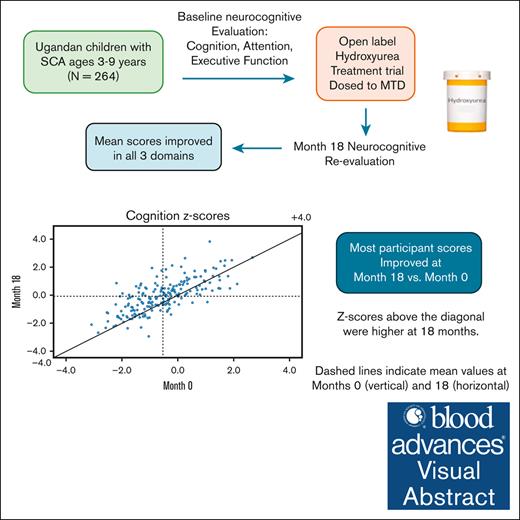
Key Points
Neurocognitive gains from hydroxyurea therapy were found among Ugandan children with SCA at month 18 vs enrollment.
Strongest predictor of improvement was baseline score, reinforcing importance of early initiation of treatment to reduce risk of decline.
Visual Abstract
Children with sickle cell anemia (SCA) frequently develop progressive neurocognitive impairment. We aimed to determine effects of hydroxyurea therapy on neurocognitive function in Ugandan children with SCA by comparing levels at enrollment to a planned 18-month interim assessment. Ugandan children (N = 264) aged 3 to 9 years attending SCA clinic were enrolled and treated in a 30-month, single-arm, open-label trial with escalation to maximum tolerated dose. Sibling controls (N = 110) without SCA underwent parallel neurocognitive testing to establish age-normalized z scores for comparison. At enrollment, mean participant age was 5.6 ± 1.2 years, with comparable socioeconomic status and caregiver education vs. controls. Month 18 mean daily hydroxyurea dose was 25.4 mg/kg. We found significantly improved participant z scores vs. baseline in all 3 neurocognitive domains tested: cognition: −0.54 ± 1.10 vs. −0.07 ± 1.16, P < .001; attention: −0.07 ± 1.01 vs. 0.27 ± 0.84, P < .001; and executive function: −0.06 ± 0.62 vs. 0.08 ± 0.72, P = .010. No month-18 measures differed from controls. Participants with SCA and cognitive impairment declined from 8.7% at enrollment to 4.0%, vs. 1.6% of controls; with decreased mean TCD velocity. Improved neurocognitive treatment z-scores were associated with higher baseline scores and socioeconomic status, hydroxyurea-associated lower TCD velocity, and hematological-induced effects, eg higher hemoglobin. Despite improvement, unadjusted neurocognitive scores remained negatively associated with age. Hydroxyurea therapy improved SCA neurocognitive function in sub-Saharan children, potentially aided by practice effects. Early treatment is needed for optimal impact. Our ongoing trial will assess impact from longer-term hydroxyurea therapy. This trial was registered at www.ClinicalTrials.gov as #NCT04750707.
Introduction
Children with sickle cell anemia (SCA) are at considerable risk of neurocognitive impairment that increases with age from cumulative insults.1-3 Vulnerability stems from chronic anemia, continuing and/or episodic hypoxia, and infections, such as endemic malaria. Severe anemia causes abnormal cerebral blood flow and stresses oxygen delivery.2,4,5 Effects may manifest as cerebrovascular injury, leading to overt stroke or, more commonly, silent cerebral infarctions (SCI) which are not associated with acute neurological events and accumulate with age.6 Nonetheless, stroke and SCI alone do not fully account for the high risk of neurocognitive impairment in pediatric SCA, as children with normal magnetic resonance imaging (MRI) findings can also have cognitive difficulties.7,8 Additional contributions to risk include malnutrition and low parental education and socioeconomic status (SES).9
Neurocognitive impairment is most consistently found in domains of cognition, attention, and executive function and compared with unaffected siblings, local, or regional performance standards.10 To date, most studies of pediatric neurocognitive impact from SCA have been performed in high-income regions, with only a modest literature base from research conducted in sub-Saharan Africa.11-13 We previously conducted a cross-sectional study, BRAIN SAFE, to assess the burden and risk of neurological and cognitive impairment in pediatric SCA in Uganda using standardized neurocognitive assessments compared with siblings without SCA, 11% of children with SCA had neurocognitive impairment.14,15 Similar to US reports, significantly lower neurocognitive function was found in children with SCA aged 5 years and older.2,16
Two recent meta-analyses of multiple pediatric cross-sectional studies, a long-term single-site registry, and a few modestly-sized trials have supported the likelihood of improved neurocognition from hydroxyurea (HU) therapy and underscored the greater impact from earlier treatment initiation.2,5,17 Nonetheless, most previous HU treatment trials have had insufficient power to discern significant predictors of improved function beyond hemoglobin (Hb) level, and none have been reported from sub-Saharan Africa where 80% of affected children live.18 Our hypothesis was that HU would stabilize or improve neurocognitive function across multiple domains and that key demographic and clinical features, including lowered transcranial Doppler (TCD) velocity from HU therapy, would identify greater likelihood of stabilization or improvement.17,19 Here, we present the neurocognitive gains detected in the 18-month interim analyses of our 30-month, open-label, single-arm HU trial, “BRAIN SAFE II,” in Ugandan children aged 3 to 9 years.
Methods
BRAIN SAFE II was designed as a 36-month trial of daily HU for children with SCA, aged 3 to 9 years, enrolled from the large Mulago Hospital Sickle Cell Clinic (MHSCC) national referral hospital in Kampala. The overall trial aim was to reduce brain-associated SCA morbidities of neurocognitive dysfunction19 (additional outcomes of overt stroke and stroke risk will be reported separately). As better non-neurological pediatric SCA outcomes have been documented for HU, ethical considerations required an open-label trial without an untreated SCA control group in parallel.20 Study HU was escalated to maximum tolerated dose (MTD) based on report of fewer non-neurological adverse outcomes, both SCA related and SCA unrelated, from MTD vs standard dosing.19,21 Premature trial end points were defined as death or acute stroke.
