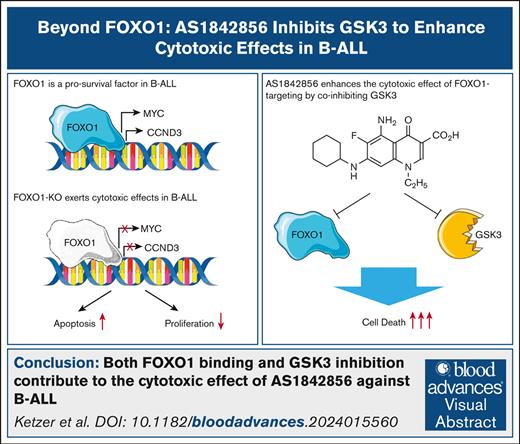
Key Points
We found that the small molecule AS1842856, previously considered a specific inhibitor of FOXO1, also directly inhibits GSK3.
Both FOXO1 binding and GSK3 inhibition contribute to the cytotoxic effect of AS1842856 against B-ALL.
Visual Abstract
Activation of the transcription factor forkhead box O1 (FOXO1) contributes to multiple pathological processes. The FOXO1 inhibitor AS1842856 demonstrated strong therapeutic effects in preclinical models of common diseases such as diabetes and anthracycline-induced heart failure. We have previously identified FOXO1 as a B-cell acute lymphoblastic leukemia (B-ALL) dependency and demonstrated in in vivo B-ALL models that AS1842856 increased the survival of animals and decreased B-ALL tumor load in all critical organ compartments, but most efficiently in the central nervous system. Here, we interrogated the underlying molecular mechanisms by comparison of the transcriptomic effects of AS1842856 and Foxo1 knockout (Foxo1-KO) in a B-ALL mouse model. Despite the significant similarity in sets of regulated genes, we identified glycogen synthase kinase (GSK) 3B inhibition as a signature enriched only in AS1842856-treated cells. Using an in vitro kinase assay and an unbiased kinome screen, we identified AS1842856 as a direct GSK3 inhibitor that ultimately stabilizes CTNNB1. CTNNB1-KO partially protected B-ALL cell lines from the cytotoxic effect of AS1842856. At the same time, using a chemical protein degradation model, we found that FOXO1 indeed contributes to the cytotoxic effect of AS1842856. We conclude that AS1842856 targets 2 B-lymphoid vulnerabilities: GSK3 and FOXO1. The unique mode of action, low toxicity, and ability to penetrate the blood-brain barrier warrant further investigation of the therapeutic potential of AS1842856 in B-ALL.
Introduction
Forkhead box class O transcription factors (FOXO1, FOXO3, FOXO4, and FOXO6) regulate cellular processes including proliferation, cell death, aging, stemness, autophagy, DNA repair, energy metabolism, and glucose homeostasis in tissue- and process-specific ways.1 FOXO1 is critical for differentiation, proliferation, and survival in early B-cell differentiation2,3 and hepatic gluconeogenesis, where it activates the transcription of key glycolytic enzymes.4 Given that hepatic gluconeogenesis drives diabetic hyperglycemia,5 FOXO1 is a therapeutic target. Consequently, the high-affinity binder AS1842856 was identified using affinity selection-mass spectrometry.6 AS1842856 suppresses the transcription of the gluconeogenic FOXO1 targets G6PC1 and PCK1 in hepatoma cells and normal liver, reducing blood glucose in db/db mice, a type 2 diabetes model. However, its molecular mechanism remains unclear. AS1842856 does neither facilitate FOXO1 nuclear export nor decrease FOXO1 expression. It has been suggested that its binding might interfere with Foxo1 interaction with insulin response elements, thereby inhibiting FOXO1-dependent transcription.6
Our interest in FOXO1 inhibition arose from paradoxical findings of high nuclear FOXO1 expression in B-cell non-Hodgkin lymphoma and B-cell acute lymphoblastic leukemia (B-ALL).7,8 This contradicts FOXO1’s essential role in apoptosis induced by (pre–)B-cell receptor (BCR) suppression in B-ALL9 and diffuse large B-cell lymphoma.10 We resolved this by proposing a Goldilocks-like effect of FOXO1, where only intermediate activity supports survival, whereas “too high” or “too low” levels induce apoptosis.7 We showed successful pharmacological FOXO1 targeting with AS1842856 in B-ALL7 and Burkitt lymphoma.11 AS1842856 mimics genetic FOXO1 depletion in many aspects, including the downregulation of the prosurvival genes CCND3 and MYC in B-ALL.
In this study, we identified glycogen synthase kinase (GSK) 3A and GSK3B as additional inhibitory targets of AS1842856 and demonstrated that the inhibition of both FOXO1 and GSK3 is necessary for the full antileukemic effect of AS1842856 in B-ALL. The leveraging of 2 B-ALL vulnerabilities and the previously shown highly efficient clearance of leukemic burden in mice, especially in the central nervous system (CNS), incentivizes the consideration of AS1842856 for further clinical investigation for the treatment of B-ALL.
Methods
Additional and detailed information on methods is provided in the supplemental Materials and methods.
Quantitative reverse transcription PCR
Isolation of RNA, complementary DNA generation, and quantitative reverse transcription polymerase chain reaction (PCR) were performed as described previously.12
Measurement of cell death and viability
In vitro kinase assay
The assessment of the GSK3B inhibitory activity was done by drug screening service of Amsbio (Amsbio LLC, Cambridge, MA) with help of GSK3β Assay Kit (catalog no. 79700).
Kinome profiling
The kinome assay was done by “KinSight Kinome Profiling Services” (AssayQuant Technologies, Marlborough, MA) using the PhosphoSens format, which included 401 kinome profiling kinases. The AS1842856 was tested at a concentration of 100 nM and adenosine triphosphate Km for each kinase.
CRISPR/Cas9 DNA editing
For CRISPR/Cas9 knockout (KO) of FOXO1 and CTNNB1, we used a tandem guide RNA (gRNA) approach as described.13
Establishing clones biallelically expressing FOXO1-dTAG fusion proteins (FPs)
Homozygous endogenous expression of FOXO1-degradation tag (dTAG) was achieved using CRISPR/Cas9 and a donor plasmid encoding FKBP12F36V.14
Results
BCR::ABL1–transformed mouse pre–B-cell model FOXO1 depletion effects comparable with human B-ALL
In our previous work,7 we demonstrated depletion of the FOXO1 gene signature in human ETV6-RUNX1 and MLLr B-ALL cell lines treated with AS1842856 and revealed similar effects of AS1842856 and small hairpin RNA–dependent FOXO1 knockdown on the expression of prosurvival FOXO1 targets including MYC and CCND3. AS1842856 did not modulate the expression of canonical FOXO1 targets including interleukin-7 receptor alpha (IL-7RA),7 indicating that binding of AS1842856 differentially modulates FOXO1 functions. Small inhibitors rarely have only 1 target,15 and some observed differences might stem from limitations of RNA interference–based experiments, particularly the delay between transduction and protein expression effects.
