Key Points
Etranacogene dezaparvovec induced stable, sustained normal/near-normal FIX levels and eliminated the need for prophylaxis over 5 years.
No late-emergent safety events were observed over 5 years.
Visual Abstract
Etranacogene dezaparvovec (CSL222, formerly AMT-061) is a recombinant adeno-associated virus serotype 5 (AAV5) vector containing the highly active factor IX (FIX) Padua variant controlled by a liver-specific promoter. This phase 2b, open-label, single-dose, single-arm, multicenter trial evaluated the efficacy and safety of etranacogene dezaparvovec. Three adult participants with severe or moderately severe hemophilia B (FIX ≤2%) and AAV5-neutralizing antibodies received a single IV dose (2 × 1013 genome copies per kg) of etranacogene dezaparvovec. The primary end point of FIX activity ≥5 IU/dL at 6 weeks was met (mean, 30.6 IU/dL). Secondary end points included bleed frequency, FIX concentrate use, and adverse events. Here, we report the end-of-study 5-year outcomes. After administration, mean (range) FIX activity increased to 40.8 IU/dL (31.3-50.2) at year 1 and was maintained at 45.7 IU/dL (39.0-51.2) at year 5. Mean annualized bleeding rate (all bleeds) was 0.14 for the cumulative follow-up period years 0 to 5. Two participants had 5 bleed-free years after treatment. Per protocol, 1 participant received episodic FIX replacement therapy after treatment for elective surgeries, 2 bleeding episodes, and 2 single self-administered infusions for unreported reasons. All participants discontinued and remained free of FIX prophylaxis. During the 5-year study period, there were no clinically significant elevations in liver enzymes, requirement for steroids, FIX inhibitor development, thrombotic complications, or late-emergent safety events in any participant. Five years after administration, etranacogene dezaparvovec was effective in adults with hemophilia B with a favorable safety profile. Participants are eligible to participate in an extension study (ClinicalTrials.gov identifier: NCT05962398) for 10-year additional follow-up. This trial was registered at www.clinicaltrials.gov as #NCT03489291.
Introduction
Hemophilia B is a rare inherited bleeding disorder characterized by clotting factor IX (FIX) deficiency resulting from mutations in the F9 gene, which leads to insufficient functional FIX expression for effective hemostasis.1,2 The current standard of care for treatment of people with hemophilia B (PwHB) is lifelong prophylactic IV factor replacement therapy, which places a burden of frequent IV injections on PwHB.1-3 The development of FIX products with an extended half-life has reduced the treatment frequency but does not provide sustained hemostatic correction.2,3 Bleeding frequency is generally correlated with FIX activity levels, and current recommended goals for trough levels are >3% to 5%.1,4 Breakthrough bleeds can still occur despite prophylactic treatment, and patients with moderate (FIX activity of 1-5 IU/dL) and severe (FIX activity level of <1 IU/dL) FIX deficiency remain at risk of bleeding events, particularly into joints, which may result in degenerative joint disease.2,4
Liver-directed adeno-associated virus (AAV) vector–based gene therapy for hemophilia B offers the possibility of a 1-time infusion resulting in long-term treatment for PwHB.5 Through gene therapy, the hemophilia phenotype may be modified by inducing endogenous production of functional FIX by the liver.3 Studies have demonstrated sustained FIX expression for at least 1 year,6-10 with some outcomes reported for patients with >10-year follow-up.11 Although the outcomes in terms of FIX activity are now known in the immediate postinfusion period, the long-term safety and durability of FIX expression after AAV-based, liver-directed gene therapy for hemophilia B remain important factors in the decision-making process.5,12-14
CSL220 (formerly known as AMT-060), a recombinant AAV vector serotype 5 (AAV5) containing the wild-type human FIX complementary DNA sequence, was first evaluated in PwHB in an ongoing phase 1/2 trial (ClinicalTrials.gov identifier: NCT02396342)6 and continues to be assessed in an ongoing extension study.15 No safety concerns were reported, and a single administration of CSL220 resulted in stable and durable expression of wild-type FIX up to 6 years in 9 of 10 participants with FIX activity ranging from 3.1 to 14.8 IU/dL in cohort 1 (5 × 1012 genome copies [gc] per kg) and 3.0 to 7.1 IU/dL in cohort 2 (2 × 1013 gc/kg) in participants who ceased prophylaxis.16 One participant in cohort 1 with FIX levels of <2 IU/dL remained on prophylaxis per protocol.6 Further clinical development resulted in the creation of etranacogene dezaparvovec (CSL222, formerly AMT-061, AAV5-Padua human FIX), in which the full AAV5 capsid sequence and most of the FIX expression cassette design features, including the promoter and other transcriptional regulatory elements and the codon optimization, were retained. However, to optimize the potency of the AAV5-based vector, a 2-nucleotide change in the transgene coding sequence was introduced to cause an R338L substitution during protein translation, leading to expression of the highly active Padua FIX variant.9,17,18 Building on the 2 × 1013 gc/kg dose confirmed in the phase 1/2 trial for CSL220, this phase 2b trial (ClinicalTrials.gov identifier: NCT03489291) was conducted to assess the efficacy of the addition of the FIX Padua variant to the vector construct in 3 participants with severe or moderately severe hemophilia B.17