Trial regulatory oversight and approvals were provided by the Makerere University School of Medicine Research and Ethics Committee (MHREC 1802), Columbia University Institutional Review Board (protocol no. AAAS8955), Mulago Hospital Institutional Review Board, and trial Data Safety and Monitoring Committee. Family- and patient-facing study documents, including neurocognitive test materials, were available in English or Luganda, the predominant local language among families at MHSCC. All study staff were native speakers in both English and Luganda.
Trial data were entered into a secure REDCap (Research Data Capture) database.
Sample size
Only a modest literature existed from prospective HU trials on neurocognitive outcomes and none from the region. Hence, the estimated trial sample size was based on previous pediatric trials for abnormal TCD findings performed in the United States and Jamaica as a separate primary outcome.22,23 A sample of 250 participants with SCA, plus 8% to accommodate expected participant loss, totaling 270, was determined to provide sufficient power (alpha = 0.05; beta = 84%) to detect significant decreased TCD time-averaged mean maximum velocity (TAMV) of ≥15 cm/s in 3 years to lower stroke risk. This sample size was estimated to provide sufficient power to detect changed neurocognitive scores of ≥0.015 z-scores with >80% power.24
Planned midpoint analysis and data capture
We chose to perform a month 18 midpoint evaluation to assess interim progress of an open-label trial testing an approved SCA medication. Trial duration was shortened to 30 months based on the results reported here.
Study procedures
Potentially eligible children were identified using the MHSCC clinical roster. Caregivers were contacted to confirm the child’s age and offer recruitment screening. Eligibility criteria included SCA confirmation by Hb electrophoresis; Hb ≥6.0 g/dL; lack of evidence of previous stroke from a stroke-focused neurological examination, the Pediatric National Institutes of Health Stroke Scale performed by a trained study physician, and other criteria (Table 1).14 At enrollment, parents were interviewed and participants examined to ensure a steady health state, that is, without pain episodes or fever in the preceding 2 weeks. Review of parental report and MHSCC records confirmed no blood transfusion within the previous 90 days.
Participant eligibility criteria
| 1. Ages 3 to 9 y (inclusive) at enrollment |
| 2. Confirmed laboratory diagnosis of HbSS or HbS-B0 thalassemia (available within 1 wk of enrollment) |
| 3. Hb ≥ 6.0 g/dL |
| 4. No history of HU use for >6 mo |
| 5. Not acutely ill at enrollment: fever, respiratory infection, sickle crisis within the previous 2 wk, or blood transfusion within the past 90 d (temporary exclusion criteria) |
| 6. Had attended the Mulago Hospital SCA clinic ≥2 times in the previous 4 y, or ≥1 time in the previous 2 y if age ≥2 y (to help ensure attendance of study visits) |
| 7. Parent and child are able to speak and understand English or Luganda (a predominant language in Kampala) |
| 8. No history of neurological abnormality known before the age of 4 mo (to avoid early non-SCA brain injury) |
| 9. Child was not currently enrolled in a clinical intervention trial |
| 10. Child and parent/legal guardian were willing to participate (if child was ≥8 y of age for assenting) |
| 11. Absence of previous stroke by standardized stroke-focused PedNIHSS examination by a study physician∗ |
| 1. Ages 3 to 9 y (inclusive) at enrollment |
| 2. Confirmed laboratory diagnosis of HbSS or HbS-B0 thalassemia (available within 1 wk of enrollment) |
| 3. Hb ≥ 6.0 g/dL |
| 4. No history of HU use for >6 mo |
| 5. Not acutely ill at enrollment: fever, respiratory infection, sickle crisis within the previous 2 wk, or blood transfusion within the past 90 d (temporary exclusion criteria) |
| 6. Had attended the Mulago Hospital SCA clinic ≥2 times in the previous 4 y, or ≥1 time in the previous 2 y if age ≥2 y (to help ensure attendance of study visits) |
| 7. Parent and child are able to speak and understand English or Luganda (a predominant language in Kampala) |
| 8. No history of neurological abnormality known before the age of 4 mo (to avoid early non-SCA brain injury) |
| 9. Child was not currently enrolled in a clinical intervention trial |
| 10. Child and parent/legal guardian were willing to participate (if child was ≥8 y of age for assenting) |
| 11. Absence of previous stroke by standardized stroke-focused PedNIHSS examination by a study physician∗ |
HbS-B0, compound heterozygote HbS and Hb Beta0 thalassemia; HbSS, homozygous HbS.
Pediatric National Institutes of Health Stroke Scale.13
Anthropometric measurements determined malnutrition from UNICEF-WHO (United Nations Children's Fund-World Health Organization) growth standards by age and sex, with malnutrition defined as lower than −2 z-scores for weight for age (wasting).14 Oxygen saturation was obtained using a portable cutaneous meter (oximeter model X004C; Arcatron Mobility, Maharashtra, India). Family SES was determined using a modified locally validated tool, the Middle Childhood Home Observation for the Measurement of the Environment, scored from 6 to 18, and caregiver educational attainment25; each of these variables can affect child neurocognitive performance.1,25 Demographic and clinical variables were collected to test relationships to the neurocognitive outcomes.
Sibling controls (N = 110) without SCA were intentionally recruited from ages 3 to 12 years, 10 to 20 candidates of each age, to establish age- or age- and sex-normalized neurocognitive z scores (depending on the measure used) through trial completion. By definition, control mean z-scores were set at 0.0 ± 1.0 for use to generate z scores for the SCA sample.26,27 Importantly, sibling data were also used to account for local social and cultural aspects; SES and caregiver educational attainment were obtained to compare the SCA sample.10,25 Controls also underwent anthropometric measurement and Hb electrophoresis to confirm lack of SCA (HbAA or HbAS) and clinical and stroke-focused examinations to ensure their good health.