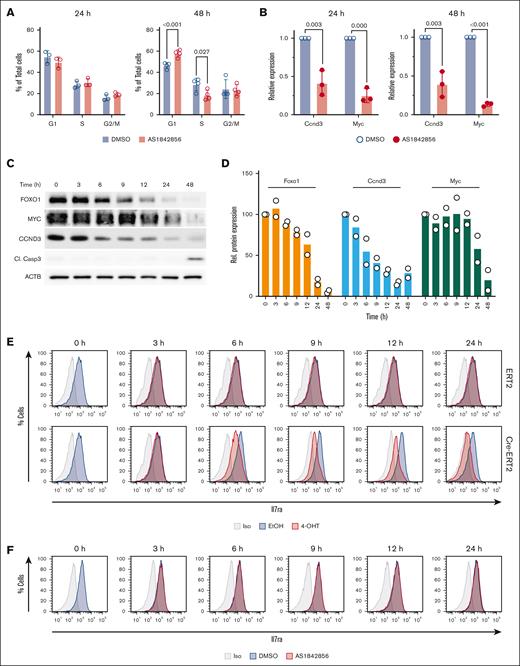
To achieve a more precise comparison of AS1842856 and genetic FOXO1 depletion effects, we used murine BCR::ABL1–transformed Foxo1fl/fl pre–B cells with tamoxifen-inducible Cre-ERT2 for conditional deletion of Foxo1.16 We first determined the sensitivity of BCR::ABL1+ mouse pre–B cells to AS1842856, finding a 50% inhibitory concentration (IC50)of 34 nM (supplemental Figure 1), comparable with human B-ALL cell lines.7 AS1842856 induced significant cell cycle arrest after 48 hours but not after 24 hours (Figure 1A), similar to human B-ALL models. Decreased Ccnd3 and Myc messenger RNA expression was observed at 24 and 48 hours, indicating that transcriptional changes precede cell cycle arrest (Figure 1A-B). This aligns with our previous observation of strong decreases in Foxo1, Myc, and Ccnd3 expression 24 hours after Foxo1-KO but first cytotoxic effects after 48 hours in the ex vivo BCR::ABL1+Foxo1fl/fl Cre-ERT2 model.12
Characterization of the effects of genetic depletion and pharmacological inhibition of FOXO1 in a BCR::ABL1+ B-ALL mouse model. (A-B) AS1842856 downregulates Myc and Ccnd3 messenger RNA expression and induces cell cycle perturbations in BCR::ABL1–transformed murine pre–B cells. (A) The cells were treated with 70 nM AS1842856. The cell cycle distribution was measured by propidium iodide staining after 24 and 48 hours (n = 3). The data are shown as mean ± standard deviation. (B) Myc and Ccnd3 expression was measured by quantitative reverse transcription PCR (n = 3) and calculated using the 2–△△Ct method. The housekeeping gene Rpl13a was used for normalization. (C-D) BCR::ABL1+ CreERT2/Foxo1fl/fl murine B-ALL cells were treated with 200 nM 4-OHT. Protein expression of FOXO1, MYC, CCND3, and Cl. Casp3 was monitored by immunoblot. A representative of 2 independent experiments is shown. (D) Quantification of immunoblots. Protein expression of FOXO1, CCND3, and MYC was divided by the corresponding beta-actin (ACTB) levels and normalized to the protein of interest-to-ACTB ratio at day 0 (100%; n = 2). (E) BCR::ABL1+ Cre-ERT2 Foxo1fl/fl murine B-ALL cells were treated with 200 nM 4-OHT or equivalent amounts of EtOH vehicle. Cells were stained for Il7ra surface expression or isotype control and analyzed by flow cytometry at the indicated time points. Data shown are representative of 3 independent experiments. (F) BCR::ABL1+ Cre-ERT2 Foxo1fl/fl murine B-ALL cells were treated with 70 nM AS1842856 or equivalent amounts of DMSO vehicle. Cells were stained for Il7ra surface expression or isotype control and analyzed by flow cytometry at the indicated time points. Data shown are representative of n = 3. Cl. Casp3, cleaved caspase-3; EtOH, ethyl alcohol; Iso, isotype; Rel, relative.
Characterization of the effects of genetic depletion and pharmacological inhibition of FOXO1 in a BCR::ABL1+ B-ALL mouse model. (A-B) AS1842856 downregulates Myc and Ccnd3 messenger RNA expression and induces cell cycle perturbations in BCR::ABL1–transformed murine pre–B cells. (A) The cells were treated with 70 nM AS1842856. The cell cycle distribution was measured by propidium iodide staining after 24 and 48 hours (n = 3). The data are shown as mean ± standard deviation. (B) Myc and Ccnd3 expression was measured by quantitative reverse transcription PCR (n = 3) and calculated using the 2–△△Ct method. The housekeeping gene Rpl13a was used for normalization. (C-D) BCR::ABL1+ CreERT2/Foxo1fl/fl murine B-ALL cells were treated with 200 nM 4-OHT. Protein expression of FOXO1, MYC, CCND3, and Cl. Casp3 was monitored by immunoblot. A representative of 2 independent experiments is shown. (D) Quantification of immunoblots. Protein expression of FOXO1, CCND3, and MYC was divided by the corresponding beta-actin (ACTB) levels and normalized to the protein of interest-to-ACTB ratio at day 0 (100%; n = 2). (E) BCR::ABL1+ Cre-ERT2 Foxo1fl/fl murine B-ALL cells were treated with 200 nM 4-OHT or equivalent amounts of EtOH vehicle. Cells were stained for Il7ra surface expression or isotype control and analyzed by flow cytometry at the indicated time points. Data shown are representative of 3 independent experiments. (F) BCR::ABL1+ Cre-ERT2 Foxo1fl/fl murine B-ALL cells were treated with 70 nM AS1842856 or equivalent amounts of DMSO vehicle. Cells were stained for Il7ra surface expression or isotype control and analyzed by flow cytometry at the indicated time points. Data shown are representative of n = 3. Cl. Casp3, cleaved caspase-3; EtOH, ethyl alcohol; Iso, isotype; Rel, relative.
Using the inducible Cre-ERT2 system, we assessed FOXO1 protein expression dynamics and its targets in the first 12 hours after 4-hydroxytamoxifen (4-OHT) treatment. Most pronounced FOXO1 protein downregulation was detected after 12 hours, with a clear decrease visible at 6 hours (Figure 1C). CCND3 expression closely mirrored FOXO1, whereas MYC expression declined after 12 hours (Figure 1C-D). Consistent with previous data,12 apoptosis became apparent at 48 hours after induction of Foxo1 deletion.
Next, we compared the pharmacological and genetic Foxo1 depletion effects on surface protein expression of its canonical target Il7ra using flow cytometry.16 Il7ra expression dynamics precisely followed FOXO1 expression decrease after 4-OHT treatment. Decreased surface expression became apparent 6 hours after treatment and gradually increased until 24 hours (Figure 1E). Strikingly, treatment with 70 nM AS1842856 yielded no detectable difference in Il7ra surface expression, even after 24 hours (Figure 1F).
This remarkable difference in canonical FOXO1 target gene expression warranted further investigation into AS1842856’s mechanism of action.
Comparison of transcriptomic effects of genetic Foxo1 depletion and treatment with AS1842856
Considering the intriguing difference on Il7ra expression after genetic Foxo1 depletion and its pharmacological inhibition, we set out to identify off-target functions of AS1842856 by comparing transcriptomic differences of genetic Foxo1-KO and its inhibition.
