Key Points
Br-RT before CAR-T was well tolerated with minimal toxicity in this large multicenter study.
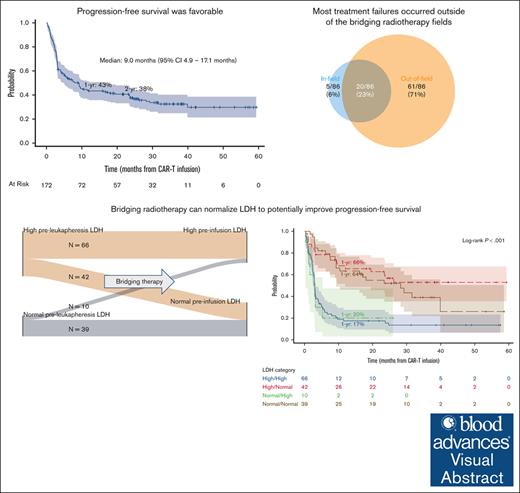
Comprehensive Br-RT and LDH normalization after RT but before CAR-T were associated with superior survival.
Visual Abstract
Despite the increasing utilization of bridging radiotherapy (Br-RT), its impact on chimeric antigen receptor T-cell therapy (CAR-T) efficacy and toxicity remains poorly characterized. We retrospectively reviewed patients with relapsed/refractory B-cell lymphomas (BCLs) who received Br-RT followed by CAR-T from 2018 to 2020 across 10 institutions. Br-RT toxicities were graded per Common Terminology Criteria for Adverse Events version 5.0, and cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) per American Society for Transplantation and Cellular Therapy Consensus Guidelines. One hundred seventy-two patients (168 large BCL) received Br-RT before axicabtagene ciloleucel (73%), tisagenlecleucel (24%), or brexucabtagene autoleucel (2%). At leukapheresis, most patients (74%) had advanced-stage disease and 39% had bulky disease measuring ≥10cm. Comprehensive Br-RT was administered to 39% and bridging systemic therapy to 35%. Among all patients, grade ≥3 Br-RT toxicity occurred in 2% (1 grade 5 toxicity), grade ≥3 CRS in 9%, and grade ≥3 ICANS in 24%. Median follow-up was 31.3 months. Two-year progression-free survival (PFS) and overall survival (OS) were 38% and 53%, respectively. On multivariable analysis, comprehensive Br-RT was associated with superior PFS (hazard ratio [HR], 0.38; P < .001) and OS (HR, 0.48; P = .011). Patients with lactate dehydrogenase (LDH) normalization after Br-RT (high pre-Br-RT LDH, normal post-Br-RT LDH) had superior PFS and OS compared with those with high post-Br-RT LDH and similar PFS and OS compared with those with normal baseline LDH. In this particularly high-risk cohort, Br-RT before CAR-T demonstrates an acceptable toxicity profile with favorable clinical outcomes compared with historical controls. Comprehensive Br-RT and LDH normalization after Br-RT may be associated with superior PFS and OS.
Introduction
CD19-targeting chimeric antigen receptor T-cell therapy (CAR-T) is a standard of care cellular immunotherapy for relapsed/refractory (R/R) aggressive B-cell lymphomas (BCLs).1-4 The process of CAR-T requires leukapheresis to obtain autologous T cells, followed by several weeks for T-cell manufacturing and release testing, administration of lymphodepleting chemotherapy, and reinfusion of the genetically engineered T cells. After leukapheresis and before lymphodepleting chemotherapy, patients may receive bridging therapy for interim disease control, cytoreduction, or symptom palliation; this may consist of chemotherapy, targeted therapy, corticosteroids, and/or radiotherapy (RT).
RT has shown potential as a bridging therapy strategy, particularly because local failure remains the predominant pattern of relapse after CAR-T.5,6 Early case series demonstrated the feasibility of bridging RT (Br-RT), although these experiences have typically been limited to small single-institution or multisite reports.7-14 Recently, the US Lymphoma CAR-T Consortium published a nationwide analysis of bridging therapies in 154 patients receiving axicabtagene ciloleucel (axi-cel); however, only 19 patients (12%) received Br-RT, limiting the ability to draw robust conclusions.15 Despite the increasing utilization of Br-RT, its impact on CAR-T efficacy and toxicity remains poorly characterized.
We report an International Lymphoma Radiation Oncology Group (ILROG)-sponsored multi-institutional analysis of Br-RT before CAR-T for patients with R/R BCL. We describe Br-RT characteristics, toxicity, and clinical outcomes after Br-RT and CAR-T. In addition, we assess variables associated with efficacy for patients who received Br-RT followed by CAR-T. We hypothesized that treating all active sites of disease with Br-RT (ie, comprehensive Br-RT) would be associated with superior outcomes, as has been suggested by previous series.11,14,16
Methods
We retrospectively reviewed records of patients with R/R BCL who received Br-RT followed by commercial CAR-T from January 2018 to December 2020 across 10 ILROG institutions. Patients who underwent leukapheresis but who did not receive CAR-T infusion were excluded. Patient and disease characteristics, previous treatments, Br-RT characteristics, and clinical outcomes were collected by each individual institution, then either deidentified or converted to a limited data set, and finally aggregated. This study was approved by the local institutional review board of each participating institution.
Baseline disease burden
Baseline disease burden was assessed before leukapheresis. All patients had preleukapheresis positron emission tomography/computed tomography (PET/CT) or CT scans, as well as brain magnetic resonance imaging in the case of central nervous system (CNS) involvement. Advanced-stage disease was defined as stage III/IV based on the Ann Arbor system, assessed before leukapheresis. The largest lesion maximum diameter and maximum standardized uptake value (SUV) were reported based on imaging reports. Some institutions did not report the numerical largest lesion size but instead whether it was ≥10 cm. Metabolic tumor volume (MTV) and total lesion glycolysis (TLG) were available from 3 institutions and were measured using the Positron Emission Tomography Response Criteria in Solid Tumors method on preleukapheresis PET/CT scans.17 Preleukapheresis (pre-Br-RT) and pre-CAR-T (post-Br-RT) infusion lactate dehydrogenase (LDH) values were recorded to identify a potential surrogate for bridging therapy response. LDH was considered elevated if it was greater than the upper limit of normal per each institution’s laboratory reference range.
Treatment
Bridging therapy was defined as any therapy (excluding corticosteroids) administered within 30 days before leukapheresis until CAR-T infusion. Comprehensive Br-RT was defined as RT to all active sites of disease seen on imaging, whereas focal Br-RT was defined as RT to less than all active sites of disease. Br-RT timing, dose/fractionation, technique, and comprehensive (vs focal) nature were at the discretion of the treating institution and reflective of multidisciplinary discussion. Patients received a single infusion of axi-cel, tisagenlecleucel (tisa-cel), or brexucabtagene autoleucel on day 0.