Neurocognitive measures
Participants with SCA underwent neurocognitive assessment at enrollment and month 18 (roughly trial midpoint) using standardized test measures, selected for comprehensive domain testing, manageable pediatric testing demands, utility over the study age range, team familiarity of administration, and validated local translations.15 To the degree possible, we selected neurocognitive measures for which a single test could be administered across all participant ages to avoid noncontinuous scoring. Of the 4 measures used, 3 had been validated in pediatric studies conducted in multiple African countries, including non-English speakers and Ugandan children.28-31 Testing staff were trained and supervised as previously described.14 The composite score defined the outcome of each measure for broader interpretation (Appendix 1).10 Impairment on any neurocognitive measure is defined by z-score below −2.27 All measures were translated into Luganda using rigorously applied standardized methods (Appendix 1).30,32
The Kaufman Assessment Battery for Children, second edition (KABC-II), for cognition used its composite index from subtests, previously reported as validated in Uganda and widely used for ages ≥5 years.33,34 Among our control group, 4-year-olds, but not 3-year-olds, demonstrated age-appropriate KABC-II testing ability. Hence, KABC-II test scores for those aged ≥4 years at baseline were included in the SCA analyses, allowing for use of a single test of cognition throughout the trial. Test of Variables of Attention (TOVA, visual version) measured attention and was confirmed as a valid test in previous Ugandan studies for ages ≥5 years using the subtests’ standard composite score (Appendix 1).15,35,36 Executive function was assessed by the following 2 different measures: (1) a multi-item caregiver checklist, the Behavioral Rating Inventory for Executive Functions (BRIEF) for ages 6 to 12 years or the preschool version, BRIEF-P, for ages 3 to 5 years, each using the global executive composite score; and (2) a direct test of executive function, Developmental Neuropsychological assessment, second edition (NEPSY-II), valid for children ages ≥5 years, with composite outcome of auditory attention and response set within the executive functioning domain. Additional information on use of the NEPSY-II is described in Appendix 1.37-39
Overall, higher scores signify better results. Because scores for the BRIEF are normally inverted (negative scores being higher), we flipped those scores (multiplied by −1.0) for consistent direction of scores reported over time.15 By convention for testing behavioral measures, z-scores for the TOVA and BRIEF/BRIEF-P were both age- and sex-normalized based on the control sample.15
In the team’s previous prospective pediatric trials of non-SCA chronic infirmities, age-normalized z-scores using these same measures for children over similar age ranges and timing of re-testing had not suggested practice effects.40 Nonetheless, we checked for evidence of practice effects by retesting a randomly selected subset of 42 (38.2%) of 110 controls at 18 months.
TCD
Standardized TCD measured cerebral arterial maximum time-averaged mean velocity (TAMV) used a portable 2 MHz nonimaging instrument (SonaraTek, Natus Medical Incorporated, Middleton, WI). Four study doctors and nurses were trained and supervised by 2 of the coauthors (D.M. and M.N.), both of whom were certified at a US academic medical center to perform and interpret pediatric TCD results. Each study staff performing TCDs performed at least 40 complete practice TCDs before independence after reaching a minimum threshold correlation coefficient for the middle cerebral arteries of Cronbach’s alpha of 0.8 between paired staff for the same child.41
We used the Stroke Prevention Trial in Sickle Cell Anemia (STOP) protocol for insonating cerebral vessels except the basilar artery, with elevated TAMV for children classified as “conditional” (170-199 cm/s) or “abnormal” (≥200 cm/s).23,42 More recently, both categories are sometimes considered as higher stroke risk.43 Quality control measures were performed as previously reported.14 TCDs without bilateral insonation of all 8 artery pairs were recorded as “inadequate,” of which none were categorized as abnormal. Participants with abnormal TCD result at enrollment or at subsequent assessments were offered blood transfusion, per standard MHSCC care. Children with abnormal TCD results had repeat testing after 1 month and then, if confirmed, every 6 months until resolution or persistence of abnormal TAMV result.19
HU trial dosing
After the baseline assessments, participants with SCA began HU therapy, determined by standard hematological parameters and followed according to local guidelines. Mean daily HU dose was initiated at 20 ± 2.5 mg/kg, with MTD escalation to a maximum of 30 mg/kg per day, having been demonstrated as safe for Ugandan children.21 The study drug was available in 100 mg and 1000 mg scored tablets for accurate, uniform daily dosing. At study visits, staff reviewed interim medical histories, drug safety, and complete blood count parameters for dose escalation, and dispensed sufficient intervisit study drug. Adherence monitoring consisted of caregiver report, residual pill counts, and laboratory trends of red cell mean corpuscular volume (MCV). Complete blood count safety monitoring was performed at baseline, month 1 and at 2-month interval visits for the initial trial year, and 3-month intervals thereafter.21 Dose was escalated by 2.5 mg/kg based on hematological criteria at a minimum of 8-week intervals, as tolerated, with a target dose to reach absolute neutrophil count of 2.0 to 4.0 × 109 per liter.18,20
Statistical analyses
Here, our primary analysis gauged HU treatment effects compared with baseline measures on neurocognitive performance. Comparisons between the SCA and control samples and the SCA at the 2 time points were carried out by 2-sided Student t test for continuous variables and by χ2 or Fisher exact test for categorical variables. Age effects on neurocognitive performance were assessed by 2-tailed Pearson and Spearman correlations using unadjusted data. Changes to neurocognitive test z-scores from baseline were evaluated by paired t tests. Bivariate and multivariate linear regression analyses were performed to identify key baseline demographic, social, and clinical variables that were associated with the change in neurocognitive measures.26,27 The bivariate analysis was used to adjust the corresponding baseline cognitive test scores. Variables that were significant in the bivariate analysis (P < .2), including score at enrollment, were selected for multivariable analysis. Analyses of the difference in raw neurocognitive scores over time for the SCA sample and the retested controls by general linear regression analysis for repeated measures provided independent assessments of each group.44 SAS version 9.4 was used for analysis.
For each neurocognitive test, SCA participant age-normalized z-scores were determined using scores generated by the sibling control sample, the latter defined as equal to 0.0 with standard deviation of 1.0 for each component and composite test measure performed (Appendix 2).26 To look for a possible learning effect from participant retesting, we analyzed the z-scores for the control group at month 0 and month 18 to look for an improvement without an intervention.