For genetic deletion, BCR::ABL1+ Cre-ERT2 Foxo1fl/fl cells were treated with 200 nM 4-OHT or an equivalent amount of the vehicle ethyl alcohol for 24 or 48 hours. Alternatively, cells were treated with either 70 nM AS1842856 or the equivalent amount of the vehicle dimethyl sulfoxide (DMSO) for 24 or 48 hours. Unsupervised hierarchical clustering revealed that cells treated with AS1842856 showed few alterations in gene expression after 24 hours of treatment, whereas Foxo1-KO immediately led to profound transcriptional changes (Figure 2A; supplemental File 1). As expected, after 48 hours, both treatments showed stronger changes in the transcriptome than 24 hours. Interestingly, when adjusted for significantly differentially regulated genes and normalized to the respective vehicle controls, the comparison of both treatments shows an overlap in many genes higher expressed after the treatments, as well as most of the strongest downregulated genes (Figure 2B). Furthermore, we also observed opposing regulation of genes by both treatments, strengthening our hypothesis that AS1842856 inhibits other targets besides FOXO1. Generally, the transcriptomic effect of Foxo1-KO was much more pronounced than treatment with AS1842856. By conducting principal component analysis, the triplicates of each group were plotted to identify patterns of transcriptomic changes introducing the maximal variance for each treatment (supplemental Figure 2). As expected, DMSO and ethyl alcohol controls clustered together with slight variance. AS1842856 treatment at 24 hours mostly matched the DMSO control but showed increased variance at 48 hours, aligning with its observed effects at 48 hours. In contrast, Foxo1-KO induced strong transcriptomic changes earlier, intensifying by 48 hours after 4-OHT treatment. Changes in the expression of canonical Foxo1 target genes at 24 hours of AS1842856 treatment were consistent with transcriptomic changes at 24 hours in the Foxo1-KO group (Figure 2C). Known Foxo1 targets, such as Rag1, Rag2, Myc, and Ccnd3, were significantly downregulated in both groups after 24 hours. In line with the flow cytometry data (Figure 1E-F), Il7r expression was downregulated only in the Foxo1-KO group. To understand the functional impact of treatment with AS1842856 and Foxo1-KO, we used gene set enrichment analysis (GSEA). For the analysis, both time points of each group were merged, to achieve a complete picture of regulated pathways (Figure 2D). As expected, “Hallmarks” gene sets of MYC- and cell cycle–dependent pathways were downregulated in both groups. However, in cells treated with AS1842856, only these 2 pathways were significantly downregulated, whereas more were upregulated (Figure 2D; supplemental File 2). In both treatments, the TP53 pathway was upregulated, most likely as a consequence of the induction of apoptosis from loss of FOXO1 function and the ensuing loss of MYC expression. Among the enriched gene sets upregulated owing to AS1842856 treatment were the mTORC1 and glycolysis pathways, reciprocal to Foxo1-KO. We have previously shown that AS1842856 inhibits mTORC1 signaling and glycolysis; however, these effects only became visible after 48 hours of treatment, demonstrating time-dependent facets of the functionality of AS1842856.17,18 In addition, Foxo1-KO led to the increase of multiple signaling pathways atypical for BCR::ABL1+ B-ALL, such as KRAS and Notch.19 Given that FOXO1 is a known regulator of pre–B-cell differentiation and identity, this change in cellular programming after its loss is not surprising.20 To further dissect the individual and shared effects of Foxo1-KO and its inhibition, we performed Venn analysis and subsequent ChIP-X Enrichment Analysis21 of transcription factor targets (Figure 2E; supplemental Files 3 and 4). As expected, genes regulated by both Foxo1-KO and AS1842856 showed significant enrichment for FOXO1 chromatin immunoprecipitation (ChIP)-sequencing targets. In line with the GSEA data, MYC ChIP-sequencing targets were highly enriched for genes regulated by Foxo1-KO only. Genes regulated by only AS1842856 included chromatin modifiers such as RACK7 and BRD4,22 but strikingly also TCF7 transcription factor targets. This is surprising, given that TCF7 was previously described to be involved in T-cell acute lymphoblastic leukemia, but not B-ALL, in which it is reportedly inactive.23 FOXO1 directly regulates TCF7 in T cells,24 but because TCF7 targets did not seem to be regulated by Foxo1-KO in our B-ALL model, we proceeded to further investigate how TCF7 targets could be regulated by AS1842856 only and which potential additional targets could be responsible for this puzzling effect.
Comparison of the transcriptomic effects of Foxo1-KO and treatment with AS1842856 in murine BCR::ABL1+ B-ALL. (A) RNA-sequencing was performed in BCR::ABL1+ Cre-ERT2 Foxo1fl/fl murine B-ALL cells treated with either 200 nM 4-OHT or an equivalent amount of vehicle EtOH or with 70 nM AS1842856 or equivalent amounts of DMSO vehicle. RNA was isolated after 24 and 48 hours. Analysis was performed using the DESeq2 workflow in RStudio (version 2024.12.1). The unsupervised hierarchical clustering of all genes found in all samples is shown (supplemental File 1). (B) Hierarchical clustering of genes significantly (P < .05; log2[FC] > 1.4) regulated was performed in both groups at 24 and 48 hours after respective treatment. (C) Volcano plot of differentially expressed genes. Significance threshold was set to adjusted P <.05 and log2(FC) >1.5. Significantly downregulated genes are shown in blue; significantly upregulated genes are shown in red. Canonical FOXO1 targets are highlighted and labeled in black. (D) Differentially expressed genes from days 1 and 2 of either Foxo1-KO or AS1842856-treated samples were pooled and subjected to GSEA for “Hallmarks” gene sets using GSEA (version 4.3.3; supplemental File 2). (E) Differentially regulated genes in BCR::ABL1+ murine B-ALL after treatment with 70 nM AS1842856 or genetic Foxo1-KO were subjected to Venn analysis. Exclusive and shared genes were cross-referenced to the ChEA 2022 database using Enrichr (supplemental Materials and methods). ChEA, ChIP-X Enrichment Analysis; FC, fold change.
Comparison of the transcriptomic effects of Foxo1-KO and treatment with AS1842856 in murine BCR::ABL1+ B-ALL. (A) RNA-sequencing was performed in BCR::ABL1+ Cre-ERT2 Foxo1fl/fl murine B-ALL cells treated with either 200 nM 4-OHT or an equivalent amount of vehicle EtOH or with 70 nM AS1842856 or equivalent amounts of DMSO vehicle. RNA was isolated after 24 and 48 hours. Analysis was performed using the DESeq2 workflow in RStudio (version 2024.12.1). The unsupervised hierarchical clustering of all genes found in all samples is shown (supplemental File 1). (B) Hierarchical clustering of genes significantly (P < .05; log2[FC] > 1.4) regulated was performed in both groups at 24 and 48 hours after respective treatment. (C) Volcano plot of differentially expressed genes. Significance threshold was set to adjusted P <.05 and log2(FC) >1.5. Significantly downregulated genes are shown in blue; significantly upregulated genes are shown in red. Canonical FOXO1 targets are highlighted and labeled in black. (D) Differentially expressed genes from days 1 and 2 of either Foxo1-KO or AS1842856-treated samples were pooled and subjected to GSEA for “Hallmarks” gene sets using GSEA (version 4.3.3; supplemental File 2). (E) Differentially regulated genes in BCR::ABL1+ murine B-ALL after treatment with 70 nM AS1842856 or genetic Foxo1-KO were subjected to Venn analysis. Exclusive and shared genes were cross-referenced to the ChEA 2022 database using Enrichr (supplemental Materials and methods). ChEA, ChIP-X Enrichment Analysis; FC, fold change.