Response assessment
Response to CAR-T was assessed at day +30 and/or +90 after CAR-T infusion, per each institution’s practice standards, with a PET/CT or CT, as well as brain magnetic resonance imaging for those with CNS involvement. The Lugano criteria were used for response assessment.18 For the purposes of this study, response was defined as the best response by day +90.
Patterns of treatment failure
Patterns of treatment failure after CAR-T infusion were classified in 2 ways: (1) failure at preexisting lesions or new lesions (with respect to disease present on preleukapheresis or preinfusion scans) and (2) failure in-field or out-of-field (with respect to Br-RT). In-field treatment failure was defined as failure within the radiation planning target volume or 95% isodose line of the radiation prescription.
Toxicity
Br-RT toxicities were graded per the Common Terminology Criteria for Adverse Events version 5.0. Only grade ≥3 toxicities, which typically require an intervention or hospitalization, were reported.
Cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) were graded per the American Society for Transplantation and Cellular Therapy criteria.19 All grades of CRS and ICANS were reported.
Statistical analysis
Descriptive statistics were used to summarize baseline characteristics, Br-RT characteristics, toxicity, response, and patterns of treatment failure. These included median/range/interquartile range (IQR) for continuous data and frequencies/percentages for categorical data.
Kaplan-Meier analysis was used to assess freedom from in-field treatment failure, progression-free survival (PFS), and overall survival (OS), measured from the date of CAR-T infusion. Survival differences were assessed using the log-rank test.
Cox proportional hazards models were generated to assess factors associated with PFS and OS. For these analyses, only patients with large BCLs (diffuse large BCL, transformed follicular lymphoma, and primary mediastinal BCL) were included. Covariates of interest included age ≥60 years, female sex, Eastern Cooperative Oncology Group (ECOG) ≥2, histology, double hit gene rearrangements, primary refractory disease, previous lines of therapy ≥3, previous transplant, advanced-stage disease, extranodal disease, CNS involvement, largest lesion maximum diameter (continuous, ≥5 cm, ≥7.5 cm, and ≥10 cm), maximum SUV ≥10, high preleukapheresis LDH, high pre-CAR-T infusion LDH, MTV, TLG, bridging systemic therapy, Br-RT equivalent dose in 2 Gy fractions (EQD2) assuming alpha/beta = 10, Br-RT to >1 site, and comprehensive Br-RT.
Multivariable Cox models were generated considering all covariates with P < .05 on univariable analysis and using stepwise variable selection. Our primary hypothesis was that comprehensive (vs focal) Br-RT would be associated with superior PFS and OS. As such, final models were forced to include at the minimum comprehensive Br-RT (primary covariate of interest) and age ≥60 years, ECOG ≥2, and advanced-stage disease (primary confounders of interest, specified a priori). Collinearity was assessed using the variance inflation factor; all variables considered for the final multivariable models were confirmed to have a variance inflation factor <5, indicating low to moderate correlation with other predictor variables. For the subset of patients with MTV and TLG data, we constructed multivariable models with MTV or TLG given that they are more quantitative measures of disease burden. We expected patients receiving comprehensive Br-RT to have a lower disease burden at baseline.
All statistical tests were 2 sided and P < .05 was considered statistically significant. Analyses were limited to cases with nonmissing data. Analyses were performed with SAS OnDemand for Academics and R version 4.2.2 (Vienna, Austria).
Results
Patient and disease characteristics
Baseline characteristics of the 172 patients who received Br-RT and CAR-T are presented in Table 1; 168 patients had large BCL (diffuse large BCL, N = 122 [71%]). Median age was 62 years, and 31 patients (18%) had an ECOG performance status ≥2. Patients received a median of 3 previous lines of therapy (range, 1-8), including 2 previous lines in 75 patients (44%) and 3 previous lines in 55 patients (32%). At leukapheresis, 127 patients (74%) had advanced-stage disease, 116 (67%) had extranodal disease, and 17 (10%) had CNS involvement. Preleukapheresis (pre-Br-RT) and preinfusion (post-Br-RT) LDH were elevated in 113 (66%) and 76 patients (44%), respectively. Median largest lesion maximum diameter was 6.7 cm; largest lesion was ≥7.5 cm and ≥10 cm in 49% and 39%, respectively. CAR-T constructs administered included axi-cel (n = 126, 73%), tisa-cel (n = 42, 24%), and brexucabtagene autoleucel (n = 4, 2%); 60 patients (35%) also had bridging systemic therapy, excluding steroids.