Results
Enrollment was conducted from March to December 2021, under Uganda Ministry of Health COVID-19 pandemic clinical guidelines. A total of 270 of 334 randomly screened children receiving care at MHSCC who met preliminary eligibility criteria were enrolled, pending Hb electrophoresis. Subsequently, 5 children without SCA were disqualified (Figure 1). One family withdrew their child before starting the study drug. Overall, 264 children began the study HU. Later on, 3 additional families withdrew (for social reasons) and 1 child was lost to follow-up. All trial assessments were performed as scheduled. Provision of the study HU and travel cost coverage contributed to excellent retention (>98%) and attendance at study visits. A total of 254 participants reached the planned midpoint assessment of 18 months.
CONSORT (Consolidated Standards of Reporting Trials) diagram for the planned month 18 analysis.
CONSORT (Consolidated Standards of Reporting Trials) diagram for the planned month 18 analysis.
Mean age at enrollment for the SCA group was 5.6 ± 1.72 years, 137 (51.9%) female participants, with mean Hb of 7.8 ± 1.2 g/dL, and 24 (9.2%) were malnourished by WHO weight-for-age standards (Table 2). The mean age of sibling control sample was significantly older due to intentional recruitment of children from ages 3 to 12 years. Controls had higher mean Hb (12.5 ± 1.1 g/dL, P <.001) and comparable proportion of malnutrition; mean SES and caregiver education did not differ by group.
Demographic, clinical, and laboratory data of the sibling controls vs SCA samples at enrollment (month 0)
| . | Controls (N = 110) . | SCA Month 0 (N = 264) . | P value . |
|---|---|---|---|
| Age (y) | 7.6 ± 3.0 | 5.6 ± 1.2 | <.001 |
| Sex (female) | 63 (57.3%) | 137 (51.9%) | .34 |
| Malnutrition∗ (%) | n = 79 3 (3.8%) | n = 262 24 (9.2%) | .16 |
| Hb (g/dL) | n = 105 12.5 ± 1.1 | 7.8 ± 1.2 | <.001 |
| Socioeconomic scale† | 7.1 ± 2.6 | 6.6 ± 2.3 | .12 |
| Caregiver education‡ | |||
| Lower | 41 (37.3%) | 102 (38.6%) | .81 |
| Higher | 69 (62.7%) | 162 (61.4%) |
| . | Controls (N = 110) . | SCA Month 0 (N = 264) . | P value . |
|---|---|---|---|
| Age (y) | 7.6 ± 3.0 | 5.6 ± 1.2 | <.001 |
| Sex (female) | 63 (57.3%) | 137 (51.9%) | .34 |
| Malnutrition∗ (%) | n = 79 3 (3.8%) | n = 262 24 (9.2%) | .16 |
| Hb (g/dL) | n = 105 12.5 ± 1.1 | 7.8 ± 1.2 | <.001 |
| Socioeconomic scale† | 7.1 ± 2.6 | 6.6 ± 2.3 | .12 |
| Caregiver education‡ | |||
| Lower | 41 (37.3%) | 102 (38.6%) | .81 |
| Higher | 69 (62.7%) | 162 (61.4%) |
End points and AEs
By month 18, 3 families withdrew for social reasons and 1 child was lost to follow-up. Adverse clinical and laboratory events are as tabulated (Appendix 3). Six participants had prematurely reached a predetermined trial end point: 4 with acute stroke and 2 deaths without evident stroke (details to be reported separately). No additional strokes occurred after month 18; stroke incidence in the 30-month trial was 0.62 per 100 participant years. One death occurred from fatal cardiopulmonary arrest early in the trial; a second participant died at home without known antecedent illness. One participant had an acute stroke in the initial trial month (Figure 1). All other serious adverse events (AEs) were hospitalizations for >7 days for pain crises and/or serious infections. Ill visits were handled by the study team’s medical personnel, enabling evaluation and documentation of acute illnesses throughout the trial. SCA-related AEs were primarily painful crises requiring hospital admission. Most non-SCA–related AEs were upper respiratory infections, potentially associated with the concurrent COVID-19 pandemic peak in Uganda. Only 9 participants had severe malarial infections, probably due to the relative protection from HU therapy.45 AEs related to the study drug were limited to asymptomatic dose-limiting cytopenias.
Clinical and laboratory trial data
HU dose was escalated to MTD at mean 25.4 ± 3.1 mg/kg using standard criteria.21 Demographic, clinical, and laboratory indices at month 18 included improved oxygen saturation, Hb and HbF levels, and other HU-responsive laboratory values (Table 3). Mild dose-limited cytopenias required standard temporary hold and dose adjustment. By consensus decision of 2 MHSCC hematologists who were not study staff, 1 child underwent a splenectomy for persistent cytopenias associated with chronic hypersplenism which had precluded continued HU therapy. She resumed the study drug after uncomplicated surgical recovery.