The interim efficacy and safety data for this phase 2b clinical trial of etranacogene dezaparvovec have previously been reported for the 26-week and 3-year time points.17,19 The primary end point was met, with mean 30.6 IU/dL FIX activity levels of ≥5 IU/dL at 6 weeks after dosing, measured by 1-stage assay,17 and this was maintained at 3 years.19 This level of FIX expression was achieved despite all trial participants having AAV5-neutralizing antibodies (AAV NAbs; mean titer at screening = 39) before treatment.19 NAbs may inhibit the ability of the AAV vector to deliver the therapeutic gene to its target cell, rendering gene transfer ineffective or inefficient.13,20-22 Therefore, individuals with preexisting NAbs against AAV are excluded from most AAV-based gene therapy clinical trials.23,24 Nevertheless, it has been shown that CSL220 was able to induce therapeutic levels of FIX in patients with preexisting AAV5 NAbs, and based on these results, patients with preexisting AAV5 NAbs were not excluded from the CSL222 phase 2b trial.23
Based on the results of the interim analysis of the phase 2b trial, the pivotal phase 3 HOPE-B (ClinicalTrials.gov identifier: NCT03569891) trial was initiated in 54 people with severe or moderately severe hemophilia B.25 The primary end point of HOPE-B was met, with a significant reduction of the annualized bleeding rate (ABR) of 64% observed for months 7 to 18 after infusion compared with lead-in prophylaxis26; this significantly reduced ABR was maintained up to month 24.25 Mean FIX activity was stable at 24 months, with a mean expression of 36.7 IU/dL, and long-term follow-up continues in this cohort, with FIX levels of 38.6 IU/dL sustained at 3 years after treatment.27
Here, we report the final analyses of the phase 2b study at 5 years after treatment, to demonstrate the favorable durability, efficacy, and safety profile of etranacogene dezaparvovec over the entire duration of the trial. To our knowledge, this is the longest duration of the FIX Padua variant gene expression reported to date and the first study to publish data on AAV5 gene therapy in AAV NAb-positive patients.
Methods
Study design and participants
This phase 2b, open-label, single-arm, multicenter trial evaluated the efficacy and safety of a single IV dose (2 × 1013 gc/kg) of etranacogene dezaparvovec and is listed with the following registry: ClinicalTrials.gov identifier: NCT03489291. The methods of this study and the design, characterization, and manufacture of etranacogene dezaparvovec (CSL222) have all been described in detail previously.17,19,28
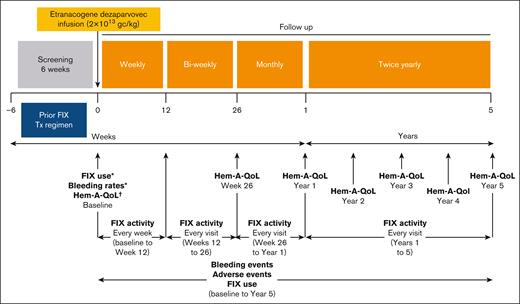
As a brief overview, 3 adult males with moderately severe to severe hemophilia B (FIX activity of ≤2 IU/dL of normal) receiving prophylactic FIX were recruited, treated with a single IV dose (2 × 1013 gc/kg) of etranacogene dezaparvovec, and followed up for a total of 5 years (Figure 1).17,19 Participants were given a dose of standard–half-life FIX (40 IU/kg) for the FIX recovery assessment before etranacogene dezaparvovec administration and were given an additional dose if FIX activity levels were <5% at day 3 after treatment to provide sufficient FIX coverage in the initial days after gene therapy. Preexisting AAV5 NAb seropositivity was assessed but not used as an exclusion criterion. Key exclusion criteria included a history of FIX inhibitors, active hepatitis B/C infection, uncontrolled HIV infection, and evidence of advanced liver fibrosis; full inclusion/exclusion criteria are listed in supplemental Table 1.17,19
Overview of the phase 2b study design. After an initial screening period, during which time the participants received their usual standard of care or FIX replacement therapy, patients received a single IV dose of 2 × 1013 gc/kg etranacogene dezaparvovec. FIX levels were assessed weekly for the first 6 weeks, before assessments were reduced to biweekly until week 26 after infusion, when they reduced to monthly up to month 12, and twice-yearly up to the study completion of month 60. Quality-of-life assessments (Hem-A-QoL) were performed at week 26, and then annually from year 1 to year 5. Bleeding events, AEs, and any exogenous FIX use were reported throughout the study period. ∗Data from the year before screening were collected retrospectively using medical records. †Data were collected on the day of dosing. Tx, treatment.
Overview of the phase 2b study design. After an initial screening period, during which time the participants received their usual standard of care or FIX replacement therapy, patients received a single IV dose of 2 × 1013 gc/kg etranacogene dezaparvovec. FIX levels were assessed weekly for the first 6 weeks, before assessments were reduced to biweekly until week 26 after infusion, when they reduced to monthly up to month 12, and twice-yearly up to the study completion of month 60. Quality-of-life assessments (Hem-A-QoL) were performed at week 26, and then annually from year 1 to year 5. Bleeding events, AEs, and any exogenous FIX use were reported throughout the study period. ∗Data from the year before screening were collected retrospectively using medical records. †Data were collected on the day of dosing. Tx, treatment.