Baseline characteristics
| N = 172; preleukapheresis, unless specified . | n (%) or median (IQR) . |
|---|---|
| Age, y | 62 (51-68) |
| Sex | |
| Male | 98 (57) |
| Female | 74 (43) |
| Race | |
| White | 131 (76) |
| Asian | 10 (6) |
| Black | 9 (5) |
| Other | 5 (3) |
| Unknown | 17 (10) |
| ECOG | |
| 0 | 43 (25) |
| 1 | 96 (56) |
| 2 | 26 (15) |
| 3 | 5 (3) |
| Unknown | 2 (1) |
| Histology | |
| DLBCL | 122 (71) |
| DLBCL (transformed FL) | 32 (19) |
| PMBCL | 13 (8) |
| Mantle cell lymphoma | 3 (2) |
| T-cell/histiocyte-rich large B-cell lymphoma | 1 (<1) |
| Burkitt lymphoma | 1 (<1) |
| Cell of origin∗ | |
| GCB | 101 (60) |
| ABC | 49 (29) |
| Unknown | 18 (11) |
| Double hit (FISH)∗ | |
| Yes | 42 (25) |
| No | 113 (67) |
| Unknown | 13 (8) |
| Primary refractory | 75 (43) |
| Previous no. of lines of therapy | 3 (2-3) |
| 1 | 2 (1) |
| 2 | 75 (44) |
| 3 | 55 (32) |
| 4 | 16 (9) |
| 5 | 15 (9) |
| 6 | 4 (2) |
| 7 | 3 (2) |
| 8 | 2 (1) |
| Previous transplant | 23 (13) |
| Stage | |
| Early (I-II) | 45 (26) |
| Advanced (III-IV) | 127 (74) |
| Extranodal disease | |
| Yes | 116 (67) |
| No | 49 (28) |
| Unknown | 7 (4) |
| CNS involvement | |
| Yes | 17 (10) |
| No | 155 (90) |
| Elevated preleukapheresis LDH | |
| Yes | 113 (66) |
| No | 57 (33) |
| Unknown | 2 (1) |
| Elevated preinfusion LDH | |
| Yes | 76 (44) |
| No | 83 (48) |
| Unknown | 13 (8) |
| Largest lesion maximum diameter, cm† | 6.7 (4.1-12.2) |
| Largest lesion ≥5 | 98 (66) |
| Largest lesion ≥7.5 | 73 (49) |
| Largest lesion ≥10 | 64 (39) |
| Maximum SUV ≥10‡ | 116 (81) |
| CAR-T construct | |
| Axi-cel | 126 (73) |
| Tisa-cel | 42 (24) |
| Brexucabtagene autoleucel | 4 (2) |
| Lymphodepletion regimen | |
| Fludarabine/cyclophosphamide | 143 (83) |
| Bendamustine | 28 (16) |
| Cyclophosphamide | 1 (<1) |
| Bridging systemic therapy (excluding steroids) | 60 (35) |
| N = 172; preleukapheresis, unless specified . | n (%) or median (IQR) . |
|---|---|
| Age, y | 62 (51-68) |
| Sex | |
| Male | 98 (57) |
| Female | 74 (43) |
| Race | |
| White | 131 (76) |
| Asian | 10 (6) |
| Black | 9 (5) |
| Other | 5 (3) |
| Unknown | 17 (10) |
| ECOG | |
| 0 | 43 (25) |
| 1 | 96 (56) |
| 2 | 26 (15) |
| 3 | 5 (3) |
| Unknown | 2 (1) |
| Histology | |
| DLBCL | 122 (71) |
| DLBCL (transformed FL) | 32 (19) |
| PMBCL | 13 (8) |
| Mantle cell lymphoma | 3 (2) |
| T-cell/histiocyte-rich large B-cell lymphoma | 1 (<1) |
| Burkitt lymphoma | 1 (<1) |
| Cell of origin∗ | |
| GCB | 101 (60) |
| ABC | 49 (29) |
| Unknown | 18 (11) |
| Double hit (FISH)∗ | |
| Yes | 42 (25) |
| No | 113 (67) |
| Unknown | 13 (8) |
| Primary refractory | 75 (43) |
| Previous no. of lines of therapy | 3 (2-3) |
| 1 | 2 (1) |
| 2 | 75 (44) |
| 3 | 55 (32) |
| 4 | 16 (9) |
| 5 | 15 (9) |
| 6 | 4 (2) |
| 7 | 3 (2) |
| 8 | 2 (1) |
| Previous transplant | 23 (13) |
| Stage | |
| Early (I-II) | 45 (26) |
| Advanced (III-IV) | 127 (74) |
| Extranodal disease | |
| Yes | 116 (67) |
| No | 49 (28) |
| Unknown | 7 (4) |
| CNS involvement | |
| Yes | 17 (10) |
| No | 155 (90) |
| Elevated preleukapheresis LDH | |
| Yes | 113 (66) |
| No | 57 (33) |
| Unknown | 2 (1) |
| Elevated preinfusion LDH | |
| Yes | 76 (44) |
| No | 83 (48) |
| Unknown | 13 (8) |
| Largest lesion maximum diameter, cm† | 6.7 (4.1-12.2) |
| Largest lesion ≥5 | 98 (66) |
| Largest lesion ≥7.5 | 73 (49) |
| Largest lesion ≥10 | 64 (39) |
| Maximum SUV ≥10‡ | 116 (81) |
| CAR-T construct | |
| Axi-cel | 126 (73) |
| Tisa-cel | 42 (24) |
| Brexucabtagene autoleucel | 4 (2) |
| Lymphodepletion regimen | |
| Fludarabine/cyclophosphamide | 143 (83) |
| Bendamustine | 28 (16) |
| Cyclophosphamide | 1 (<1) |
| Bridging systemic therapy (excluding steroids) | 60 (35) |
ABC, activated B-cell like; DLBCL, diffuse large B-cell lymphoma; FISH, fluorescence in situ hybridization; FL, follicular lymphoma; GCB, germinal center B-cell like; PMBCL, primary mediastinal B-cell lymphoma.
Excluding 3 mantle cell and 1 Burkitt lymphoma cases.
Largest lesion maximum diameter (cm), largest lesion ≥5 cm, and largest lesion ≥7.5 cm were unavailable for 23 patients; however, largest lesion ≥10 cm was unavailable for only 6 patients.
Unavailable for 29 patients.
Br-RT details
Br-RT characteristics are presented in Table 2. Median time from leukapheresis to Br-RT start was 4 days (IQR, –9 to 11), and median time from Br-RT completion to CAR-T infusion was 15 days (IQR, 10-24). Comprehensive Br-RT was given to 67 patients (39%). The comprehensive Br-RT group had a lower disease burden at baseline (supplemental Table 1).