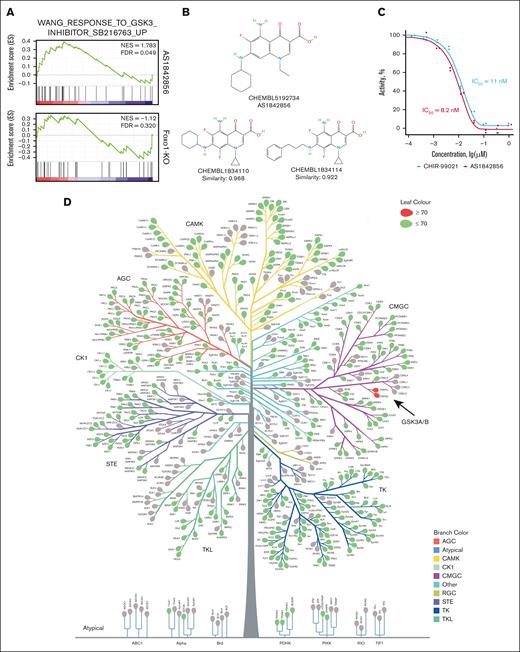
AS1842856 is a potent inhibitor of GSK3A and GSK3B
To isolate the apparent off-target effects of AS1842856, we performed GSEA with genes exclusively regulated by AS1842856 or Foxo1-KO for all curated gene sets available in the molecular signature database25,26 (supplemental File 2). Filtering for gene sets containing data from small molecular weight inhibitors, we found significant enrichment for the gene set “WANG_RESPONSE_TO_GSK3_INHIBITOR_SB216763_UP” only after the treatment with AS1842856 but not after Foxo1-KO (Figure 3A). This is in line with a recent publication on the acceleration of GSK3A/B exocytosis by AS1842856. However, this effect was observed only at a high concentration of 0.5 μM.27 In contrast, the IC50 of AS1842856 in most B-ALL cell lines is in the range of 1 to 40 nM,7 suggesting an alternative mechanism of GSK3 inhibition. To clarify this, we used the SwissTargetPrediction tool (http://www.swisstargetprediction.ch/; accessed on 25 October 2023).28,29 Based on the high structural similarity to the other quinolizidinone carboxylic acid derivatives CHEMBL1834110 and CHEMBL1834114,30 which directly inhibit GSK3B at IC50 of 600 nM and 45 nM, respectively (BindingDB https://www.bindingdb.org/rwd/bind/index.jsp31; accessed on 22 January 2024), GSK3B was identified as the only likely (probability, 0.691) target of AS1842856 (Figure 3B). To confirm this, we used an in vitro kinase assay. As a positive control, we chose the most selective, clinically tested inhibitor of GSK3, CHIR-99021/Laduviglusib/CT99021.32 AS1842856 and CHIR-99021 were nearly equally potent in inhibiting GSK3B enzymatic activity in vitro (IC50 = 8.2 nM and 11 nM, respectively; Figure 3C).
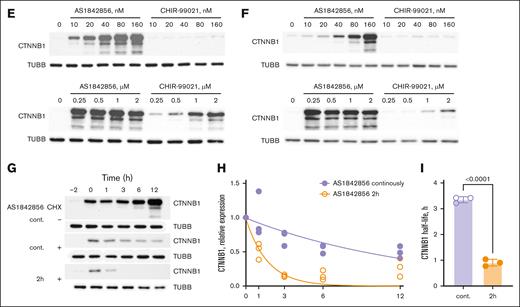
AS1842856 is an inhibitor of GSK3B. (A) GSEA of genes exclusively regulated by either Foxo1-KO or AS1842856 were subjected to GSEA for the “c2” curated database as described in Figure 2D. The result for the gene set “WANG_RESPONSE_TO_GSK3_INHIBITOR_SB216763_UP” in both conditions is shown. (B) Structural similarity of AS1842856 to quinolizidinone carboxylic acid derivates that inhibit GSK3B. To predict the pharmacological activities of AS1842856, we used the SwissTargetPrediction tool (http://www.swisstargetprediction.ch/). The prediction was made by the high structural similarity of AS1842856 to quinolizidinone carboxylic acid derivatives. The 2 most similar compounds are shown (BindingDB and PubCHEM). (C) Investigation of GSK3B-inhibitory activity was performed by in vitro kinase luminescent assay. The highly specific GSK3B inhibitor CHIR-99021 was used as a positive control (n = 2). (D) An unbiased kinome screen was performed. Kinases inhibited by AS1842856 >70% are shown as red leaves. AS1842856 was tested at concentration 100 nM and adenosine triphosphate Km for each kinase. (E-F) RS4;11 (E) and 018Z cells (F) were treated with indicated compounds or vehicle (DMSO) for 24 hours. The expression of CTNNB1 or TUBB (loading control) was determined by immunoblot. The most representative of 3 independent experiments is shown. (G-I) AS1842856 reversibly inhibits CTNNB1 degradation. 018Z cells were cont. incubated with 100 nM AS1842856. After 2-hour incubation with AS1842856, CHX was added at a concentration of 50 μg/mL. In 1 of the groups (2 hours), AS1842856 was washed out before the addition of CHX. (G) The most representative of 3 independent experiments is shown (n = 3). (H) The immunoblots were quantified by ImageJ 1.53k software (https://imagej.net/ij). The relative CTNNB1 expression was calculated as (CTNNB1E/TUBBE)/(CTNNB1C/TUBBC), in which E are the results of measurements in experimental and C in control (time point 0) groups. The data are shown as mean and scattered experimental points (n = 3). (I) The CTNNB1 half-life was calculated with the help of GraphPad Prism 10.4.1 software (nonlinear regression; absolute IC50). CHX, cycloheximide; cont., continuously; FDR, false discovery rate; NES, normalized enrichment score.
AS1842856 is an inhibitor of GSK3B. (A) GSEA of genes exclusively regulated by either Foxo1-KO or AS1842856 were subjected to GSEA for the “c2” curated database as described in Figure 2D. The result for the gene set “WANG_RESPONSE_TO_GSK3_INHIBITOR_SB216763_UP” in both conditions is shown. (B) Structural similarity of AS1842856 to quinolizidinone carboxylic acid derivates that inhibit GSK3B. To predict the pharmacological activities of AS1842856, we used the SwissTargetPrediction tool (http://www.swisstargetprediction.ch/). The prediction was made by the high structural similarity of AS1842856 to quinolizidinone carboxylic acid derivatives. The 2 most similar compounds are shown (BindingDB and PubCHEM). (C) Investigation of GSK3B-inhibitory activity was performed by in vitro kinase luminescent assay. The highly specific GSK3B inhibitor CHIR-99021 was used as a positive control (n = 2). (D) An unbiased kinome screen was performed. Kinases inhibited by AS1842856 >70% are shown as red leaves. AS1842856 was tested at concentration 100 nM and adenosine triphosphate Km for each kinase. (E-F) RS4;11 (E) and 018Z cells (F) were treated with indicated compounds or vehicle (DMSO) for 24 hours. The expression of CTNNB1 or TUBB (loading control) was determined by immunoblot. The most representative of 3 independent experiments is shown. (G-I) AS1842856 reversibly inhibits CTNNB1 degradation. 018Z cells were cont. incubated with 100 nM AS1842856. After 2-hour incubation with AS1842856, CHX was added at a concentration of 50 μg/mL. In 1 of the groups (2 hours), AS1842856 was washed out before the addition of CHX. (G) The most representative of 3 independent experiments is shown (n = 3). (H) The immunoblots were quantified by ImageJ 1.53k software (https://imagej.net/ij). The relative CTNNB1 expression was calculated as (CTNNB1E/TUBBE)/(CTNNB1C/TUBBC), in which E are the results of measurements in experimental and C in control (time point 0) groups. The data are shown as mean and scattered experimental points (n = 3). (I) The CTNNB1 half-life was calculated with the help of GraphPad Prism 10.4.1 software (nonlinear regression; absolute IC50). CHX, cycloheximide; cont., continuously; FDR, false discovery rate; NES, normalized enrichment score.