Demographic, clinical, and laboratory data for the SCA sample at month 0 vs month 18
| . | SCA at month 0 (N = 264) . | SCA at month 18 (N = 254) . | Differences (month 18-month 0) (N = 254) . | P value . |
|---|---|---|---|---|
| Age (y) | 5.6 ± 1.2 | 7.1 ± 1.7 | ||
| Sex (female) | 137 (51.9%) | 131 (52.0%) | NS | |
| Malnutrition∗ (%) | 24 (9.2) | n = 241 19 (7.9) | NS | |
| Oxygen saturation (in %) | n = 263 95.9 ± 6.8 | 97.4 ± 2.0 | n = 253 1.4 ± 7.1 | .002 |
| HU dose (mg/kg) | n = 263 20.2 ± 0.9 | n = 253 25.4 ± 3.1 | n = 252 5.2 ± 3.1 | <.001 |
| Hb (g/dL) | 7.8 ± 1.2 | 8.8 ± 1.3 | 1.0 ± 1.2 | <.001 |
| Fetal Hb (in %) | n = 256 11.9 ± 8.1 | 23.7 ± 11.2 | n = 242 12.0 ± 10.7 | <.001 |
| MCV (fL) | 81.0 ± 8.9 | 96.9 ± 10.8 | 15.9 ± 8.8 | <.001 |
| WBC (×109/L) | 13.8 ± 4.2 | 9.6 ± 3.2 | −4.1 ± 4.2 | <.001 |
| ANC (×109/L) | 5.6 ± 2.3 | 3.9 ± 1.8 | −1.7 ± 2.5 | <.001 |
| ARC (×109/L) | n = 262 344.7 ± 120.0 | 212.2 ± 101.6 | −130.1 ± 122.8 | <.001 |
| Platelets (×109/L) | n = 263 443.7 ± 153.0 | 368.2 ± 168.4 | n= 253 −76.1 ± 191.9 | <.001 |
| TCD TAMV† (cm/s) | n = 257 148.4 ± 29.2 | n = 246 130.9 ± 21.3 | n = 242 −17.5 ± 22.2 | <.001 |
| TCD category‡, n (%) | ||||
| Abnormal | 15 (5.7) | 4 (1.6) | Kappa = 0.24 (95% confidence interval, 0.12-0.36) | <.001 |
| Conditional | 42 (15.9) | 10 (3.9) | ||
| Normal | 191 (72.4) | 228 (89.8) | ||
| Inadequate | 16 (6.1) | 12 (4.7) |
| . | SCA at month 0 (N = 264) . | SCA at month 18 (N = 254) . | Differences (month 18-month 0) (N = 254) . | P value . |
|---|---|---|---|---|
| Age (y) | 5.6 ± 1.2 | 7.1 ± 1.7 | ||
| Sex (female) | 137 (51.9%) | 131 (52.0%) | NS | |
| Malnutrition∗ (%) | 24 (9.2) | n = 241 19 (7.9) | NS | |
| Oxygen saturation (in %) | n = 263 95.9 ± 6.8 | 97.4 ± 2.0 | n = 253 1.4 ± 7.1 | .002 |
| HU dose (mg/kg) | n = 263 20.2 ± 0.9 | n = 253 25.4 ± 3.1 | n = 252 5.2 ± 3.1 | <.001 |
| Hb (g/dL) | 7.8 ± 1.2 | 8.8 ± 1.3 | 1.0 ± 1.2 | <.001 |
| Fetal Hb (in %) | n = 256 11.9 ± 8.1 | 23.7 ± 11.2 | n = 242 12.0 ± 10.7 | <.001 |
| MCV (fL) | 81.0 ± 8.9 | 96.9 ± 10.8 | 15.9 ± 8.8 | <.001 |
| WBC (×109/L) | 13.8 ± 4.2 | 9.6 ± 3.2 | −4.1 ± 4.2 | <.001 |
| ANC (×109/L) | 5.6 ± 2.3 | 3.9 ± 1.8 | −1.7 ± 2.5 | <.001 |
| ARC (×109/L) | n = 262 344.7 ± 120.0 | 212.2 ± 101.6 | −130.1 ± 122.8 | <.001 |
| Platelets (×109/L) | n = 263 443.7 ± 153.0 | 368.2 ± 168.4 | n= 253 −76.1 ± 191.9 | <.001 |
| TCD TAMV† (cm/s) | n = 257 148.4 ± 29.2 | n = 246 130.9 ± 21.3 | n = 242 −17.5 ± 22.2 | <.001 |
| TCD category‡, n (%) | ||||
| Abnormal | 15 (5.7) | 4 (1.6) | Kappa = 0.24 (95% confidence interval, 0.12-0.36) | <.001 |
| Conditional | 42 (15.9) | 10 (3.9) | ||
| Normal | 191 (72.4) | 228 (89.8) | ||
| Inadequate | 16 (6.1) | 12 (4.7) |
Bold values indicate significance at P < .05.
ANC, absolute neutrophil count; ARC, absolute reticulocyte count; MCV, mean corpuscular volume; NS, not significant.
Defined as weight-for-age z score below −2.0 by WHO global standards.13
TCD mean time-averaged maximum velocity, excluding inadequate scans.
Per international standards for children with SCA.5
Primary outcomes
Neurocognitive function
To determine the impact from HU therapy, participants with SCA were tested at enrollment and at month 18 by age-appropriate measures of cognition, attention, and executive function. The test was attempted in Luganda by 94% of the participants, precluding meaningful comparison of scores by language. At enrollment, participants with SCA had lower mean neurocognitive z scores for cognition than the controls (Appendix 4). Among the SCA sample, 18 of 208 (8.7%) had z-scores for cognition below −2.0 (“impaired”), compared with 1 of 64 controls (1.6%).
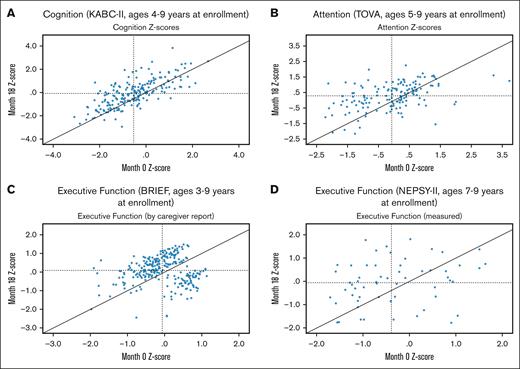
At month 18, 8 of 202 (4.0%) participants with SCA were cognitively impaired, including 5 (27.8%) of those originally impaired (one of the 18 had reached a premature end point of acute stoke). Most importantly, at month 18, the mean z-scores of the SCA sample significantly improved vs baseline in all 4 measures (Table 4), with no differences from the controls. Despite adjustment for multiple testing by Bonferroni correction, all measures remained significantly improved. Heterogeneity of initial and treatment-associated z-scores was expected (Figure 2A-D), given the range of disease-associated complications and backgrounds of the participants.16,46
Neurocognitive mean z scores comparing the composite scores for the SCA sample at months 0 and 18
| Composite test (age in y) . | SCA at month 0 . | SCA at month 18 . | Difference∗ . | P value . |
|---|---|---|---|---|
| KABC-II MPI (≥4 y) | n = 208† | n = 202† | n = 201 | |
| Cognition | −0.54 ± 1.10 | −0.07 ± 1.16 | 0.45 ± 0.84 | <.001 |
| TOVA D’ (≥5 y) | n = 152 | n = 150 | n = 146 | |
| Attention | −0.07 ± 1.01 | 0.27 ± 0.84 | 0.35 ± 0.97 | <.001 |
| BRIEF GEC (all ages)‡ | n = 264 | n = 254 | n = 254 | |
| Executive function | −0.06 ± 0.62 | 0.08 ± 0.72 | −0.15 ± 0.92 | .010 |
| NEPSY-II AAR (≥7 y) | n = 60 | n = 62 | n = 59 | |
| Executive function | −0.38 ± 0.93 | −0.09 ± 0.97 | 0.32 ± 1.18 | .035 |
| Composite test (age in y) . | SCA at month 0 . | SCA at month 18 . | Difference∗ . | P value . |
|---|---|---|---|---|
| KABC-II MPI (≥4 y) | n = 208† | n = 202† | n = 201 | |
| Cognition | −0.54 ± 1.10 | −0.07 ± 1.16 | 0.45 ± 0.84 | <.001 |
| TOVA D’ (≥5 y) | n = 152 | n = 150 | n = 146 | |
| Attention | −0.07 ± 1.01 | 0.27 ± 0.84 | 0.35 ± 0.97 | <.001 |
| BRIEF GEC (all ages)‡ | n = 264 | n = 254 | n = 254 | |
| Executive function | −0.06 ± 0.62 | 0.08 ± 0.72 | −0.15 ± 0.92 | .010 |
| NEPSY-II AAR (≥7 y) | n = 60 | n = 62 | n = 59 | |
| Executive function | −0.38 ± 0.93 | −0.09 ± 0.97 | 0.32 ± 1.18 | .035 |
Bold values indicate significance at P < .5.