Long-term follow-up end points
See Figure 1 for the long-term follow-up schedule. End points, including the results of the primary efficacy end point, have been reported in detail previously.17,19 As a brief overview, the primary efficacy end point was FIX activity of ≥5 IU/dL by 6 weeks after treatment.17 FIX activity was assessed using a 1-stage activated partial thromboplastin time–based assay (SynthASil reagent) and a chromogenic assay (BIOPHEN FIX method). Bleeding event data and FIX concentrate use were recorded prospectively by participants using diary entries, which were reviewed by investigators. Medical records were used to obtain baseline FIX replacement data. Health-related quality of life was assessed as an exploratory end point using the hemophilia-specific quality-of-life index (Hem-A-QoL).29 The Hem-A-QoL is a disease-specific tool that measures the impact of hemophilia on health-related quality of life across 10 domains, with lower scores indicating less impairment because of hemophilia in the past 4 weeks.
Safety assessments included the occurrence of adverse events (AEs), and abdominal ultrasounds to monitor liver health. Long-term assessments included anti-AAV5 antibody titers (total and NAbs), presence of antibodies to FIX (including FIX inhibitors), liver enzymes (alanine aminotransferase [ALT], and aspartate transaminase [AST]), α-fetoprotein, and routine blood and coagulation parameters. Methods for the abovementioned analyses have been reported previously.17,19,23,30
Data analysis
The data presented here were collected from study initiation on 24 July 2018 to 31 October 2023, representing study completion at 5 years of posttreatment follow-up. No statistical hypothesis testing was conducted because of the study sample size (N = 3). All results are reported using descriptive statistics. All authors had access to primary clinical trial data.
Ethics
The study was approved by the institutional review board/institutional ethics committee at each center and all participants provided written informed consent. The trial was performed according to the Declaration of Helsinki and the principles of good clinical practice. The study was supported by the US Food and Drug Administration and the European Medicines Agency to address the change of transgene product, and to inform the dose choice for etranacogene dezaparvovec in the phase 3 trial.17,19 The biopharmaceutical companies, UniQure and CSL Behring, collaborated with the authors on the study design, data collection, data analysis, and/or data interpretation. All authors have access to the primary clinical trial data.
Results
Study population
Of the 3 participants, 1 had moderate-severe hemophilia (FIX = 1 IU/dL) and 2 had severe hemophilia (FIX of <1 IU/dL). The mean age of the participants was 46.7 years (range, 43-50) at enrollment. Before the study, all participants were on long-term prophylaxis with an extended half-life FIX product. All participants tested positive for AAV5 NAbs before etranacogene dezaparvovec dosing, with a mean AAV5 NAb titer of 25 (range, 20-33) on the day of infusion. Two participants had controlled HIV, and all 3 had previously resolved hepatitis C virus at baseline. Full baseline demographics and disease characteristics of the participants can be found in Table 1.
Participant baseline demographics and disease characteristics
| Characteristic . | Participant 1 . | Participant 2 . | Participant 3 . |
|---|---|---|---|
| Age at enrolment, y | 43 | 50 | 47 |
| Weight, kg | 89 | 81 | 82 |
| HIV status | Nonreactive | Reactive (controlled) | Reactive (controlled) |
| HBV status | Negative | Negative | Negative |
| HCV status | Resolved | Resolved | Resolved |
| Baseline FIX activity levels, IU/dL | 1 | <1 | <1 |
| Prescreening FIX treatment | Prophylaxis (EHL) | Prophylaxis (EHL) | Prophylaxis (EHL) |
| ABR during 1 year before screening∗ | 3 | 1 | 5† |
| AAV5 NAb status at screening (titer)‡ | Positive (48) | Positive (44) | Positive (25) |
| AAV5 NAb status at day of dosing (titer)‡ | Positive (22) | Positive (33) | Positive (20) |
| Characteristic . | Participant 1 . | Participant 2 . | Participant 3 . |
|---|---|---|---|
| Age at enrolment, y | 43 | 50 | 47 |
| Weight, kg | 89 | 81 | 82 |
| HIV status | Nonreactive | Reactive (controlled) | Reactive (controlled) |
| HBV status | Negative | Negative | Negative |
| HCV status | Resolved | Resolved | Resolved |
| Baseline FIX activity levels, IU/dL | 1 | <1 | <1 |
| Prescreening FIX treatment | Prophylaxis (EHL) | Prophylaxis (EHL) | Prophylaxis (EHL) |
| ABR during 1 year before screening∗ | 3 | 1 | 5† |
| AAV5 NAb status at screening (titer)‡ | Positive (48) | Positive (44) | Positive (25) |
| AAV5 NAb status at day of dosing (titer)‡ | Positive (22) | Positive (33) | Positive (20) |
Table reproduced from von Drygalski et al.17,19
EHL, extended half-life; HBV, hepatitis B virus; HCV, hepatitis C virus.
Total bleeds (treated + untreated).