Br-RT characteristics
| Characteristic . | n (%) or median (IQR) . |
|---|---|
| By patient (N = 172) | |
| Interval from leukapheresis to Br-RT start, d | 4 (–9 to 11)∗ |
| Interval from Br-RT end to CAR-T infusion, d | 15 (10-24) |
| Comprehensive Br-RT (to all active lesions) | 67 (39) |
| Nodal/extranodal | |
| Nodal | 61 (35) |
| Extranodal | 67 (39) |
| Mixed | 26 (15) |
| Unknown | 18 (10) |
| Maximum diameter of largest treated lesion (cm)† | 6 (3.5-10.5) |
| Concurrent systemic therapy | |
| Yes | 39 (23) |
| No | 116 (67) |
| Unknown | 17 (10) |
| RT technique | |
| 3D-CRT | 77 (45) |
| IMRT/VMAT | 69 (40) |
| 3D-CRT/IMRT mix | 5 (3) |
| Electron therapy | 3 (2) |
| Stereotactic radiosurgery | 2 (1) |
| Proton therapy | 1 (<1) |
| IMRT/Proton therapy mix | 1 (<1) |
| Unknown | 14 (8) |
| No. of sites treated with Br-RT | |
| 1 | 139 (81) |
| 2 | 20 (12) |
| 3 | 8 (5) |
| 4 | 5 (3) |
| ByBr-RTsite (N = 223) | |
| Sites treated | |
| Abdomen/pelvis | 78 (35) |
| Head/neck | 50 (22) |
| Thorax | 30 (13) |
| Extremity/soft tissue | 27 (12) |
| Spine/paraspinal | 14 (6) |
| CNS‡ | 13 (6) |
| Focal brain | 7 |
| Whole brain | 2 |
| Optic nerve | 3 |
| Leptomeningeal disease | 1 |
| Axilla | 11 (5) |
| Total dose, Gy§ | 25 (20-30.6) |
| No. of fractions§ | 10 (5-15) |
| Dose per fraction, Gy§ | 2.8 (2-4) |
| EQD2, Gy (alpha/beta = 10)§ | 29.3 (21-33) |
| Most common regimens§ | |
| 30 Gy/10 fractions | 35 (16) |
| 20 Gy/5 fractions | 29 (13) |
| 20 Gy/10 fractions | 9 (4) |
| 37.5 Gy/15 fractions | 9 (4) |
| Characteristic . | n (%) or median (IQR) . |
|---|---|
| By patient (N = 172) | |
| Interval from leukapheresis to Br-RT start, d | 4 (–9 to 11)∗ |
| Interval from Br-RT end to CAR-T infusion, d | 15 (10-24) |
| Comprehensive Br-RT (to all active lesions) | 67 (39) |
| Nodal/extranodal | |
| Nodal | 61 (35) |
| Extranodal | 67 (39) |
| Mixed | 26 (15) |
| Unknown | 18 (10) |
| Maximum diameter of largest treated lesion (cm)† | 6 (3.5-10.5) |
| Concurrent systemic therapy | |
| Yes | 39 (23) |
| No | 116 (67) |
| Unknown | 17 (10) |
| RT technique | |
| 3D-CRT | 77 (45) |
| IMRT/VMAT | 69 (40) |
| 3D-CRT/IMRT mix | 5 (3) |
| Electron therapy | 3 (2) |
| Stereotactic radiosurgery | 2 (1) |
| Proton therapy | 1 (<1) |
| IMRT/Proton therapy mix | 1 (<1) |
| Unknown | 14 (8) |
| No. of sites treated with Br-RT | |
| 1 | 139 (81) |
| 2 | 20 (12) |
| 3 | 8 (5) |
| 4 | 5 (3) |
| ByBr-RTsite (N = 223) | |
| Sites treated | |
| Abdomen/pelvis | 78 (35) |
| Head/neck | 50 (22) |
| Thorax | 30 (13) |
| Extremity/soft tissue | 27 (12) |
| Spine/paraspinal | 14 (6) |
| CNS‡ | 13 (6) |
| Focal brain | 7 |
| Whole brain | 2 |
| Optic nerve | 3 |
| Leptomeningeal disease | 1 |
| Axilla | 11 (5) |
| Total dose, Gy§ | 25 (20-30.6) |
| No. of fractions§ | 10 (5-15) |
| Dose per fraction, Gy§ | 2.8 (2-4) |
| EQD2, Gy (alpha/beta = 10)§ | 29.3 (21-33) |
| Most common regimens§ | |
| 30 Gy/10 fractions | 35 (16) |
| 20 Gy/5 fractions | 29 (13) |
| 20 Gy/10 fractions | 9 (4) |
| 37.5 Gy/15 fractions | 9 (4) |
3D-CRT, 3-dimensional conformal radiotherapy; IMRT, intensity modulated radiotherapy; VMAT, volumetric modulated arc therapy.
Negative number (−9) indicates radiotherapy was started before leukapheresis.
Unavailable for 38 patients.
Thirteen sites treated among 8 patients.
Excluding 1 patient with missing dose/fractionation details.
Br-RT was delivered to 223 total sites, most commonly the abdomen/pelvis (n = 78, 35%), head/neck (n = 50, 22%), thorax (n = 30, 13%), and extremity/soft tissue (n = 27, 12%). Median EQD2 was 29.3 Gy (IQR, 21-33). The most common regimen was 30 Gy in 10 fractions (n = 35, 16%). Granular Br-RT details by body site and dose/fractionation are presented in supplemental Table 2.
Response rate to CAR-T and patterns of treatment failure
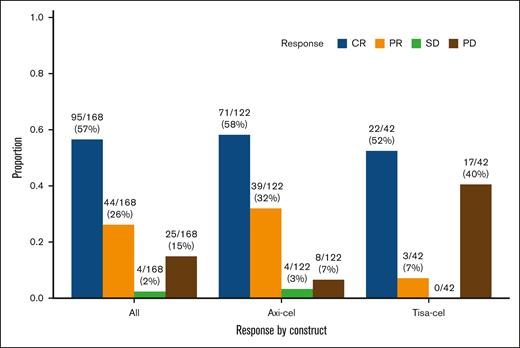
The response rate to CAR-T (complete response or partial response) was 139 of 168 (83%), including 110 of 122 (90%) after axi-cel and 25 of 42 (60%) after tisa-cel (Figure 1). Disease response was not assessable for 4 patients because of early death in the absence of disease progression (see Toxicity section below).
Best response to CAR-T by 3 months (N = 168 evaluable). CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease.
Best response to CAR-T by 3 months (N = 168 evaluable). CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease.
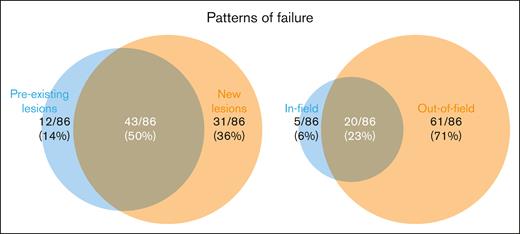
Median follow-up was 31.3 months (range for survivors, 3.4-59.4 months). Ninety-two patients (53%) experienced disease progression after CAR-T, of whom 86 had scans available for assessment of patterns of treatment failure (Figure 2). Of these 86 patients, the pattern of progression was predominantly new lesions (n = 74, 86% of failures) and out-of-field (N = 81, 94% of failures). In-field failure occurred in 25 patients (29% of failures), of which 5 were isolated in-field failures. Details of the in-field failures are presented in supplemental Table 3. Two-year freedom from in-field failure was 80% (95% confidence interval [CI], 71-86) (supplemental Figure 1). Higher Br-RT EQD2 was not significantly associated with lower in-field failure (hazard ratio [HR], 0.98/Gy; 95% CI, 0.95-1.01; P = .24).
Patterns of treatment failure (N = 86 evaluable) with respect to lesions present from preleukapheresis scans up to CAR-T infusion (preexisting lesions, new lesions, or both; left) and with respect to Br-RT fields (in-field, out-of-field, or both; right).