For each measure, the same participants were compared between the 2 time points.
BRIEF GEC, Behavior Rating Inventory of Executive Function Global Executive Composite; KABC-II MPI, Kaufman Assessment Battery for Children, second edition, Mental Processing Index; NEPSY-II AAR, Developmental Neuropsychological Assessment; TOVA, Test of Variables of Attention.
The same participant sample was compared in the analysis of each measure.
17 (8.2%) were cognitively impaired (z score below −2.0) at enrollment compared with 8 (4.0%) at month 18.
BRIEF scores were flipped (multiplying by −1.0) for consistency in direction of change across measures used.14
Scatter plots of each of 4 neurocognitive tests of the SCA. Dashed lines represent the group mean at enrollment (vertical) and month 18 (horizontal). Panels A-D depict data from the month 18 testing for cognition (panel A), attention (panel B), executive function by caregiver report (panel C) and by direct test (panel D).
Scatter plots of each of 4 neurocognitive tests of the SCA. Dashed lines represent the group mean at enrollment (vertical) and month 18 (horizontal). Panels A-D depict data from the month 18 testing for cognition (panel A), attention (panel B), executive function by caregiver report (panel C) and by direct test (panel D).
Next, we evaluated the characteristics associated with improved neurocognitive performance. The z-scores of both cognition and attention at month 18 were strongly correlated with baseline performance (r = 0.72 and 0.46, respectively, both P < .001), with a similar trend for executive function (NEPSY-II r = 0.24, P = .06). Additional individual predictors of improved performance at month 18 included TCD velocity (r = 0.66, P < .001), female sex, higher SES and/or caregiver educational attainment, higher Hb, and lower white blood cell (WBC) (data not revealed). However, due to the strong influence from baseline on month 18 outcomes, Table 5 demonstrates bivariate and multivariate analyses adjusted for baseline score. Under those conditions, only SES, TCD velocity, Hb, WBC, and absolute reticulocyte count were associated with 1 to 2 of the outcomes.
Bivariate and multivariate analyses of improved neurocognitive z scores at month 18 from enrollment among the SCA sample for each of the 4 measures
| Cognition (n = 201), ages ≥4 y at enrolment . | ||||
|---|---|---|---|---|
| Predictors at mo 18 . | Bivariate analysis (adjusted for score at enrollment) . | Multivariable analysis . | ||
| Coefficient (SE) . | P value . | Coefficient (SE) . | P value . | |
| Score at enrollment | −0.28 (0.053) | <.001 | ||
| SES∗ | 0.054 (0.024) | .03 | 0.05 (0.025) | .048 |
| Platelets (×109/L) | −0.001 (0.0004) | .03 | −0.0007 (0.0004) | .047 |
| Attention (n = 146), ages ≥5 y at enrolment | ||||
| Predictors at month 18 | Bivariate analysis (adjusted for score at enrollment) | Multivariable analysis | ||
| Coefficient (SE) | P value | Coefficient (SE) | P value | |
| Score at enrollment | −0.69 (0.065) | <.001 | ||
| WBC (×106/L) | −0.038 (0.015) | .015 | −0.003 (0.024) | .91 |
| ARC (×106/L) | −0.001 (0.0005) | .014 | −0.001 (0.0006) | .15 |
| Executive function (n = 254), all ages at enrolment | ||||
| Predictors at month 18 | Bivariate analysis (adjusted for score at enrollment) | Multivariable analysis | ||
| Coefficient (SE) | P value | Coefficient (SE) | P value | |
| Score at enrollment | −0.93 (0.071) | <.001 | ||
| SES∗ | 0.048 (0.020) | .015 | 0.05 (0.020) | .015 |
| Executive function (n = 59), ages ≥7 y at enrolment | ||||
| Predictors at month 18 | Bivariate analysis (adjusted for score at enrollment) | Multivariable analysis | ||
| Coefficient (SE) | P value | Coefficient (SE) | P value | |
| Score at enrollment | −0.60 (0.146) | <.001 | ||
| Hb (g/dL) | 0.26 (0.114) | .025 | 0.32 (0.121) | .012 |
| TCD TAMV (cm/s)† | 0.006 (0.004) | .144 | 0.009 (0.004) | .032 |
| Cognition (n = 201), ages ≥4 y at enrolment . | ||||
|---|---|---|---|---|
| Predictors at mo 18 . | Bivariate analysis (adjusted for score at enrollment) . | Multivariable analysis . | ||
| Coefficient (SE) . | P value . | Coefficient (SE) . | P value . | |
| Score at enrollment | −0.28 (0.053) | <.001 | ||
| SES∗ | 0.054 (0.024) | .03 | 0.05 (0.025) | .048 |
| Platelets (×109/L) | −0.001 (0.0004) | .03 | −0.0007 (0.0004) | .047 |
| Attention (n = 146), ages ≥5 y at enrolment | ||||
| Predictors at month 18 | Bivariate analysis (adjusted for score at enrollment) | Multivariable analysis | ||
| Coefficient (SE) | P value | Coefficient (SE) | P value | |
| Score at enrollment | −0.69 (0.065) | <.001 | ||
| WBC (×106/L) | −0.038 (0.015) | .015 | −0.003 (0.024) | .91 |
| ARC (×106/L) | −0.001 (0.0005) | .014 | −0.001 (0.0006) | .15 |
| Executive function (n = 254), all ages at enrolment | ||||
| Predictors at month 18 | Bivariate analysis (adjusted for score at enrollment) | Multivariable analysis | ||
| Coefficient (SE) | P value | Coefficient (SE) | P value | |
| Score at enrollment | −0.93 (0.071) | <.001 | ||
| SES∗ | 0.048 (0.020) | .015 | 0.05 (0.020) | .015 |
| Executive function (n = 59), ages ≥7 y at enrolment | ||||
| Predictors at month 18 | Bivariate analysis (adjusted for score at enrollment) | Multivariable analysis | ||
| Coefficient (SE) | P value | Coefficient (SE) | P value | |
| Score at enrollment | −0.60 (0.146) | <.001 | ||
| Hb (g/dL) | 0.26 (0.114) | .025 | 0.32 (0.121) | .012 |
| TCD TAMV (cm/s)† | 0.006 (0.004) | .144 | 0.009 (0.004) | .032 |
Bold values indicate significance at P < .5.