This participant experienced a further spontaneous bleed in the left hip between screening and enrolment (bleed onset: study day −30) that is not represented in their ABR for the year before screening.
AAV5 NAb data considered positive if titer was ≥2. Luciferase cell-based assay.
Stable increase in endogenous FIX activity
Elevations in FIX activity remained stable from year 1 (mean, 40.77 IU/dL; standard deviation [SD], 9.45; range, 31.3-50.2) to year 5 (mean, 45.7 IU/dL [SD, 6.18; range, 39.0-51.2]; Figure 2). At 5 years, participants 1 and 3 were maintaining FIX activity in the nonhemophilia range (≥40 IU/dL), at 46.8 IU/dL and 51.2 IU/dL, respectively, whereas participant 2 was close to the nonhemophilia range at 39 IU/dL. One-stage and chromogenic FIX activity by year and per participant is shown in supplemental Table 2.
Sustained FIX activity over 5-year follow-up after etranacogene dezaparvovec administration. FIX activity was sustained across the 5 years of follow-up for all study participants, increasing to a mean (SD; range) 30.57% (6.97; 23.9-37.8) at week 6 that remained stable and in the nonhemophilia range from year 1 (40.77% [9.45; 31.3-50.2]) to year 5 (45.7% [6.18; 39.0-51.2]). ∗HemosIL SynthASil reagent. aPTT, activated partial thromboplastin time.
Sustained FIX activity over 5-year follow-up after etranacogene dezaparvovec administration. FIX activity was sustained across the 5 years of follow-up for all study participants, increasing to a mean (SD; range) 30.57% (6.97; 23.9-37.8) at week 6 that remained stable and in the nonhemophilia range from year 1 (40.77% [9.45; 31.3-50.2]) to year 5 (45.7% [6.18; 39.0-51.2]). ∗HemosIL SynthASil reagent. aPTT, activated partial thromboplastin time.
Reduction in bleeds and FIX use
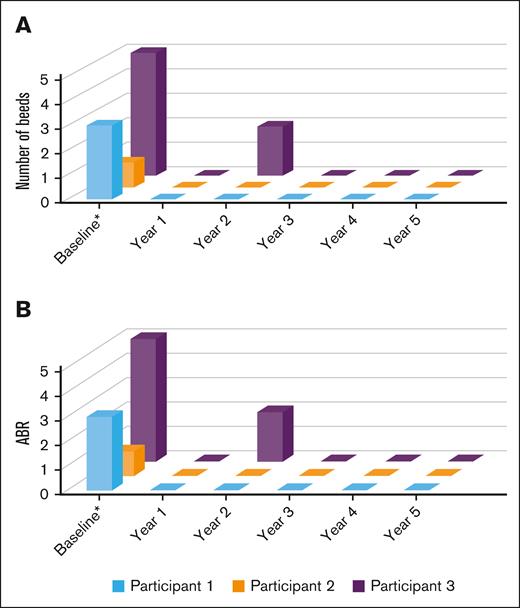
Bleeding rates decreased considerably in all participants after etranacogene dezaparvovec treatment, with cumulative mean ABRs of 0.22 for years 0 to 3, 0.17 for years 0 to 4, and 0.14 for years 0 to 5. None of the participants experienced bleeding events after year 2 (Figure 3), and there were no joint bleeds throughout the entire study period. Overall, percentage bleed reductions relative to before etranacogene dezaparvovec treatment were 100% in 2 participants (ABRs of 0 throughout 5 years of follow-up) and 92% in 1 participant at year 5. Participant 3 had a mean ABR of 0.42 over the 5-year follow-up period after treatment. This participant experienced 2 bleeding events (1 spontaneous and 1 traumatic) in year 2, both of which were successfully managed with a self-administered single on-demand exogenous FIX infusion. This participant also received episodic FIX infusions for the management of 3 hip surgeries, as described in the safety section hereafter, and 2 single self-administered infusions for unreported reasons.
Total number of bleeds and ABR at baseline and during 5-year follow-up. (A) Total number of bleeds per participant, per year at baseline and during 5-year follow-up after etranacogene dezaparvovec administration. Participants 1 and 2 did not report any bleeding events during the 5-year follow-up period, and participant 3 experienced 2 bleeds during year 2. No bleeding episodes were reported from year 3 to year 5 for all participants. (B) ABR by participant at baseline and during 5 years of follow-up after etranacogene dezaparvovec administration; ABR was 0 for all participants from years 3 to 5. ∗Data collected retrospectively 1 year before screening from medical records.
Total number of bleeds and ABR at baseline and during 5-year follow-up. (A) Total number of bleeds per participant, per year at baseline and during 5-year follow-up after etranacogene dezaparvovec administration. Participants 1 and 2 did not report any bleeding events during the 5-year follow-up period, and participant 3 experienced 2 bleeds during year 2. No bleeding episodes were reported from year 3 to year 5 for all participants. (B) ABR by participant at baseline and during 5 years of follow-up after etranacogene dezaparvovec administration; ABR was 0 for all participants from years 3 to 5. ∗Data collected retrospectively 1 year before screening from medical records.