To estimate the specificity of this unexpected effect and to identify other targets that might contribute to the cytotoxic effect of AS1842856, we performed kinome profiling. Of 401 kinases, 17 were inhibited at ≥30% (variability threshold; supplemental Figure 3), whereas only 2 kinases, GSK3A and GSK3B, were inhibited at ≥70% (Figure 3D; supplemental File 5). Notably, all kinase hits >50% inhibition, with the exception of PIM3, were within the cyclin-dependent kinase (CDK), MAPK, GSK, and cdc-like kinase (CLK) group. Importantly, we used AS1842856 for profiling at a concentration of 100 nM, which is lower than the maximum concentration (Cmax) in mouse plasma but 10 to 25 times higher than the IC50 determined in in vitro cytotoxic experiments. Hence, we conclude that the observed biological effects of AS1842856 in B-ALL at low nanomolar concentrations are predominantly caused by the direct inhibition of GSK3A/B and its binding to FOXO1.
Given that inhibitors of GSK3 stabilize CTNNB1,33 we compared the effects of AS1842856 and CHIR-99021 on CTNNB1 protein expression in the B-ALL cell lines RS4;11 (Figure 3E) and 018Z (Figure 3F) as a functional readout of GSK3 inhibition. Despite performing equally in the in vitro kinase assay, AS1842856 significantly outperformed CHIR-99021 in CTNNB1 stabilization. The increase in CTNNB1 expression was already visible at the lowest AS1842856 concentration (10 nM) in both cell lines, whereas cells treated with CHIR-99021 only showed a similar increase at a concentration of 250 to 500 nM (Figure 3E-F).
Next, we investigated the dynamics of CTNNB1 stabilization by cycloheximide chase assay (Figure 3G-I). We found that 2 hours before incubation with AS1842856 was sufficient to achieve a measurable expression of CTNNB1, which then increased continuously in the presence of AS1842856. Blocking translation by cycloheximide for 2 hours after induction of CTNNB1 expression prevented an increase of CTNNB1 expression, followed by its slow decrease (half-life, 3.35 hours), when AS1842856 was left in the medium. Removal of AS1842856 before adding cycloheximide (CXH) decreased CTNNB1 half-life to 0.90 hours, which corresponds to the half-life of CTNNB1 in most human cells that do not have Wnt/CTNNB1-activating mutations (50-60 minutes).34,35 We conclude that AS1842856 is a selective, reversible inhibitor of GSK3A and GSK3B. In addition, these results explain the modulation of TCF7 transcription factor targets exclusively by AS1842856 but not Foxo1-KO, given that stabilized CTNNB1 binds to TCF/LEF transcription factors to exert transcriptional effects.36
CTNNB1 stabilization contributes to the cytotoxic effect of AS1842856 against B-ALL
Recently published preliminary data indicate that CTNNB1 stabilization by GSK3 inhibitors is the main mechanism of their cytotoxic effect in B-ALL.36 To understand the role of CTNNB1 for the cytotoxic effect of AS1842856 in B-ALL, we inactivated this gene by CRISPR/Cas9 tandem gRNA editing. We were aware that CRISPR-dependent DNA editing induces a DNA damage response and may ultimately facilitate the selection of TP53-deficient clones37,38 that are less sensitive to apoptosis. To overcome this limitation, we avoided the selection of CTNNB1-deficient clones in our loss-of-function experiments and used bulk cultures instead to preserve the intrinsic heterogeneity of tumor cell populations, which is also maintained in cell lines.39
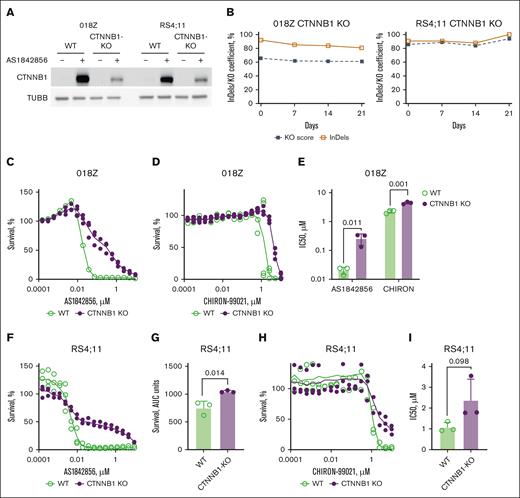
Given that CTNNB1 protein is naturally expressed at very low levels in 018Z and RS4;11 B-ALL cells, we achieved increased expression by GSK3 inhibition using AS1842856 to control the efficiency of the CTNNB1-KO. In both cell lines, stabilization of CTNNB1 by AS1842856 conferred much higher expression levels of CTNNB1 in wild-type (WT) than in edited KO cells, indicating the decrease of WT-CTNNB1 expression in transfected bulk cultures (Figure 4A). In addition, we monitored the dynamics of the population containing inactivating indels (KO score; Figure 4B). The KO scores in 018Z and RS4;11 cell lines did not change significantly during the period of observation and fluctuated in a range of 61% to 66% and 86% to 94%, respectively.
CTNNB1 contributes to the cytotoxic effect of AS1842856. (A-B) 018Z and RS4;11 cell lines were CRISPR/Cas9 edited with tandem CRISPR-RNA (crRNA) targeting CTNNB1. (A) The efficiency of CTNNB1 loss-of-function editing was measured by immunoblotting after 24-hour incubation with AS1842856 at a concentration of 100 nM to stabilize CTNNB1 (n = 2). (B) Dynamic of CTNNB1 inactivating mutations in CRISPR/Cas9-edited RS4;11 and 018Z cell lines. The proportion of inactivating mutations (KO score) was monitored by quantification of Sanger chromatograms of the edited region using the ICE (Inference of CRISPR Edits) CRISPR analysis tool (https://ice.synthego.com, accessed on 13 June 2024; Synthego Corporation, Redwood City, CA). (C-I) CTNNB1-KO decreases the sensitivity of B-ALL to AS1842856 and CHIR-99021. The cytotoxic effect of the inhibitors on the survival of 018Z (C-E) and RS4;11 cell lines (F-I) was analyzed with the help of 6-day MTT assay. In panels E,H-I, the dose-effect data were fitted by nonlinear regression model and IC50 values and AUC values were calculated with the help of GraphPad Prism software, version 10.30 (San Diego, CA). The significance of IC50 or AUC differences was calculated by 2-sided nonpaired Student t test (n = 3).AUC, area under survival curve.
CTNNB1 contributes to the cytotoxic effect of AS1842856. (A-B) 018Z and RS4;11 cell lines were CRISPR/Cas9 edited with tandem CRISPR-RNA (crRNA) targeting CTNNB1. (A) The efficiency of CTNNB1 loss-of-function editing was measured by immunoblotting after 24-hour incubation with AS1842856 at a concentration of 100 nM to stabilize CTNNB1 (n = 2). (B) Dynamic of CTNNB1 inactivating mutations in CRISPR/Cas9-edited RS4;11 and 018Z cell lines. The proportion of inactivating mutations (KO score) was monitored by quantification of Sanger chromatograms of the edited region using the ICE (Inference of CRISPR Edits) CRISPR analysis tool (https://ice.synthego.com, accessed on 13 June 2024; Synthego Corporation, Redwood City, CA). (C-I) CTNNB1-KO decreases the sensitivity of B-ALL to AS1842856 and CHIR-99021. The cytotoxic effect of the inhibitors on the survival of 018Z (C-E) and RS4;11 cell lines (F-I) was analyzed with the help of 6-day MTT assay. In panels E,H-I, the dose-effect data were fitted by nonlinear regression model and IC50 values and AUC values were calculated with the help of GraphPad Prism software, version 10.30 (San Diego, CA). The significance of IC50 or AUC differences was calculated by 2-sided nonpaired Student t test (n = 3).AUC, area under survival curve.