Bivariate analysis tested for predictors listed in Table 2, adjusted for the z score at enrollment. Variables that were significant in the bivariate analysis (P < .2) were selected for multivariable analysis. Only predictors with significant effects are listed.
Complete data are available on request.
ARC, absolute reticulocyte count.
Socioeconomic scale, per locally validated scale.24
TCD velocity mean time-averaged maximum velocity.
We confirmed our a priori prediction that baseline neurocognitive z scores strongly affected the outcome scores for the participants with SCA. We also evaluated clinically relevant influences on baseline z scores (Appendix 5). Univariate positive associations were found with SES and Hb, with negative associations found with male sex (for z-scored measures not adjusting for sex), TCD velocity, WBC, and absolute neutrophil count. Reductions in WBC, absolute neutrophil count, and platelet count are each predictable dose-related responses to HU exposure.21 Of these variables, only SES and TCD velocity held up to bivariate and multivariate analyses, with SES associated across multiple measures, as has been reported for neurocognitive performance in pediatric SCA.9 Colinearity was likely in multivariate analyses to diminish the association of several HU-responsive hematological parameters, for example, Hb and HbF.
Because the cohort’s z scores were age-normalized to evaluate age effects on performance at baseline and month 18, we used raw scores for the 3 measures that were valid across the age range (KABC-II, TOVA, and BRIEF). At both study time points, lower scores on cognition with age were found, although attention and executive function improved with age; controls demonstrated similar trends (Appendix 6).
To check for possible practice effects for the SCA sample at month 18, a randomly selected subset of controls (n = 42; 38.2%) underwent retesting.47-49 Of the 4 measures used, controls’ age-normalized z-scores were higher than their initial results. Only 1, the KABC-II test for cognition, suggested a potential practice effect (Appendix 7). We also analyzed raw test results from the SCA and retested controls at both study time points (Appendix 7). However, these data do not account for the significant age difference between samples (Table 2), or the potential aging and/or schooling effects within the samples. In the absence of an untreated SCA group as disease-specific control (which was deemed unethical), we cannot eliminate the potential for any practice effects.
TCD
Similar to other global HU treatment trials, month 18 TCD TAMV significantly improved from baseline. Mean TAMV decreased from 148.4 ± 29.2 to 130.9 ± 21.3 cm/s (mean change 17.5 ± 22.2 cm/s), with markedly reduced proportions of participants in the abnormal and conditional categories (Table 2). Participants with abnormal TCD and Hb <9.0 g/dL were offered transfusions, which no parent or participant rejected. Scarcity of ABO-matched blood units occasionally delayed transfusions. Consistent with our previous study findings, no TCD velocities of <70 cm/s were found in middle or anterior cerebral arteries at either time point.14 As mentioned previously, TCD velocity was associated solely with improved executive function among the 4 neurocognitive measures.
Discussion
Worldwide, over 400 000 children are born with SCA, the most severe genotype of sickle cell disease, with most living in sub-Saharan Africa.18 Without disease-modifying intervention, pediatric SCA neurocognitive function declines with age, with consequences including reduced educational achievement and employment potential, even without overt or silent cerebral infarcts.2,8,14,50 To date, reported HU trial impact on disease manifestations in sub-Saharan Africa has focused on reducing acute systemic complications and abnormal TCD results. Our neurocognitive results from the planned 18-month midpoint evaluation of our open-label, pediatric HU intervention trial with MTD dosing in Uganda used age-normalized participant with SCA z-scored measures generated through a sibling sample without SCA to control for SES and social/cultural variability.8,10 Use of z-scores also enabled direct comparison to each participant’s previous performance across the same test instruments.