All 3 participants discontinued continuous FIX prophylaxis within 1 to 4 days after treatment. The mean annualized exogenous FIX use, excluding use for invasive procedures, decreased from 306 205 IU/y in the year before screening (as per medical record data) to 605 IU/y over the entire 5-year study period, and to 342 IU/y in the postcontinuous prophylaxis period. For episodic FIX use, the 2 participants with ABRs of 0 had an annualized mean FIX use of 0 IU/y, whereas the participant with 2 bleeding events had an annualized mean FIX use of 1026 IU/y (Table 2).
Exogenous FIX consumption, excluding invasive procedures
| Year . | Annualized FIX consumption, IU/y . | |||
|---|---|---|---|---|
| Participant . | Mean . | |||
| 1 . | 2 . | 3 . | N = 3 . | |
| Baseline | 223 429∗ | 261 186∗ | 434 000∗ | 306 205∗ |
| 1† | 0 | 0 | 1705 | 568.3 |
| 2 | 0 | 0 | 3400 | 1133.3 |
| 3 | 0 | 0 | 0 | 0 |
| 4 | 0 | 0 | 0 | 0 |
| 5 | 0 | 0 | 0 | 0 |
| Year . | Annualized FIX consumption, IU/y . | |||
|---|---|---|---|---|
| Participant . | Mean . | |||
| 1 . | 2 . | 3 . | N = 3 . | |
| Baseline | 223 429∗ | 261 186∗ | 434 000∗ | 306 205∗ |
| 1† | 0 | 0 | 1705 | 568.3 |
| 2 | 0 | 0 | 3400 | 1133.3 |
| 3 | 0 | 0 | 0 | 0 |
| 4 | 0 | 0 | 0 | 0 |
| 5 | 0 | 0 | 0 | 0 |
Data collected retrospectively 1 year before screening from medical records.
Postcontinuous FIX prophylaxis defined as a combination of 3 events: (1) study drug administered, (2) uncontaminated FIX activity (after treatment) has since been observed to be ≥5%, and (3) the patient has not used exogenous FIX for some continuous time period of at least 15 days after study drug administration.
Safety
AEs
A total of 84 treatment-emergent AEs were reported during the 5-year follow-up period, most of which were mild or moderate in intensity (96%), with 55 mild treatment-emergent AEs, 26 moderate, and 3 severe. In year 1, treatment-related AEs (TRAEs) were reported in only 1 participant; as previously reported, participant 1 had 2 TRAEs: a headache and a transient elevation of C-reactive protein that resolved without treatment.17 None of the participants experienced TRAEs or serious TRAEs in years 2 to 5. Participant 3 experienced 2 serious AEs (worsening avascular necrosis in the left and right hip); however, these were considered unrelated to treatment with etranacogene dezaparvovec. This participant underwent 3 hip surgeries during the study; he required a femoral head decompression on the left hip on day 197 and arthroplasties on his left and right hip on day 720 and day 1672, respectively. Per protocol, the participant received perioperative FIX infusions, with dosing and duration of treatment tailored to their endogenous FIX levels at the time of each surgery reported in supplemental Table 3 (see further details in the supplemental Results).
FIX inhibitor levels remained below the limit of detection throughout the 5 years after etranacogene dezaparvovec treatment, and there were no thrombotic events, treatment-related transaminase elevations, requirement for steroids, or cancer diagnoses reported throughout the study. Measurements of liver enzymes (mean ALT and AST) over 5 years for the 3 participants are shown in supplemental Figure 1.
Immune and inflammatory markers
As previously reported,19 all 3 participants demonstrated rapid elevation of total anti-AAV5 immunoglobulin G (IgG) and IgM titers within 1 week of gene transfer. At 5 years, all participants demonstrated sustained and elevated anti-AAV5 IgG antibody titers. AAV5 NAbs rose to titers of 36 450 (the maximum limit of detection of the assay) by week 2 after administration and remained >36 450 for the remainder of the posttreatment period. No participant exhibited a clinically significant cytotoxic T-cell–mediated immune response to the capsid. No clinically relevant changes in inflammatory biomarkers were observed.
Patient-reported outcomes
The mean Hem-A-QoL total transformed scores for participants 1, 2, and 3 decreased from baseline to year 5 by 14.7, 7.4, and 3.6, respectively (Table 3), indicating a consistent overall improvement in participants’ quality of life. Individual participants' scores shown by domain are provided in supplemental Figure 2.
Hem-A-QoL total transformed score
| Year . | Total transformed score∗ . | ||
|---|---|---|---|
| Participant . | |||
| 1 . | 2 . | 3 . | |
| Baseline | 26.7 | 13.0 | 13.7 |
| 1 | 11.4 | 5.0 | 21.7 |
| 2 | 19.6 | 5.0 | 14.0 |
| 3 | 8.3 | 5.0 | 15.5 |
| 4 | 12.5 | 7.7 | 21.7 |
| 5 | 12.0 | 5.6 | 10.1 |
| Year . | Total transformed score∗ . | ||
|---|---|---|---|
| Participant . | |||
| 1 . | 2 . | 3 . | |
| Baseline | 26.7 | 13.0 | 13.7 |
| 1 | 11.4 | 5.0 | 21.7 |
| 2 | 19.6 | 5.0 | 14.0 |
| 3 | 8.3 | 5.0 | 15.5 |
| 4 | 12.5 | 7.7 | 21.7 |
| 5 | 12.0 | 5.6 | 10.1 |
Lower scores indicate better quality of life.