In line with previous findings from other groups, we conclude that the B-ALL cell lines are not dependent on CTNNB1 and KO cells provide a viable model.40 Next, we used CTNNB1-deficient bulk cultures of 018Z and RS4;11 to investigate the role of CTNNB1 in the cytotoxic effect of AS1842856 and CHIR-99021 (positive control; Figure 4C-I). CTNNB1-KO 018Z cells were more than 13-fold less sensitive to AS1842856 than WT 018Z (IC50 = 264 nM and 20 nM, respectively). CTNNB1-KO 018Z cells were also less sensitive to CHIR-99021 at IC50 of 4.5 μM and 2.2 μM, respectively. In RS4;11 CTNNB1-KO cell lines, the dose-response curve had a multiphasic pattern that did not allow calculation of IC50 (Figure 4F). To estimate the statistical significance of the apparent increase of resistance in CTNNB1-KO RS4;11 cells, we analyzed the area under dose-response curve (www.graphpad.com/support/faqid/2031/; accessed on 26 July 2024; Figure 4F-G). The CTNNB1-KO also protected RS4;11 cells from CHIR-99021 treatment (Figure 4H-I). We conclude that GSK3 inhibition, as indicated by CTNNB1 accumulation, contributes to the cytotoxic effect of AS1842856 and serves as a viable therapeutic strategy in B-ALL.
FOXO1 contributes to the cytotoxic effect of AS1842856
We have previously shown that FOXO1-overexpression can rescue B-ALL cells from the cytotoxic effect of AS1842856.7 However, because FOXO1 depletion in contrast to CTNNB1 demonstrably induces cell death in mouse and human B-cell leukemia, it is challenging to create a relevant loss-of-function model to assess the specific contribution of FOXO1 to the effect of AS1842856.
To this end, we set out to generate a model that would lose FOXO1 dependency and allow inducible depletion of the FOXO1 protein. Although inducible Foxo1-KO induces cell death in BCR::ABL1–transformed pro–B cells,12,16 the generation of Foxo1-independent clones in the same model by CRISPR/Cas9 DNA editing is feasible.41 To confer inducible FOXO1 depletion, we took advantage of the allele-specific knockin of a dTAG.42 In particular, we knocked in the FKBP12F36V dTAG in-frame directly in front of the FOXO1 stop codon. The inserted construct also contained the fluorescent marker enhanced green fluorescent protein (supplemental Figure 4A) for sorting of the knockin cells. We selected 3 clones expressing FOXO1-dTAG on both alleles as validated by immunoblot and PCR (supplemental Figure 4B-C). Using fluorescence microscopy, we demonstrated normal nuclear and cytoplasmic expression for the FOXO1-dTAG FP.7 Treatment with the protein kinase B (AKT) inhibitor MK2206 facilitated the nuclear translocation of the FOXO1-dTAG protein, which is characteristic of WT FOXO1 (supplemental Figure 4D). To initiate the degradation of FOXO1-dTAG, we compared 2 commercially available proteolysis-targeting chimeras (PROTACs). dTAG-13, a FKBP12F36V-specific PROTAC that recruits the E3 ubiquitin ligase cereblon,42 and dTAGV-1, engaging the von Hippel-Lindau E3 ligase complex.14 Given that dTAG-13 performed better, as measured by the calculation of FOXO1-dTAG FP expression after treatment with the PROTACs at equimolar concentrations, we used it for further experiments (supplemental Figure 4E-F).
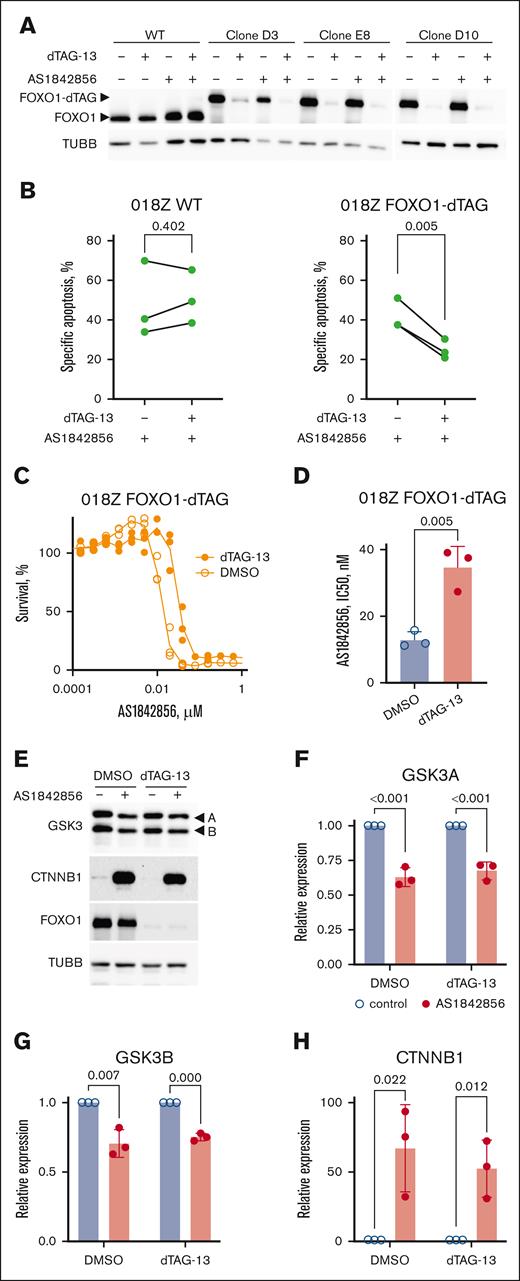
dTAG-13 strongly reduced FOXO1 expression in all 3 clones. AS1842856 did not affect FOXO1 expression either alone or in combination with dTAG-13 (Figure 5A). FOXO1 degradation in the clones did not reduce the expression of the essential FOXO1 prosurvival targets CCND3 and MYC7,12 (supplemental Figure 5A). Regardless of the PROTAC used, FOXO1 depletion did not induce apoptosis in any of the clones (supplemental Figure 5B-C). We assumed that the clones might have lost their dependency on FOXO1 in the process of CRISPR/Cas9 DNA editing. To prove this, we assessed the dependence on FOXO1-dTAG alleles compared with WT-FOXO1. To this end, we examined the dynamics of FOXO1/FOXO1-dTAG alleles with inactivating indels (KO score) after CRISPR/Cas9-mediated editing as we described previously43 (supplemental Figure 5D-E). We additionally controlled for nonspecific CRISPR/Cas9 effects by targeting the heart-specific gene troponin I type 3, which is nonessential for B-ALL cell lines.43 FOXO1 targeting continuously decreased FOXO1/FOXO1-dTAG KO score, in 018Z and 018Z-FOXO1-dTAG cells in a similar manner, whereas by targeting the troponin I type 3 locus KO score remained unchanged or even increased (supplemental Figure 5F-G). Given that our data indicated intact functionality of the FOXO1-dTAG FP, we reasoned that small, but still detectable by immunoblot, amounts of the protein might be sufficient to support cell survival and transcriptional activity. Given that either downregulation or upregulation of FOXO1 induces cell death in B-ALL,7,9 we investigated the effect of FOXO1-dTAG degradation on the sensitivity of 018Z FOXO1-dTAG cells to the AKT inhibitor MK2206. FOXO1 depletion decreased the IC50 of MK2206 by more than 100-fold (supplemental Figure 5H). Next, we assessed the effect of FOXO1-dTAG degradation on the cell death, induced by AS1842856. The cytotoxic effect of AS1842856 in FOXO1-dTAG clones was comparable with that observed in WT 018Z cells. However, the absence of FOXO1 significantly reduced cell death in the clones (Figure 5B). This partial dependency of the cytotoxic effect of AS1842856 on FOXO1 was confirmed by MTT assay. FOXO1-dTAG degradation significantly decreased the cytotoxic effect of AS1842856 (Figure 5C-D). Thus, our chemical degradation experiments support the involvement of both FOXO1-dependent and -independent processes in the cytotoxic effect of AS1842856.