Patterns of treatment failure (N = 86 evaluable) with respect to lesions present from preleukapheresis scans up to CAR-T infusion (preexisting lesions, new lesions, or both; left) and with respect to Br-RT fields (in-field, out-of-field, or both; right).
PFS and OS
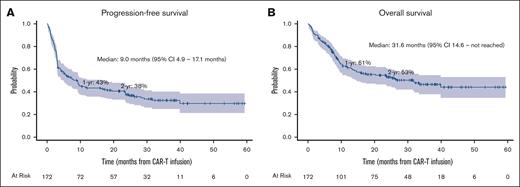
One- and 2-year PFS rates were 43% (95% CI, 36-51) and 38% (95% CI, 30-45), respectively (Figure 3A). Comprehensive Br-RT was associated with superior PFS (HR, 0.38; 95% CI, 0.22-0.63, P < .001) in a multivariable model with age ≥60 years, ECOG ≥2, advanced-stage disease, CNS involvement, largest lesion maximum diameter ≥10 cm, and high pre-CAR-T infusion LDH (Table 3). The strength of the association between comprehensive Br-RT and PFS did not vary across different variables. In particular, there was no interaction between comprehensive Br-RT and any of the following: advanced-stage disease, largest lesion maximum diameter ≥10 cm, high preinfusion LDH, and CAR-T construct (interaction P = .38, .66, .46, and .65, respectively).
Cox regression for PFS and OS among patients with DLBCL, transformed FL, and PMBCL (N = 167)
| Variable . | PFS, univariable . | PFS, multivariable (N = 150, 99 events) . | OS, univariable . | OS, multivariable (N = 150, 73 events) . | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) . | P value . | HR (95% CI) . | P value . | HR (95% CI) . | P value . | HR (95% CI) . | P value . | |
| Age ≥60 years | 1.54 (1.03-2.28) | .034 | 1.84 (1.21-2.80) | .0044 | 1.33 (0.85-2.08) | .22 | 1.67 (1.03-2.71) | .037 |
| Female | 1.12 (0.76-1.65) | .56 | 1.35 (0.87-2.10) | .18 | ||||
| ECOG ≥2 | 1.84 (1.17-2.92) | .0089 | 1.04 (0.65-1.67) | .87 | 2.14 (1.28-3.56) | .0035 | 1.28 (0.75-2.19) | .36 |
| Histology (reference: DLBCL) | ||||||||
| Transformed FL | 1.00 (0.61-1.61) | .99 | 1.14 (0.66-1.97) | .63 | ||||
| PMBCL | 1.08 (0.54-2.15) | .84 | 1.42 (0.68-2.98) | .36 | ||||
| Double hit | 0.95 (0.61-1.49) | .82 | 1.21 (0.74-1.98) | .46 | ||||
| Primary refractory | 0.93 (0.63-1.37) | .72 | 1.25 (0.81-1.94) | .32 | ||||
| Previous lines of therapy ≥3 | 1.58 (1.07-2.33) | .021 | 1.79 (1.13-2.83) | .013 | ||||
| Previous transplant | 1.15 (0.68-1.92) | .61 | 0.61 (0.30-1.27) | .19 | ||||
| Advanced stage | 2.37 (1.44-3.95) | <.001 | 1.21 (0.66-2.24) | .54 | 2.20 (1.21-3.99) | .0094 | 1.29 (0.64-2.57) | .48 |
| Extranodal disease | 2.01 (1.26-3.20) | .0032 | 1.44 (0.87-2.38) | .16 | ||||
| CNS involvement | 2.54 (1.41-4.55) | .0018 | 2.74 (1.35-5.59) | .0055 | 2.35 (1.24-4.45) | .0091 | 2.29 (1.01-5.15) | .047 |
| Largest lesion maximum diameter, cm | 1.07 (1.03-1.10) | <.001 | 1.10 (1.06-1.14) | <.001 | ||||
| Largest lesion ≥5 cm | 1.32 (0.86-2.04) | .21 | 1.99 (1.15-3.42) | .014 | ||||
| Largest lesion ≥7.5 cm | 1.83 (1.21-2.75) | .0038 | 2.45 (1.51-3.96) | <.001 | ||||
| Largest lesion ≥10 cm | 2.46 (1.66-3.64) | <.001 | 1.63 (1.04-2.55) | .032 | 3.04 (1.93-4.77) | <.001 | 1.93 (1.11-3.34) | .019 |
| Maximum SUV ≥10 | 1.20 (0.71-2.02) | .51 | 1.83 (0.93-3.57) | .078 | ||||
| High preleukapheresis LDH | 1.65 (1.08-2.52) | .021 | 2.06 (1.22-3.48) | .0072 | ||||
| High preinfusion LDH | 3.37 (2.240-5.08) | <.001 | 2.57 (1.63-4.04) | <.001 | 4.88 (2.93-8.13) | <.001 | 3.29 (1.85-5.87) | <.001 |
| Bridging systemic therapy | 2.09 (1.42-3.09) | <.001 | 2.14 (1.38-3.33) | <.001 | ||||
| EQD2 (Gy) | 0.99 (0.97-1.00) | .087 | 0.99 (0.97-1.01) | .24 | ||||
| EQD2 ≥29.3 Gy (median) | 0.69 (0.47-1.01) | .058 | 0.69 (0.44-1.06) | .092 | ||||
| Br-RT to >1 site | 1.14 (0.71-1.83) | .60 | 1.40 (0.83-2.37) | .21 | ||||
| Comprehensive Br-RT | 0.34 (0.22-0.52) | <.001 | 0.38 (0.22-0.63) | <.001 | 0.30 (0.18-0.53) | <.001 | 0.45 (0.25-0.83) | .011 |
| Variable . | PFS, univariable . | PFS, multivariable (N = 150, 99 events) . | OS, univariable . | OS, multivariable (N = 150, 73 events) . | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) . | P value . | HR (95% CI) . | P value . | HR (95% CI) . | P value . | HR (95% CI) . | P value . | |
| Age ≥60 years | 1.54 (1.03-2.28) | .034 | 1.84 (1.21-2.80) | .0044 | 1.33 (0.85-2.08) | .22 | 1.67 (1.03-2.71) | .037 |
| Female | 1.12 (0.76-1.65) | .56 | 1.35 (0.87-2.10) | .18 | ||||