Discussion
Here, we present the completed 5-year follow-up from this phase 2b study of liver-directed gene therapy for hemophilia B, in which 3 participants with endogenous FIX activity of ≤1 IU/dL received a single IV dose of 2 × 1013 gc/kg of etranacogene dezaparvovec. All 3 participants experienced sustained clinically relevant increases in FIX activity and were expressing endogenous FIX activity within the mild or normal FIX range at 5 years after treatment (mean, 45.7 IU/dL), with a low incidence of treatment-related AEs. This supports the previously reported interim analyses after 26 weeks and 3-year follow-up, during which rapid increases in FIX activity were observed, resulting in mean FIX activities of 31 IU/dL by week 6, 47 IU/dL at week 26, and 37 IU/dL at year 3.17,19 This successful maintenance of increased FIX activity resulted in significant reductions of ABRs for spontaneous, joint, and traumatic bleeding episodes for all participants. Of particular note, over the 5-year follow-up period, 2 of the participants did not experience any bleeds, and the other participant achieved 3 bleed-free years. To add further novelty, all 3 participants in this study were positive for preexisting AAV NAbs yet still demonstrated not only an initial FIX activity correction but sustained FIX expression at 5 years, associated with a maintained correction of the bleeding phenotype.
Liver health is an essential aspect of successful hemophilia gene therapy.31 AAV-based gene therapy for hemophilia can be associated with mild to moderate elevations in serum ALT levels and may be accompanied with an immune response and loss of factor expression.13 In this study, however, no steroids were required and no FIX inhibitors were detected, indicating the participants did not exhibit a clinically significant immune response to the treatment. In addition, no treatment-related transaminase elevations were reported, indicating good liver health after treatment with etranacogene dezaparvovec in these 3 participants. These observations align with subsequent observations made in the phase 3 HOPE-B study, in which 11 of 54 study participants developed transaminase elevations, of whom 9 participants received reactive glucocorticoid treatment for immune suppression.26 This suggests an overall low rate and manageable burden of immune reactions, representing the lowest rate ever reported in hemophilia A or B gene therapy trials.32 Although this trial and the successive HOPE-B trial planned to administer steroids reactively in case of liver enzyme elevations, a previous trial using a Padua transgene in an AAV vector administered glucocorticoids prophylactically, and reported a rate of 20% ALT and AST elevations despite preemptive steroid administration.33 In addition, a phase 2 trial investigating another AAV-based Padua variant gene therapy fidanacogene dezaparvovec for hemophilia B reported a rate (20%) of liver enzyme elevations requiring prednisone treatment,9 which increased to a rate of 53% of patients experiencing liver enzyme elevations and 62% of patients requiring glucocorticoids for liver enzyme elevation or decreased FIX levels (or both) during the phase 3 pivotal study.32
Furthermore, the incidence rate of serious AEs was low, with no serious AEs being considered related to etranacogene dezaparvovec treatment. Importantly, no cases of thrombotic events were reported immediately after transduction with the hyperactive FIX R338L Padua variant,17 which is associated with a rare X-linked thrombophilia,34 and this 5-year follow-up in 3 participants confirms no increased risk of thrombosis after transduction of the Padua variant in people with severe and moderately severe hemophilia B with resulting FIX levels of <60% of normal. Together these results indicate that the AAV5 vector etranacogene dezaparvovec has a favorable safety profile. However, longer-term follow-up studies are needed, and all participants from this study (N = 3) have enrolled in the extension study for an additional 10 years (CSL222_3003; ClinicalTrials.gov identifier: NCT05962398).15,35
Although this study evaluated a small number of participants (N = 3), the efficacy and safety of etranacogene dezaparvovec have been further characterized in the ongoing phase 3 HOPE-B study, the largest hemophilia B gene therapy cohort to date. As per this study, HOPE-B included participants with titers of preexisting AAV5 NAbs.27 Consistent with this study, the interim outcomes from HOPE-B at 36 months have demonstrated stable FIX activity (mean, 38.6 IU/dL) and ABR reduced by 64% after infusion. The safety outcomes have also been favorable and consistent with previously presented data. Together, these data ultimately led to the approval of etranacogene dezaparvovec gene therapy as the first gene therapy for hemophilia B.36-38
Stability of FIX expression is a key consideration in the decision-making process for patients and physicians. The data from this study demonstrate the long durability of FIX expression with etranacogene dezaparvovec and build on the results of the phase 1 study 6-year follow-up outcomes with AMT-060 (5 × 1012 gc/kg). At year 6 in the phase 1 study, no new safety events were identified, FIX activity per the 1-stage activated partial thromboplastin time assay remained stable (mean [SD], 7.5 IU/dL [6.4] in cohort 1, and 5.5 IU/dL [1.5] in cohort 2, respectively), and 9 of 10 participants remained free from prophylaxis.16 Of note, FIX expression in participants was approximately sixfold greater in this study with etranacogene dezaparvovec than with AMT-060 in the phase 1 study, and all participants were able to stop prophylaxis. This improved efficacy observed with etranacogene dezaparvovec, using the same vector dose as AMT-060, can be attributed to the enhancement of the gene therapy design to include the naturally occurring, highly active FIX-Padua variant. To continue to evaluate the long-term durability and safety of AMT-060, the phase 1/2 extension study (ClinicalTrials.gov identifier: NCT05360706) will follow up participants for 10 years, resulting in a total follow-up period of 15 years after AMT-060 administration,15 in line with the extension study for this study with etranacogene dezaparvovec.35