The cytotoxicity of AS1842856 in B-ALL depends on FOXO1 protein but extends beyond its transcriptional inhibition. (A) Treatment with dTAG-13 but not AS1842856 induces degradation of FOXO1-dTAG FP. FOXO1-dTAG clones were treated with dTAG-13 and/or AS1842856, at concentrations of 500 nM and 100 nM, respectively; 24 hours after treatment, the cells were harvested and FOXO1/FOXO1-dTAG expression was measured by immunoblot using anti-FOXO1 antibody. (B) Apoptosis induction in 018Z WT and FOXO1-dTAG clones was measured by propidium iodide (PI)/annexin V-allophycocyanin (APC) staining and subsequent flow cytometry 3 days after treatment with dTAG-13 at a concentration of 500 nM, expressed as percentage of PI+, annexin V–positive, and PI+/annexin V–positive cells (n = 3). Specific apoptosis was calculated by normalization to respective DMSO controls. Statistical significance was analyzed by ratio paired t test calculating the difference between treatment with AS1842856 and AS1842856 + dTAG-13 (WT, P = .402; FOXO1-dTAG clones, P = .005; n = 3). (C-D) Effect of FOXO1 degradation on the sensitivity of B-ALL cells to AS1842856. The cytotoxic effect was measured by a 6-day MTT test. To this end, 018Z FOXO1-dTAG cells were incubated in complete medium and treated with 500 nM of dTAG-13 or vehicle (DMSO). Two hours later, AS1842856 was added at gradually decreasing concentrations with a dilution factor of 2. The dose-effect data were fitted by nonlinear regression model. The statistical significance of the IC50 values differences was calculated by Student t test (D). (E-H) 018Z-FOXO1-dTAG cells were treated for 24 hours with 30 nM of AS1842856 (+) or vehicle DMSO (−). FOXO1 degradation was initiated by treatment with 500 nM of dTAG-13. (E) Expression of GSK3A, GSK3B, CTNNB1, FOXO1-dTAG FP, and TUBB was measured by immunoblot. The most representative of 3 independent experiments is shown. (F-H) Expression of GSK3A, GSK3B, and CTNNB1 was quantified by ImageJ software. To calculate the relative protein expression intensities of immunoblot signals (n = 3), results were normalized to TUBB. The statistical significance was analyzed by 2-sided, nonpaired t test.
The cytotoxicity of AS1842856 in B-ALL depends on FOXO1 protein but extends beyond its transcriptional inhibition. (A) Treatment with dTAG-13 but not AS1842856 induces degradation of FOXO1-dTAG FP. FOXO1-dTAG clones were treated with dTAG-13 and/or AS1842856, at concentrations of 500 nM and 100 nM, respectively; 24 hours after treatment, the cells were harvested and FOXO1/FOXO1-dTAG expression was measured by immunoblot using anti-FOXO1 antibody. (B) Apoptosis induction in 018Z WT and FOXO1-dTAG clones was measured by propidium iodide (PI)/annexin V-allophycocyanin (APC) staining and subsequent flow cytometry 3 days after treatment with dTAG-13 at a concentration of 500 nM, expressed as percentage of PI+, annexin V–positive, and PI+/annexin V–positive cells (n = 3). Specific apoptosis was calculated by normalization to respective DMSO controls. Statistical significance was analyzed by ratio paired t test calculating the difference between treatment with AS1842856 and AS1842856 + dTAG-13 (WT, P = .402; FOXO1-dTAG clones, P = .005; n = 3). (C-D) Effect of FOXO1 degradation on the sensitivity of B-ALL cells to AS1842856. The cytotoxic effect was measured by a 6-day MTT test. To this end, 018Z FOXO1-dTAG cells were incubated in complete medium and treated with 500 nM of dTAG-13 or vehicle (DMSO). Two hours later, AS1842856 was added at gradually decreasing concentrations with a dilution factor of 2. The dose-effect data were fitted by nonlinear regression model. The statistical significance of the IC50 values differences was calculated by Student t test (D). (E-H) 018Z-FOXO1-dTAG cells were treated for 24 hours with 30 nM of AS1842856 (+) or vehicle DMSO (−). FOXO1 degradation was initiated by treatment with 500 nM of dTAG-13. (E) Expression of GSK3A, GSK3B, CTNNB1, FOXO1-dTAG FP, and TUBB was measured by immunoblot. The most representative of 3 independent experiments is shown. (F-H) Expression of GSK3A, GSK3B, and CTNNB1 was quantified by ImageJ software. To calculate the relative protein expression intensities of immunoblot signals (n = 3), results were normalized to TUBB. The statistical significance was analyzed by 2-sided, nonpaired t test.
Finally, we investigated the contribution of FOXO1 in the regulation of CTNNB1 stability. FOXO1 directly interacts with CTNNB1 to activate the expression of genes that protect against oxidative stress.44 In pancreatic adenocarcinoma, FOXO1 attenuated canonical Wnt/CTNNB1 signaling by activating the transcription of the long noncoding RNA LINC01197, which directly binds to and inactivates CTNNB1.45 To clarify whether FOXO1 inactivation may contribute to the effect of AS1842856 on CTNNB1 stability, we examined the effect of chemical (Figure 5E) and CRISPR/Cas9-dependent FOXO1-KO (supplemental Figure 5D) on CTNNB1 expression. Neither of these approaches had a detectable effect on CTNNB1 expression. Given that AS1842856 has been reported to decrease GSK3A and GSK3B levels by accelerating their exocytosis in neuronal cells,46 we addressed the impact of this mechanism in B-ALL. AS1842856 significantly downregulated the expression of both GSK3 isoforms in 018Z cells independently on the FOXO1 expression status (Figure 5E-H). The effect of AS1842856 was controlled by measuring CTNNB1 expression (Figure 5H).
We concluded that, in addition to direct inhibition of enzymatic activity, AS1842856 also decreases the expression of GSK3A and GSK3B at the protein level.
Discussion
We found that the FOXO1 inhibitor AS1842856, first identified in 2010 and used as a molecular probe to investigate FOXO1’s role in various pathological conditions, is also a potent specific direct inhibitor of GSK3A and GSK3B kinase activity. The reason this has gone unnoticed likely stems from the high similarity of FOXO1 and GSK3 regulation and function. Both are AKT-regulated substrates that similarly modulate carbohydrate and lipid metabolism,47,48 but via distinct mechanisms. For example, GSK3B degrades PDX1 in β-cells, impairing insulin promoter activation.49 In addition, GSK3B augments FOXO1-driven transcription of key metabolic genes in the liver.50 Both FOXO1 and GSK3 block β-cell proliferation, but FOXO1 by inducing differentiation,51 whereas GSK3 blocks proliferation by facilitating NFAT nuclear export.52
Correspondingly, AS1842856 and canonical GSK3 inhibitors reduce hepatic gluconeogenesis, enhance glucose utilization, and promote β-cell regeneration in diabetic models.6,51 AS1842856 also provides cardioprotective effects, ameliorating diabetic cardiomyopathy and oxidative stress.53 Similarly, GSK3B inhibition enhances cardiomyocyte proliferation via Wnt/CTNNB1 activation.54 In B-ALL, we demonstrated that GSK3 inhibition and CTNNB1 stabilization in part underlie AS1842856’s cytotoxicity. Future research should clarify whether the same mechanism exists in newer FOXO1 inhibitors.55
Our FOXO1-dTAG system did not reproduce the dependency of B-ALL on FOXO1, allowing assessment of FOXO1’s role in the cytotoxic effect of AS1842856. B-ALL is dependent on FOXO1, as shown in human cell lines by CRISPR/Cas9 (supplemental Figure 6), small hairpin RNA knockdown,7 and transgenic mouse models.17,56 This dependency in pre–B cells and B-ALL follows the Goldilocks principle, in which only medium “just right” dosages of FOXO1 support cell proliferation, whereas both upregulation and downregulation halt proliferation or induce apoptosis. This Goldilocks-type dependency applies not only to FOXO1 but also to AKT, which phosphorylates FOXO1 and facilitates its nuclear export, serving as FOXO1’s main regulator in B-ALL.7,9,57,58
The thresholds of this Goldilocks interval can shift toward AKT activation/FOXO1 deactivation as an adaptation mechanism. Low FOXO1 expression in B-ALL is associated with chemotherapy resistance and relapses.59 Similarly, phosphoinositide-3-kinase (PI3K)-AKT pathway activation in B-ALL blast cells at diagnosis correlates with poor response to induction chemotherapy, and AKT activation reduced apoptosis induced by antitumor drugs in B-ALL cell lines.60 In our experiments, Foxo1-KO in the mouse B-ALL model increased expression of Lck proto-oncogene and Csf1r (colony-stimulating factor 1 receptor; 6.4-fold and 8.5-fold, respectively). Both are oncogenic factors in B-ALL that activate PI3K-AKT.61,62 This adaptive PI3K-AKT activation might contribute to shifting the Goldilocks interval to function at low but detectable FOXO1-dTAG FP levels that remain after degradation.