| ECOG ≥2 | 1.84 (1.17-2.92) | .0089 | 1.04 (0.65-1.67) | .87 | 2.14 (1.28-3.56) | .0035 | 1.28 (0.75-2.19) | .36 |
| Histology (reference: DLBCL) | ||||||||
| Transformed FL | 1.00 (0.61-1.61) | .99 | 1.14 (0.66-1.97) | .63 | ||||
| PMBCL | 1.08 (0.54-2.15) | .84 | 1.42 (0.68-2.98) | .36 | ||||
| Double hit | 0.95 (0.61-1.49) | .82 | 1.21 (0.74-1.98) | .46 | ||||
| Primary refractory | 0.93 (0.63-1.37) | .72 | 1.25 (0.81-1.94) | .32 | ||||
| Previous lines of therapy ≥3 | 1.58 (1.07-2.33) | .021 | 1.79 (1.13-2.83) | .013 | ||||
| Previous transplant | 1.15 (0.68-1.92) | .61 | 0.61 (0.30-1.27) | .19 | ||||
| Advanced stage | 2.37 (1.44-3.95) | <.001 | 1.21 (0.66-2.24) | .54 | 2.20 (1.21-3.99) | .0094 | 1.29 (0.64-2.57) | .48 |
| Extranodal disease | 2.01 (1.26-3.20) | .0032 | 1.44 (0.87-2.38) | .16 | ||||
| CNS involvement | 2.54 (1.41-4.55) | .0018 | 2.74 (1.35-5.59) | .0055 | 2.35 (1.24-4.45) | .0091 | 2.29 (1.01-5.15) | .047 |
| Largest lesion maximum diameter, cm | 1.07 (1.03-1.10) | <.001 | 1.10 (1.06-1.14) | <.001 | ||||
| Largest lesion ≥5 cm | 1.32 (0.86-2.04) | .21 | 1.99 (1.15-3.42) | .014 | ||||
| Largest lesion ≥7.5 cm | 1.83 (1.21-2.75) | .0038 | 2.45 (1.51-3.96) | <.001 | ||||
| Largest lesion ≥10 cm | 2.46 (1.66-3.64) | <.001 | 1.63 (1.04-2.55) | .032 | 3.04 (1.93-4.77) | <.001 | 1.93 (1.11-3.34) | .019 |
| Maximum SUV ≥10 | 1.20 (0.71-2.02) | .51 | 1.83 (0.93-3.57) | .078 | ||||
| High preleukapheresis LDH | 1.65 (1.08-2.52) | .021 | 2.06 (1.22-3.48) | .0072 | ||||
| High preinfusion LDH | 3.37 (2.240-5.08) | <.001 | 2.57 (1.63-4.04) | <.001 | 4.88 (2.93-8.13) | <.001 | 3.29 (1.85-5.87) | <.001 |
| Bridging systemic therapy | 2.09 (1.42-3.09) | <.001 | 2.14 (1.38-3.33) | <.001 | ||||
| EQD2 (Gy) | 0.99 (0.97-1.00) | .087 | 0.99 (0.97-1.01) | .24 | ||||
| EQD2 ≥29.3 Gy (median) | 0.69 (0.47-1.01) | .058 | 0.69 (0.44-1.06) | .092 | ||||
| Br-RT to >1 site | 1.14 (0.71-1.83) | .60 | 1.40 (0.83-2.37) | .21 | ||||
| Comprehensive Br-RT | 0.34 (0.22-0.52) | <.001 | 0.38 (0.22-0.63) | <.001 | 0.30 (0.18-0.53) | <.001 | 0.45 (0.25-0.83) | .011 |
DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma; PMBCL, primary mediastinal B-cell lymphoma.
Boldface indicates significant P values <0.05.
Among patients with assessable preleukapheresis MTV (N = 74) and TLG (N = 71), comprehensive Br-RT remained associated with superior PFS after controlling for either measure of tumor bulk (supplemental Table 4). There was no interaction between comprehensive Br-RT and either MTV or TLG (interaction P = .28 and .23, respectively).
One- and 2-year OS rates were 61% (95% CI, 53-68) and 53% (95% CI, 45-60), respectively (Figure 3B). Comprehensive Br-RT was associated with superior OS (HR, 0.45; 95% CI, 0.25-0.83; P = .011) on multivariable analysis (Table 3).
Among patients who received comprehensive Br-RT (N = 67), there was no difference in PFS or OS based on the overall cohort’s median EQD2 (ie, higher RT dose was not associated with improved PFS or OS) (supplemental Figure 2).
Prognostic value of LDH changes after bridging therapy
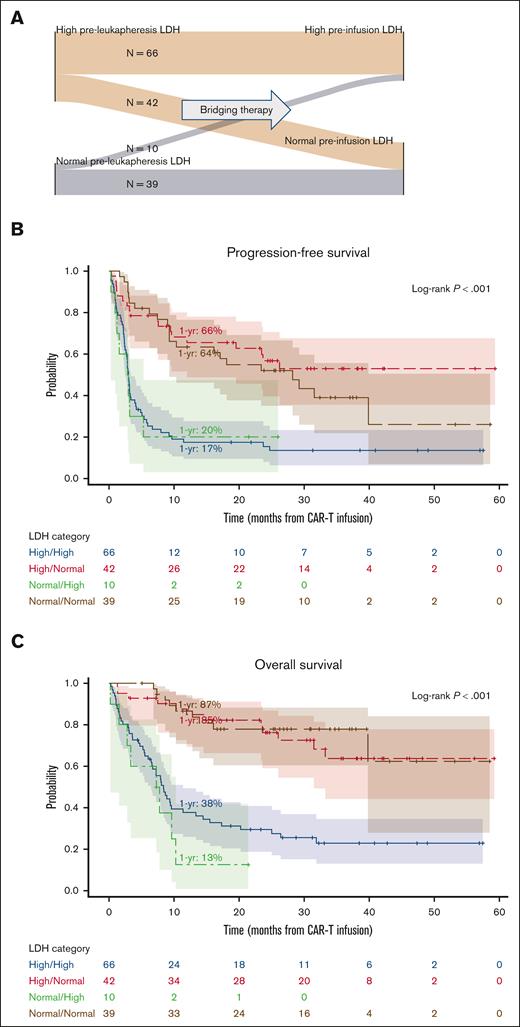
We assessed the impact of change in LDH between leukapheresis and before CAR-T infusion. Pairwise values of preleukapheresis (pre-Br-RT) and pre-CAR-T infusion (post-Br-RT) LDH were available for 157 patients, creating 4 groups: 66 patients (42%) had high pre-Br-RT LDH and high post-Br-RT LDH (high/high), 42 patients (29%) had LDH normalization after Br-RT (high/normal), 10 patients (6%) developed increased LDH after Br-RT (normal/high), and 39 patients (25%) maintained normal LDH throughout the peri-Br-RT process (normal/normal) (Figure 4A). Patients with LDH normalization after bridging therapy (ie, high/normal group) had superior PFS, OS, and complete response rates and lower grade ≥3 ICANS rates than the 2 groups with elevated post-Br-RT LDH (ie, high/high and normal/high groups) (Figure 4B-C; supplemental Table 5) and fared similarly to the normal/normal group. For example, 1-year PFS was 66% (high/normal), 17% (high/high), 20% (normal/high), and 64% (normal/normal) (P < .001). The high/normal LDH group had the highest rate of comprehensive Br-RT receipt (57%).