Durability of FIX expression has also been demonstrated with other gene therapies using an AAV8 vector for the treatment of adults with moderately severe or severe hemophilia B. Stable FIX activity has been observed in a study of 10 participants who received infusion with the FIX transgene scAAV2/8-LP1-hFIXco.8,10,39 A dose-dependent increase in FIX activity of 1 to 6 IU/dL of the normal value was observed within 4 months after a single vector infusion, and remained stable for >10 years.10,39 For the 3 dose cohorts (2 × 1011 vector genome per kg [vg/kg; N = 2], 6 × 1011 vg/kg [N = 2], and 2 × 1012 vg/kg [N = 6]), mean FIX activity (1-stage) was reported to be 1.7, 2.3, and 4.9 IU/dL, respectively, at a median follow-up of 10.7 years.39 Both scAAV2/8-LP1-hFIXco and AMT-060 share the same DNA cassette featuring wild-type FIX, and only differ by capsid, suggesting that durability of etranacogene dezaparvovec may demonstrate similar or longer FIX expression beyond the 5 years reported here. Of note, a single patient (with preexisting AAV5 NAbs) has returned to continuous FIX prophylaxis at ∼30 months after treatment with etranacogene dezaparvovec in the phase 3 pivotal HOPE-B trial over 4 years after treatment.40 Fidanacogene elaparvovec (approved in the United States and Europe in 202441,42), an AAVrh74-based vector also expressing FIX-Padua variant, was evaluated in the BENEGENE-2 (ClinicalTrials.gov identifier: NCT03861273) phase 3 trial in adult male patients without preexisting AAV NAbs,43 in which the mean FIX activity, also measured by 1-stage assay using SynthASil reagent, was 27.8 IU/dL at week 12 (n = 44) and 26.6 IU/dL at month 24 (n = 39); 6 (13%) participants returned to continuous FIX prophylaxis before month 24.32
Despite recent key advances in gene therapy, most PwHB are still treated with regular factor infusions, which can be a high treatment burden, with patients being preoccupied with managing FIX levels to prevent bleeds and missing out on physical activities.44,45 Together, these factors can negatively affect a patient’s quality of life. Therefore, a 1-time treatment of gene therapy for hemophilia B, which can alleviate the burden of bleeds and enable patients to take part in activities without having to plan or worry, has the potential to change patients’ lives.46,47 In this study, all participants stopped FIX prophylaxis after a single treatment with etranacogene dezaparvovec. In addition, the Hem-A-QoL scores improved across all participants at the 5-year time point. These results correlate with the patient-reported outcomes in the phase 3 HOPE-B trial at 2 years after treatment, which concluded that gene therapy improved health-related quality of life in several Hem-A-QoL domains, including treatment, feelings, work/school, and future.48 Intriguingly, the domain “dealing with hemophilia” scores increased after gene therapy for the 2 individuals that remained 5-year bleed free (supplemental Figure 2). This domain requests patients to evaluate how they were able to recognize and manage a bleed; potentially causing inappropriate and inconsistent responses over time in participants who are not experiencing any bleed after treatment with gene therapy. This ambiguity undermines the validity of the domain as a measure of quality of life for patients who have undergone gene therapy. Nonetheless, improvement of the Hem-A-QoL scores across all participants indicates the net positive effect of treatment with etranacogene dezaparvovec.
In conclusion, the presented data from the phase 2b study, generated and analyzed up to 5 years (completion of study), demonstrate a continued positive safety and efficacy profile of etranacogene dezaparvovec treatment with sustained clinical benefits, including increased FIX activity maintained over 5 years, and no late-emergent safety events. To our knowledge, this is the first trial in which individuals with detectable AAV NAbs were intentionally treated and we now report 5 years of experience to support the durable nature of FIX expression in AAV NAb-positive participants treated with etranacogene dezaparvovec.
Acknowledgments
This study was supported by CSL Behring. Medical writing assistance was provided by Lucy Craggs, Claire Crouchley, and Cara Valvona on behalf of Bioscript Group, Macclesfield, United Kingdom, in accordance with Good Publication Practice guidelines, and funded by CSL Behring.
Authorship
Contribution: A.v.D., E.G., A.G., G.C., N.S.K., S.U.L., F.W.G.L., W.A.M., M.R., and S.W.P. were study investigators; A.v.D., E.G., A.G., and S.W.P. enrolled and treated participants and conducted clinical follow-up; P.E.M. and S.L.Q., both full-time employees of CSL Behring, were involved in the interpretation of the data and the development of the manuscript; the study sponsor, CSL Behring, collaborated with the authors on the study design, data collection, data analysis, and data interpretation; and all authors provided critical feedback on the manuscript and approved the final version of the manuscript before submission.
