territorial governments of Canada.
Authorship
Contribution: H.M. conceived the idea and supervised the study; E.I., E.S.L., and K.M. performed the experimental work; and all authors contributed to the data analysis and wrote the manuscript.
Conflict-of-interest disclosure: H.M. is an inventor on the US patent 9880084, which was used in this work. The remaining authors declare no competing financial interests.
Correspondence: Hongshen Ma, Department of Mechanical Engineering, The University of British Columbia, 2054-6250 Applied Science Lane, Vancouver, BC, Canada V6T 1Z4; email: hongma@mech.ubc.ca.
References
Author notes
Data generated and analyzed during this study are available on reasonable request from the corresponding author, Hongshen Ma (hongma@mech.ubc.ca).
The full-text version of this article contains a data supplement.