Prognostic value of LDH changes after bridging therapy among all evaluable patients (N = 157). (A) Sankey diagram depicting preleukapheresis (prebridging therapy) and preinfusion (postbridging therapy) LDH (high vs normal for each). (B) PFS and (C) OS stratified by LDH category.
Prognostic value of LDH changes after bridging therapy among all evaluable patients (N = 157). (A) Sankey diagram depicting preleukapheresis (prebridging therapy) and preinfusion (postbridging therapy) LDH (high vs normal for each). (B) PFS and (C) OS stratified by LDH category.
Among patients who received Br-RT alone without systemic bridging therapy (n = 103), similar findings were observed, noting there were only 3 patients in the normal/high group (supplemental Figure 3; supplemental Table 5). One-year PFS was 74% (high/normal), 17% (high/high), and 69% (normal/normal) (P < .001).
Toxicity
Grade ≥3 nonhematologic toxicity related to Br-RT occurred in 3 of 155 evaluable patients (2%); toxicity was unavailable for 17 patients (1 institution). One patient died, as further described below. Two patients developed grade 3 mucositis after radiation to extranodal head/neck lesions: the first patient developed grade 3 mucositis requiring a patient-controlled analgesia pump after 27 Gy in 9 fractions (with concurrent methotrexate, leucovorin, and cytarabine), and the other patient developed grade 3 mucositis and trismus requiring temporary total parenteral nutrition after 45 Gy in 30 fractions (without concurrent systemic therapy).
Four patients (2%) experienced early death after CAR-T in the absence of disease progression and before response assessment. All 4 patients died from complications of sepsis in the setting of sigmoid diverticulitis and perforation, pancolitis, prolonged neutropenia, or small bowel perforation. The early deaths were determined to be unrelated to Br-RT except for the death secondary to the small bowel perforation, which was possibly exacerbated by Br-RT. This patient had a large mesenteric mass with transmural bowel involvement and a known fistula before bridging therapy. The patient then underwent bridging gemcitabine-oxaliplatin-rituximab followed by an abbreviated course of Br-RT (22.5 Gy in 15 fractions delivered, 30 Gy in 20 fractions planned; stopped early owing to readiness of CAR-T product) to the mesenteric mass. After CAR-T infusion, the patient developed sepsis. Subsequent imaging demonstrated frank bowel perforation along with shrinkage of the irradiated lesion. The patient ultimately died at day +33 after CAR-T.
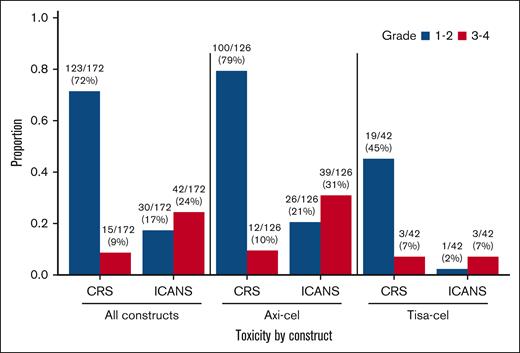
Toxicity from CAR-T is shown in Figure 5 and supplemental Table 6. Grade ≥3 CRS occurred in 15 patients (9%), including 12 of 126 (10%) after axi-cel and 3 of 42 (7%) after tisa-cel. Grade ≥3 ICANS occurred in 42 patients (24%), including 39 of 126 (31%) after axi-cel and 3 of 42 (7%) after tisa-cel. There were no grade 5 CRS or ICANS events.
Discussion
We report the largest experience of Br-RT before CAR-T in 172 patients with R/R BCL treated across 10 ILROG institutions. In a notably high-risk population, we demonstrate favorable 2-year PFS and OS (38% and 53%, respectively) with no evidence of synergistic toxicity between Br-RT and CAR-T. The CAR-T toxicity rates, PFS, and OS we observed are comparable with those seen in the seminal CAR-T trials and large consortia in which Br-RT was typically not used and bulky disease ≥10 cm was less common. Bulky disease was present in 39% of patients in our study compared with 8% in JULIET, 17% in ZUMA-1, and 23% in the US Lymphoma CAR-T Consortium.2,20,21 Our findings are noteworthy given the association of bulky disease with more severe CAR-T toxicity and poorer lymphoma-specific outcomes.20,22 These findings suggest that Br-RT may have the potential to improve clinical outcomes without an associated increase in severe treatment-related toxicities. Furthermore, patients who received comprehensive Br-RT and achieved LDH normalization after Br-RT demonstrated superior clinical outcomes. These observations may help guide future Br-RT strategies and identify LDH normalization as a possible surrogate to measure Br-RT response, respectively.
Without Br-RT, local treatment failure is a predominant pattern of disease progression after CAR-T with nearly all patients having a component of local failure (86%) at the time of progression and approximately one-third (36%) exhibiting strictly local treatment failures.5,6 In our report, most treatment failures (71%) occurred outside of the Br-RT fields, and only 6% of treatment failures were isolated in-field. This suggests a significant change in the mechanism of treatment failure after CAR-T. This finding may also support incorporating local therapies, such as Br-RT, to improve CAR-T efficacy.
Further optimization of RT with CAR-T is needed, including refinement of patient selection, dose/fractionation (high-dose ablative vs low-dose immune priming), target field size (comprehensive vs focal), and RT timing (bridging vs consolidative RT).23 Br-RT dose/fractionation schemes were notably heterogeneous in our study, ranging from 4 Gy in 2 fractions to 54 Gy in 30 fractions. Despite the various dosing schemas, the 2-year freedom from in-field failure was high (80%).
Currently, there are 2 contrasting approaches to Br-RT dosing: (1) high-dose RT (ablative) to optimize local control vs (2) low-dose RT (immune priming), which may be sufficient given that CAR-T will follow Br-RT.24 Either or both approaches may potentially augment CAR-T efficacy. Although benefits of ablative doses vs low doses of Br-RT cannot be teased out based on our study, clinical trials are underway to test various approaches (ClinicalTrials.gov identifiers: NCT06104592, NCT05800405, NCT06004167, and NCT05574114).