To our knowledge, no substantial HU trial on neurocognition in sub-Saharan Africa or other low- or middle-income country has been previously reported. Our main interim findings from a large pediatric sample with SCA aged 3 to 9 years at enrollment were the following: (1) neurocognitive function significantly improved in each of 4 measures assessing cognition, attention, and executive function to levels similar to sibling controls, potentially aided by practice effects. Concomitantly, the proportion of participants with cognitive impairment reduced by more than twofold; (2) regression analyses identified baseline neurocognitive scores as the major factor for all neurocognitive outcomes. In addition to sex and SES, effects on neurocognitive function were found from HU-responsive variables, including lower TCD velocity, higher Hb, and other HU-responsive hematological parameters; and (3) despite cognitive improvement with HU therapy, our measure remained negatively associated with age. Overall trial stroke incidence was similar to that reported in a Nigerian stroke prevention trial.51
Several large US-based, multi-site natural history studies have consistently demonstrated a negative trajectory in neurocognitive function, starting early and progressing through childhood and into adulthood, along with accumulation of higher vascular flow rates, SCI, stroke, and ongoing social risk factors.1,3,6,9,52-54 These studies have used repeated measurement with the same age-appropriate measures, providing a strong argument that the natural history pattern of neurocognitive function in childhood SCA involves a downward trajectory over time. SCA studies in United States have also documented HU effects on stabilization of cognitive performance, with greater effects from earlier age of HU initiation.2 Taken together, the finding of stable or improved neurocognition after HU therapy is more likely due to treatment effects than to practice effects of measurement. Decline of cognition for the Ugandan SCA sample by age at both time points and for sibling controls suggests that effects from environmental contributions, for example, serious infections, low school quality, family poverty, and/or caregiver educational attainment, may have contributed to the decline despite HU therapy.9 Whether prolonged HU therapy will further affect the SCA age-associated decline will be assessed at 30-month trial completion.
Our Uganda-based results are consistent with modestly-sized, previous US-based pediatric HU trials aimed at prospectively ameliorating SCA-associated cognitive decline through HU, transfusion, or a pharmacologic strategy to enhance Hb.55-58 The BRAIN SAFE II trial had sufficient power to detect impact from multiple different modifiers. Our observation of heterogeneity of initial and treatment-associated z-scores was expected, given the range of disease-associated complications and backgrounds of the participants.16,46
Mechanism(s) by which HU improves neurocognitive function seems to include short- or long-term increased Hb to improve cerebral blood flow, likely also reducing risks from abnormal cerebrovascular injury and improved oxygenation.4,59 HU also reduces severity and incidence of acute chest syndrome/pneumonia and acute malaria, thereby potentially decreasing acute cerebral risk from hypoxia, inflammation, and worsened anemia.4,45 Consistent with the importance of HU effects on cerebral blood flow through increased Hb, a Nigerian cross-sectional analyses reportedly a correlation between abnormal cerebrovascular blood flow detected by TCD and lower executive function.60 Higher Hb may also affect neurocognitive performance through improved well-being. The predictive value of preintervention on postintervention performance has been established for neurocognitive function in children with or without other chronic health conditions (eg, autism).61-63
Limitations included the unplanned exclusion of 3-year-old participants from cognitive testing, as controls of that age were not developmentally ready for the measure used. Some of the measures could not be used over the full age range of the sample. Nonetheless, abundant statistical power remained for detecting differences from HU therapy. Heterogeneity of initial and treatment-associated z-scores was expected, given the range of disease-associated complications and backgrounds of the participants.16,46 As MRI is not standard care in Uganda and only a subset of participants underwent imaging, we did not assess neurocognitive scores based on MRI findings. Higher z-scores in a subsample of retested controls suggested potential practice effects in 2 measures in the modestly-sized retested subsample. Practice effects are more likely with test coaching or with a learned correct answer, neither of which applied here.49 Moreover, the controls lacked SCA, hence subtracting scores of participants with SCA based on retested controls, although in the same direction, it would not have accurately accounted for the results. Hence, we did not account for this potential effect. Improved neurocognitive function remained significant despite correction for multiple testing.
Here, we provide interim results from a longer HU trial and have prospectively demonstrated that HU therapy improved neurocognitive function in a large sample of Ugandan children with SCA at the initial 18 months of our 30-month HU trial. Despite the potential for some practice effects in 1 of the 4 measures used, our findings add to other well-documented benefits of HU on pediatric SCA, including stabilized silent cerebral infarcts and improved TCD. Whether neurocognitive effects further improve after an additional 12 months to trial completion remain to be assessed. Our data add direct evidence to the growing literature that HU treatment reverses the broad age-dependent decline in neurocognitive function in children without disease-modifying therapy and identifies risk effects for children living in sub-Saharan Africa (SSA). Furthermore, our use of age- and sex-normalized z-scores derived from non-SCA sibling controls could support future investigation for the generalization of these findings across pediatric populations with SCA across settings in SSA and elsewhere.
Acknowledgments
The authors gratefully acknowledge the support of the outstanding study team and from Global Health Uganda and the pediatric staff of the MHSCC who served as key clinical links to the trial. Most importantly, they thank the many families who supported their child’s trial participation. The authors also thank the Data Safety and Monitoring Committee for their important trial role.
The work was supported by grants from the Fogarty International Center and the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health R01HD096559 (R.I. and N.S.G.) and the Fogarty International Center research training program D43TW010928 (John, R.I.). Study drug (Siklos) was donated by Theravia (formerly AddMedica, France). The TOVA Company provided instruments at a discounted rate for this research.
S.K.N. is a PhD candidate at Makerere University. This work is submitted in partial fulfillment of the requirement for the PhD.
The funders, donors, or sponsors had no role in designing, gathering, analyzing, or interpreting the data. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does the mention of trade names, commercial products, or organizations imply endorsement by the US government.
Authorship
Contribution: P.B., S.K.N., R.I., R.O., C.N., C.R., and N.S.G. designed the trial; S.K.N., V.M., D.M., B.W., R.N., P.K., M.K., G.N., S.M., and M.N. implemented the study procedures; D.K., J.S., Z.J., and I.R.T. analyzed the results and made the figures; and N.S.G. and R.I. wrote the paper.
Conflict-of-interest disclosure: The members of the Safety Monitoring Committee and the authors have no financial interest in hydroxyurea or any other product used in this trial.
Correspondence: Nancy S. Green, Department of Pediatrics, Columbia University Irving Medical Center, 630 West 168 St, Black Building 241, New York, NY 10032; email: nsg11@cumc.columbia.edu.
References
Author notes
Presented in abstract form at the 65th annual meeting of the American Society of Hematology, San Diego, CA, 9 to 12 December 2023.
Data that support the findings of this study, including deidentified individual participant data, will be made available 1 year after the publication date on reasonable request from the corresponding author, Nancy S. Green (nsg11@cumc.columbia.edu).
The study protocol is available online.
The full-text version of this article contains a data supplement.