Conflict-of-interest disclosure: A.v.D. serves as a consultant for BioMarin, Regeneron, Pfizer, Bioverativ/Sanofi, Sobi, CSL Behring, Novo Nordisk, Pfizer, Spark Therapeutics, Takeda, Genentech, and uniQure; and is a cofounder and member of the board of directors of Hematherix LLC, a biotech company that is developing activated superFactor V therapy for bleeding complications. E.G. serves as a consultant for BioMarin, CSL Behring, and Genzyme. A.G. serves as a consultant for Bioverativ, Genentech/Roche, BioMarin, and uniQure; and reports a speaker bureau role with Bioverativ and Genentech/Roche. G.C. reports grant/research support from CSL Behring, Pfizer, and Sobi; and a speaker bureau role with Bayer, BioMarin, BIOVIIIx, CSL Behring, Roche, Sobi, Grifols, LFB Biopharmaceuticals, Novo Nordisk, Werfen, Kedrion, and uniQure. N.S.K. serves as a consultant for uniQure, BioMarin, and Novo Nordisk. S.U.L. serves as a consultant for uniQure. F.W.G.L. reports grant/research support from CSL Behring, Shire/Takeda, Roche, and Sobi; and serves as a consultant for uniQure, Takeda, Novo Nordisk, and BioMarin. W.A.M. reports grant/research support from Bayer, Biotest, Takeda, LFB Biopharmaceuticals, Octapharma, Novo Nordisk, Pfizer, and uniQure; and serves as a consultant for Bayer, BioMarin, Freeline, LFB Biopharmaceuticals, Octapharma, Novo Nordisk, Pfizer, and uniQure. M.R. reports research funding to employers from Bayer, BioMarin, CSL Behring, Genentech, Grifols, HEMA Biologics, LFB Biopharmaceuticals, Novo Nordisk, Octapharma, Pfizer, Sanofi, Spark Therapeutics, Takeda, and uniQure; serves as a consultant for Catalyst Biosciences, CSL Behring, Genentech, HEMA Biologics, Kedrion, Novo Nordisk, Pfizer, Sanofi, Takeda, and uniQure; and serves on the board of directors for Foundation for Women and Girls with Blood Disorders, Partners in Bleeding Disorders Education, and Thrombosis and Hemostasis Societies of North America. P.E.M. and S.L.Q. are employees of CSL Behring. S.W.P. reports grants/contracts from Siemens and YewSavin; reports consulting fees from ApcinteX/Centessa, ASC Therapeutics, Bayer, BioMarin, CSL Behring, Freeline, Genentech Inc/Roche, HEMA Biologics, LFB Biopharmaceuticals, Metagenomi, Novo Nordisk, Poseida Therapeutics, Precision BioSciences, Pfizer, Regeneron/Intellia, Sanofi, Spark Therapeutics Inc, Takeda, and uniQure; and has participated on the scientific advisory boards for GeneVentiv and Equilibra Bioscience.
Correspondence: Annette von Drygalski, Division of Hematology/Oncology, Department of Medicine, University of California San Diego, 9333 Genesee Ave, Suite 310, San Diego, CA 92121; email: avondrygalski@health.ucsd.edu.
References
Author notes
Individual participant data will not be shared. CSL Behring can provide scientific researchers access to deidentified participant data collected in clinical trials to improve participant care to support the advancement of medical science. Data are available on request from the corresponding author, Annette von Drygalski (avondrygalski@health.ucsd.edu).
The full-text version of this article contains a data supplement.


![Sustained FIX activity over 5-year follow-up after etranacogene dezaparvovec administration. FIX activity was sustained across the 5 years of follow-up for all study participants, increasing to a mean (SD; range) 30.57% (6.97; 23.9-37.8) at week 6 that remained stable and in the nonhemophilia range from year 1 (40.77% [9.45; 31.3-50.2]) to year 5 (45.7% [6.18; 39.0-51.2]). ∗HemosIL SynthASil reagent. aPTT, activated partial thromboplastin time.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/9/14/10.1182_bloodadvances.2024015291/2/m_blooda_adv-2024-015291-gr2.jpeg?Expires=1764955843&Signature=MOff~VpIGCScfZie0WxtzT2ki6oo2VqMhRFzH5nEhRztLox~4W0I2xa4gpB7sUnhqWWwgcfee2o~ZaUdKrr2S-P1Q6U7eYYRppMlrmxuOt3NS-8w9RvJuO94WSvZcljtACfBwYfptTz2mzKnj~wPlto9PpHW24NlBmvl8ubJhpItdfCha~ndApDBqLsFap77pHnyTTChDWQXSSA5BcBJgOhjMq9xal7~xAOcsCvVoAxhtf0dQnYGuc2y-~sbfEQPMX62Q0MSy579yZqalM16xB5ePz9Y7NrLfK8JxBBUILwX2h-xmmxUC9bsj-1SO1iuQPgMfeCcqSBO8O1A~3rLsQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)