The identification of AS1842856 as a GSK3 inhibitor raised questions about FOXO1’s role in the cytotoxic effect. Using the dTAG system, we have shown that FOXO1 modulation significantly contributes to, but is not the only mechanism in, AS1842856’s cytotoxic effect. One limitation is that GSK3 kinase inhibition likely occurs earlier than FOXO1-inhibition, as demonstrated by CTNNB1 accumulation within an hour. However, the order of effects does not reduce AS1842856’s therapeutic potential as a dual inhibitor for B-ALL treatment.
Regarding AS1842856’s specificity, it may also inhibit other prosurvival kinases at nontoxic plasma concentrations, although much less than GSK3A/B. These include proproliferative kinases PIM363 and CDK6,64 prosurvival kinase CDK18 (upregulation responsible for poly(ADP-Ribose) polymerase (PARP) inhibitor resistance65), and DYRK1B, which protects tumor cells from tumoricidal macrophages46 and increases radiotherapy resistance.66 However, inhibition of any kinase other than GSK3A/B was <55% at 100 nM, higher than B-ALL cells’ IC50. In addition, CTNNB1-KO pushed AS1842856’s cytotoxic effects outside a therapeutic window, supporting GSK3’s central role in the compound’s cytotoxic effect. In previous studies, AS1842856 significantly reduced tumor burden, especially in CNS of high-risk B-ALL mouse models,7 supporting its potential against CNS-involved B-ALL, a subtype with particularly poor clinical outcomes.67
In conclusion, AS1842856 is a potent dual inhibitor of FOXO1 and GSK3. Given the virtually identical spectrum of pharmacological activities of GSK3 inhibitors and putative FOXO1 inhibitors, our findings add to the rationale for using this compound to efficiently treat a broad spectrum of diseases dependent on GSK3 and FOXO1 function, including B-ALL, particularly with CNS involvement. Our study also supports the pharmacological potential of protein binders that modulate protein functions rather than solely inhibit them.
Acknowledgments
The authors are grateful to Hassan Jumaa for providing the mouse BCR::ABL1–transformed pre–B-cell lines, Ralf Marienfeld for the fruitful discussions and the help with short tandem repeat analysis of the cell lines, Lüder H. Meyer for the donation of 018Z cell line, and Scott W. Hiebert for helping to establish the dTAG system.
Authorship
Contribution: F.K., U.B., A.U., and T.W. contributed to conceptualization; F.K., U.B., D.G., and A.K. contributed to methodology; U.B., F.K., D.G., and A.K. performed the investigation; F.K., U.B., D.G., and A.K. were responsible for data curation; F.K. and A.U. prepared original draft; A.U., U.B., F.K., and T.W. reviewed and edited the manuscript; and A.U. and T.W. supervised the study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Thomas Wirth, Institute of Physiological Chemistry, University of Ulm, Albert-Einstein-Allee 11, 89081 Ulm, Germany; email: thomas.wirth@uni-ulm.de; Alexey Ushmorov, Institute of Physiological Chemistry, University of Ulm, Albert-Einstein-Allee 11, 89081 Ulm, Germany; email: alexey.ushmorov@uni-ulm.de; and Franz Ketzer, Institute of Clinical Chemistry and Pathobiochemistry, TUM School of Medicine and Health, Technical University of Munich, Ismaninger Strße 22, 81675 Munich, Germany; email: franz.ketzer@tum.de.
References
Author notes
F.K. and U.B. contributed equally to this study.
The RNA-sequencing data reported in this study are available at the Gene Expression Omnibus repository (accession number GSE283254).
Original data are available on request from the corresponding author, Frank Ketzer (franz.ketzer@tum.de).
The full-text version of this article contains a data supplement.

![Comparison of the transcriptomic effects of Foxo1-KO and treatment with AS1842856 in murine BCR::ABL1+ B-ALL. (A) RNA-sequencing was performed in BCR::ABL1+ Cre-ERT2 Foxo1fl/fl murine B-ALL cells treated with either 200 nM 4-OHT or an equivalent amount of vehicle EtOH or with 70 nM AS1842856 or equivalent amounts of DMSO vehicle. RNA was isolated after 24 and 48 hours. Analysis was performed using the DESeq2 workflow in RStudio (version 2024.12.1). The unsupervised hierarchical clustering of all genes found in all samples is shown (supplemental File 1). (B) Hierarchical clustering of genes significantly (P < .05; log2[FC] > 1.4) regulated was performed in both groups at 24 and 48 hours after respective treatment. (C) Volcano plot of differentially expressed genes. Significance threshold was set to adjusted P <.05 and log2(FC) >1.5. Significantly downregulated genes are shown in blue; significantly upregulated genes are shown in red. Canonical FOXO1 targets are highlighted and labeled in black. (D) Differentially expressed genes from days 1 and 2 of either Foxo1-KO or AS1842856-treated samples were pooled and subjected to GSEA for “Hallmarks” gene sets using GSEA (version 4.3.3; supplemental File 2). (E) Differentially regulated genes in BCR::ABL1+ murine B-ALL after treatment with 70 nM AS1842856 or genetic Foxo1-KO were subjected to Venn analysis. Exclusive and shared genes were cross-referenced to the ChEA 2022 database using Enrichr (supplemental Materials and methods). ChEA, ChIP-X Enrichment Analysis; FC, fold change.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/9/14/10.1182_bloodadvances.2024015560/3/m_blooda_adv-2024-015560-gr2.jpeg?Expires=1764994499&Signature=teMbh2V0dD8rVnwg~iWK-4e7WpuaKwx0-ZP~8AG4Bh1cWoxzUFiUm5iLHx8Mcu5~D5VUllvLPFwFwDQJv1rasc~VAM6~rvd~Qn57IUOQYOtaoFowyov0XPuUgVWsPZEwsrH6injf8Tr~eAvxB0otjjJQG3hcT5JALigxlJCJ2J9xScJgrcefmAVr-GmG9fEIDG35YyxNsRvL~gFL2dRNfThTFA-ad-vnmX1TREwaooy~Bg1lFEbigDhobaeA5tyD8AE1DcPM1Ey8FMAJecEG7V~sVC7FbAjXoFTqMV7wQt7V80MMwXxWI9bbv0EA4Rrai-h2zUbWcsZAJoQyIbbXuA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)