Another unknown is the percentage of lymphoma that should be encompassed with Br-RT. Early data suggest that patients who receive comprehensive Br-RT targeting all active sites of disease may have superior PFS and OS compared with patients who receive focal Br-RT.14 Furthermore, Saifi et al16 found better event-free survival after comprehensive Br-RT vs no bridging therapy among patients with limited disease (<5 sites). However, there is potential selection bias because comprehensive Br-RT use could correlate with disease burden (ie, diffuse disease cannot be treated with comprehensive Br-RT whereas limited stage disease would be more likely managed with comprehensive Br-RT). Our study demonstrates that comprehensive Br-RT was associated with better PFS and OS even after controlling for multiple measures of disease severity, including advanced-stage disease, lesion size, and preinfusion LDH, as well as after controlling for MTV or TLG in the subset of patients with available PET measurements. We acknowledge that this finding should be interpreted cautiously given that the comprehensive Br-RT group had lower disease burden at baseline, and that we lacked the sample size to adjust for all these differences. We attempted propensity matching but, given the small sample size and patient heterogeneity, balanced groups could not be generated. Nevertheless, our study suggests that, although there is certainly selection bias, comprehensive Br-RT may be preferable if it can be delivered safely.
One argument may be that comprehensive Br-RT is not necessary, but rather we should use Br-RT to achieve a sufficient extent of cytoreduction before CAR-T. Patients with increased tumor burden, defined as MTV >147.5cc, had inferior PFS and OS after CAR-T.22 Hubbeling et al25 found that a reduction in MTV after bridging therapy was associated with superior PFS and less ICANS. Notably, patients with a reduction in MTV fared similarly to those with initially and persistently low MTV.
On exploratory analysis, we found that patients with elevated LDH who achieve LDH normalization after Br-RT had better PFS and OS, lower grade ≥3 ICANS rates, and more frequently received comprehensive Br-RT than patients who retained, or developed, elevated post-Br-RT LDH. These patients fared similarly to those with persistently normal LDH before and after Br-RT. As such, we hypothesize that Br-RT, specifically comprehensive Br-RT, may neutralize risk factors in at-risk patients with LDH response acting as a potential surrogate for treatment response. Of note, Hubbeling et al9 also found that, among 41 patients, Br-RT produced significant reductions in LDH, along with lesion diameter, SUV, and MTV, each of which is a predictor of poor post-CAR-T outcomes. It may not be feasible to perform both pre-Br-RT and post-Br-RT PET/CT scans to measure peri-Br-RT cytoreduction given cost constraints and PET resources. Therefore, LDH normalization may be an attractive, cost-efficient surrogate to monitor tumor burden peri-Br-RT and provide prognostic information.
This study is limited by its retrospective nature, heterogeneity in patient population and follow-up procedures, and missing data. There may be selection bias, given that we excluded patients who underwent leukapheresis but not CAR-T infusion; these patients may have experienced greater Br-RT toxicity. Similarly, there was no comparator group of patients not receiving Br-RT. Moreover, certain variables were unavailable, including laboratory values other than LDH (eg, ferritin), whether patients were bridged with corticosteroids, and hematologic and grade 1-2 Br-RT toxicities.
To the best of our knowledge, this is the most comprehensive analysis to date of patients receiving Br-RT before CAR-T. Many of these patients had very unfavorable disease characteristics, including bulky disease, extranodal disease, and elevated LDH before CAR-T. Our findings provide a strong rationale to support Br-RT before CAR-T. In particular, comprehensive Br-RT and LDH normalization after Br-RT were associated with superior outcomes. Our findings suggest that targeting LDH normalization may be a useful clinical end point. The ideal degree of lymphoma control before CAR-T as well as optimization of Br-RT before CAR-T warrants additional study.
Acknowledgments
The authors thank all patients for their contributions to make this project possible.
E.A.C. is supported by a Lymphoma Research Foundation Career Development Award. B.I. and J.Y. are supported by a Leukemia & Lymphoma Society Translational Research Program Award (6654-22). T.J.R. is supported by research funding from a Clinician Scientist Development grant from the American Cancer Society (ASTRO-CSDG-23-1155803-01-RMC).
Authorship
Contribution: N.Y.-R., J.P.P., C.M.W., S.J.S., E.A.C., and N.B.F. conceived and designed the study; N.Y.-R., J.A.B., H.H., A.J.S., C.L., C.C.P., J.C.Y., J.C., S. Sinha, S. Sun, G.S., S.B., and N.B.F. collected patient information; N.Y.-R., J.P.P., S.Z., E.A.C., and N.B.F. performed data analysis and interpretation; N.Y.-R., J.P.P., E.A.C., and N.B.F. drafted the manuscript; and all authors participated in manuscript writing and review and provided final approval of the manuscript.
Conflict-of-interest disclosure: E.A.C. reports research funding from Genmab, AbbVie, AstraZeneca, CARGO, Genentech/Roche, and Nurix, and has consulted for AstraZeneca, BeiGene, Genmab, and AbbVie. N.M.D. reports research funding from Bristol Myers Squibb and serves on the advisory board for Miltenyi Biotec. S.J.S. reports research funding from Genentech/Roche, Genmab, and Nurix; has consulted for AbbVie, AstraZeneca, BeiGene, BioNTech, Genentech, Genmab, Janssen, Kite Pharmaceuticals, Legend Biotech, MorphoSys, and Novartis; and serves on steering committees for Caribou Biosciences and Novartis. P.M.R. reports research funding from Genentech and Seagen; has consulted for Caribou Biosciences and Kite Gilead; and has received speaking fees from Kite Gilead. M.J.D. reports research funding from Kite Gilead, Incyte, and Eli Lilly, and has consulted for and provides advisory to Kite Gilead and Novartis. The remaining authors declare no competing financial interests.
Correspondence: Nicholas B. Figura, Department of Radiation Oncology, H. Lee Moffitt Cancer Center, 12902 USF Magnolia Dr, Tampa, FL 33612; email: nicholas.figura@moffitt.org; and Elise A. Chong, Perelman Center for Advanced Medicine, 3400 Civic Center Blvd, South Pavilion Extension, 12th Floor, Philadelphia, PA 19104; email: elise.chong@pennmedicine.upenn.edu.
References
Author notes
N.Y.-R. and J.P.P. contributed equally to this study.
Original data are available on request from the corresponding author, Nicholas B. Figura (nicholas.figura@moffitt.org).
The full-text version of this article contains a data supplement.